Abstract
JCV causes progressive multifocal leukoencephalopathy (PML) in immunocompromised patients. The mechanism of JCV reactivation and immunity in a transplanted immune system remains unclear. We prospectively studied 30 patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT), and collected blood and urine samples pre-HSCT, 3, 6, and 12–18 months after HSCT. Pre-HSCT, JCV DNA was detected in 7/30 urine, 5/30 PBMC and 6/30 plasma samples. While JC viruria remained stable after HSCT with detection in 5/21 samples, viremia was detected in only 1/22 plasma and none of 22 PBMC samples 12–18 months after HSCT. Prevalence of anti-JCV IgG was 83% pre-HSCT and decreased to 72% at 12–18 months. Anti-JCV IgM was rarely detected. JCV-specific CD4+ and CD8+ T cell responses increased 12–18 months after HSCT. While JC viruria correlated directly with detection of anti-JCV IgG, the cellular immune response to JCV measured by ELISpot was inversely correlated with anti-JCV IgG response. The diagnosis of acute myelogenous leukemia and age groups were two independent patient factors associated with significantly reduced cellular immune responses to JCV. This prospective study in HSCT patients provides a model of interactions between the host immune response and viral activation in multiple compartments during the recovery of the immune system.
Introduction
JC virus (JCV) causes progressive multifocal leukoencephalopathy (PML) in immunocompromised patients (1, 2). Up to 80% of the general populations is seropositive for JCV and both the humoral and cellular immune responses are necessary for containment of viral proliferation (3, 4). Thus, immunocompromised patients, including those with hematological malignancies requiring allogeneic hematopoietic stem cell transplantation (HSCT), are at increased risk for developing PML. Indeed, PML was initially described in 3 patients with hematological malignancies in 1958 (5). Currently, many more patients survive HSCT due in part to improved long-term immunosuppression treatment they receive post transplantation. Among all published reports of transplant recipients with PML, HSCT patients make up the largest group; up to 8% of PML patients have hematological cancers (6, 7). The incidence rate of PML in patients with HSCT was estimated at 35.4 in 100,000 person-years (8). Furthermore, PML can develop as early as 1.5 months or as late as years after transplantation and is associated with myeloablative conditioning regimen used to wipe out the HSCT recipient cells in preparation for transplantation (7, 9). The median survival time for HSCT recipient with PML is less than 2 years (7). Thus, PML is devastating in HSCT patients as there is no effective therapy for this disease.
While studies have examined the host immune responses to JCV in patients with PML, little is known of the host-viral interactions prior to PML onset (10-12). Of importance, better understanding of how the host immune responses control viral proliferation is crucial in order to prevent the development of PML. Even though the cellular immune system cannot eradicate chronic viruses, immune surveillance prevents active infection under normal immune conditions. Reactivation of chronically latent viruses remains a major complication after HSCT(13). It is unclear when JCV reactivation occurs or, in HSCT, how the transplanted immune system interacts with JCV in the infected host to maintain viral latency. Thus, we designed a prospective study to analyze host immune responses to JCV prior to HSCT and examine the dynamic changes as the transplanted immune system reconstitutes and expands its anti-viral armamentarium.
Methods
Study subjects and samples
This study was approved by the Dana Farber Harvard Cancer Center Institutional Review Board. Adult patients were enrolled consecutively from April 2008 to July 2010 as they presented for allogeneic HSCT at Beth Israel Deaconess Medical Center. Thirty healthy volunteers were also enrolled. All subjects were consented to the study. Blood and urine samples were obtained pre-HSCT, 3 months, 6 months, and 12–18 months post-HSCT. Plasma and peripheral blood mononuclear cells (PBMC) were isolated as previously reported (12). Aliquots of PBMC, plasma and urine were stored at −80°C for DNA extraction.
DNA Extraction and Quantitative PCR (qPCR) for JCV
Total DNA was extracted from PBMC using the QIAamp DNA Blood Mini Kit (Qiagen, CA) and from plasma and urine samples using the Qiagen MinElute kit following the manufacturer’s instructions. JCV DNA was detected and quantified by quantitative PCR (qPCR) using standard TaqMan assay conditions and Large T primers as previously described (14). Each sample was run in triplicate, on an ABI 7300 Real-time PCR System. JCV DNA viral loads in PBMC were expressed in copies per μg of DNA used for qPCR, and in plasma and in urine were expressed in copies per ml of urine or plasma used for extraction. A sample was considered positive if at least 2/3 replicate wells showed positive amplification with a limit of detection of 188 copies per ml for urine and plasma and 10 copies per μg for PBMC.
Cellular Immune Response to JCV
a) Intracellular Cytokine Staining
After 10–14 days in culture with JCV VP1 peptides, 1×106 lymphocytes were incubated in RPMI 1640 with 12% FBS medium, with a VP1 peptide pool (2 μg/ml), or with PMA and ionomycin (1 μg/ml and 5 μg/ml, respectively) at 37°C for 6 hour s. After the first hour, all samples received monensin (GolgiStop; BD Bioscience). Cells were stained with fluorescently conjugated antibodies specific for human CD4 (clone L200) and CD8 (clone SK1), then fixed, permeabilized (BD Cytofix/Cytoperm) and stained with antibodies specific for IFN-γ (clone B27) and CD3 (clone SK7). All antibodies were obtained from BD Biosciences. Data were acquired on a FACS Calibur flow cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar Inc.). ICS result was considered positive when the percentage of IFN-γ producing CD4+ or CD8+ T cells was equal to or greater than two times the baseline value. The ICS results were reported after subtraction of baseline value as previously described (12).
b) ELISpot
1×105 lymphocytes were incubated on 96-well MultiScreen HTS plates (Millipore) coated with anti-human IFN-γ (5 μg/ml) in RPMI 1640 with 12% FBS medium, with a VP1 peptide pool (2 μg/ml), or with phytohemagglutinin (PHAM) (10 μg/ml) at 37°C overnight. After washing, the plates were incubated with anti-human IFN-γ biotin (Biosource) for 2 hours at room temperature, washed, and incubated with streptavidin (Southern Biotechnology). The plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphatechromogen (Pierce) and analyzed on an Immunospot Analyzer (Cellular Technology Limited). Results were considered positive when the number of spot forming units (SFU) was greater than 50 per 106 cells after subtraction of baseline, and greater than three times the baseline value. Results were reported after subtraction of baseline values.
c) Tetramer Staining
Tetramer stainings were performed in human leukocyte antigen (HLA)-A*0201+ study subjects. Lymphocytes were stimulated with the HLA-A*0201-restricted JCV VP1 epitopes, VP1p36 or VP1p100, and stained with a fluorescently conjugated tetramer specific to HLA-A*0201/VP1p36 or HLA-A*0201/VP1p100 as previously described (15). Data were acquired on a FACS Calibur flow cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar Inc.).
Humoral Immune Response to JCV
Anti-JCV IgM, IgG, and IgA were quantified by ELISA as previously described (16). All samples were run in duplicate and the mean values are reported.
Statistical Analysis
Due to the longitudinal nature of the data which were collected at 4 time intervals of interest (pre-HSCT, 3 months, 6 months, 12 –18 months), we first used a smoothing cubic spline function to graphically examine the underlying trend of the dependent variables (e.g. percentages of JCV-specific CD4+ T– lymphocytes) through time. Potential nonlinear effect of time was visualized especially at the last time interval. The 4 different categorical study time points were used to model the time effect. The within-subject correlation in the data was taken into account in the modeling using linear mixed-effects model. We used compound symmetry or AR as the variance-covariance structure. We also adjusted for the potential confounders with the 6 covariates (transplant type, conditioning regimen, graft versus host, presence of concurrent viremia, age, diagnosis of acute myelogenous leukemia) in the linear mixed-effects models. We found that the dependent variables were largely non-normal, and the covariates to be mostly statistically non-significant; therefore, we also used nonparametric test, Kruskal—Wallis, to assess the effect of time. The inference on the time effect was similar in both the parametric (with linear mixed-effects model), and nonparametric analysis. We chose to report the results from the nonparametric tests. We also used Spearman correlation coefficients to assess the association between JC viremia, viruria, and cellular and humoral immune response parameters. We used the SAS software version 9.12 for all of the analyses.
Results
Subjects characteristics
We enrolled a total of 30 patients undergoing allogeneic HSCT at Beth Israel Deaconess Medical Center. Patients were followed up to 18 months after HSCT. Two patients died in the initial 100 days after transplantation, and 5 other patients passed away prior to reaching the last study time point. No patient developed PML. Two-thirds of these patients were male and the most frequent indication for HSCT was acute myelogenous leukemia (Table 1). While 6 patients had umbilical cord transplantation, others had matched unrelated or matched related donors. The majority (22/30) had myeloablative conditioning. High resolution HLA typing was performed on all study subjects. Fourteen patients were HLA-A*0201 positive, for which we have previously mapped JCV immune-dominant epitopes. Tetramer staining assays for these epitopes were performed on samples from the HLA-A*0201 positive patients. Donor leukocytes were obtained and tested for the presence of JCV DNA by qPCR. JCV was detected at a quantity below our limit of detection in only one donor sample. The recipient of this sample did not have any detectable JCV DNA in blood or urine samples. Eleven patients had evidence of active viral replication during the course of the study, including 7 with cytomegalovirus (CMV) in blood, 3 with Epstein Barr virus (EBV) in blood, 2 with varicella zoster (VZV) reactivation in skin, 1 with human herpes virus 6 (HHV6) in cerebral spinal fluid (CSF), and 1 detection of parainfluenza virus from bronchial lavage. Two patients had both CMV and EBV and 1 had both EBV and VZV.
Table 1. Study subjects characteristics.
| Number of study subjects |
|
|---|---|
| Indication for transplant | |
| Acute Myelogenous Leukemia | 13 |
| Multiple Myeloma | 6 |
| Chronic Lymphoid Leukemia | 3 |
| Chronic Myeloid Leukemia | 2 |
| Non Hodgkin’s Lymphoma | 2 |
| Acute Lymphoid Leukemia | 1 |
| Others | 3 |
|
| |
| Gender | |
| Male | 20 |
| Female | 10 |
|
| |
| Types of transplant | |
| Matched Unrelated Donor | 13 |
| Matched Related Donor | 11 |
| Umbilical Cord Blood | 6 |
|
| |
| Conditioning regimens | |
| Myeloablative | 22 |
| Reduced Intensity | 8 |
|
| |
| GVHD | |
| GVHD during study period | 11 |
| No GVHD during study period | 19 |
|
| |
| Concurrent Viremia | |
| Viremia during study period | 11 |
| No viremia during study period | 19 |
GVHD: Graft versus host disease; Concurrent viremia: study subjects with diagnosis of another viral infection during the study period, including herpes simplex, Epstein Barr virus, cytomegalovirus, and parainfluenza.
Decreased prevalence of JC viremia post HSCT
Blood and urine samples were collected at 4 different time points: pre-HSCT (median —27, range −77 to −6 days); 3 months (median 97, range 100 to 120 days); 6 months (median 183, range 121 to 220 days), 12–18 months (median 387, range 221 to 529 days) post-HSCT. Pre-HSCT, JCV DNA was detected in 7/30 (23%) urine, 5/30 (17%) PBMC, 6/30 (20%) plasma samples (Table 2). Fifteen (50%) of the 30 study patients had detectable JCV DNA in at least one sample in either the plasma or in the PBMC during the time of the study. In plasma, the number of patients with detectable JCV DNA decreased after transplantation to 4 (18%) at 3 months, zero at 6 months and one patient (5%) at 12–18 months (DNA viral load mean 565, range 270 to 2,900 copies/ml). In PBMC, the detection of JCV DNA peaked at 19% at 6 months after transplant and also decreased at 12–18 months (DNA viral load mean 56, range 17 to 250 copies/μg). While JCV DNA was detected in plasma and PBMC of different patients in our study, it is usually not detected in the blood of healthy individuals (17). Detection of JCV DNA in urine at the 4 study time points ranged from 23–39% (DNA viral load mean 2.8 × 107, range 200 to 3.2 × 108 copies/ml), similar to the detection frequency in healthy individuals (18). Fourteen (47%) of the 30 patients had at least one urine sample positive for JCV DNA during the study period. Of these, 6 patients (43%) had detectable JCV DNA in at least 3 if not all 4 study time points. Detection of JCV DNA in either blood or urine of these patients was not correlated with any clinical symptom of viral infection or the diagnosis of GVHD, and did not affect post transplant survival in this cohort.
Table 2. Prevalence of JCV DNA in blood and urine, anti-JCV antibodies, and cellular immune responses.
| JCV DNA Detection by qPCR | Anti-JCV Antibodies | Cellular Immune Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| ICS | ELISpot | |||||||
| Serum | PBMC | Urine | IgM | igG | CD4+ | CD8+ | ||
| Number of positives / total numbers tested (%) | ||||||||
|
Pre
Transplant |
6/30 (20%) | 5/30 (17%) | 7/30 (23%) | 2/30 (7%) | 25/30 (83%) | 15/23 (65%) | 14/23 (61%) | 12/19 (63%) |
| 3 Months | 4/22 (18%) | 2/22 (9%) | 9/23 (39%) | 1/23 (4%) | 19/23 (83%) | 12/15 (80%) | 6/15 (40%) | 10/13 (77%) |
| 6 Months | 0/21 | 4/21 (19%) | 7/19 (37%) | 2/21 (10%) | 15/21 (71%) | 13/20 (65%) | 13/20 (65%) | 3/5 (60%) |
|
12–18
Months |
1/22 (5%) | 0/22 | 5/21 (24%) | 1/22 (5%) | 16/22 (73%) | 22/23 (96%) | 17/23 (74%) | 14/15 (93%) |
qPCR: quantitative polymerase chain reaction; PBMC: peripheral blood mononuclear cells; ICS: intracellular cytokine staining; ELISpot: enzyme linked immune spot.
JCV-specific humoral immune response remains stable over time post HSCT
While the prevalence of JCV-specific IgG (IgG) remained stable from 83% pre HSCT to 72% at 12–18 months, JCV-specific IgM (IgM) were rarely detected (Table 2). Prior to HSCT, 2 out of 30 patients had detectable IgM. While both of these patients had detectable JCV DNA in their urine at all study time points, only one had detectable JCV DNA in PBMC pre-HSCT, and neither had detectable JCV DNA in plasma at any study time point. One patient, who had detectable JCV DNA in PBMC pre-HSCT, became IgM positive at 3 months post-transplant and remained IgM positive at 6 months post-transplant. There was no serum available at 12–18 months for this patient. One patient became IgM positive at 6 months and another patient became IgM positive at the 12–18 months study point. All the patients who were IgM positive were also IgG positive, indicating viral reactivation rather than a primary infection. IgG quantity fluctuated above and below the threshold for positive response throughout the study period. One patient remained negative at all four time points despite detectable JCV DNA in the serum 3 months post-transplant. This is an example of a detectable JCV infection in a seronegative individual as reported by others (19). Four patients with negative IgG pre transplant became IgG positive in later study time points. Anti-JCV IgA (IgA) was positive in one subject at 3 months and in 2 different subjects at 6 months post-transplant. Lastly, while there were no statistically significant quantitative changes in IgG, IgM, and IgA levels over the course of the transplant, the JCV-specific antibody response correlated well each other. Spearman correlation coefficients were significantly positive for IgG to IgM (0.29, p=0.004), IgG to IgA (0.26, p= 0.01), IgA to IgM (0.42, p=<0.0001).
JCV-specific cellular immune response increases over time post-HSCT
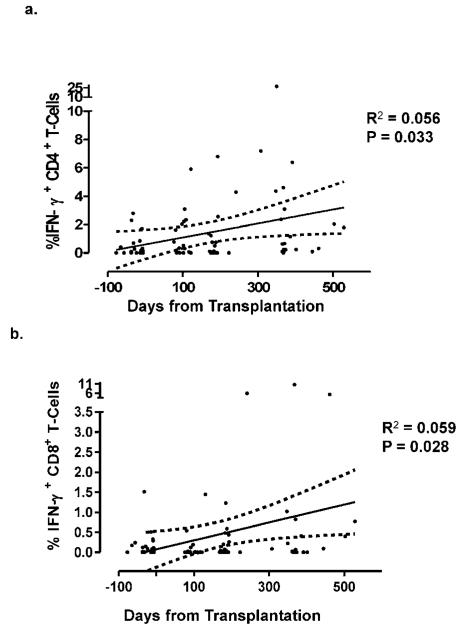
Qualitative analysis showed JCV-specific cellular immune response peaked at 12–18 months in both CD4+ T- cells and CD8+ T- cells as well as by ELISpot (Table 2). We analyzed the exact date of each sample collection and the percentage of IFN-γ-producing CD4+ and CD8+ T cells by ICS. The non-linear curve showed the quantitative increased percentage of JCV-specific CD4+ and CD8+ T cells over time (Figure 1).
Figure 1.
Increase of JCV-specific CD4+ and CD8+ T cells over time after transplantation.
The percentages of JC virus-specific CD4+ (a.) and CD8+ (b.) T cells increased after hematopoietic stem cell transplantation.
We next grouped the samples according to the 4 study time points, Pre-HSCT, 3, 6, and 12–18 months. The linear mixed-effects model with the 4 time points and 6 covariates showed the adjusted effect of time to be statistically significant (p = 0.012). Due to the small sample size of subjects with cellular or humoral immune response above baseline value, the distributions of the cellular and humoral immune response were highly non-normal. Small sample size also prevented us from having sufficient statistical power to assess the normality assumption using Shapiro-Wilk test. Therefore, we used the non-parametric Wilcoxon Rank Sum test to compare the distributions of these immune response variables. Due to the non-normality in the distribution of the dependent variable JCV-specific CD4+ and CD8+ T cells, and the non-significance of 4 of the 6 covariates, we also estimated the significance of the time effect using nonparametric Kruskal—Wallis test which yielded p-value of 0.004 for CD4+ T cells (Table 3A).
Table 3. Evolution of the cellular immune response to JC virus after HSCT.
| A. JCV-specific CD4+ T-cells | ||||
|---|---|---|---|---|
| Number of Subjects |
% IFN-γ+CD4+ T- cells (unadjusted mean ± std dev) |
Median (range) |
P (Kruskal- Wallis) |
|
|
Time from
transplant |
||||
| Pre Transplant | 23 | 0.55 ± 0.96 | 0 (0 – 5.44) | |
| 3 months | 15 | 0.89 ± 1.60 | 0.04 (0 – 7.83) | |
| 6 months | 20 | 1.01 ± 2.34 | 0 (0 – 13.43) | |
| 12 – 18 months | 23 | 2.91 ± 7.40 | 0.31 (0 – 44.12) | 0.0041 |
| Transplant type | ||||
| Cord | 6 | 1.02 ± 1.95 | 0 (0 – 8.21) | |
| MUD | 13 | 1.46 ± 3.28 | 0.03 (0 – 26.17) | |
| MRD | 11 | 1.48 ± 6.02 | 0.03 (0 – 44.12) | 0.54 |
| Conditioning | ||||
| MC | 22 | 1.00 ± 1.86 | 0.06 (0 – 9.32) | |
| RIC | 8 | 1.55 ± 4.90 | 0 (0 – 44.12) | 0.26 |
|
Graft versus host
disease |
||||
| No | 19 | 1.78 ± 5.13 | 0 (0 – 44.12) | |
| Yes | 11 | 0.88 ± 2.71 | 0.07 (0 – 26.17) | 0.76 |
|
Concurrent
viremia |
||||
| No | 19 | 1.57 ± 5.14 | 0.02 (0 – 44.12) | |
| Yes | 11 | 1.18 ± 2.83 | 0 (0 – 26.17) | 0.86 |
| Age | ||||
| <49 | 8 | 0.62 ± 1.14 | 0.01 (0 – 5.47) | |
| 49 – 58 | 8 | 2.48 ± 4.16 | 0.68 (0 – 26.17) | |
| 59 – 63 | 10 | 1.57 ± 6.20 | 0 (0 – 44.12) | |
| >63 | 4 | 0.53 ± 0.94 | 0 (0 – 3.84) | 0.0012 |
|
Diagnosis of
AML |
||||
| No | 17 | 1.61 ± 3.04 | 0.41 (0 – 26.17) | |
| Yes | 13 | 1.15 ± 5.43 | 0 (0 – 44.12) | <0.0001 |
| B. JCV-specific CD8+ T-cells | ||||
|---|---|---|---|---|
| Number of Subjects |
% IFN-γ+CD8+ T- cells (unadjusted mean ± std dev) |
Median (range) |
P (Kruskal- Wallis) |
|
|
Time from
transplant |
||||
| Pre Transplant | 23 | 0.16 ± 0.45 | 0 (0 – 3.26) | |
| 3 months | 15 | 0.06 ± 0.21 | 0 (0 – 1.40) | |
| 6 months | 20 | 0.25 ± 0.65 | 0 (0 – 4.32) | |
| 12 – 18 months | 23 | 1.12 ± 4.82 | 0 (0 – 33.96) | 0.11 |
| Transplant type | ||||
| Cord | 6 | 0.7 ± 3.14 | 0 (0 – 21.29) | |
| MUD | 13 | 0.54 ± 3.25 | 0 (0 – 33.96) | |
| MRD | 11 | 0.18 ± 0.57 | 0 (0 – 4.32) | 0.83 |
| Conditioning | ||||
| MC | 22 | 0.77 ± 3.41 | 0 (0 – 23.52) | |
| RIC | 8 | 0.31 ± 2.27 A | 0 (0 – 33.96) | 0.18 |
|
Graft versus host
disease |
||||
| No | 19 | 0.39 ± 2.56 | 0 (0 – 33.96) | |
| Yes | 11 | 0.50 ± 2.74 | 0 (0 – 23.52) | 0.90 |
|
Concurrent
viremia |
||||
| No | 19 | 0.52 ± 3.09 | 0 (0 – 33.96) | |
| Yes | 11 | 0.33 ± 1.86 | 0 (0 – 21.29) | 0.09 |
| Age | ||||
| <49 | 8 | 0.36 ± 2.11 | 0 (0 – 21.29) | |
| 49 – 58 | 8 | 1.13 ± 4.65 | 0 (0 – 33.96) | |
| 59 – 63 | 10 | 0.1 ± 0.28 | 0 (0 – 1.76) | |
| >63 | 4 | 0.08 ± 0.16 | 0 (0 – 0.67) | 0.08 |
|
Diagnosis of
AML |
||||
| No | 17 | 0.64 ± 3.18 | 0 (0 – 33.96) | |
| Yes | 13 | 0.2 ± 1.75 | 0 (0 – 21.29) | 0.003 |
HSCT: hematopoietic stem cell transplantation; Cord: umbilical cord blood transplantation; MUD: matched unrelated donor; MRD: matched related donor; MC: myeloablative conditioning; RIC: reduced intensity conditioning; AML: acute myelogenous leukemia.
We examined the distribution of age as a continuous variable relative to the immune response variables and found that age had potentially a non-linear association with these variables; therefore, we decided to categorize age into quartiles to capture the non-linear effect. Table 3A results showed the significant effect of age which could also be co-linear with the time effect. The diagnosis of acute myelogenous leukemia was also found to have significant negative effect on percentage of JCV-specific CD4+ T cells (p<0.0001). Interestingly, in this cohort, at the 12 — 18 months study time point the percentage of JCV-specific CD4+ T cells was significantly higher than that in healthy controls (p=0.0087). Conversely for JCV-specific CD8+ T cells (Table 3B), we found no significant effect of time; although, the mean estimates showed increasing trend at 6 month and 12–18- months post-HSCT compared to pre-HSCT and 3 months post-HSCT. Furthermore, the effect of age did not reach significance in JCV-specific CD8+ T cells. As in JCV-specific CD4+ T cells, we also found that the diagnosis of acute myelogenous leukemia had a negative effect on the mean percentage of JCV-specific CD8+ T cells (p=0.003). The transplant type, conditioning regimen, presence of graft versus host disease and concurrent viremia from other viruses had no significant influence on the CD4+ or CD8+ T-cell responses.
Tetramer staining for the previously mapped HLA A*0201 JCV-specific dominant epitopes VP1p36 and - p100 were performed on the blood samples of 14 HLA A*0201-positive patients. The results correlated to the presence of JCV-specific CD8+ T cells on ICS performed at the same time point (data not shown).
JC viremia correlated with selective increase in cellular immune response
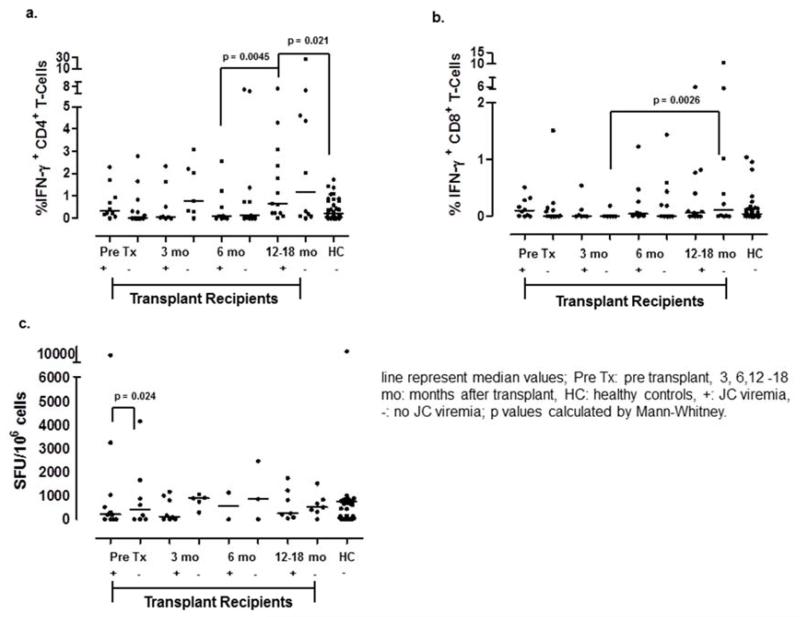
We performed stratified analysis based on the presence or absence of JC viremia. We divided the patients into the two groups (viremia (n=15) versus no viremia (n=15)) based on at least one detection of JCV in either PBMC or serum and analyzed their cellular immune responses at the four study time points (Figure 2). For comparison purposes, we included a previously published healthy control group (HC) (12). Overall, both ICS and ELISpot showed similar levels of JCV-specific cellular immune responses between the JC viremia and the no viremia groups at all study time points. The frequency of JCV-specific CD4+ T cells was significantly higher in the JC viremia group at 12–18 months compared to 6 months of the same group (p=0.045). Also, the JC viremia group 12–18 months after transplant had significantly more JCV-specific CD4+ T cells than those in the healthy control group (p=0.021). Conversely, JCV-specific CD8+ T- cell responses were significantly higher at 12–18 month in the no JC viremia group compared to 3 months (p=0.026). ELISpot demonstrated significantly stronger cellular immune response in the no viremia group pre-transplant as compared to the viremia group (p=0.024).
Figure 2.
Differences in JCV-specific CD4+ and CD8+ T cells responses in patients with or without viremia.
a.) JCV-specific CD4+ T cells significantly increased in the JC viremic subgroup at 12-18 months as compared to the same group at 6 months after transplantation.
b.) JCV-specific CD8+ T cells in the no JC viremia group at 12-18 months was higher than the same group at 3 months after transplantation.
c.) ELISpot demonstrated a significantly elevated T cell response in the subgroup without JCV viremia at the pre transplant time point as compared to those with JCV viremia.
While ICS separately assays CD4+ and CD8+ T- cell responses, ELISpot measures both responses together. JCV-specific T-cell responses by ELISpot correlated well to both JCV-specific CD4+ and CD8+ T- cell responses by ICS with Spearman correlation coefficients of 0.62 (p=<0.0001) and 0.33 (p=0.04), respectively. ICS CD4 results also correlated well to ICS CD8 results (0.28, p=0.01).
Correlations of JCV viruria, viremia, and immune responses
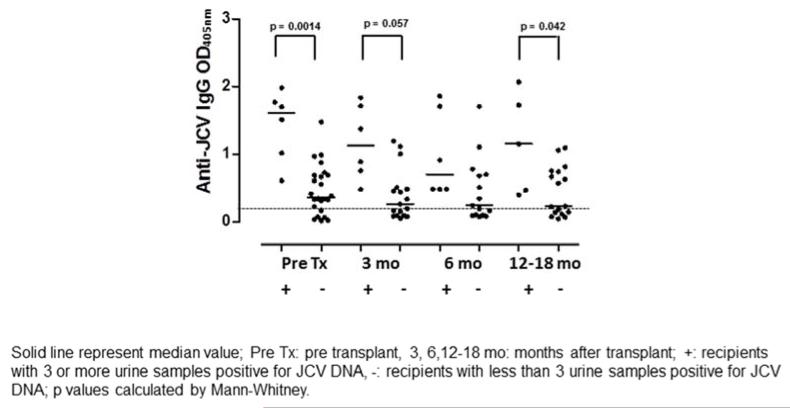
We next correlated the presence of JCV DNA in serum, PBMC, and urine to JCV-specific humoral and cellular immune responses. We detected significant positive correlations between JCV DNA load in the urine and the amount of IgG in the blood on all samples (Spearman coefficient = 0.46, p = <0.0001). Based on this finding, we further analyzed the 6 patients with with JC viruria in at least 3 of 4 study time points. Compared to all other study patients, these patients had IgG levels that were significantly elevated at the pre-transplant, 3 months, and 12–18 months (Figure 3). JCV DNA load in urine samples also correlated to the amount of IgA (Spearman coefficient = 0.24, p = 0.026). These data showed a strong association between JCV in the urine and elevated anti-JCV immunoglobulins, suggesting that JCV reactivation in the kidneys can trigger JCV-specific humoral immune response.
Figure 3.
Humoral immune response to JCV increase in patients with three or more JCV DNA positive urine samples.
Quantitatively higher levels of anti JCV-IgG were detected in the subgroup with three or more JCV positive urine samples at the pre transplant, 3, and 12 months after transplant study time points. Dashed line: cut-off for positive values (OD450nm=0.15)
In blood, we detected non-significant correlations. JCV DNA load in plasma negatively correlated with T-cell responses measured by ELISpot (Spearman coefficient = − 0.22, p = 0.12); and JCV DNA load in PBMV also negatively correlated to JCV-specific CD4+ T cells as measured by ICS (Spearman coefficient = −0.20, p = 0.08). These data showed that a weaker cellular immune response to JCV may be associated with the presences of JCV DNA in PBMC and plasma, which suggest that loss of JCV-specific cellular immune controls may result in JCV reactivation in blood.
We examined the relationships between humoral and cellular immune responses. While IgA and IgM did not have significant correlations with T-cell responses, IgG consistently inversely correlated with all T-cell responses with ELISpot (Spearman coefficient = −0.42, p = 0.0019), with ICS CD4+ T cells (Spearman coefficient = −0.20, p = 0.077), and with ICS CD8+ T cells (Spearman coefficient = −0.16, p = 0.14).
Discussion
This prospective study examines JC viral reactivation and the consequent host immune responses in a cohort of HSCT subjects. Following the same individual from pre-transplantation through at least one year after transplantation allowed us to analyze the progression of viral and host interactions in a maturing immune system. This provides an opportunity to observe JCV immune response in the initially immunosuppressed state and the dynamic progression to the restoration of immune system. In this study, we found that JCV does reactivate and the prevalence of JC viremia in patients undergoing HSCT was higher than in healthy individuals, while viruria remained comparable to those of the general population. Although the cellular immune responses to JCV were activated after bone marrow reconstitution, the transplanted adaptive immune system could not fully respond to JCV reactivation until 1 year after transplantation. While JCV reactivates independently in plasma, PBMC, and urine compartments, the presence of JCV in urine appeared to trigger a humoral immune response, and the development of JC viremia may be caused by a decrease in cellular immune response.
JCV reactivation in blood and urine of HSCT patients
The prevalence of JCV DNA in the blood of our cohort up to 6 months after bone marrow transplantation is similar to those described for HIV+ individuals without PML (17). Since JC viremia is usually not present in healthy individuals, the detection of JC viremia in HSCT patients in our study may indicate an increased risk of central nervous system seeding by JCV during systemic immunosuppression. Unlike in HIV+ individuals with AIDS, where immunosuppression generally lasts years, host immune responses in HSCT patients are most often restored by approximately one year after transplantation, shortening the total duration of immunosuppression and the consequent exposure of JCV. Therefore, the decreased exposure time to JC viremia may be why fewer HSCT patients develop PML as compared HIV+ individuals with AIDS.
It is unclear whether JCV exist in the blood as a cell-free or cell-associated virus, and whether it is attached to the cell surface or carried intracellularly. Therefore, we separately analyzed JCV detection in the serum and the PBMC samples, which showed that these constitute distinct and independent sites of viral latency and may be governed by different mechanisms of reactivation.
The decrease of JC viral load in blood was not observed in urine. Similar to prior studies, we also did not find any correlation between JCV reactivation in urine and in the blood compartments (17, 20). This suggests that JCV reactivation from latency in the kidneys tubular epithelial cells and bone marrow are independent.
JC viremia and viruria correlate with immune responses
This is the first study to follow a cohort of patients prospectively and analyze the detection of JCV in both the urine and the blood compartments and to correlate them to both host humoral and cellular immune responses. In the pre-transplant study time point, study subjects with no JC viremia had significantly higher JCV-specific CD4+ and CD8+ T-cell responses than those with JC viremia, as measured by ELISpot. These results suggest that hosts with a more efficient immune system can control JCV reactivation in blood. Furthermore, JCV detection is curtailed in both serum and PBMC in hosts with better overall immune responses, indicated by the significant rise of JCV-specific cellular immune responses as detected by ICS at 12–18 months study time point, where patients with JC viremia had significantly higher JCV-specific CD4+ T-cell response than those detected in healthy controls, who do not have detectable JCV DNA in blood. However, despite the above differences in cellular immune responses by the JC viremic group, the majority of the time, patients with JC viremia did not have significantly different levels of cellular immune responses than patients without viremia. This may be due to the small sample size of our study. Alternatively, this may also indicate that while HSCT restores host immune responses, the cellular immune response, especially CD8+ T cells, is less effective than in healthy individuals. In addition, the lack of IFN-γ expression on CD4+ or CD8+ T cells in those patients with JCV viremia may reflect a Th2 response (21). Future studies analyzing IL-10 and other Th2 cytokines response in these patients will expand our understanding of JCV-specific cellular immune responses.
While the detection of JCV DNA in the urine remained relatively constant throughout the course of bone marrow transplantation, the JC viral load in urine correlated directly with the quantity of both IgG and IgA. Study subjects with JCV in at least three urine samples had significantly higher quantities of IgG than those with fewer urine samples positive for JCV at three out of four study time points — indicating JCV activity in the kidney may trigger humoral immune response. Furthermore, the humoral immune responses were not influenced by JC viremia, indicating that these responses were not able to control and stop JC viral replication.
Clinical factors associated with JCV-specific cellular immune response
We have attempted to identify risk factors that significantly influence JCV-specific cellular immune response in this cohort of study subjects. Our data demonstrate that both CD4+ and CD8+ T-cell responses dramatically increase with time from transplantation. Furthermore, the recovery of CD4+ T-cell responses to JCV occurs earlier and is stronger than those of CD8+ T cells. Age, as separated into the four groups in our study, was identified as a significant factor, where those younger than 49 years and those older than 63 years have a lower CD4+ T-cell-mediated cellular immune response to JCV. This could be due to that patients in these two groups undergoing HSCT may have serious malignancies with immune dysfunction, even after transplantation. In addition, immune-senescence may also play a role in the low cellular immune responses to JCV in the older age group. Lastly, those patients with acute myelogenous leukemia have difficulties generating JCV-specific cellular immune responses, indicating the severe immunosuppression associated with this disease.
Correlations between humoral and cellular immune responses
This is the first study to measure both cellular and humoral immune responses against JCV at the same time points, allowing for analysis of correlations between these two arms of the immune responses. We used both ICS and ELISpot assays for detection of JCV-specific cellular immune responses. While the strong correlations of the data obtained from both assays indicate consistency and reproducibility of our data, each assay also provided unique insights to the dynamic changes of JCV-specific cellular immune response in the course of HSCT. The JCV-specific cellular immune response, as tested by both ICS and ELISpot, correlated negatively with anti-JCV IgG. This suggests that the cellular immune response is able to control JCV replication, and consequently decrease the overall antigenic stimulation, resulting in the associated decrease in production of IgG.
Future studies
The consecutive enrollment of patients who present for bone marrow transplantation allows for a realistic representation of the at-risk population. However, the design includes heterogeneity in the study population. Our cohort of patients had different types of hematologic malignancies, bone marrow sources, and different conditioning regimens. While we attempted to correlate host immune responses to the presences of JCV DNA in the same study time point, the expanse of our four study time points did not allow for examination of time lag influences of viral reactivation on immune responses. In addition, while use of steroid and other immunosuppressants can contribute to host immune responses, our cohort was too small to examine this question. Future studies examining JCV-specific immune responses in a more homogenous population with closer time points may offer further insights to factors that are associated with robust responses. Furthermore, the total T cell counts or the amount of IgG in each patient was not examined in this study because we chose to focus on the specific anti-JCV functions of both B and T cells. Future studies examining total IgG levels and T cell counts along with functional studies will help us understand better the kinetics of immune responses post transplantation.
Conclusions
In this prospective study in HSCT patients, we detected JCV viral reactivations in blood and urine, analyzed JCV-specific humoral and cellular immune responses, and correlated the findings. Our data demonstrated that JC viremia is prevalent in HSCT patients even prior to transplant and does not curtail until 12–18 months after transplant. Furthermore, while the humoral immune responses are linked to the presence of JCV in urine, these responses cannot control viral replications in blood or urine. In addition, the cellular immune responses were not fully available until close to one year after transplant. Therefore, HSCT patients are at increased risk for JC viral dissemination to the brain and development of PML, especially those with acute myelogenous leukemia, and those in the older and younger age groups.
Acknowledgement
This work was conducted with support from NIH grants R01 NS 047029 and NS 074995, NS R56 041198, and K24 NS 060950 to IJK, and NIH grant K08 NS 064215-01 to CST.
Footnotes
Financial disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferenczy MW, Marshall LJ, Nelson CD, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25:471–506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV) Adv Exp Med Biol. 2006;577:19–45. doi: 10.1007/0-387-32957-9_2. [DOI] [PubMed] [Google Scholar]
- 4.Egli A, Infanti L, Dumoulin A, et al. Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors. J Infect Dis. 2009 doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 5.Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leukoencephalopathy. Brain. 1958;81:93–127. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Koralnik IJ, Schellingerhout D, Frosch MP. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 14-2004. A 66-year-old man with progressive neurologic deficits. N Engl J Med. 2004;350:1882–1893. doi: 10.1056/NEJMcpc030038. [DOI] [PubMed] [Google Scholar]
- 7.Mateen FJ, Muralidharan R, Carone M, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol. 2011;70:305–322. doi: 10.1002/ana.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amend KL, Turnbull B, Foskett N, Napalkov P, Kurth T, Seeger J. Incidence of progressive multifocal leukoencephalopathy in patients without HIV. Neurology. 2010;75:1326–1332. doi: 10.1212/WNL.0b013e3181f73600. [DOI] [PubMed] [Google Scholar]
- 9.Pelosini M, Focosi D, Rita F, et al. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of literature. Ann Hematol. 2008;87:405–412. doi: 10.1007/s00277-007-0411-6. [DOI] [PubMed] [Google Scholar]
- 10.Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 11.Du Pasquier RA, Schmitz JE, Jean-Jacques J, et al. Detection of JC virus-specific cytotoxic T lymphocytes in healthy individuals. J Virol. 2004;78:10206–10210. doi: 10.1128/JVI.78.18.10206-10210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85:7256–7263. doi: 10.1128/JVI.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharfan-Dabaja MA, Ayala E, Greene J, Rojiani A, Murtagh FR, Anasetti C. Two cases of progressive multifocal leukoencephalopathy after allogeneic hematopoietic cell transplantation and a review of the literature. Bone Marrow Transplant. 2007;39:101–107. doi: 10.1038/sj.bmt.1705548. [DOI] [PubMed] [Google Scholar]
- 14.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121:217–221. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Tan CS, Broge TA, Jr., Seung E, et al. Detection of JC virus-specific immune responses in a novel humanized mouse model. PLoS One. 2013;8:e64313. doi: 10.1371/journal.pone.0064313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viscidi RP, Khanna N, Tan CS, et al. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin Infect Dis. 2011;53:711–715. doi: 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253, 260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz RB, Thompson HC, Mueller JF, Cohen JA, Dynan WS. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis. 1993;167:13–20. doi: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- 19.Major EO, Frohman E, Douek D. JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med. 2013;368:2240–2241. doi: 10.1056/NEJMc1214233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, et al. Comprehensive investigation of the presence of JC virus in AIDS patients with and without progressive multifocal leukoencephalopathy. J Med Virol. 1997;52:235–242. doi: 10.1002/(sici)1096-9071(199707)52:3<235::aid-jmv1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Perkins MR, Ryschkewitsch C, Liebner JC, et al. Changes in JC virus-specific T cell responses during natalizumab treatment and in natalizumab-associated progressive multifocal leukoencephalopathy. PLoS Pathog. 2012;8:e1003014. doi: 10.1371/journal.ppat.1003014. [DOI] [PMC free article] [PubMed] [Google Scholar]