Summary
Objectives
Modern functional neuroimaging provides opportunities to visualize activity of the entire brain, making it an indispensable diagnostic tool for epilepsy. Various forms of non-invasive functional neuroimaging are now also being performed as research tools in animal models of epilepsy and provide opportunities for parallel animal/human investigations into fundamental mechanisms of epilepsy and identification of epilepsy biomarkers.
Methods
Recent animal studies of epilepsy using positron emission tomography, tractography, and functional magnetic resonance imaging were reviewed.
Results
Epilepsy is an abnormal emergent property of disturbances in neuronal networks which, even for epilepsies characterized by focal seizures, involve widely distributed systems, often in both hemispheres. Functional neuroimaging in animal models now provides opportunities to examine neuronal disturbances in the whole brain that underlie generalized and focal seizure generation as well as various types of epileptogenesis.
Significance
Tremendous advances in understanding the contribution of specific properties of widely distributed neuronal networks to both normal and abnormal human behavior have been provided by current functional neuroimaging methodologies. Successful application of functional neuroimaging of the whole brain in the animal laboratory now permits investigations during epileptogenesis and correlation with deep brain EEG activity. With the continuing development of these techniques and analytical methods, the potential for future translational research on epilepsy is enormous.
Keywords: PET, MRI, EEG-fMRI, tractography
Current treatment for epilepsy suppresses seizures, but does not alter the underlying epileptogenic process or associated comorbid conditions. A major priority of current research on epilepsy is disease modification with a goal to cure or prevent epilepsy and its consequences. Modern neuroimaging now permits visualization of structural and functional abnormalities of the entire brain, noninvasively, and has revolutionized clinical diagnosis. Functional neuroimaging, in particular, also offers the opportunity to elucidate aberrant pathophysiological mechanisms and neuronal connections that might provide insights into novel therapeutic targets and biomarkers. However, research on animal models remains essential for obtaining correlative invasive electrophysiological, anatomical, and molecular/cellular data, and for studying the process of epileptogenesis.
Systemic functional neuroimaging is now being performed as a research tool with animal models of epilepsy and provides unique opportunities for parallel animal/human investigations needed to develop interventions that will reverse or prevent the causes of disability experienced by people with epilepsy. There are numerous challenges associated with preclinical imaging, including influences of anesthesia, blood sampling, and kinetic evaluation for PET, and limited resolution, which eventually will require a certain degree of standardization if data are to be compared among laboratories and correlated with human investigations.
Here we review findings from studies in the English literature retrieved from PubMed, as well as those presented by the authors at WONOEP XII, that have utilized small animal PET, diffusion-weighted MRI and tractography, and fMRI in animal models of epilepsy. As small animal PET is a relatively new technology in the epilepsy field, only a limited number of papers have been published. Therefore, all papers through October 2013 related to PET imaging in rodents and epilepsy are discussed in the review. Considering the extensive number of animal epilepsy studies that have incorporated various MRI methods, a comprehensive review of MRI was beyond the scope of this paper. Rather, this review considered all publications on diffusion-weighted imaging (DWI), tractography, connectomics, and fMRI in rodents and epilepsy through December 2013.
Small animal Positron Emission Tomography imaging
Since the advent of dedicated small animal PET in the mid-nineties, the use of molecular imaging in pre-clinical research has increased exponentially and has become a critically important tool for biomedical sciences.1 Firstly, it allows sensitive quantification of a virtually unlimited range of molecular targets in vivo, which is unique for nuclear imaging. Secondly, it gives non-invasive 3D brain image information allowing for follow-up and serial scanning in contrast to terminal studies. Thirdly, for the reasons mentioned above implementation into clinical applications is enhanced. This neuroimaging technique has reliably revealed abnormalities in many epilepsy syndromes in pre-clinical and clinical studies.2–4
Progression in small animal PET of epileptogenesis with FDG
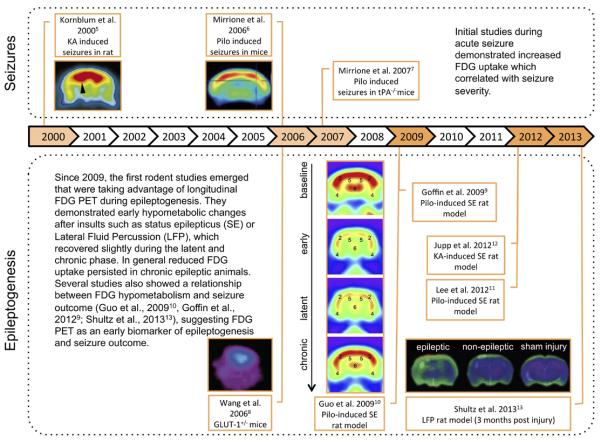
Over the last two decades, small animal PET studies have emerged in the field of epilepsy. In particular, temporal lobe epilepsy (TLE) models have been widely used to study epileptogenesis and chronic epilepsy. Initially, the focus of several studies was 18F-fluorodeoxyglucose (FDG) PET to investigate alterations in brain glucose metabolism related to brain activation. The first studies have demonstrated strong increases in brain glucose metabolism induced by acute seizures provoked by excitotoxins such as kainic acid and pilocarpine in normal rat and mice with FDG small animal PET.5,6 (Fig. 1, top) This technique may help understand mechanisms of seizure generation in models such as transgenic tissue plasminogen activator knock out mice, where a unique pattern of FDG uptake is seen following pilocarpine administration, possibly explaining the reduced sensitivity to seizures compared to wild type mice.7 Further, FDG PET was used to investigate alterations in chronic epilepsy models. In a mouse model of Glut-1 haploinsufficiency, a human genetic condition associated with epilepsy, reduced FDG brain uptake was found.8 This is also the general finding in models of TLE such as post-status epilepticus or models of posttraumatic epilepsy such as Lateral Fluid Percussion injury (LFP),9–13 in agreement with observations in TLE patients.
Fig. 1.
Progression in small animal PET of epileptogenesis with FDG. Abbreviations: KA, kainic acid; LFP, Lateral Fluid Percussion; pilo, pilocarpine; tPA, tissue Plasminogen Activator; GLUT, glutamate transporter. All images are reproduced with permission.
A significant advantage of animal models is that they allow the investigation of different stages of epileptogenesis preceding the manifestation of chronic epilepsy, which is difficult to do in patients. These studies have shown that brain glucose hypometabolism occurs early in the processes of limbic epileptogenesis in post-status epilepticus rat models.9–12 (Fig. 1, bottom). Interestingly, Guo et al10 found that early hypometabolism in the entorhinal cortex correlated with the later development of spontaneous recurrent seizures. Also in the LFP model, hypometabolism in the ipsilateral hippocampus was able to predict the epileptic outcome whereas cortical structural damage detected on MRI was not related to seizure susceptibility.13 Jupp et al.12 investigated potential confounding effects of cell loss in the post-status epilepticus model and concluded that the observed hypometabolism does not merely reflect cell loss and brain atrophy but may represent cellular mechanisms occurring early during epileptogenesis.
New PET ligands for small animal PET studies
Currently, small animal PET studies have taken advantage of the opportunity to study a wide range of biological processes beyond brain glucose metabolism. Dysfunctional GABAergic neurotransmission and more particularly decreased GABAA/benzodiazepine receptors (GABAA/cBZR) have been demonstrated in patients with TLE using flumazenil PET.14,15 These findings have been reproduced in the kindling model16 and in the post Kainic Acid-induced Status Epilepticus (KASE) model.17 In the latter model, Dedeurwaerdere and colleagues18 investigated this further using in vitro autoradiography and in vivo small animal PET. Combining in vivo PET imaging techniques with autoradiography allows detailed evaluation of the spatial and temporal changes. Cross-sectional 3H-flumazenil autoradiography demonstrated time dependent and subregional hippocampal changes in GABAA/cBZR density with an initial increase 24h after SE followed by a persistent decrease 2 to 6 weeks after SE in most hippocampal regions except for the stratum moleculare of the dentate gyrus. Secondly, new fluorinated flumazenil radioligands were evaluated,19 as these offer the practical advantage of more widespread clinical applications as an on-site cyclotron is not required and they allow a more efficient preclinical use as more animals can be scanned from the same radiosynthesis. Next, non-invasive quantification methods were developed following validation with invasive protocols employing arterial blood sampling.20 GABAA/cBZR density (Bmax) and affinity (Kd) were non-invasively measured using the partial saturation method according to Delforge et al.21 This showed that decreases in GABAA/cBZR density in vivo occurred independent of morphological brain deformations on MRI or histology 6 weeks after SE.20 (Fig. 2A)
Fig. 2.
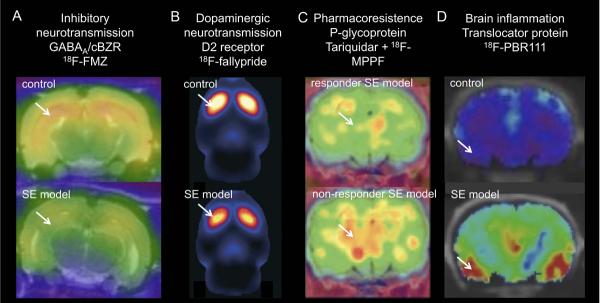
Application of new PET tracers in epilepsy models. A) Decrease in 18F-FMZ PET binding in epileptic rats (kainic acid-induced SE model) compared to control rats. Coronal PET/MRI images are shown at the level of the ventral hippocampus. B) Decrease in 18F-fallypride binding in epileptic animals (pilocarpine-induced SE model) indicative of reduced dopamine D2/3 receptor availability. Sagittal PET image through the rat striatum. C) Increased k1 of P-gp substrate 18F-MPPF in non-responders to phenobarbital (model for drug resistance) after tariquidar (P-gp inhibitor) treatment compared to responder epileptic rats (electrical-induced SE). Mean parametric map of unidirectional blood-brain clearance (k1) superimposed on a coronal rat brain histological atlas at the level of the dorsal hippocampus. D) Increased 18F-PBR111 binding 7 days post kainic acid-induced SE. Coronal PET/CT images of control and SE model. Arrows indicate altered tracer uptake. Abbreviations: SE, status epilepticus. Images reproduced with permission.22, 25,26
In another application of receptor/ligand imaging, decreased availability of D2/3 dopamine receptors was recently demonstrated with 18F-fallypride PET in a post-status epilepticus model.22 (Fig. 2B) This study is setting the scene for longitudinal and drug interaction preclinical studies related to dopaminergic neurotransmission.
Pharmacoresistance to anti-seizure drugs (ASDs) is a major issue in refractory epilepsy patients. P-glycoprotein (P-gp) has been proposed as an efflux transporter at the blood brain barrier (BBB) mediating drug resistance. Small animal PET studies in post-status epilepticus models have investigated the contribution of P-gp towards this phenomenon (Fig. 2C). In these studies, several PET tracers that are P-gp substrates or inhibitors (18FMPPF, 11C-verapamil, 11C-quinidine and 11C-laniquidar) were tested.24–27 Administration of tariquidar, (a P-gp inhibitor) before scanning, enhanced discrimination of group differences in kinetic influx/efflux rate constants (k1 and k2) of 11C-quinidine and 11C-verapamil between control and epilepsy groups indicating that P-gp activity is altered in epilepsy models.25, 27 In addition, pretreatment with tariquidar allowed differentiating between responders and non-responders in a model of pharmacoresistent epilepsy with [11C]quinidine and 18FMPPF (Fig. 2C).25,26 A small difference in baseline unidirectional blood-brain clearance (k1) between control, seizure-free and pharmacoresistant epilepsy patients has recently been demonstrated with PET imaging in patients.23 Whereas in animal studies tariquidar treatment generally augmented the increase in tracer kinetics in pharmacoresistant individuals, this increase was on the contrary attenuated in pharmacoresistant patients. More studies will be needed to corroborate the benefit of these P-gp PET ligands in predicting drug resistance in patients.
Brain inflammation has emerged as an important player in epileptogensis.28,29 Dedeurwaerdere and colleagues30 have performed studies with autoradiography and PET imaging to define a spatiotemporal pattern of brain inflammation in the post-status epilepticus model. For the in vivo measurements, 18F-PBR111 was used; this is a second generation and highly specific PET ligand for the translocator protein (TSPO, formerly known as the peripheral benzodiazepine receptor). As a definite brain region without TSPO changes (reference region) has not been identified so far, a simplified method was proposed based on the ratio of brain to plasma radioligand activity. Brain inflammatory changes were found with autoradiography and PET in the latent period (1 week post SE) of epileptogenesis (Fig. 2D). Increased TSPO binding was found in hippocampal, but also extrahippocampal limbic brain regions and olfactory bulb,30 which although attenuated was persistent up to the chronic phase (6–12 weeks post SE) (S. Dedeurwaerdere, unpublished).
These examples underscore the potential of small animal PET for investigating key mechanisms related to brain activation, neurotransmitter systems, drug resistance and brain inflammation in both epileptogenesis and established epilepsy. Although the development of new PET ligands is not a sinecure, it is anticipated that the number of radioligands for brain targets will continue raise in the future. Therefore, it is to be expected that small animal PET imaging in epilepsy research will continue to expand in the future. The current and future studies will provide the tools and biomarkers to advance molecular imaging in preclinical epilepsy research towards studying the evolution of these changes over time, investigating the interdependence of these imaging changes with multi-modal imaging, and evaluating the effect of disease-modifying interventions on the imaging biomarkers in the same animals.
Diffusion Weighted Imaging (DWI) and Tractography in rodent epilepsy models
MRI represents a non-invasive medical and research tool with the ability to identify early pathophysiological changes involved in epileptogenesis, monitor disease progression, and assess the effectiveness of possible therapies. In particular, advances in DWI-based methods, such as tractography, may provide sensitive in vivo measures of the subtle changes in brain connectivity involved in the epileptogenic process.31–33
DWI quantifies the diffusivity of water molecules in brain tissue.31,32 As water diffusivity in axons is directionally limited (anisotropic) relative to grey matter, DWI-based approaches are particularly sensitive to changes in white matter.31 The most common and basic DWI measures include the magnitude of water diffusion in a given direction (apparent diffusion coefficient; ADC), the anisotropy of water diffusion (fractional anisotropy; FA), and the mean diffusivity (MD) of water molecules across a number of directions.31 Previous studies in rodent models of epilepsy have applied these DWI measures in an attempt to provide insight into epileptogenesis with mixed and sometimes contradicting results. For example, in SE models of TLE, both decreases and increases in ADC have been reported during acute stages post-SE.34–37 ADC then returns to baseline by one week post-SE, with a consequent increase in ADC at more chronic time-points.36 These ADC abnormalities have been associated with a number of pathophysiological processes that may be important in epilepsy including edema, metabolic abnormalities, axonal injury, and neuronal loss34–36. Acute and chronic changes in ADC have also been reported in the hippocampus after rats were given LFP and have been associated with mossy fibre sprouting, EEG abnormalities, and increased seizure susceptibility after a chemoconvulsant challenge.38,39 Notably, changes in FA have also been reported in rodent models of epilepsy and may have relevance to epileptogenesis.40,41 However, although these basic DWI measures are capable of detecting changes after brain insult, they may not be sensitive to the more subtle changes in brain connectivity that are thought to occur in epileptogenesis.32
Tractography is an advanced DWI-based imaging method that allows for the detailed 3D reconstruction of brain white matter tracts, and thus holds great promise as a research tool to assess inter- and intra-structural connectivity changes in epileptogenesis.32,42 Indeed, an initial study applying tractography in rat models of genetic spike-wave epilepsy reports changes in corpus callosum and somatosensory cortex connectivity that may be related to the onset of spike-wave discharges.43 Tractography requires the acquisition of DWI data, the estimation of fiber orientations within each imaging voxel, and the application of a tracking algorithm based on the fiber orientations.31,42 To date the large majority of tractography studies utilize the diffusion tensor imaging (DTI) tractography model, including the abovementioned animal genetic epilepsy study, despite its well-documented limitations.42 Namely, DTI is only able to determine a single fiber orientation for each imaging voxel.42 Given that up to 90% of white matter voxels may contain fibers in different orientations,42,44 DTI greatly underestimates the degree of connectivity within the brain and often fails to accurately identify major white matter tracts.42 This was recently demonstrated by Farquharson and colleagues, where DTI-based tractography methods failed to reconstruct corticospinal pathways.40 However, constrained spherical deconvolution (CSD)-based tractography, a higher order-tractography model able to estimate numerous fiber orientations for each imaging voxel, was found to be superior than DTI methods, and consistently reconstructed the corticospinal tract as biologically expected.42 Taken together, although tractography represents a promising research tool in epilepsy, the limitations with DTI must be considered in the design and interpretation of experiments, and future studies are still required to rigorously assess the use of tractography methods as epileptogenic biomarkers.
In light of these needs, Shultz and colleagues have recently utilized serial CSD-based tractography, in conjunction with more conventional neuroimaging techniques, behavioral testing, and video-EEG analysis, to investigate epileptogenesis in rat models of acquired epilepsy.13, 45 Consistent with the clinical findings comparing CSD and DTI tractography, CSD-based tractography provides more biologically relevant tracts than DTI (see Fig. 3) in the rat brain. Furthermore, in the LFP rat model of post-traumatic epilepsy and the post-SE model of TLE, CSD-based tractography identified clear changes associated with the later development of epilepsy and neurobehavioral deficits. These preliminary results demonstrate the ability of CSD-based tractography to detect alterations in rat models of acquired epilepsy, and support the use of CSD-based tractography in future studies. However, it is important to emphasize that even higher-order tractography methods remain limited (e.g. spatial resolution), require further validation, and must be interpreted with caution.46
Fig. 3.
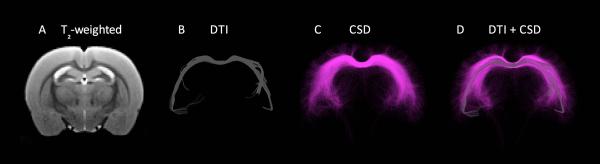
Example of diffusion tensor imaging (DTI)-based versus constrained spherical deconvolution (CSD)-based tractography in the rat corpus callosum.43 DTI (B) is the most commonly used tractography method despite well-documented limitations that result in the underestimation of tracts.40 Advances in higher-order tractography models, such as CSD (C), provide more sensitive and accurate representations of white matter tracts, and might be applied in future studies investigating brain connectivity in epileptogenesis.
Functional MRI (fMRI)
Based on the principle that neural activity leads to increased blood flow and changes in blood oxygenation levels, otherwise known as the blood oxygenation level dependent (BOLD) signal, fMRI in animal studies allows for the investigation of the location and networks of brain function.47,48 As epilepsy involves abnormalities in neural activity and circuitry, EEG-fMRI has been a particularly informative tool in both basic and clinical epilepsy research48 Of note, there are several challenges associated with acquiring simultaneous EEG during fMR image acquisition in animal epilepsy models. These include eliminating MR-induced artifact in EEG recordings, difficulties inducing seizures in anesthetized animals, and choosing the anesthetic that least alters systemic and cerebral physiology.49 Animal studies offer several advantages over human EEG-fMRI studies. For example, ictal studies are not limited by movement artifact since they can be performed on anesthetized and paralyzed animals. There is greater control over the specific seizure type and onset. It is also possible to obtain microelectrode recordings simultaneously with fMRI to better understand the mechanisms associated with seizure-induced BOLD response. Lastly, it is possible to perform more invasive physiological studies and obtain tissue/whole brain samples to better interrogate underlying physiologic, molecular, and genetic mechanisms.
The use of fMRI in animal models has provided insight into the local and network properties underlying seizure generation. In addition, they have improved our understanding of local and distant effects of interictal and ictal discharges, leading to a better understanding of interictal cognitive impairment associated with epilepsy.
Generalized epilepsy and generalized seizures
Most animal EEG-fMRI studies of generalized spike-wave seizures have relied on the Wistar AlbinoGlaxo rat of Rijswijk (WAG/Rij), a model of absence seizures. Spike-wave seizures in this model are associated with BOLD signal increases in bilateral regions in the cerebral cortex and thalamus.50,51 Notably, anterior cortical areas show the greatest BOLD signal increases, which are also the regions showing the greatest activity in electrophysiological recordings of spike-wave seizures.52,53 A more recent study focused on subcortical BOLD signal changes in this model (Fig. 4).54 In addition to EEG-fMRI, laser Doppler cerebral blood flow, local field potential, and multiunit activity recordings were obtained to better understand the basis of the observed BOLD signal changes. Prominent BOLD signal increases were seen in somatosensory barrel cortex and the thalamus, whereas BOLD signal decreases were seen in basal ganglia, a pattern similar to that seen with human generalized seizures.55–57 Notably, the observed cortical and thalamic BOLD signal increases were associated with the expected increases in electrophysiological activity and cerebral blood flow.54 On the other hand, the basal ganglia BOLD signal decreases were associated with cerebral blood flow decreases despite increases in neuronal electrical activity (Fig. 4). This suggests that the BOLD signal reflects electrophysiological activity in the cerebral cortex and thalamus, whereas this may not be the case in the basal ganglia. Thus, caution is warranted when interpreting BOLD signals in the basal ganglia.
Fig. 4.
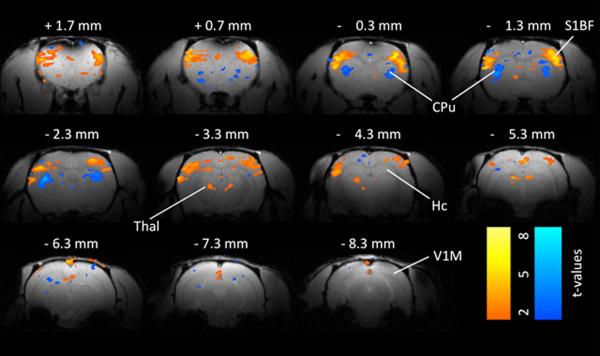
Example of BOLD fMRI changes 2– 4 s after spike-wave discharge onset in a WAG/Rij rat. A. Somatosensory cortex and thalamus (Thal) show prominent increases in BOLD signal during the spike-wave discharges. Prominent BOLD decreases are present in the caudate-putamen (CPu). Smaller changes are seen in other areas. Simultaneous EEG acquired during fMRI was used to identify images obtained 2– 4 s after SWD onset for comparison with baseline images obtained immediately before start of SWDs. Color bars indicate t values for increases (warm colors) and decreases (cold colors). Threshold value t > 2. Abbreviations: CPu caudate-putamen, Hc hippocampus, S1BF somatosensory barrel cortex, Thal thalamus, V1 primary visual cortex. Reproduced with permission. Modified from Mishra et al.51 with permission.
Studies of generalized tonic-clonic seizures have primarily used rats and agents such as kainic acid, pentylenetetrazole, and bicuculline (reviewed by Blumenfeld48). These studies also showed seizure-associated widespread cortical increases, maximal in the somatosensory cortex, as well as in the thalamus and other subcortical structures.58–60 These findings confirm that “generalized” seizures do not affect the entire brain similarly. Following seizures, widespread BOLD signal decreases were observed.59 Interestingly, one study showed that pre-ictal BOLD signal increases in the somatosensory cortex (S1, S2) and thalamus occur several seconds before seizure onset.59 This supports the existence of a pre-ictal state that precedes seizure onset that may serve as a target for future therapeutic intervention in an effort to abort seizures.
Focal epilepsy
Compared to generalized epilepsies and secondarily generalized seizures, few studies of focal seizures have been performed. Early studies of focal seizures relied on the local application of penicillin to cerebral cortex to product focal seizures in pigs61 or rats62 and associated BOLD signal increases in same region. In a porcine study of focal interictal discharges localized BOLD signals were also seen in the location of the discharge, however, they preceded the electrical activity.63 A study of spontaneous hippocampal seizures produced by intraperitoneal injection of kainic acid showed robust BOLD signal increases bilaterally in the hippocampus.64 Notably, a small number of electrographic seizures did not produce a measurable BOLD response, suggesting that neuronal activity and the BOLD responses may become decoupled at times in this animal model.
Another animal study of focal seizures induced by hippocampal stimulation showed increased BOLD signal in the hippocampus, thalamus and septum, but decreases in orbitofrontal, cingulate, and retrosplenial cortex during partial seizures.65 The BOLD signal decreases were associated with cortical slow waves, which were interpreted to reflect a depressed cortical state resembling sleep or coma, thus providing a possible measure of altered awareness during mesial temporal seizures. In the same study, secondarily generalized, convulsive seizures were associated with neocortical fast polyspike activity as well as BOLD signal increases in the regions that showed decreases during mesial temporal seizures, highlighting a different mechanism for altered consciousness during generalized seizures.65
Functional connectivity
Few animal studies have examined resting state functional connectivity in animal models of epilepsy. The first was performed using Genetic Absence Epilepsy Rats from Strasbourg (GAERS), a model of absence seizures.66 Simultaneous intracranial EEG-fMRI showed discharges originating from primary somatosensory cortex associated with strong BOLD activation in this area. Several functional connectivity measures derived from fMRI data (Granger causality and Dynamic Causal Modeling) failed to show any functional connectivity. It was argued that local hemodynamics vary between cerebral regions in this model, thus making it difficult to find temporal correlations in the fMRI data.
A recent study examined resting state functional connectivity in WAG/Rij rats, a model of absence seizures.67 In this study, cortical regions showing BOLD signal increases associated with spike-wave discharges were used as seeds for subsequent connectivity analysis. Striking differences between WAG/Rij and nonepileptic control rats (Wistar) were observed, with the strongest connectivity observed in the bilateral somatosensory and adjacent cortices, which was where the spike-associated BOLD signal changes were maximal. These data confirm that long-term changes in epileptic networks are present in this animal model in between seizures. Such alterations could possibly underlie interictal behavioral and cognitive abnormalities seen in persons with epilepsy.
One study of focal seizures examined resting state functional connectivity following seizures produced by tetanus toxin injection into right primary motor cortex of rats.68 These animals displayed spontaneous facial motor seizures that persisted for many weeks. Resting state connectivity maps of the right and left sensorimotor cortices were obtained 7, 21, 49, and 70 days following toxin injection. In the epileptic brain, increased interhemispheric functional connectivity of both sensorimotor cortices was seen at day 7, extending into the adjacent secondary somatosensory and medial cingulate cortices as well as other areas. These changes returned to control levels by day 70. Additionally, decreased interhemispheric functional connectivity of both sensorimotor cortices was seen, starting at day 21 and recovering later.68
Conclusions
Epilepsy is an abnormal emergent property of disturbances in neuronal networks which, even for epilepsies characterized by focal seizures, involve widely distributed systems, often in both hemispheres. Systemic functional neuroimaging now provides opportunities to examine neuronal disturbances in the whole brain that underlie various types of epileptogenesis and epileptic seizure generation.
Although FDG brain uptake is a fairly generic physiological measurement, recent studies suggest some characteristic patterns could serve as a biomarker of epileptogenesis. Drug occupancy PET imaging could provide biomarkers for novel drug targets and facilitate clinical trials. Novel PET tracers could provide insights into molecular mechanisms. DWI methods allow for the in vivo study of brain connectivity. More basic DWI measures have already been utilized to detect changes in animal models of epilepsy, but tractography in particular holds great promise as a future research tool to assess abnormal inter- and intra-structural connections in epileptogenesis. Functional MRI studies in animals offer the advantage of performing more invasive studies to better understand network, cellular, molecular, and genetic mechanisms underlying the generation of seizures as well as the remote effects of seizures on the brain. EEG-fMRI studies in animal models of epilepsy have already provided fundamental insights into mechanisms underlying the generation of seizures that, to date, have not been possible in human studies.
In our quest to find true disease-modifying therapies, we need non-invasive biomarkers that represent underlying neurobiological mechanisms. This will require translational animal/human validation at several levels: Does the imaging biomarker reflect a pathological process, a protective compensatory mechanism, or an epiphenomenon? How does the imaging biomarker correlate with the temporal process of epileptogenesis? How does the imaging biomarker change following therapy?
The studies described above are but a few examples of work now underway that will permit extensive animal/human parallel investigations, using patients to identify specific abnormalities of human epilepsy, subsequent studies in animal models to identify fundamental neuronal mechanisms that are not easily examined in a clinical setting, followed by selective studies with patients to validate results of animal research. Given tremendous advances in understanding the contribution of specific properties of widely distributed neuronal networks to both normal and abnormal human behavior being elucidated by the new field of connectomics, the potential for future research on epilepsy is enormous.
Supplementary Material
Acknowledgements
Dr. Dedeurwaerdere received support from Fonds voor Wetenschappelijk Onderzoek (FWO)(1514412N), ERA-NET Neuron Epilepsy-TBI (G.A009.13N) and Bijzonder Onderzoeksfonds (BOF) Universiteit Antwerpen. Dr. Engel received support from NIH grants P01 NS02808, R01 NS33310, U01 NS42372, and P20 NS 80181, The Epilepsy Therapy Project, CURE, the Epilepsy Foundation, and the Resnick Foundation. Dr. Shultz received funding from the Australian National Health and Medical Research Council and the Canadian Institutes of Health Research. Dr. Federico received funding from the Canadian Institutes of Health Research and Epilepsy Canada.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Virdee K, Cumming P, Caprioli D, et al. Applications of positron emission tomography in animal models of neurological and neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36:1188–216. doi: 10.1016/j.neubiorev.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Dedeurwaerdere S, Jupp B, O'Brien TJ. Positron Emission Tomography in basic epilepsy research: a view of the epileptic brain. Epilepsia. 2007;48(Suppl 4):56–64. doi: 10.1111/j.1528-1167.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 3.Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Semin Nucl Med. 2008;38:227–39. doi: 10.1053/j.semnuclmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien TJ, Jupp B. In-vivo imaging with small animal FDG-PET: a tool to unlock the secrets of epileptogenesis? Exp Neurol. 2009;220:1–4. doi: 10.1016/j.expneurol.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Kornblum HI, Araujo DM, Annala AJ, Tatsukawa KJ, Phelps ME, Cherry SR. In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET) Nat Biotechnol. 2000;18:655–60. doi: 10.1038/76509. [DOI] [PubMed] [Google Scholar]
- 6.Mirrione MM, Schiffer WK, Siddiq M, Dewey SL, Tsirka SE. PET imaging of glucose metabolism in a mouse model of temporal lobe epilepsy. Synapse. 2006;59:119–21. doi: 10.1002/syn.20216. [DOI] [PubMed] [Google Scholar]
- 7.Mirrione MM, Schiffer WK, Fowler JS, Alexoff DL, Dewey SL, Tsirka SE. A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. Neuroimage. 2007;38:34–42. doi: 10.1016/j.neuroimage.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Pascual JM, Yang H, et al. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–79. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 9.Goffin K, Van Paesschen W, Dupont P, Van Laere K. Longitudinal microPET imaging of brain glucose metabolism in rat lithium-pilocarpine model of epilepsy. Exp Neurol. 2009;217:205–9. doi: 10.1016/j.expneurol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Gao F, Wang S, et al. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009;162:972–9. doi: 10.1016/j.neuroscience.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Lee EM, Park GY, Im KC, et al. Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia. 2012;53:860–9. doi: 10.1111/j.1528-1167.2012.03432.x. [DOI] [PubMed] [Google Scholar]
- 12.Jupp B, Williams J, Binns D, et al. Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia. 2012;53:1233–44. doi: 10.1111/j.1528-1167.2012.03525.x. [DOI] [PubMed] [Google Scholar]
- 13.Shultz SR, Cardamone L, Liu YR, et al. Can structural or functional changes following traumatic brain injury in the rat predict epileptic outcome? Epilepsia. 2013;54:1240–50. doi: 10.1111/epi.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivash L, Gregoire MC, Lau EW, et al. 18F-Flumazenil: A gamma-Aminobutyric Acid A-Specific PET Radiotracer for the Localization of Drug-Resistant Temporal Lobe Epilepsy. J Nucl Med. 2013;54:1270–7. doi: 10.2967/jnumed.112.107359. [DOI] [PubMed] [Google Scholar]
- 15.Van Paesschen W. Qualitative and quantitative imaging of the hippocampus in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimaging Clin N Am. 2004;14:373–400. vii. doi: 10.1016/j.nic.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Liefaard LC, Ploeger BA, Molthoff CF, et al. Changes in GABAA receptor properties in amygdala kindled animals: in vivo studies using [11C]flumazenil and positron emission tomography. Epilepsia. 2009;50:88–98. doi: 10.1111/j.1528-1167.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 17.Syvanen S, Labots M, Tagawa Y, et al. Altered GABAA receptor density and unaltered blood-brain barrier transport in a kainate model of epilepsy: an in vivo study using 11C-flumazenil and PET. J Nucl Med. 2012;53:1974–83. doi: 10.2967/jnumed.112.104588. [DOI] [PubMed] [Google Scholar]
- 18.Vivash L, Tostevin A, Liu DS, et al. Changes in hippocampal GABAA/cBZR density during limbic epileptogenesis: relationship to cell loss and mossy fibre sprouting. Neurobiol Dis. 2011;41:227–36. doi: 10.1016/j.nbd.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Dedeurwaerdere S, Gregoire MC, Vivash L, et al. In-vivo imaging characteristics of two fluorinated flumazenil radiotracers in the rat. Eur J Nucl Med Mol Imaging. 2009;36:958–65. doi: 10.1007/s00259-009-1066-4. [DOI] [PubMed] [Google Scholar]
- 20.Vivash L, Gregoire MC, Bouilleret V, Berard A, Wimberley C, Binns D, Roselt P, Katsifis A, Myers DE, Hicks RJ, O'Brien TJ, Dedeurwaerdere S. In vivo measurement of hippocampal GABAA/cBZR density with [(18)F]- flumazenil PET for the study of disease progression in an animal model of temporal lobe epilepsy. PLoS One. 2014;9:e86722. doi: 10.1371/journal.pone.0086722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delforge J, Pappata S, Millet P, et al. Quantification of benzodiazepine receptors in human brain using PET, [11C]flumazenil, and a single-experiment protocol. J Cereb Blood Flow Metab. 1995;15:284–300. doi: 10.1038/jcbfm.1995.34. [DOI] [PubMed] [Google Scholar]
- 22.Yakushev IY, Dupont E, Buchholz HG, et al. In vivo imaging of dopamine receptors in a model of temporal lobe epilepsy. Epilepsia. 2010;51:415–22. doi: 10.1111/j.1528-1167.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- 23.Feldman M, Asselin MC, Liu J, Wang S, McMahon A, Anton-Rodriguez J, Walker M, Symms M, Brown G, Hinz R, Matthews J, Bauer M, Langer O, Thom M, Jones T, Vollmar C, Duncan JS, Sisodiya SM, Koepp MJ. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol. 2013;12:777–785. doi: 10.1016/S1474-4422(13)70109-1. [DOI] [PubMed] [Google Scholar]
- 24.Syvanen S, Luurtsema G, Molthoff CF, et al. (R)-[11C]verapamil PET studies to assess changes in P-glycoprotein expression and functionality in rat blood-brain barrier after exposure to kainate-induced status epilepticus. BMC Med Imaging. 2011;11:1. doi: 10.1186/1471-2342-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syvanen S, Russmann V, Verbeek J, et al. [(11)C]quinidine and [(11)C]laniquidar PET imaging in a chronic rodent epilepsy model: Impact of epilepsy and drug-responsiveness. Nucl Med Biol. 2013;40:764–75. doi: 10.1016/j.nucmedbio.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Bartmann H, Fuest C, la Fougere C, et al. Imaging of P-glycoprotein-mediated pharmacoresistance in the hippocampus: proof-of-concept in a chronic rat model of temporal lobe epilepsy. Epilepsia. 2010;51:1780–90. doi: 10.1111/j.1528-1167.2010.02671.x. [DOI] [PubMed] [Google Scholar]
- 27.Bankstahl JP, Bankstahl M, Kuntner C, Stanek J, Wanek T, Meier M, Ding XQ, Müller M, Langer O, Löscher W. A novel positron emission tomography imaging protocol identifies seizure-induced regional overactivity of P-glycoprotein at the blood-brain barrier. J Neurosci. 2011;31:8803–8811. doi: 10.1523/JNEUROSCI.6616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dedeurwaerdere S, Friedman A, Fabene PF, et al. Finding a better drug for epilepsy: antiinflammatory targets. Epilepsia. 2012;53:1113–8. doi: 10.1111/j.1528-1167.2012.03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dedeurwaerdere S, Callaghan PD, Pham T, et al. PET imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy. EJNMMI Res. 2012;2:60. doi: 10.1186/2191-219X-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Engel J, Jr, Thompson PM, Stern JM, Staba RJ, Bragin A, Mody I. Connectomics and epilepsy. Curr Opin Neurol. 2013;26:186–94. doi: 10.1097/WCO.0b013e32835ee5b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernhardt BC, Hong S, Bernasconi A, Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci. 2013;7:624. doi: 10.3389/fnhum.2013.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelhorn T, Hufnagel A, Weise J, Baehr M, Doerfler A. Monitoring of acute generalized status epilepticus using multilocal diffusion MR imaging: early prediction of regional neuronal damage. AJNR Am J Neuroradiol. 2008;28:321–327. [PMC free article] [PubMed] [Google Scholar]
- 35.van Eijsden P, Notenboom RG, Wu O, et al. In vivo 1H magnetic resonance spectroscopy, T2-weighted and diffusion-weighted MRI during lithium-pilocarpine-induced status epilepticus in the rat. Brain Res. 2004;1030:11–18. doi: 10.1016/j.brainres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Wall CJ, Kendall EJ, Obenaus A. Rapid alterations in diffusion-weighted images with anatomic correlates in a rodent model of status epilepticus. AJNR Am J Neuroradiol. 2000;21:1841–1852. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong J, Petroff OAC, Prichard JW, Gore JC. Changes in water diffusion and relaxation properties of rat cerebrum during status epilepticus. Magn Reson Med. 1993;30:241–246. doi: 10.1002/mrm.1910300214. [DOI] [PubMed] [Google Scholar]
- 38.Kharatishvili I, Immonen R, Grohn O, Pitkänen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain. 2007;130:3155–3168. doi: 10.1093/brain/awm268. [DOI] [PubMed] [Google Scholar]
- 39.Frey L, Lepkin A, Schickedanz A, Huber K, Brown MS, Serkova N. ADC mapping and T1-weighted signal changes on post-injury MRI predict seizure susceptibility after experimental traumatic brain injury. Neurol Res. 2013 doi: 10.1179/1743132813Y.0000000269. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parekh MB, Carney PR, Sepulveda H, Norman W, King M, Mareci TH. Early MR diffusion and relaxation changes in the parahippocampal gyrus precede the onset of spontaneous seizures in an animal model of chronic limbic epilepsy. Exp Neurol. 2010;224:258–270. doi: 10.1016/j.expneurol.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sierra A, Laitinen T, Lehtimaki K, Rieppo L, Pitkanen A, Grohn O. Diffusion tensor MRI with tract-based spatial statistics and histology reveals undiscovered lesioned areas in kainate model of epilepsy in rat. Brain Struct Funct. 2011;216:123–135. doi: 10.1007/s00429-010-0299-0. [DOI] [PubMed] [Google Scholar]
- 42.Farquharson S, Tournier JD, Calamante F, et al. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 2013;118:1367–77. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- 43.Chahboune H, Mishra AM, DeSalvo MN, et al. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22099. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shultz SR, Zheng P, Wright DK, et al. WONOEP XII meeting planner. Quebec, Canada: 2013. Tractography and magnetic resonance spectroscopy in acquired epilepsy models. [Google Scholar]
- 46.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and dont's of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 48.Blumenfeld H. Functional MRI studies of animal models in epilepsy. Epilepsia. 2007;48(Suppl 4):18–26. doi: 10.1111/j.1528-1167.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 49.Mirsattari SM, Ives JR, Leung LS, Menon RS. EEG monitoring during functional MRI in animal models. Epilepsia. 2007;48(Suppl 4):37–46. doi: 10.1111/j.1528-1167.2007.01240.x. [DOI] [PubMed] [Google Scholar]
- 50.Nersesyan H, Hyder F, Rothman DL, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004;24:589–99. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- 51.Tenney JR, Duong TQ, King JA, Ferris CF. FMRI of brain activation in a genetic rat model of absence seizures. Epilepsia. 2004;45:576–82. doi: 10.1111/j.0013-9580.2004.39303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:1480–95. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004;24:1057–68. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- 54.Mishra AM, Ellens DJ, Schridde U, et al. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:15053–64. doi: 10.1523/JNEUROSCI.0101-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aghakhani Y, Bagshaw AP, Benar CG, et al. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain: a journal of neurology. 2004;127:1127–44. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- 56.Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–22. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 57.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15236–40. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brevard ME, Kulkarni P, King JA, Ferris CF. Imaging the neural substrates involved in the genesis of pentylenetetrazol-induced seizures. Epilepsia. 2006;47:745–54. doi: 10.1111/j.1528-1167.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 59.DeSalvo MN, Schridde U, Mishra AM, et al. Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage. 2010;50:902–9. doi: 10.1016/j.neuroimage.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cerebral cortex. 2008;18:1814–27. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Opdam HI, Federico P, Jackson GD, et al. A sheep model for the study of focal epilepsy with concurrent intracranial EEG and functional MRI. Epilepsia. 2002;43:779–87. doi: 10.1046/j.1528-1157.2002.04202.x. [DOI] [PubMed] [Google Scholar]
- 62.Mirsattari SM, Wang Z, Ives JR, et al. Linear aspects of transformation from interictal epileptic discharges to BOLD fMRI signals in an animal model of occipital epilepsy. Neuroimage. 2006;30:1133–48. doi: 10.1016/j.neuroimage.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Makiranta M, Ruohonen J, Suominen K, et al. BOLD signal increase preceeds EEG spike activity--a dynamic penicillin induced focal epilepsy in deep anesthesia. Neuroimage. 2005;27:715–24. doi: 10.1016/j.neuroimage.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 64.Airaksinen AM, Hekmatyar SK, Jerome N, et al. Simultaneous BOLD fMRI and local field potential measurements during kainic acid-induced seizures. Epilepsia. 2012;53:1245–53. doi: 10.1111/j.1528-1167.2012.03539.x. [DOI] [PubMed] [Google Scholar]
- 65.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:9066–81. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.David O, Guillemain I, Saillet S, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS biology. 2008;6:2683–97. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra AM, Bai X, Motelow JE, et al. Increased resting functional connectivity in spike-wave epilepsy in WAG/Rij rats. Epilepsia. 2013;54:1214–22. doi: 10.1111/epi.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Otte WM, Dijkhuizen RM, van Meer MP, et al. Characterization of functional and structural integrity in experimental focal epilepsy: reduced network efficiency coincides with white matter changes. PloS one. 2012;7:e39078. doi: 10.1371/journal.pone.0039078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.