Abstract
Background
Improvements in obstructive sleep apnea syndrome (OSAS) severity may be associated with improved pharyngeal fluid mechanics following adenotonsillectomy (AT). The study objective is to use image-based computational fluid dynamics (CFD) to model changes in pharyngeal pressures after AT, in obese children with OSAS and adenotonsillar hypertrophy.
Methods
Three-dimensional models of the upper airway from nares to trachea, before and after AT, were derived from magnetic resonance images obtained during wakefulness, in a cohort of 10 obese children with OSAS. Velocity, pressure, and turbulence fields during peak tidal inspiratory flow were computed using commercial software. CFD endpoints were correlated with polysomnography endpoints before and after AT using Spearman’s rank correlation (rs).
Results
Apnea hypopnea index (AHI) decrease after AT was strongly correlated with reduction in maximum pressure drop (dPTAmax) in the region where tonsils and adenoid constrict the pharynx (rs=0.78, P = 0.011), and with decrease of the ratio of dPTAmax to flow rate (rs = 0.82, P = 0.006). Correlations of AHI decrease to anatomy, negative pressure in the overlap region (including nasal flow resistance), or pressure drop through the entire pharynx, were not significant. In a subgroup of subjects with more than 10% improvement in AHI, correlations between flow variables and AHI decrease were stronger than in all subjects.
Conclusions
The correlation between change in dPTAmax and improved AHI suggests that dPTAmax may be a useful index for internal airway loading due to anatomical narrowing, and may be better correlated to AHI than direct airway anatomic measurements.
Keywords: Magnetic Resonance Imaging, Humans, Computer Simulation, Airway Resistance, Pediatrics
Obstructive sleep apnea syndrome (OSAS) is a respiratory disorder characterized by narrowing of the pharyngeal airway, resulting in repeated episodes of flow limitation or complete cessation, associated with oxygen desaturation and sleep disruption (Stradling and Davies, 2004). OSAS is common in children and may affect 1–4% of the general pediatric population (Lumeng and Chervin, 2008) and up to 50% of obese children(Kalra et al., 2005; Marcus et al., 1996; Silvestri et al., 1993). Magnetic resonance imaging (MRI) studies of the upper airway (UA) confirm that children with OSAS frequently have adenotonsillar hypertrophy and a structurally narrowed pharynx (Arens et al., 2002; Arens et al., 2001) located in the region where the tonsils and adenoid overlap (Arens et al., 2003). The American Academy of Pediatrics (AAP) recommends considering adenotonsillectomy surgery (AT) as the first treatment for children with OSAS (American Academy of Pediatrics, 2002). But the persistence of OSAS after AT is common particularly in obese children and may reach 50% (Mitchell and Boss, 2009; Mitchell and Kelly, 2007; Suen et al., 1995; Tal et al., 2003), which suggests that a clinical tool for predicting the outcome of AT would be valuable.
Computational fluid dynamics (CFD) is an engineering tool that can simulate detailed three-dimensional air flow dynamics and the resulting pressure distributions driven by anatomical narrowing, and may be helpful for comparing different surgical approaches to airway restriction (Mylavarapu et al., 2013). The effects of AT on airway pressure drop and flow resistance have been modeled by CFD based on pre- and post- surgery MRI (Mihaescu et al., 2008). However, few patients were studied so that statistical analysis could not be performed. But image-based CFD model endpoints have been strongly correlated to clinical benefit of mandibular advancement devices (De Backer et al., 2007; Zhao et al., 2013), and more weakly correlated with polysomnography endpoints (Cisonni et al., 2013; Vos et al., 2007; Wootton et al., 2014).
In the present retrospective cohort study, we hypothesize that CFD model endpoints will correlate well with treatment response of AT as measured by a decrease in the apnea-hypopnea index (AHI) from an overnight polysomnography study. MRI, physiologic data, and CFD data were utilized to calculate the velocity and air pressure distribution in ten obese children with OSAS, before and after AT. Based on these models we analyzed the correlation between AT treatment response and airway geometrical changes, minimum surface pressure, local air pressure drops in different airway segments, and airway pressure-flow ratio.
METHODS
Subjects and procedures
The study was approved by the Committee of Clinical Investigations at Albert Einstein College of Medicine (Protocol #2005-578). Informed consent was obtained from each subject and or/parent. Ten obese adolescent subjects were recruited at the Children’s Hospital at Montefiore using the following criteria: (1) diagnosed with OSAS by overnight polysomnography, (2) normal development and intact adenoid and tonsils, (3) and adenotonsillar hypertrophy indicating AT surgery. Subjects were a subset of subjects from a larger study of anatomical effects of AT (Nandalike et al., 2013). Before and after surgery all subjects underwent polysomnography and MRI. Mean interval between AT and second polysomnography was 4.4 months, and between AT and second MRI was 4.8 months. Overnight polysomnography (Xltek, Oakville, ON, Canada) was performed at the Sleep Disorders Center at the Children’s Hospital at Montefiore. OSAS was determined if the obstructive apnea hypopnea index (AHI) was ≥2/hour. Resting respiratory flow rate was measured in supine position with a pneumotachometer (RSS 100HR Research; Hans Rudolph) on the day of each imaging study. After discarding unusually short or long breaths at least ten normal tidal breaths were averaged, and average maximum inspiratory flow rate calculated. Bilateral nasal resistance curves were measured using anterior rhinomanometry (NR-6 Research, GM Instruments). Three measurements of four breaths with at least 150 Pa pressure drop were acquired (Clement and Gordts, 2005). Average Rohrer coefficients for each nasal passage were used to calculate nasal resistance and air pressure in the choanae at peak inspiratory flow.
Subjects underwent upper airway MRI, and T-1 & T-2 weighted axial images were obtained in the Department of Radiology at the Children’s Hospital at Montefiore, using a Philips 3.0 Tesla Achieva scanner with 16 channel surface array coil (SENSE XL; Philips Medical System, Best, The Netherlands) as previously described.(Nandalike et al., 2013) Subjects were awake during imaging and positioned supine with head in neutral position, i.e., the Frankfort plane perpendicular to the table. The slice thickness was 3 mm with 0.3 mm gap and 0.5×0.5mm pixel size.
Seventeen subjects from the original study were excluded in cases where nasal resistance data was not available both before and after surgery, typically due to nasal obstruction, and in cases where subject motion in an MRI study made accurate surface reconstruction for CFD modeling impossible. All subjects not excluded (N=10) were studied as a single cohort with one-time follow-up.
Image Processing and CFD Mesh Generation
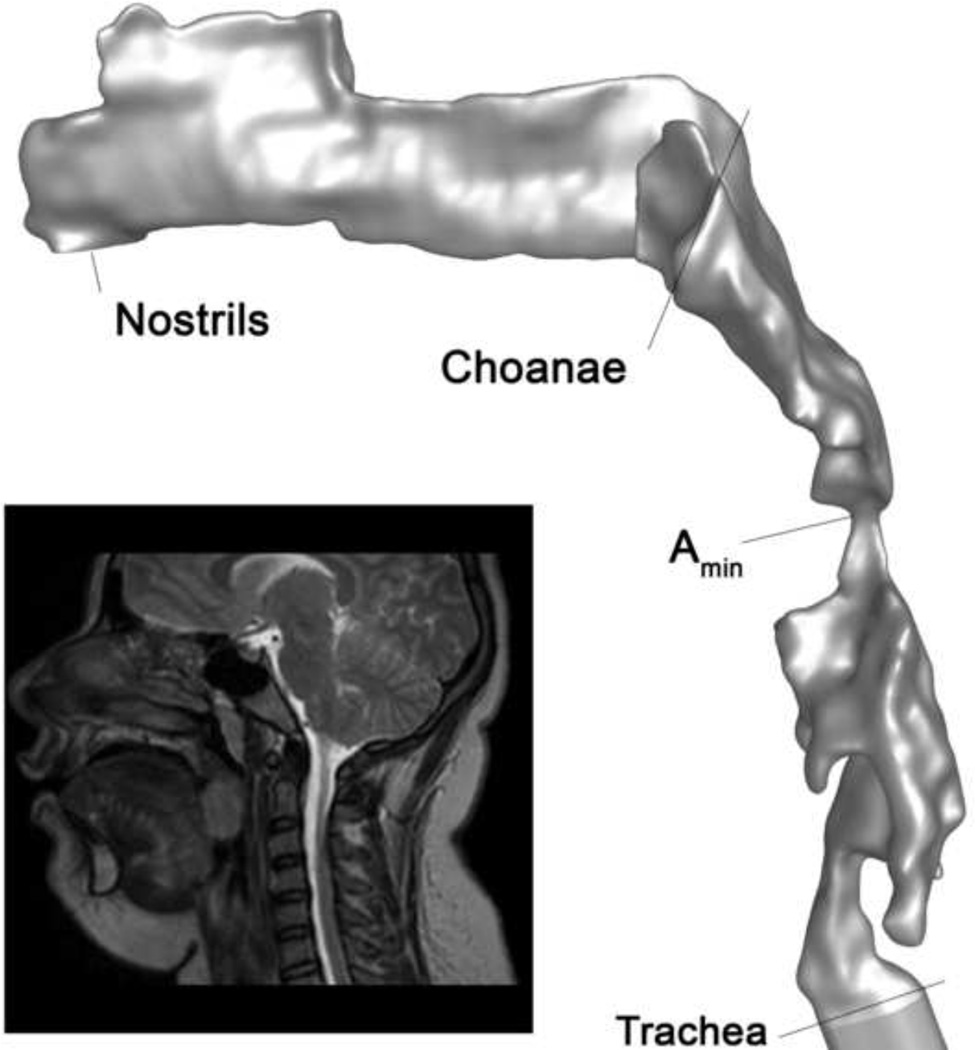
The airway from nares to trachea was reconstructed from the axial image stack using commercial medical imaging software (MIMICS, Materialise). Segmented slices were reconstructed with a volume-preserving smoothing algorithm to generate a three-dimensional model (Figure 1). Airway cross-sections perpendicular to the airway direction were defined at the choanae, at the minimum cross-section of the region where tonsils and adenoids constrict the airway, and the trachea. The 3D model was imported into commercial CFD meshing software (Gambit, A NSYS, Lebanon, NH, USA) to create an unstructured tri/tetrahedral mesh with 1.6 to 2.6 million cells.
Figure 1.
3D upper airway model of subject 4 pre-surgery based on reconstructed segmented MR axial images. Anatomical locations along the airway model are shown for reference. Amin is the location of minimum cross-section where tonsils and adenoids constrict the pharynx; in this subject Amin is located between the tonsils in the retrolingual pharynx. Inset: midline sagittal MR image of subject 4.
Fluid Mechanics Parameters and Model Solution
A commercial CFD package (Fluent ANSYS 14, ANSYS) was used to solve the flow governing equations, ignoring airway wall motion. Both pre and post surgery flow simulations were performed at the maximum inspiratory flow rate computed from pre surgery waveform flow data. Due to the area restriction, a turbulent jet is expected downstream of the restriction (Young, 1979; Young and Tsai, 1973), so a low Reynolds number k-ω turbulence model was used to solve for turbulence quantities (Wilcox, 1998). The computer simulation pressure field accuracy has been verified with experimental data from in vitro (Xu et al., 2006) and in vivo (Wootton et al., 2014) airway models.
Endpoints and Statistical Methods
Several endpoints were derived from the CFD models. Area-averaged air pressures were computed at the choanae (PCH) and trachea (PTR), as well as representative cross-sections in the nasopharynx, velopharynx, retrolingual pharynx, and hypopharynx. Minimum airway wall pressure in the region where tonsils and adenoids constrict the airway (PTA) and minimum pressure in the pharynx (PPM) were identified. Pressure drop from the choanae through the maximum constriction area (dPTAmax = PCH - PTA) was derived from the CFD model. Minimum airway pressure in the region where tonsils and adenoids maximally constrict the airway (Pmin) was computed from the measured nasal resistance and the pressure drop: Pmin = −dPrhino – dPTAmax. Pressures and pressure drops require a clinical measurement of volume flow rate V̇ and are somewhat sensitive to V̇, therefore the pressure drop to flow ratio was also computed from the CFD data:
| (1) |
An anatomical-based estimate of PQRTAmax, ignoring viscous losses and secondary flow effects, was derived from Bernoulli’s equation:
| (2) |
where Amin and ACH are the minimum area where tonsils and adenoids constrict the pharynx, and the choanae cross-section area, respectively, and ρ is the density of air.
Clinical, anatomical, and CFD endpoint improvements were normalized by their pre-surgery values, e.g. AHI decrease = (AHIpre-AT – AHIpost-AT)/AHIpre-AT. Spearman’s correlation coefficients (rs) and p-values were computed between AHI decrease as a measure of OSAS severity, and CFD and anatomical endpoint improvement (Matlab).
RESULTS
Subjects and clinical outcomes
Demographic and sleep study values are shown in Table 1. Mean subject age was 13.4±1.7 years; 5 subjects were female. Before AT, five subjects had severe OSAS (AHI≥10), four had moderate OSAS (5<AHI<10) and one had mild OSAS (2≤AHI≤5). Seven subjects had improved AHI following AT, one subject had minimal improvement in AHI (7% reduction in AHI, and still severe OSAS), and two actually had worse AHI.
Table 1.
Subject characteristics
| Patient Number |
Age | Gender | Pre BMI |
Pre AHI |
Post AHI |
AHI Decrease |
max inspiration flow rate (l/min) |
|---|---|---|---|---|---|---|---|
| 1 | 11 | F | 31.9 | 6.2 | 0 | 100.0% | 12.2 |
| 2 | 16 | F | 40.7 | 8.3 | 0.2 | 97.6% | 14.8 |
| 3 | 12 | F | 58.2 | 69.2 | 10.9 | 84.2% | 14.0 |
| 4 | 13 | F | 39.1 | 22.5 | 3.8 | 83.1% | 19.6 |
| 5 | 12 | M | 28.6 | 5.7 | 1.2 | 78.9% | 15.8 |
| 6 | 15 | M | 30.8 | 12.8 | 4.1 | 66.7% | 6.6 |
| 7 | 15 | F | 50.7 | 4.8 | 1.7 | 64.6% | 25.9 |
| 8 | 14 | M | 41 | 10.7 | 10 | 6.5% | 27.6 |
| 9 | 15 | M | 30.6 | 12.3 | 15.8 | −28.5% | 10.9 |
| 10 | 12 | M | 43.8 | 7.1 | 10.2 | −43.7% | 32.3 |
CFD Model and Anatomical Changes
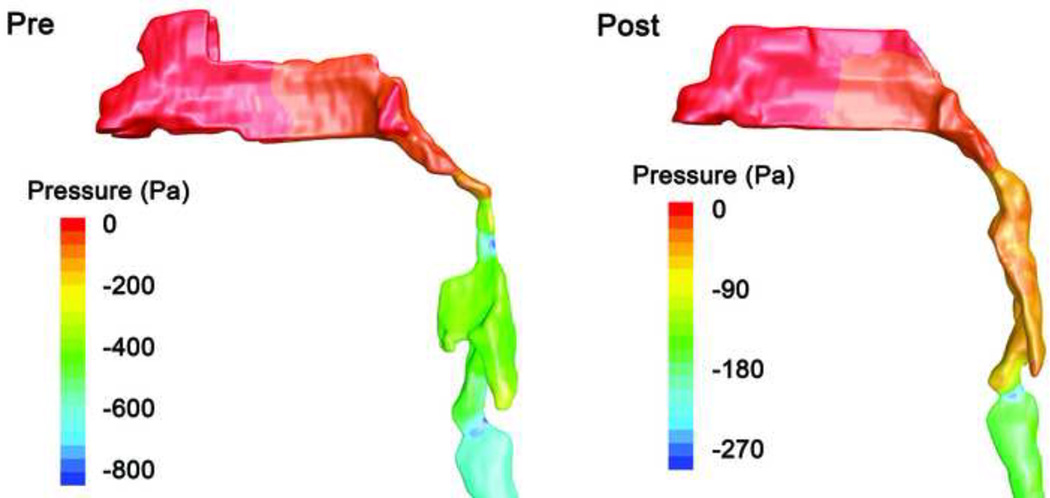
CFD simulations show a qualitatively similar pressure distribution in most cases (Figure 2). During inspiration, pressure falls between the nares and the relatively narrow nasal passages, and then a rapid pressure decrease occurs in the narrow region of the pharynx where tonsils and adenoids constrict the airway. The pressure drop in the pharynx can be primarily explained by the Bernoulli principle: as cross-sectional area decreases, airflow velocity increases and the pressure falls due to conservation of energy. Downstream of the minimum area there is usually a modest rise in the pressure as the air speed falls in the retrolingual pharynx. A downstream pressure minimum is also observed at the larynx. The same qualitative pressure distribution is observed after AT (Figure 3). In most subjects, because pressure recovery in the retrolingual airway downstream of the minimum area is rather small, the minimum pressure Pmin is representative of the airway pressure loading both at the minimum cross-section and in the retrolingual pharynx downstream of the area minimum and upstream of the hypopharynx (Figures 2 and 3). Similarly, the pressure drop from choanae to area minimum, dPTAmax, is also representative of the pressure difference between the choanae and the retrolingual pharynx.
Figure 2.
Pressure contours on the UA surface of subject 1 before and after AT surgery (lateral view; note larger pressure scale in pre-surgery image). Anterior-posterior restriction in the nasopharynx and oropharynx is reduced after AT. Pressure drop was low (about 50 Pa) in the nasal passages of this subject. Before surgery there was a rapid drop in pressure (averaged over the airway cross-section) to Pmin = −620 Pa in the retrolingual pharynx where tonsils restrict the airway, then mild pressure recovery to −400 Pa. After surgery the minimum cross-section area in the overlap region increased, and as a result the minimal pressure increased to Pmin = −60 Pa. The pressure in the retrolingual pharynx is well represented by Pmin in each model.
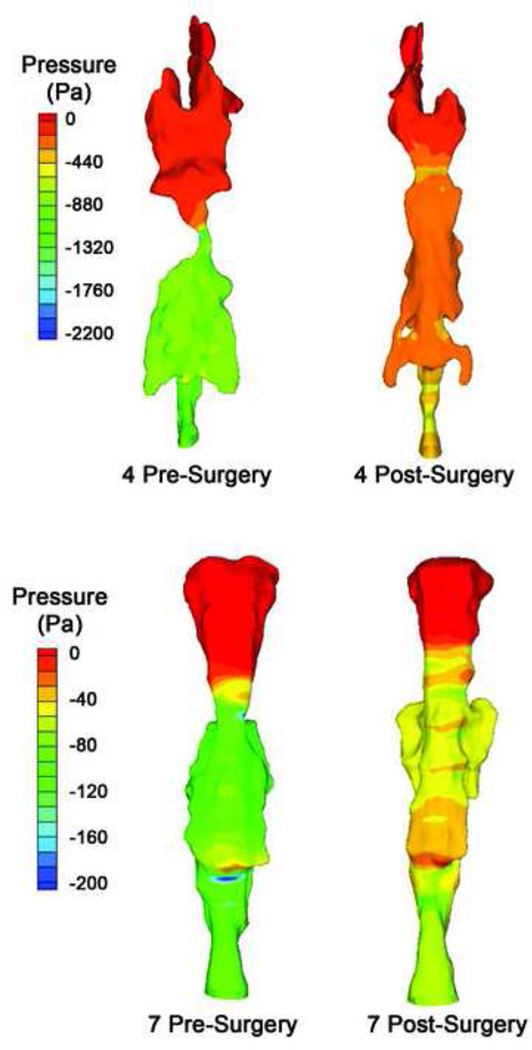
Figure 3.
Pressure contour on the UA surface of subjects 4 (top) and 7 (bottom), before and after AT surgery. Surgery relieved both lateral and anterior-posterior (not visible in this view) restriction of the airway. These two subjects actually had decreased UA volume after surgery, due to decreased retrolingual airway volume. But increased minimum cross-section area and more gradual narrowing caused increased Pmin and increased retrolingual airway pressure in both subjects.
Anatomical and CFD endpoints before vs. after surgery are summarized in Table 2. The UA volume increased in 8 of 10 subjects, and minimum cross-section area increased following AT in 9 of 10 subjects. As a result of reduced airway restriction, dPTAmax and PQRTAmax decreased in all subjects with increased minimum cross-sectional area (Table 2).
Table 2.
Upper airway model parameters and results
| Patient Number |
Volume increase |
Min area increase |
dPTAmax decrease |
PQRTAmax decrease |
PQRTA (Bernoulli) decrease |
|---|---|---|---|---|---|
| 1 | 17.9% | 156.2% | 96.2% | 94.7% | 93.6% |
| 2 | 51.0% | 20.7% | 92.1% | 99.8% | 95.6% |
| 3 | 14.0% | 87.5% | 74.7% | 93% | 72.2% |
| 4 | −21.8% | 122.6% | 80.6% | 80.6% | 80.0% |
| 5 | 23.9% | 26.9% | 54.6% | 45.5% | 48.7% |
| 6 | 42.4% | 62.3% | 64.6% | 62.4% | 61.8% |
| 7 | −8.7% | 29.5% | 47.6% | 46.7% | 41.8% |
| 8 | 23.5% | −21.6% | −120.2% | −102.6% | −115.2% |
| 9 | 63.9% | 15.2% | 36.2% | 53.2% | 24.3% |
| 10 | 46.1% | 47.0% | 67.6% | 48% | 54.8% |
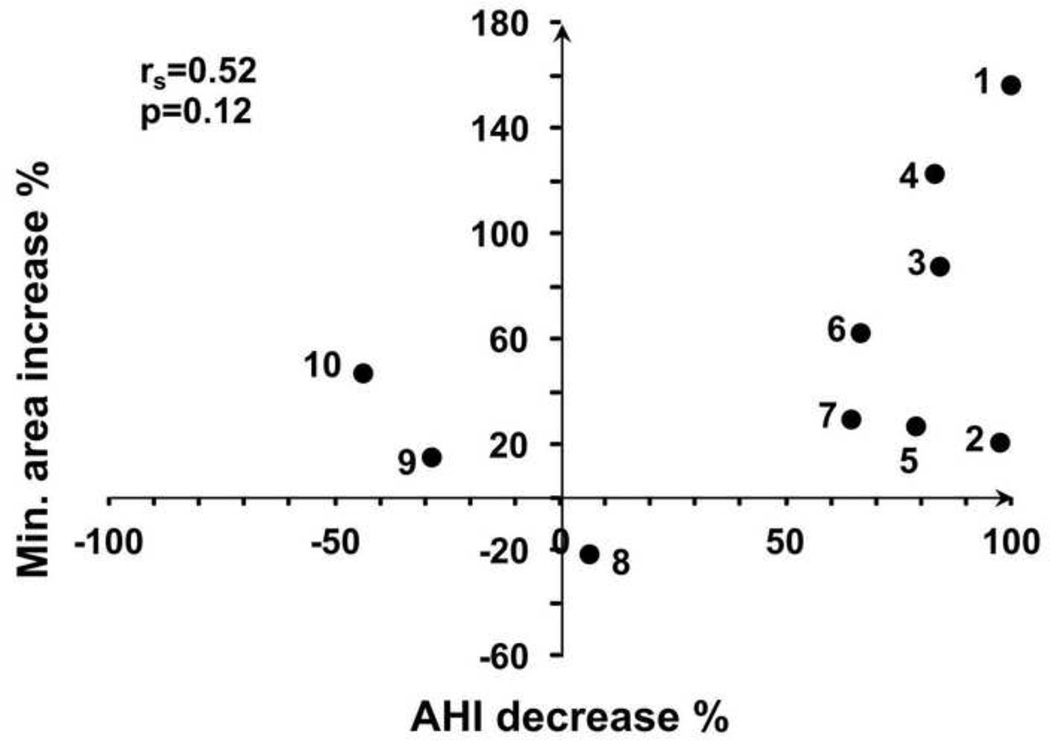
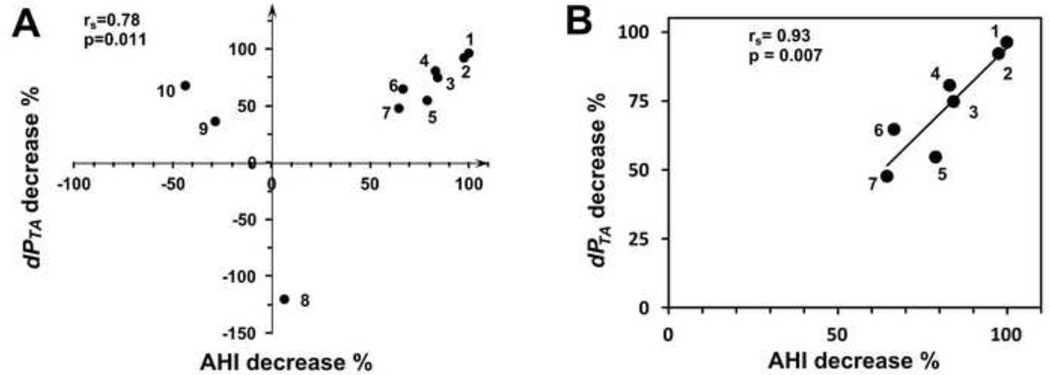
Correlations with AHI improvement are summarized in Table 3. After surgery the UA volume of most subjects increased, but subjects 4 and 7 had decreased volume even though their AHI improved. Thus increased UA volume did not correlate significantly with AHI improvement (Table 3). AT surgery increased the minimum cross-section area in all subjects with improved AHI, and correlation between cross-section improvement and AHI change was higher but not significant (Figure 4). In comparison to anatomical endpoints, several CFD endpoints correlated more strongly with clinical improvement. Significant correlation was found between dPTAmax decrease and AHI decrease (Figure 5). Even the two subjects who had smaller UA volume after surgery had decreased dPTAmax due to increased minimum cross-section.
Table 3.
Spearman correlation coefficients of selected anatomical and flow model endpoints to AHI decrease.
| Endpoint | All Subjects (N=10) |
>10% improved AHI Subjects (N=7) |
||
|---|---|---|---|---|
| rs | p | rs | P | |
| Airway Volume Increase | −0.35 | 0.331 | 0.21 | 0.662 |
| Minimum Area Increase | 0.53 | 0.123 | 0.32 | 0.498 |
| dPTAmax Decrease | 0.78 | 0.012 | 0.93 | 0.007 |
| PQRTAmax Decrease | 0.75 | 0.018 | 0.86 | 0.024 |
| PQRTA Bernoulli Decrease | 0.82 | 0.007 | 0.89 | 0.012 |
| Pmin Increase | 0.24 | 0.514 | 0.07 | 0.906 |
Figure 4.
Correlation between the percentage increase in minimum cross section area and the percentage decrease in AHI.
Figure 5.
Correlation between the percentage decrease in airway pressure from choanae to area minimum, dPTAmax, and the percentage decrease in AHI. A. All subjects. B. Subjects with >10% improvement in AHI after surgery (moderately to completely successful surgery). Correlation is approximately linear in this subgroup of patients.
The pressure-flow ratio (PQR) is a pressure drop normalized by the flow rate, and may indicate decreased stability of the overlap region; when PQR is high, a small change in flow rate produces a large increase in pressure load that the airway may not be able to resist. A decrease in AHI correlates to decreased PQRTAmax (Table 3). A slightly stronger correlation was found between PQRTA Bernoulli decrease and AHI decrease. For all three of the pressure endpoints dPTAmax, PQRTAmax, and PQRTA Bernoulli, there appears to be a strong, approximately linear correlation to improvement in AHI in a subgroup of subjects with >10% improvement in AHI (Figure 5), leading to improved correlation coefficients (Table 3).
In contrast to pressure drop from choanae to minimum area, the increase in minimum pressure in the airway, Pmin, which combines the effects of nasal resistance and pharyngeal area restriction, did not correlate significantly to decrease in AHI (rs=0.24, p=0.5).
DISCUSSION
The current study evaluated the utility of CFD as a tool to assess the efficacy of AT in obese children with OSAS. Decreases in both dPTAmax and PQRTAmax were correlated to AHI decrease. These correlations are consistent with CFD studies of adults (Cisonni et al., 2013; Lucey et al., 2010; Vos et al., 2007) and children (Van Holsbeke et al., 2013; Wootton et al., 2014) correlating pharyngeal pressure drops to OSAS severity. The strong correlation between dPTAmax and AHI decreases support further development of patient-specific CFD as a potential clinical tool for surgical planning in patient populations where residual OSAS is more common, such as obese patients. The subgroup analysis showed even stronger correlation between changes in dPTAmax and AHI, but it also showed that CFD was not an accurate predictor of the benefits of TA surgery for 30% of subjects in this study, suggesting that at least one additional endpoint may be needed to for a surgical planning tool.
The strength of correlation of AHI change with PQRTAmax as estimated by Bernoulli’s equation (Equation 2) is also promising. Although CFD provides more accurate PQR data because it accounts for viscous and turbulent losses and secondary shape effects, CFD is time-consuming and requires technical training and often expensive software. Bernoulli's method requires only the known flow rate and the cross-sectional area at the choanae and the minimum area; in spite of its limitations PQRTA Bernoulli was a significant indicator of OSAS in this subject group, and would be more convenient for clinical implementation; a recent CFD study of adult subjects also supports this approach (Lucey et al., 2010). The approximately linear correlation of pressure drop reduction to AHI improvement in subjects with moderately or completely successful AT surgery is intriguing, and suggests that image-based fluid dynamics, if combined with one or more other parameters that predict neutral or worse response to AT surgery, may be useful for guiding the volume of tissue removal in AT surgery.
The relationship between decrease in dPTAmax and decrease in AHI can suggest a similar correlation to UA volume increase should hold. DeBacker et al (De Backer et al., 2007) found a correlation between the UA volume and AHI. However, in our work UA volume and minimum cross section area had weaker correlations with AHI. For example, surgery was successful in two subjects whose UA volume was reduced after surgery. According to Bernoulli’s principle airway pressure drop can be estimated by the following formula ΔP =0.5ρ V̇2(AD−2− AU−2) where ρ is air density, V̇ is volumetric flow rare, AU is the upstream cross section area and AD is the downstream cross section area. The pressure drop depends on the relationship of cross section areas upstream and downstream as well as flow rate, and neither UA volume nor a single minimum area can fully account for this change. For example, post-surgery subjects 4 and 7, which have smaller volume compared to pre-surgery, have less severe and more gradual area restrictions, and no strong pressure minimum (Figure 3). Thus the intraluminal surface pressure post surgery is higher than pre surgery.
The correlation between pressure drop (dPTAmax) improvement and AHI improvement is consistent with published models of the airway with mandibular advancement devices (De Backer et al., 2007; Zhao et al., 2013), which modeled only pressure changes in the pharyngeal airway and did not include the effect of nasal passage pressure drop. In contrast there was no significant correlation with minimum internal airway pressure Pmin, which included the effect of nasal resistance as measured by anterior rhinomanometry. In the balance of forces (Remmers et al., 1978) and Pcrit (Schwartz et al., 1988) models of airway collapse, negative pharyngeal air pressure during inspiration is a load that tends to collapse the airway; if the airway is passive, flow limitation and obstruction would tend to increase with nasal resistance. But upper airway collapse is also related to the function of pharyngeal dilator muscles (Block et al., 1984; Remmers et al., 1978), which act to stiffen or distend the collapsible pharyngeal airway during inspiration. Dilator muscle activity is pressure sensitive (Horner et a l., 1991; Hwang et al., 1984; Mathew et al., 1982a, b) and is modulated by pharyngeal mechanosensors located in the nasopharynx and oropharynx (Horner et al., 1991). The lower correlation of AHI to pressures influenced by nasal resistance suggests that most obese children are able to compensate for the variable effects of nasal resistance, while the correlation of dPTAmax to AHI suggests that these patients are not able to fully respond to further pressure drops within the constricted pharynx. We speculate that airway restriction from adenotonsillar hypertrophy could interfere with the pressure-sensing function of pharyngeal mechanosensors, and/or directly inhibit the function of pharyngeal dilators to widen the overlap region.
We recently used image-based CFD models to compare CFD endpoints to polysomnography endpoints in fifteen obese children with OSAS and fifteen BMI, gender, and age-matched control subjects (Wootton et al., 2014). That study found significant direct correlation between dPTAmax and polysomnography endpoints, but the correlation was weaker (0.48). The stronger correlation in this study may be attributed to normalizing endpoints to pre-surgical values, which may compensate for other contributing factors that aren’t evident from MR imaging. Both studies are also consistent in showing lower correlation of Pmin to OSAS severity.
Van Holsbeke et al (Van Holsbeke et al., 2013) found a direct correlation between UA volume and AHI. However, in our work we didn’t find a significant direct correlation between AHI and UA volume. This is reasonable given the small sample size and the number of factors involved in airway collapse. Airway collapse is the result of joint effects of internal airway pressure, passive tissue compliance, and activation of the airway muscles. When AHI and CFD endpoints are normalized by pre-surgical values, patient-to-patient variability due to these factors was reduced enough to give correlation. Along with the small size of this study, there may be some concern that the study population is biased compared to the original patient population (Nandalike et al., 2013). But comparing this subpopulation to the whole study population, the percent reductions of adenoid volume (−6% vs. 12%) and tonsil volume (87% vs. 88%), as well as the correlation between change in adenoid + tonsil volume and AHI (r=0.66 vs. r=0.57), were similar so we believe that there is no inadvertent bias in this study group.
Several publications have compared CFD and anatomical endpoints before and after AT surgery. Mihaescu et al (Mihaescu et al., 2008) published a case study showing reduced pressure drops following AT, consistent with the current study. But in a subgroup of eight subjects modeled by Van Holsbeke et al (Van Holsbeke et al., 2013) before and after AT surgery, no significant differences were reported. To our knowledge the current study is the first to report a significant correlation between changes in CFD model endpoints and improvements in OSAS severity after AT surgery.
CONCLUSION
In this study, the utility of CFD endpoints to evaluate the effect of anatomical changes in the upper airway following AT in children with OSAS was evaluated. Airflows and pressure gradients were calculated on 3D models from MRI images, and demonstrated improvements in airway fluid dynamics after AT in these subjects. We noted higher and more significant correlations between decreased AHI and decreased pressure drop or pressure-flow ratio between the choanae and the reg ion where tonsils and adenoid constrict the pharynx, in comparison to changes in AHI and upper airway anatomical parameters, such as UA volume and minimum cross section area. CFD was shown to be a potentially useful modality for the clinical assessment of the mechanical properties of the upper airway after AT in children with OSAS. We suggest that it could also be useful a tool for simulating outcomes of other surgical approaches in other phenotypes of OSAS in both children and adults. between the choanae and the reg ion where tonsils and adenoid constrict the pharynx, in comparison to changes in AHI and upper airway anatomical parameters, such as UA volume and minimum cross section area. CFD was shown to be a potentially useful modality for the clinical assessment of the mechanical properties of the upper airway after AT in children with OSAS. We suggest that it could also be useful a tool for simulating outcomes of other surgical approaches in other phenotypes of OSAS in both children and adults.
Acknowledgments
Funding
Support given by U. S. National Institutes of Health Grants 5 R01 HD053693 and 5 R01 HL105212, and U. S. National Science Foundation Grant 959915. Academic software license discounts were provided by Ansys Inc and Materialise Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors report no competing interests.
REFERENCE LIST
- American Academy of Pediatrics, So. P.P., Subcommittee on Obstructive Sleep Apnea Syndrome. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–712. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- Arens R, McDonough JM, Corbin AM, Hernandez EM, Maislin G, Schwab RJ, Pack AI. Linear dimensions of the upper airway structure during development: assessment by magnetic resonance imaging. Am J Respir Crit Care Med. 2002;165:117–122. doi: 10.1164/ajrccm.165.1.2107140. [DOI] [PubMed] [Google Scholar]
- Arens R, McDonough JM, Corbin AM, Rubin NK, Carroll ME, Pack AI, Liu J, Udupa JK. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2003;167:65–70. doi: 10.1164/rccm.200206-613OC. [DOI] [PubMed] [Google Scholar]
- Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- Block AJ, Faulkner JA, Hughes RL, Remmers JE, Thach B. Clinical conference in pulmonary disease. Factors influencing upper airway closure. Chest. 1984;86:114–122. doi: 10.1378/chest.86.1.114. [DOI] [PubMed] [Google Scholar]
- Cisonni J, Lucey AD, Walsh JH, King AJ, Elliott NS, Sampson DD, Eastwood PR, Hillman DR. Effect of the velopharynx on intraluminal pressures in reconstructed pharynges derived from individuals with and without sleep apnea. J Biomech. 2013;46:2504–2512. doi: 10.1016/j.jbiomech.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Clement PA, Gordts F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–179. [PubMed] [Google Scholar]
- De Backer JW, Vanderveken OM, Vos WG, Devolder A, Verhulst SL, Verbraecken JA, Parizel PM, Braem MJ, Van de Heyning PH, De Backer WA. Functional imaging using computational fluid dynamics to predict treatment success of mandibular advancement devices in sleep-disordered breathing. J Biomech. 2007;40:3708–3714. doi: 10.1016/j.jbiomech.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JC, StJohn WM, Bartlett D., Jr Afferent pathways for hypoglossal and phrenic responses to changes in upper airway pressure. Respir Physiol. 1984;55:341–354. doi: 10.1016/0034-5687(84)90056-2. [DOI] [PubMed] [Google Scholar]
- Kalra M, Inge T, Garcia V, Daniels S, Lawson L, Curti R, Cohen A, Amin R. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13:1175–1179. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- Lucey AD, King AJ, Tetlow GA, Wang J, Armstrong JJ, Leigh MS, Paduch A, Walsh JH, Sampson DD, Eastwood PR, Hillman DR. Measurement, reconstruction, and flow-field computation of the human pharynx with application to sleep apnea. IEEE Trans Biomed Eng. 2010;57:2535–2548. doi: 10.1109/TBME.2010.2052808. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–183. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: af ferent pathways. J Appl Physiol. 1982a;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on respiratory frequency. Respir Physiol. 1982b;49:223–233. doi: 10.1016/0034-5687(82)90075-5. [DOI] [PubMed] [Google Scholar]
- Mihaescu M, Murugappan S, Gutmark E, Donnelly LF, Kalra M. Computational modeling of upper airway before and after adenotonsillectomy for obstructive sleep apnea. Laryngoscope. 2008;118:360–362. doi: 10.1097/MLG.0b013e31815937c1. [DOI] [PubMed] [Google Scholar]
- Mitchell RB, Boss EF. Pediatric obstructive sleep apnea in obese and normal-weight children: impact of adenotonsillectomy on quality-of-life and behavior. Dev Neuropsychol. 2009;34:650–661. doi: 10.1080/87565640903133657. [DOI] [PubMed] [Google Scholar]
- Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137:43–48. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Mylavarapu G, Mihaescu M, Fuchs L, Papatziamos G, Gutmark E. Planning human upper airway surgery using computational fluid dynamics. J Biomech. 2013;46:1979–1986. doi: 10.1016/j.jbiomech.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Nandalike K, Shifteh K, Sin S, Strauss T, Stakofsky A, Gonik N, Bent J, Parikh SR, Basilla M, Nikova M, Muzumdar H, Arens R. Adenotonsillectomy in Obese Children With Obstructive Sleep Apnea Syndrome: Magnetic Resonance Imaging Findings and Considerations. Sleep. 2013;36:841–847. doi: 10.5665/sleep.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993;16:124–129. doi: 10.1002/ppul.1950160208. [DOI] [PubMed] [Google Scholar]
- Stradling JR, Davies RJ. Sleep. 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax. 2004;59:73–78. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen JS, Arnold JE, Brooks LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg. 1995;121:525–530. doi: 10.1001/archotol.1995.01890050023005. [DOI] [PubMed] [Google Scholar]
- Tal A, Bar A, Leiberman A, Tarasiuk A. Sleep characteristics following adenotonsillectomy in children with obstructive sleep apnea syndrome. Chest. 2003;124:948–953. doi: 10.1378/chest.124.3.948. [DOI] [PubMed] [Google Scholar]
- Van Holsbeke C, Vos W, Van Hoorenbeeck K, Boudewyns A, Salgado R, Verdonck PR, Ramet J, De Backer J, De Backer W, Verhulst SL. Functional respiratory imaging as a tool to assess upper airway patency in children with obstructive sleep apnea. Sleep Med. 2013;14:433–439. doi: 10.1016/j.sleep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Vos W, De Backer J, Devolder A, Vanderveken O, Verhulst S, Salgado R, Germonpre P, Partoens B, Wuyts F, Parizel P, De Backer W. Correlation between severity of sleep apnea and upper airway morphology based on advanced anatomical and functional imaging. J Biomech. 2007;40:2207–2213. doi: 10.1016/j.jbiomech.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wilcox D. Turbulence Modeling for CFD. 2nd ed. La Canada, CA: DCW Industries Inc; 1998. [Google Scholar]
- Wootton DM, Luo H, Persak SC, Sin S, McDonough JM, Isasi CR, Arens R. Computational fluid dynamics endpoints to characterize obstructive sleep apnea syndrome in children. J Appl Physiol. 2014;116(1985):104–112. doi: 10.1152/japplphysiol.00746.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Sin S, McDonough JM, Udupa JK, Guez A, Arens R, Wootton DM. Computational fluid dyanmics modeling of the upper airway of children with obstructive sleep apnea syndrome in steady flow. Journal of Biomechanics. 2006;39:2043–2054. doi: 10.1016/j.jbiomech.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Young DF. Fluid Mechanics of Arterial Stenoses. Journal of Biomechanical Engineering. 1979;101:157–175. [Google Scholar]
- Young DF, Tsai FY. Flow characteristics in models of arterial stenoses. I Steady flow. Journal of Biomechanics. 1973;6:395–410. doi: 10.1016/0021-9290(73)90099-7. [DOI] [PubMed] [Google Scholar]
- Zhao M, Barber T, Cistulli P, Sutherland K, Rosengarten G. Computational fluid dynamics for the assessment of upper airway response to oral appliance treatment in obstructive sleep apnea. J Biomech. 2013;46:142–150. doi: 10.1016/j.jbiomech.2012.10.033. [DOI] [PubMed] [Google Scholar]