Abstract
The purpose of this study was to explore the feasibility of using a Web-based tool to provide tailored symptom management strategies for persons living with HIV (PLWH) and to estimate the effect size of the tool for future studies. Testing the components of the Web-based system was done by incorporating a repeated measures design measuring the outcomes of symptom frequency and intensity, use of symptom management strategies, and engagement with health care providers. We recruited 42 PLWH; participants were enrolled in the study for 12 weeks and were asked to use the system and complete the questionnaires every 2 weeks. Our results showed that participants who used the strategies were more likely to have a decrease in symptom frequency and intensity. Findings from this feasibility study provide preliminary evidence for the use of a Web-based HIV symptom management tool with self-management strategies for individuals living with HIV infection.
Keywords: persons living with HIV, symptom frequency, symptom intensity, Web-based tools
In the United States, an estimated 1.1 million people are living with HIV due, in part, to successful medications to treat the disease. As a result, HIV has evolved from an acute illness to a chronic illness and may lead to a number of physiological and psychological symptoms that potentially impact the lives of people living with HIV (PLWH; (Centers for Disease Control and Prevention, 2009). Moreover, many HIV-related symptoms frequently go unrecognized by health care providers; they are often underreported by patients and are often undertreated (Hughes, 2004). Patients’ symptom experiences and symptom management are strongly related to HIV disease progression and clinical factors (Spirig, Moody, Battegay, & De Geest, 2005). For instance, the prevalence of individual signs and symptoms, as well as the intensity of the symptoms, influences patients’ decisions to seek care. Symptoms have also contributed to reduced adherence to medications, which increases the likelihood of resistance to medication regimens and exacerbation of symptoms (Siegel, Schrimshaw, & Dean, 1999). These factors may also reduce a person’s quality of life (Lorenz, Cunningham, Spritzer, & Hays, 2006).
Much work has documented the use and relevance of various symptom management strategies for PLWH. This work includes fatigue (Corless et al., 2002), neuropathy (Nicholas et al., 2007), anxiety (Kemppainen et al., 2006), body changes associated with lipodystrophy (Nicholas, Kirksey, Corless, & Kemppainen, 2005), depression (Eller et al., 2005), and quality of life (Holzemer, Hudson, Kirksey, Hamilton, & Bakken, 2001). In a review of self-management of chronic illness, investigators reported that patient education and skills for self-management were both physically and psychologically beneficial (Coster & Norman, 2009).
While much work has documented the success of paper-based tools for symptom management, Web-based behavior change interventions offer an innovative strategy for symptom self-management. Information technology makes tailored communication possible by allowing for individual-specific information to be provided to each patient (Kreuter, Oswald, Bull, & Clark, 2000). Behavior change interventions can be effectively communicated by Web-based applications, and the interventions are at least as effective as traditional face-to-face and paper-based behavior change interventions (Wantland, Portillo, Holzemer, Slaughter, & McGhee, 2004). The purpose of our study was to explore the feasibility of using a Web-based tool to provide tailored symptom management strategies for PLWH and to estimate the effect size of the tool for future studies.
Methods
Study Design
We conducted a repeated measures study to test the components of the new Web-based system on symptom frequency and intensity, use of symptom management strategies, and engagement with health care providers. We hypothesized that after exposure to the system for 12 weeks, there would be a decrease in symptom intensity and frequency; these were our primary outcomes. In addition, for secondary outcomes we hypothesized that after use of the system during the study period, there would be an improvement in (a) patient perceptions about engaging with their health care providers, (b) adherence, and (c) quality of life measures.
Description of the System
We built a Web-based symptom management tool for PLWH based on a symptom management manual validated by researchers at the University of California at San Francisco (UCSF) School of Nursing. The paper-based manual was tested in a randomized controlled trial to determine how well nurses using the manual could assess the symptom frequency and intensity for 775 PLWH for 3 months. Findings from the study showed improved helpfulness rating scores in participants who used the symptom management manual and a greater significant reduction in symptom intensity scores compared to the control group (Wantland et al., 2008).
Using the paper based-manual, which included strategies for 21 symptoms, we developed and iteratively refined a Web-based Symptom Self-Management tool for PLWH (UCSF, 2004). Due to funding constraints, the Web-based system provided tailored strategies for only six symptoms: (a) depression, (b) anxiety, (c) fatigue, (d) diarrhea, (e) neuropathy, and (f) nausea. To develop the system, we first did usability testing through a heuristic evaluation with five experts in human computer interaction (Schnall et al., 2011). Following that process we conducted end-user usability testing with PLWH. Findings from the usability testing were used to refine the user interface and functionality of the system. The goal of the usability testing was not to refine the content of the symptom management strategies. Based on the feedback from the usability testing we refined the system and launched the Web-based tool for a 12-week feasibility study to obtain effect size estimates on the change in symptom frequency and intensity.
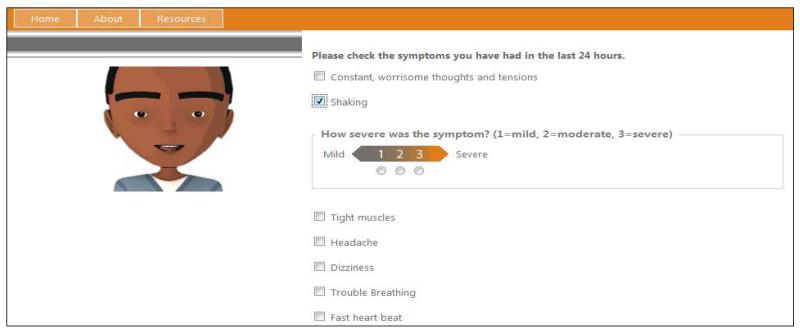
The system was designed so that once users logged in, they were guided by an avatar through a series of questions ascertaining the nature and severity of their symptoms (Figure 1). Once the evaluation was completed, the avatar would recommend self-management strategies for managing specific symptoms, including anything from taking a nap to changing the diet or contacting a care provider. Visit information was stored in the database and, upon the next login, further tailored questions and responses were provided based on the change in symptom intensity or frequency.
Figure 1.
Screenshot of Web-based symptom management tool.
Study Procedures
Following institutional review board approval, we recruited 42 participants from the adult HIV clinic at NewYork Presbyterian Hospital in the Washington Heights community of New York City. We placed posters describing the study around the clinic and potential subjects contacted the project coordinator via telephone or email to set up a mutually acceptable time for the consent process and access to the Web applications as needed. Participation was voluntary and in no way affected the care of any participant.
Participants were enrolled in the study for 12 weeks and were asked to use the system and complete the questionnaires every 2 weeks. Reminder emails were sent to participants every 2 weeks. The initial use of the Web-based system took about 1 hour. Subsequent use of the system took about 30 minutes per visit per participant. Participants were able to use the system from home or could use computers at our study site to access the system.
Sample
Inclusion criteria were that participants: (a) were able to read and write in English, (b) provide written informed consent, and (c) were at least 18 years of age. Participants needed to be comfortable using a computer as operationalized by their willingness to participate in the study and use a computer. Exclusion criteria were an inability to communicate in English and cognitive delay as defined by the inability to provide written informed consent.
Study Measures
At the first visit, we collected demographic information on all of our participants including age, gender, race, educational background and health literacy using the Questions for Limited Health Literacy tool (Chew et al., 2008). We also asked participants about their HIV illness characteristics, including years living with HIV, an AIDS diagnosis, and other co-morbid conditions. Symptom intensity was assessed by the HIV Sign and Symptom CheckList-Revised (Holzemer et al., 2001). The checklist identified the 72 most commonly experienced symptoms and their intensity in HIV-infected adults. For purposes of our study, we only included symptom checklist items that were related to the six symptoms included in our Web-based system. Each of the symptoms was scored on a scale of 0 to 3. A score of 0 indicated that in the previous 24 hours, the participant had not experienced that symptom, and a score of 3 that the participant experienced that symptom severely. The mean daily symptom intensity score was computed by summing each symptom intensity score and dividing by the total number of items related to the symptom category (e.g., depression). The total score reliability estimate in previous studies was 0.97 (Webel, 2010).
In addition, we administered the Engagement with Health Care Provider Scale, scoring on this scale is such that a lower score indicated improved patient/provider engagement (Bakken et al., 2000), the modified AIDS Clinical Trials Group (ACTG) adherence questionnaire (Chesney et al., 2000; Holzemer et al., 2006), and the Medical Outcomes Study Short Form-12 health survey (SF-12; (Ware, Kosinski, & Keller, 1996) at the first visit and all subsequent visits. The SF-12 was comprised of the following scales: physical functioning, role limitations due to physical health, role limitations due to emotional health, energy, emotional well-being, general health, and social functioning.
Data Analysis
Responses to the questionnaires were entered into SAS version 9.2 software (SAS Institute Inc., Cary, NC). http://www.jpsmjournal.com/article/S0885-3924(08)00133-4/fulltext-bib14 Descriptive statistics (i.e., means, standard deviations, frequencies, and percentages) were used to examine demographic characteristics of the sample and the frequency and intensity of the symptoms. We created a symptom summary score, which included the six symptoms in our study, and individual symptom scores for each of the six symptoms.
Following analysis of the demographic characteristics, we calculated model-based estimated mean values with standard errors at week 0 and week 12 for all outcome variables. The model-based estimates were calculated using a generalized estimating equation (GEE) model with a negative binomial distribution for symptom frequency and a GEE linear model for symptom intensity scores over time. The GEE procedure was a convenient and general approach that allowed for analysis of repeated measurements or other correlated observations for both continuous and categorical outcomes (Liang & Zeger, 1986).
Ongoing measurement enabled us to assess the stability of patient self-management processes and the predictability of outcomes controlling for demographic and illness characteristic differences. This method provided average score estimates at different time points, which were adjusted by between-subject differences. These repeated-measures data allowed us to describe changes in symptom experience over time as well as to asses any net benefits in the patients’ reports about engaging with their health care providers and adherence to their medications.
Results
Study Sample
The mean age for the sample participants (n = 42) was 50.0 years (SD = 11.3; range = 26-66 years of age); 66.7% (n = 28) were male, 31.0% (n = 13) female, and 2.4% (n = 1) transgender male to female. Forty-six percent (n = 31) of participants were African American/Black, 1.5% (n = 1) White, 1.5% (n = 28) multiracial, and 13.4% (n = 9) self-described as other. Education background included 19.4% who had completed eleventh grade or less, 28.4% who had a high school education, and 15% who had completed at least a 2-year college degree. The HIV illness characteristics found the mean years living with HIV to be 15.6 years (SD = 6.6); 40.5% (n = 17) had an AIDS diagnosis; 15.2% (n = 10) reported also being infected with hepatitis C; 27.3% (n = 18) reported hypertension; and 27.3% (n =18) reported depression.
Symptom Frequency and Intensity
At baseline, the most frequently reported symptom was fatigue, followed by anxiety. Using a negative binomial model, we found a trend toward a decrease in symptom frequency over time for all symptoms except diarrhea (Table 1). The estimated effect sizes, with standard errors, are presented in Tables 1, 2, and 3. For symptom frequency, the effect size was calculated as the ratio of symptom frequency (i.e., relative risk) at week 12 divided by frequency at week 0 (baseline). For example, the average number of all symptom frequency at week 0 and week 12 was 7.94 and 2.97, respectively. Therefore, the estimated effect size was 0.37. For symptom intensity score, the effect size was calculated as the difference of intensity score between week 12 and week 0. The average symptom intensity at week 0 and week 12 was 12.50 and 4.10, respectively. Therefore, the estimated effect size was −8.41. Using a linear model, we found a trend toward a decrease in symptom intensity over time for all symptoms except diarrhea (Table 2).
Table 1. Model-based Estimated Means for Symptom Frequency.
| Variable | Week 0 | Week 12 | Effect size (RR) |
Standard Error |
|---|---|---|---|---|
| All | 7.94 | 2.97 | 0.37 | 0.19 |
| Neuropathy | 0.67 | 0.39 | 0.58 | 0.26 |
| Nausea | 0.11 | 0.08 | 0.68 | 0.53 |
| Fatigue | 2.01 | 0.78 | 0.39 | 0.26 |
| Anxiety | 2.28 | 1.13 | 0.49 | 0.14 |
| Depression | 2.14 | 0.87 | 0.41 | 0.18 |
| Diarrhea | 0.08 | 0.11 | 1.42 | 1.47 |
Note. A smaller effect size suggests that a larger sample will be needed in future studies to detect a significant difference from the intervention. RR = relative risk
Table 2. Model-based Estimated Means for Symptom Intensity.
| Variable | Week 0 | Week 12 | Effect size (difference) |
Standard Error |
|---|---|---|---|---|
| All | 12.50 | 4.10 | −8.41 | 4.09 |
| Neuropathy | 1.38 | 0.68 | −0.70 | 0.44 |
| Nausea | 0.12 | 0.10 | −0.02 | 0.07 |
| Fatigue | 3.29 | 0.86 | −2.43 | 1.20 |
| Anxiety | 3.62 | 1.52 | −2.09 | 0.93 |
| Depression | 3.92 | 0.88 | −3.04 | 1.60 |
| Diarrhea | 0.13 | 0.18 | 0.04 | 0.18 |
Note: A smaller effect size suggests that a larger sample will be needed in future studies to detect a significant difference from the intervention.
Table 3. Model-based Estimated Means for Secondary Outcome Measures.
| Variable | Week 0 | Week 16 | Effect size (difference) |
Standard Error |
|---|---|---|---|---|
| Physical functioning | 68.54 | 76.06 | 7.52 | 7.85 |
| Role limitations due to physical health | 60.32 | 47.74 | −12.59 | 13.28 |
| Role limitations due to emotional health | 67.73 | 60.14 | −7.60 | 15.46 |
| Energy | 64.45 | 64.13 | −0.32 | 6.16 |
| Emotional well-being | 74.44 | 73.51 | −0.93 | 7.57 |
| General health | 63.46 | 64.93 | 1.48 | 4.24 |
| Social functioning | 73.54 | 75.63 | 2.09 | 6.83 |
| Engaging with health care provider | 18.65 | 20.24 | 1.59 | 1.31 |
| Adherence | 0.38 | 0.34 | −0.03 | 0.22 |
Note. A smaller effect size suggests that a larger sample will be needed in future studies to detect a significant difference from the intervention.
Quality of Life and Engaging with Health Care Providers
For these measures, the effect size was calculated as the difference of the score between week 12 and week 0. Compared to those who were exposed to the strategies, those who were not exposed to the strategies had scores on role limitations due to emotional problems, physical functioning, general health, and engaging with health care providers that were lower over time (Table 3). For example, the engaging with health care providers score was 18.65 at week 0 and 20.24 at week 12, so the estimated effect size was 1.59.
Compared to those who were exposed to the intervention, those who were not exposed to the strategies had scores on role limitations due to physical functioning, emotional well-being, and adherence that were higher over time. For example, the physical functioning score was 68.54 at week 0 and 76.06 at week 12, so the estimated effect size was 7.52.
Discussion
The information provided in our study demonstrated the feasibility of a Web-based tool for symptom management for PLWH. Our findings provided information that will help researchers and health care providers be more aware of self-management strategies that can help PLWH make informed choices (Balas et al., 1996). While computerized and Web-based programs and interventions have been made available for many chronic illnesses, including HIV, the long-term efficacy of such interventions are rare, failing to sustain successful behavior change outcomes (Grant et al., 2008). Thus, a goal of our research was to develop a tailored Web-based system that would encourage sustainable use.
Symptom frequency and intensity improved over time for our study participants with the exception of diarrhea. Diarrhea was measured by a single question, which may have limited its reliability. Likewise, our secondary outcome measures supported the use of the tool. In particular, engaging with health care providers improved, which was similar to earlier findings by Balas et al. (1996), who found that interactive patient instruction, education, and therapeutic programs helped individuals improve their health; at the same time, health care delivery processes were also improved.
Role limitations due to emotional problems and emotional well-being did not improve, which was surprising because symptoms related to depression and anxiety did improve. Further research is needed to understand the lack of congruence between the primary and secondary outcomes. Moreover, adherence did not improve in our study, which was unexpected given that in HIV treatment, adherence to a treatment plan has been associated with patients’ perceived levels of engagement and satisfaction with their providers. These findings, however, were consistent with a recent review suggesting that effective medication adherence interventions tended to require more than 12 weeks (Rueda et al., 2006). Moreover, our study participants were likely providing truthful reports of adherence because of evidence that participants in computer administered interviews perceive a Web-based survey to be more neutral and private than either face-to-face interviews or paper-based surveys; this can increase patients’ willingness to disclose non-adherence (Bangsberg, Bronstone, & Hofmann, 2002). Another possibility would be that studies have shown that available adherence measures have limitations, raising the possibility that our measure was not adequate (Liu et al., 2001).
The goal of our study was to determine whether the Web-based symptom management tool was a feasible intervention and appropriate for further testing. Findings from our study will enable researchers to assess whether or not the ideas and findings can be relevant and sustainable. Key areas of focus for feasibility studies include acceptability, demand, implementation, practicality, adaption, integration, expansion, and limited-efficacy testing. In our study, we demonstrated the acceptability, demand, and practicality of this intervention (Bowen et al., 2009).
These findings can be used by researchers to shape our tool into a sustainable intervention that can be further tested in a randomized controlled trial. One purpose of a feasibility study is to demonstrate the ability to recruit and retain study participants, which was successfully achieved in our project. We recruited 42 participants in 30 days, demonstrating the interest of PLWH in Web-based tools to address their HIV-related symptoms. Attrition in our study was similar to other studies with PLWH; 79.6% of participants completed the study. We oversampled our population because we had targeted a 25% attrition rate. It may be that those who dropped out of the study were no longer experiencing HIV-related symptoms.
Future studies with a larger sample size and a longer treatment time are needed to assess the potential effects of this Web-based symptom management system. The study limitations included a small sample from a single site. Our sample was a convenience sample and so may not be fully representative of the larger population of PLWH. In addition, because participants self-selected, our study sample may include those who are more willing to use Web-based technology than those in the general population. Finally, all of our surveys were self-administered and thus are subject to the biases inherent in this method of data collection. In particular, our study did not include validation of medical co-morbidities and HIV illness information.
Conclusion
Our study provides preliminary evidence for the use of a Web-based HIV symptom management system for PLWH. Overall, our results showed that participants who used the strategies were more likely to have a decrease in symptom frequency and intensity during a 12-week period of time. Importantly, the findings from this feasibility study showed that participants were willing to use the Web-based tool and the use of the targeted symptom-specific strategies produced a reduction in symptom frequency and intensity.
Key Considerations.
Web-based tools have the potential to allow patients to self-manage their symptoms.
Patients are interested in and willing to use the Web-based tool to manage their HIV-related symptoms.
Use of the targeted symptom-specific strategies can produce a reduction in symptom frequency and intensity.
Acknowledgments
This work was supported by: P30NR010677 (PI: Bakken) and P30 NR010677-03S1 (Feasibility Study PI: Schnall). Dr. Rebecca Schnall is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081, formerly the National Center for Research Resources, Grant Number KL2 RR024157. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Dr. Suzanne Bakken for her thoughtful review of this manuscript and Ms. Martha Rodriguez for her data collection efforts on behalf of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement. The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
Rebecca Schnall, Columbia University School of Nursing, New York, New York, USA.
Dean Wantland, Rutgers, The State University of New Jersey, College of Nursing, Newark, New Jersey, USA.
Olivia Velez, Columbia University Department of Biomedical Informatics, New York, New York, USA.
Kenrick Cato, Columbia University School of Nursing, New York, New York, USA.
Haomiao Jia, Clinical Biostatistics (in Nursing), Columbia University School of Nursing and School of Public Health, New York, New York, USA.
References
- Bakken S, Holzemer WL, Brown MA, Powell-Cope GM, Turner JG, Inouye J, Corless IB. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care STDS. 2000;14(4):189–197. doi: 10.1089/108729100317795. doi:10.1089/108729100317795. [DOI] [PubMed] [Google Scholar]
- Balas EA, Austin SM, Mitchell JA, Ewigman BG, Bopp KD, Brown GD. The clinical value of computerized information services. A review of 98 randomized clinical trials. Archives of Family Medicine. 1996;5(5):271–278. doi: 10.1001/archfami.5.5.271. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Bronstone A, Hofmann R. A computer-based assessment detects regimen misunderstandings and nonadherence for patients on HIV antiretroviral therapy. AIDS Care. 2002;14(1):3–15. doi: 10.1080/09540120220097892. doi:10.1080/09540120220097892. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Fernandez M. How we design feasibility studies. American Journal of Preventive Medicine. 2009;36(5):452–457. doi: 10.1016/j.amepre.2009.02.002. doi:10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cases of HIV Infection and AIDS in the United States and Dependent Areas, 2007. 2009 http://www.cdc.gov/hiv/topics/surveillance/basic.htm.
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. doi:10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Vanryn M. Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of General Internal Medicine. 2008;23(5):561–566. doi: 10.1007/s11606-008-0520-5. doi:10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corless IB, Bunch EH, Kemppainen JK, Holzemer WL, Nokes KM, Eller LS, Chou FY. Self-care for fatigue in patients with HIV. Oncology Nursing Forum. 2002;29(5):E60–E69. doi: 10.1188/02.ONF.E60-E69. doi:10.1188/02.ONF.E60-E69. [DOI] [PubMed] [Google Scholar]
- Coster S, Norman I. Cochrane reviews of educational and self-management interventions to guide nursing practice: A review. International Journal of Nursing Studies. 2009;46(4):508–528. doi: 10.1016/j.ijnurstu.2008.09.009. doi:10.1016/j.ijnurstu.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Eller LS, Corless I, Bunch EH, Kemppainen J, Holzemer W, Nokes K, Nicholas P. Self-care strategies for depressive symptoms in people with HIV disease. Journal of Advanced Nursing. 2005;51(2):119–130. doi: 10.1111/j.1365-2648.2005.03474.x. doi:10.1111/j.1365-2648.2005.03474.x. [DOI] [PubMed] [Google Scholar]
- Grant RW, Wald JS, Schnipper JL, Gandhi TK, Poon EG, Orav EJ, Middleton B. Practice-linked online personal health records for type 2 diabetes mellitus: A randomized controlled trial. Archives of Internal Medicine. 2008;168(16):1776–1782. doi: 10.1001/archinte.168.16.1776. doi:10.1001/archinte.168.16.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzemer WL, Bakken S, Portillo CJ, Grimes R, Welch J, Wantland D, Mullan JT. Testing a nurse-tailored HIV medication adherence intervention. Nursing Research. 2006;55(3):189–197. doi: 10.1097/00006199-200605000-00005. doi:10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Holzemer WL, Hudson A, Kirksey KM, Hamilton MJ, Bakken S. The revised Sign and Symptom Check-List for HIV (SSC-HIVrev) Journal of the Association of Nurses in AIDS Care. 2001;12(5):60–70. doi: 10.1016/s1055-3290(06)60263-x. doi:10.1016/S1055-3290(06)60263-X. [DOI] [PubMed] [Google Scholar]
- Hughes A. Symptom management in HIV-infected patients. Journal of the Association of Nurses in AIDS Care. 2004;15(5 Suppl):7S–13S. doi: 10.1177/1055329004269477. doi:10.1177/1055329004269477. [DOI] [PubMed] [Google Scholar]
- Kemppainen JK, Eller LS, Bunch E, Hamilton MJ, Dole P, Holzemer W, Tsai YF. Strategies for self-management of HIV-related anxiety. AIDS Care. 2006;18(6):597–607. doi: 10.1080/09540120500275726. doi:10.1080/09540120500275726. [DOI] [PubMed] [Google Scholar]
- Kreuter MW, Oswald DL, Bull FC, Clark EM. Are tailored health education materials always more effective than non-tailored materials? Health Education Research. 2000;15(3):305–315. doi: 10.1093/her/15.3.305. doi:10.1093/her/15.3.305. [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi:10.1093/biomet/73.1.13. [Google Scholar]
- Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, Wenger NS. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. doi:10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Lorenz KA, Cunningham WE, Spritzer KL, Hays RD. Changes in symptoms and health-related quality of life in a nationally representative sample of adults in treatment for HIV. Quality of Life Research. 2006;15(6):951–958. doi: 10.1007/s11136-005-6010-x. doi:10.1007/s11136-005-6010-x. [DOI] [PubMed] [Google Scholar]
- Nicholas PK, Kemppainen JK, Canaval GE, Corless IB, Sefcik EF, Nokes KM, Gallagher DM. Symptom management and self-care for peripheral neuropathy in HIV/AIDS. AIDS Care. 2007;19(2):179–189. doi: 10.1080/09540120600971083. doi:10.1080/09540120600971083. [DOI] [PubMed] [Google Scholar]
- Nicholas PK, Kirksey KM, Corless IB, Kemppainen J. Lipodystrophy and quality of life in HIV: Symptom management issues. Appied Nursing Research. 2005;18(1):55–58. doi: 10.1016/j.apnr.2004.09.012. doi:10.1016/j.apnr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, Glazier RH. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Collaboration. 2006:3. doi: 10.1002/14651858.CD001442.pub2. CD001442. doi:10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnall R, Wantland D, Velez O, Yen P-Y, Cato K, Bakken S. Integrating avatars into a symptom management system for PLWH; Abstract presented at the AMIA Annual Symposium; Washington, DC. 2011.Oct, [Google Scholar]
- Siegel K, Schrimshaw EW, Dean L. Symptom interpretation: Implications for delay in HIV testing and care among HIV-infected late middle-aged and older adults. AIDS Care. 1999;11(5):525–535. doi: 10.1080/09540129947686. doi:10.1080/09540129947686. [DOI] [PubMed] [Google Scholar]
- Spirig R, Moody K, Battegay M, De Geest S. Symptom management in HIV/AIDS: Advancing the conceptualization. Advances in Nursing Science. 2005;28(4):333–344. doi: 10.1097/00012272-200510000-00005. [DOI] [PubMed] [Google Scholar]
- University of California San Francisco Self care symptom management strategies: Manual for people living with HIV/AIDS. 2004 Retrieved from www.bms.com/documents/STF/manual/resource_12.pdf.
- Wantland DJ, Holzemer WL, Moezzi S, Willard SS, Arudo J, Kirksey KM, Huang E. A randomized controlled trial testing the efficacy of an HIV/AIDS symptom management manual. Journal of Pain and Symptom Management. 2008;36(3):235–246. doi: 10.1016/j.jpainsymman.2007.10.011. doi:10.1016/j.jpainsymman.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of Web-based vs. non-Web-based interventions: a meta-analysis of behavioral change outcomes. Journal of Medical Internet Research. 2004;6(4):e40. doi: 10.2196/jmir.6.4.e40. doi:10.2196/jmir.6.4.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Jr., Kosinski M, Keller SD. A 12-Item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. doi:10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–1040. doi: 10.1080/09540120903214389. doi:10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]