Abstract
Non-Hodgkin lymphomas (NHL) disproportionately affect older patients who uncommonly receive allogeneic hematopoietic cell transplantation (HCT). We analyzed CIBMTR data on 1248 patients ≥40 years receiving reduced-intensity conditioning (RIC) or non-myeloablative (NMA) HCT for aggressive (n=668) and indolent (n=580) NHL. Aggressive lymphoma was more frequent in the oldest cohort [(age 40–54) 49% vs. (55–64) 57% vs. (≥65) 67% p=0.0008]; fewer patients ≥65 had prior autografting [26% vs. 24% vs. 9%; p=0.002)]. Rates of relapse, acute and chronic GVHD and non-relapse mortality (NRM) at one year were similar [22%, 95% confidence interval (CI) 19–26%; 27%, 95% CI 23–31%; 34%, 95% CI 24–44%]. Progression-free (PFS) and overall (OS) survival at 3 years was slightly lower in older cohorts [OS:54%, 95% CI 50–58%; 40%, 95% CI 36–44%; 39%, 95% CI 28–50%; p<0.0001]. Multivariate analysis revealed no significant effect of age on acute or chronic GVHD or relapse. Age ≥55 years, Karnofsky performance status <80, and HLA-mismatch adversely impacted NRM, PFS, and OS. Disease status at HCT, but not histologic subtype, worsened NRM, relapse, PFS and OS. Even for patients ≥55 years, OS still approached 40% at 3 years suggesting HCT effects long-term remissions and remains underutilized in qualified older patients with NHL.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) for patients with non-Hodgkin lymphoma (NHL) is increasingly used for patients with high-risk and relapsed/refractory disease1. As over one-half of cases are diagnosed in those older than 65 years, this represents a growing population of patients for whom allogeneic HCT could provide long-term disease free survival and improve outcomes2. It is postulated that conventional myeloablative conditioning prior to HCT is not feasible for the vast majority of older patients due to limited physiologic resilience and accompanying comorbidities. Hence non-myeloablative (NMA) and reduced intensity conditioning (RIC) strategies have made HCT available to less fit individuals who have relapsed or poor-risk hematologic malignancies amenable to allogeneic HCT. Recent reports show acceptable non-relapse mortality (NRM) rates of 10–20% and 2–3 year progression-free survival reported from 25–75% depending on the NHL subtype. However, data specific to older patients with NHL remains limited3–7.
We recently examined the influence of age on outcomes in older patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) in first complete remission (CR) and found similar outcomes compared to younger patients when given RIC HCT regimens8. In this analysis, we examined the same question in those receiving a RIC or NMA allogeneic HCT for NHL of aggressive or indolent histologies to define post-HCT outcomes in older patients and to evaluate patient, disease and treatment characteristics influencing these outcomes.
Patients and methods
Data for this analysis were submitted to the Center for International Blood and Transplant Research (CIBMTR), a voluntary working group of more than 450 transplant centers worldwide who contribute data on consecutive allogeneic HCT to a statistical center housed at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) in Minneapolis. Patients are followed longitudinally with yearly follow-up. Computerized checks for errors and on-site audits of participating centers ensure data quality. Physician review of data and additional requested information from reporting centers are included. Observational studies conducted by the CIBMTR are performed with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Patient selection
Patients 40 years or older receiving a RIC or NMA HCT between 2001–2007 for aggressive [(diffuse large B cell (n=202), mantle cell (279), immunoblastic/anaplastic B/T cell (52), peripheral T cell (60), peripheral T cell lymphoma NOS (25), Burkitt (4), other (46)] and indolent [small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) (156), follicular (387), marginal zone (13), other (24)] NHL were included. Patients were classified as being in first (n=87) or second (231) complete remission, first (478) or second (304) partial remission (CR1/2 or PR1/2), resistant (RD, 304) disease as known prior to HCT. Grafts were from a related or unrelated donor (URD) and cord blood grafts were not studied. Patients receiving prior autografts were included.
A total of 1248 cases were identified; 668 patients with aggressive and 580 with indolent NHL were treated at 165 centers. There were 1119 patients with B-cell and 106 patients with T-cell histology (3 patients were not classifiable). Ages ranged from 40 to 75 years and were divided into 3 age cohorts for analysis: 40–54 years (n= 614); 55–64 years (n= 552) and ≥ 65 years (n= 82). Previously established criteria for donor:recipient HLA matching were used to define well-matched, partially-matched or mismatched categories9. Preparative regimens were classified as either RIC or NMA. RIC included any regimen consisting of: 1) ≤500 cGy total body irradiation (TBI) as a single fraction or ≤800 cGy if fractionated; 2) ≤9 mg/kg busulfan oral (or intravenous equivalent); 3) <140 mg/m2 melphalan; 4) <10mg/kg thiotepa; or 5) BEAM regimen (carmustine, etoposide, cytarabine, and melphalan)10–11. Other regimens were classified as NMA where prompt hematopoietic recovery could reasonably be expected without transplantation within 28 days12. T-cell depletion accomplished via ex vivo or in vivo methods was included.
Study end points and definitions
Primary outcomes were overall (OS) and progression-free (PFS) survival defined as survival from allogeneic HCT without death or without disease progression or relapse. NRM was defined as any death in the first 28 days post-transplant or any death after day 28 without documented NHL progression or relapse. All data were censored at date of last reported follow-up. Secondary endpoints included neutrophil recovery defined as time to absolute neutrophil count of ≥ 500 neutrophils/mcL sustained for three consecutive days and the cumulative incidence of acute (grades II–IV) and chronic graft-versus-host disease (GVHD) as defined by consensus criteria13–14.
Statistical analysis
Patient-, disease-, and transplant-related variables were compared between age cohorts using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Univariate probabilities of PFS and OS were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood’s formula. Probabilities of neutrophil recovery, acute and chronic GVHD, NRM and relapse were calculated using cumulative incidence curves to accommodate competing risks. Ninety-five percent confidence intervals (95% CI) for all probabilities and p-values of pairwise comparisons were derived from pointwise estimates and calculated using standard techniques.
Age groups’ influence on neutrophil recovery was compared using logistic regression. Cox proportional hazards regression models were used for all other outcomes. The proportional hazards assumption was tested for all variables. Variables were stratified in the model when the proportionality assumption did not hold. Patient-, disease-, and transplant-related variables were considered in the model building procedure, but as recipient age was the main interest of this study, it was included in all steps of model building. Separate analyses of CR1 vs. CR2 and PR1 vs. PR2 patients identified no significant differences in major endpoints and these groups were combined for subsequent analysis. Patient-related variables considered were gender and Karnofsky performance status (KPS) of < 80 vs. ≥ 80. Disease-related variables included histology subtype (aggressive vs. indolent), disease status at time of HCT (CR1/2+ vs. PR1/2+ vs. refractory) and time from diagnosis to HCT (<2 years vs. ≥2 years). International prognostic index (IPI) was considered, but LDH was not reported for 69% of the patients and thus it could not be assigned. Extranodal sites and number of pre-transplant therapies were considered as well. Transplant-related variables included: year of transplant (2001–2004 vs. 2005–2007), donor/recipient CMV serostatus (−/− vs. −/+ vs. +/− vs. +/+), HLA matching (HLA matched sibling vs. well-matched URD vs. partially matched URD vs. mismatched URD), stem cell source [bone marrow vs. peripheral blood (PBSC)], GVHD prophylaxis [cyclosporine (CSA) ± methotrexate (MTX) ± other vs. tacrolimus (Tac) ± MTX ± other vs. T-cell depletion], donor-recipient gender match (M-M vs. M-F vs. F-M vs. F-F); and conditioning regimen intensity (RIC vs. NMA). Risk factors with p < 0.05 were included in the models. The potential interaction between the main effect of age and all significant covariates were examined. To analyze any possible impact of specific histology on outcomes, we divided patients into 5 histological groups (diffuse large B cell, mantle cell, SLL/CLL, follicular, and other and included them in the models for relapse/progression, PFS and OS. All computations were performed using the statistical package SAS version 9.1.
Results and outcomes
Patient Characteristics
Table 1 summarizes patient-, disease-, and transplant-related variables across age groups. Of 1248 patients, 614 (49%) were age 40–54, 552 (44%) were age 55–64 years and 82 (7%) were ≥ 65 years. Most patients were transplanted for aggressive NHL subtypes which were most often seen in the oldest cohort of patients (49% 40–54 years vs. 57% 55–64 vs. 67% ≥65, p=0.0008). The oldest group also had the lowest incidence of prior autologous HCT (26% 40–54 vs. 24% 55–64 vs. 9% ≥65 years, p=0.002). 225 patients with aggressive and 73 patients with indolent NHL had a previous autologous HCT. Though not statistically differing across the age groups, 23–30% of all patients were transplanted with refractory disease including 30% in the oldest group. Use of an HLA-matched related sibling (MRD) or URD was similar across the age groups. The remaining variables of gender, performance status, interval from diagnosis to transplant, donor-recipient sex match, donor-recipient CMV serostatus, and donor-recipient HLA match were balanced across age cohorts. GVHD prophylaxis most often included a calcineurin inhibitor with or without methotrexate, but 37–46% of patients received T-cell depletion, particularly those in the oldest cohort. Median follow-up for the 3 cohorts ranged from 47–56 months.
Table 1.
Characteristics of 1,248 patients aged ≥ 40 years receiving RIC or NMA allogeneic HCT for NHL
| Patient Characteristics |
40–54 N (%) |
55–64 N (%) |
≥ 65 N (%) |
p-value |
|---|---|---|---|---|
| Number of patients | 614 | 552 | 82 | |
| Number of centers | 120 | 112 | 45 | |
| Age, median (range) | 49 (40–54) | 59 (55–64) | 67 (65–75) | <0.0001 |
| Male sex | 406 (66) | 359 (65) | 64 (78) | 0.06 |
| Karnofsky score prior transplant ≥80% | 512 (83) | 481 (87) | 71 (87) | 0.24 |
| Missing | 38 (6) | 30 (5) | 2 (2) | |
| Prior autologous transplant | 160 (26) | 131 (24) | 7 (9) | 0.002 |
| NHL Histology* | 0.0008 | |||
| Aggressive | 299 (49) | 314 (57) | 55 (67) | |
| Indolent | 315 (51) | 238 (43) | 27 (33) | |
| Disease status at transplant | 0.79 | |||
| CR1/CR2+ | 173 (28) | 160 (29) | 19 (23) | |
| PR1/PR2+ | 233 (38) | 218 (39) | 32 (39) | |
| Resistant disease | 155 (25) | 126 (23) | 25 (30) | |
| Unknown or untested sensitivity | 53 (9) | 48 (9) | 6 (7) | |
| Interval from diagnosis to transplant, | 0.12 | |||
| < 2 years | 217 (35) | 167 (30) | 31 (38) | |
| ≥ 2 years | 397 (65) | 385 (70) | 51 (62) | |
| Donor-recipient sex match | 0.04 | |||
| M-M | 262 (43) | 230 (42) | 51 (62) | |
| M-F | 122 (20) | 109 (20) | 9 (11) | |
| F-M | 144 (23) | 129 (23) | 13 (16) | |
| F-F | 86 (14) | 84 (15) | 9 (11) | |
| Donor-recipient CMV serostatus | 0.41 | |||
| −/− | 193 (31) | 142 (26) | 19 (23) | |
| −/+ | 171 (28) | 171 (31) | 24 (29) | |
| +/+ | 173 (28) | 170 (31) | 31 (38) | |
| +/− | 60 (10) | 55 (10) | 6 (7) | |
| Unknown | 17 (3) | 14 (3) | 2 (2) | |
| Graft source | 0.53 | |||
| Bone marrow | 84 (14) | 78 (14) | 15 (18) | |
| Peripheral blood | 530 (86) | 474 (86) | 67 (82) | |
| HLA Match | 0.29 | |||
| HLA-identical sibling | 262 (43) | 208 (38) | 29 (35) | |
| Unrelated donor, well-matched | 222 (36) | 234 (42) | 39 (48) | |
| Unrelated donor, partially matched | 95 (15) | 76 (14) | 9 (11) | |
| Unrelated donor, mismatched | 15 (2) | 17 (3) | 1 (1) | |
| Unrelated donor, missing | 20 (3) | 17 (3) | 4 (5) | |
| Year of transplant | <0.0001 | |||
| 2001–2004 | 363 (59) | 243 (44) | 36 (44) | |
| 2005–2007 | 251 (41) | 309 (56) | 46 (56) | |
| Conditioning regimen intensity | 0.05 | |||
| Reduced intensity | 333 (54) | 260 (47) | 43 (52) | |
| Nonmyeloablative | 281 (46) | 292 (53) | 39 (48) | |
| Conditioning regimen | 0.25 | |||
| TBI≤200cGy | 111 (18) | 129 (23) | 17 (21) | |
| TBI>200cGy | 21 (3) | 22 (4) | 4 (5) | |
| Alkylator only (Cy, Mel, Bu, Thio), no TBI | 443 (72) | 359 (65) | 57 (70) | |
| Other | 39 (6) | 42 (8) | 4 (5) | |
| GVHD prophylaxis | 0.43 | |||
| CSA±MTX±other | 205 (33) | 162 (29) | 19 (23) | |
| Tac±MTX±other | 179 (29) | 172 (31) | 25 (30) | |
| T-cell depletion** | 230 (37) | 218 (39) | 38 (46) | |
| Median follow-up of survivors, months | 56 (3–111) | 47 (2–111) | 47 (2–86) |
Abbreviations: CR:complete remission; PR:partial remission; HLA:human leukocyte antigen; M:male; F:female; CMV: cytomegalovirus; Cy:cyclophosphamide; Mel:melphalan; Bu:busulfan; Thio:thiotepa; TBI: total body irradiation; CSA: cyclosporine A; MTX: methotrexate; Tac:tacrolimus.
Detailed in Methods: Patient selection
Includes in vivo (anti-thymocyte globulin or alemtuzumab) and ex vivo T-cell depletion.
Neutrophil Recovery and GVHD
Neutrophil recovery by day 28 was similar across age groups (Table 2). Multivariate analysis demonstrated that older patients (Odds ratio (OR) 0.32, 95% CI 0.14–0.75; p=0.0085, KPS<80% (OR 0.28, 95% CI 0.21–1.85; p=0.0002), and refractory disease status at HCT (OR 0.25, 95% CI 0.10–0.60; p=0.002) were associated with lower likelihood of prompt engraftment. Use of PBSC grafts vs. marrow was associated with faster neutrophil recovery (OR 3.09, 95% CI 1.64–5.85; p=0.0005).
Table 2.
Univariate analysis of outcome for all patients aged ≥ 40 years receiving RIC or NMA allogeneic HCT for NHL
| Age group | 40–54 | 55–64 | ≥ 65 | ||||
|---|---|---|---|---|---|---|---|
| Outcome Event | N | Prob (95%CI) | N | Prob (95%CI) | N | Prob (95%CI) | P-valuea |
| Neutrophil Engraftment | 614 | 552 | 82 | ||||
| @ 28 days | 96 (95–97)% | 96 (94–97)% | 89 (82–95)% | 0.09 | |||
| Acute GVHD Grades II–IV | 614 | 552 | 82 | ||||
| @ 100 days | 35 (31–39)% | 34 (30–38)% | 33 (23–44)% | 0.92 | |||
| Chronic GVHD | 614 | 551 | 82 | ||||
| @ 3 years | 56 (52–60)% | 54 (49–58)% | 48 (37–59)% | 0.39 | |||
| Non-relapse mortality | 605 | 552 | 82 | ||||
| @ 100 days | 13 (10–16)% | 17 (14–20)% | 18 (11–28)% | 0.11 | |||
| @ 1 year | 22 (19–26)% | 27 (23–31)% | 34 (24–44)% | 0.05 | |||
| Progression/Relapse | 605 | 552 | 82 | ||||
| @ 3 years | 28 (24–32)% | 33 (29–37)% | 33 (23–44)% | 0.22 | |||
| Progression-free Survival | 605 | 552 | 82 | ||||
| @ 3 years | 44 (39–48)% | 32 (28–36)% | 27 (17–37)% | <0.0001 | |||
| Overall Survival | 614 | 552 | 82 | ||||
| @ 3 years | 54 (50–58)% | 40 (36–44)% | 39 (28–50)% | <0.0001 | |||
Pointwise p-value.
The incidence of grades II–IV acute GVHD (33–35% by day 100, p=0.92) and chronic GVHD (48–56% by three years, p=0.39) was similar across all age subgroups (Table 2). Multivariate analysis demonstrated no impact of age on acute or chronic GVHD. Multivariate analysis did, however, show a higher risk of acute GVHD in those not in CR at time of HCT (PR1/PR2 RR 1.37, 95% CI 1.08–1.75, p=0.01; Resistant RR 1.46, 95% CI 1.12–1.91, p=0.0005) and when using a RIC vs. a NMA regimen (RR 1.31, 95% CI 1.08–1.59; p=0.007). Use of a non-CSA GVHD prophylaxis regimen was associated with less acute GVHD (Tac±MTX±other RR 0.76, 95% CI 0.60–0.96, p=0.02; T-cell depletion RR 0.45, 95% CI 0.35–0.57, p<0.001). Compared to matched sibling HCT, URD and HLA mismatch (URD well matched 1.45, 95% CI 1.20–1.75, p=0.002; URD partially matched 1.75, 95% CI 1.35–2.26, p<0.001) and RIC conditioning regimens (RR 1.29, 95% CI 1.32–3.36; p<0.002) were all associated with higher incidence of chronic GVHD.
Non-relapse Mortality
Cumulative incidence of NRM did not differ among the age cohorts either at day 100 (13–18%; p=0.11) or 1 year (22–34%; p=0.05) (Table 2). Univariate analyses stratified by disease histology (aggressive vs. indolent) also showed no impact of age on NRM at these same time points (Table 3). As too few patients in the oldest cohort (n=7) had prior autologous HCT, we performed a separate analysis in the two younger cohorts comparing outcomes in those with or without prior autologous HCT. We observed higher 3 year NRM if patients had received an autograft before their allogeneic HCT (prior autograft 42%, 95% CI (25–31) vs. 28%, 95% CI (37–48), p<0.0001). In multivariate analysis, older age was associated with worse NRM (55–64 years RR 1.52, 95% CI 1.24–1.86, p<0.001; ≥65 years RR 1.57, 95% CI 1.08–2.29, p=0.02), although NRM was similar in the two older age cohorts (Table 4). Lower KPS, advanced disease at HCT, and less favorable HLA match also adversely impacted NRM.
Table 3.
Univariate outcomes by disease histology for patients aged ≥ 40 years receiving RIC or NMA allogeneic HCT for NHL
| 40–54 | 55–64 | ≥ 65 | |||||
|---|---|---|---|---|---|---|---|
| Outcome Event | N | Prob (95%CI) | N | Prob (95%CI) | N | Prob (95%CI) | P-valuea |
| Aggressive NHL | |||||||
| Non-relapse mortality | 297 | 314 | 55 | ||||
| @ 100 days | 13 (9–17)% | 16 (12–20)% | 24 (13–36)% | 0.15 | |||
| @ 1 year | 22 (17–27)% | 26 (22–32)% | 38 (26–51)% | 0.05 | |||
| Progression / Relapse | 297 | 314 | 55 | ||||
| @ 3 years | 34 (28–39)% | 36 (31–42)% | 29 (18–42)% | 0.55 | |||
| Progression-free Survival | 297 | 314 | 55 | ||||
| @ 3 years | 38 (32–44)% | 30 (25–35)% | 25 (14–37)% | 0.04 | |||
| Overall Survival | 299 | 314 | 55 | ||||
| @ 3years | 50 (44–56)% | 37 (31–43%) | 34 (21–47)% | 0.003 | |||
| Indolent NHL | |||||||
| Non-relapse mortality | 308 | 238 | 27 | ||||
| @ 100 days | 13 (9–17)% | 18 (13–23)% | 8 (1–20)% | 0.10 | |||
| @ 1 year | 23 (18–27)% | 27 (22–33)% | 24 (10–42)% | 0.46 | |||
| Progression / Relapse | 308 | 238 | 27 | ||||
| @ 3 years | 23 (18–27)% | 28 (22–34)% | 41 (23–61)% | 0.11 | |||
| Progression-free Survival | 308 | 238 | 27 | ||||
| @ 3 years | 49 (43–55)% | 35 (29–41)% | 30 (13–50)% | 0.003 | |||
| Overall Survival | 315 | 238 | 27 | ||||
| @ 3 years | 58 (52–63)% | 44 (38–51)% | 50 (30–79)% | 0.013 | |||
Pointwise p-value.
Table 4.
Multivariate analysis of NRM, relapse/progression, progression-free and overall survival after RIC or NMA allogeneic HCT for NHL
| N | Relative Risk (95% CI) | p-value* | Overall p-value | ||
|---|---|---|---|---|---|
| Non-relapse Mortality | |||||
| Age | |||||
| 40–54 | 614 | 0.0002 | |||
| 55–64 | 552 | 1.52 (1.24–1.86) | <.0001 | ||
| ≥ 65 | 82 | 1.57 (1.08–2.29) | 0.0189 | ||
| Significant covariates | |||||
| KPS | |||||
| ≥ 80 | 1064 | 0.0001 | |||
| < 80 | 114 | 1.87 (1.40–2.50) | <.0001 | ||
| NHL disease status | |||||
| CR1/CR2+ | 352 | <.0001 | |||
| PR1/PR2+ | 483 | 1.27 (0.99–1.63) | 0.0576 | ||
| Resistant disease | 306 | 1.90 (1.45–2.49) | <.0001 | ||
| HLA match | |||||
| HLA identical sibling | 499 | <.0001 | |||
| URD well matched | 495 | 1.36 (1.07–1.71) | 0.0116 | ||
| URD partially matched | 180 | 2.30 (1.74–3.03) | <.0001 | ||
| URD mismatched | 33 | 2.9 (1.76–4.77) | <.0001 | ||
| Relapse | |||||
| Age | |||||
| 40–54 | 614 | 0.059 | |||
| 55–64 | 552 | 1.29 (1.05–1.59) | 0.0176 | ||
| ≥ 65 | 82 | 1.18 (0.78–1.77) | 0.4321 | ||
| Significant covariates | |||||
| NHL disease status | |||||
| CR1/CR2+ | 352 | <.0001 | |||
| PR1/PR2+ | 483 | 1.67 (1.27–2.19) | 0.0002 | ||
| Resistant disease | 306 | 2.73 (2.04–3.64) | <.0001 | ||
| GVHD prophylaxis | |||||
| CSA+/−MTX+/−other | 386 | <.0001 | |||
| Tac+/−MTX+/−other | 376 | 0.88(0.67–1.16) | 0.3607 | ||
| T-cell depletion | 486 | 1.52 (1.18–1.95) | 0.0011 | ||
| Progression Free Survival | |||||
| Age | |||||
| 40–54 | 614 | 0.0001 | |||
| 55–64 | 552 | 1.37 (1.18–1.59) | <.0001 | ||
| ≥ 65 | 82 | 1.34 (1.01–1.76) | 0.0397 | ||
| Significant covariates | |||||
| KPS | |||||
| ≥ 80 | 1064 | <.0001 | |||
| < 80 | 114 | 1.63 (1.30–2.05) | <.0001 | ||
| NHL disease status | |||||
| CR1/CR2+ | 352 | <.0001 | |||
| PR1/PR2+ | 483 | 1.45 (1.21–1.75) | <.0001 | ||
| Resistant disease | 306 | 1.45 (1.88–2.78) | <.0001 | ||
| GVHD prophylaxis | |||||
| CSA+/−MTX+/−other | 386 | 0.0001 | |||
| Tac+/−MTX+/−other | 376 | 0.87 (0.72–1.06) | 0.1655 | ||
| T-cell depletion | 486 | 1.26 (1.05–1.50) | 0.0129 | ||
| HLA match | |||||
| HLA identical sibling | 499 | <.0001 | |||
| URD well matched | 495 | 1.13 (0.96–1.34) | 0.1468 | ||
| URD partially matched | 180 | 1.39 (1.12–1.72) | 0.0029 | ||
| URD mismatched | 33 | 2.28 (1.56–3.32) | <.0001 | ||
| Overall Survival | |||||
| Age | |||||
| 40–54 | 614 | <.0001 | |||
| 55–64 | 552 | 1.47 (1.25–1.72) | <.0001 | ||
| ≥ 65 | 82 | 1.47 (1.09–1.98) | 0.0127 | ||
| Significant covariates | |||||
| NHL disease status | |||||
| CR1/CR2+ | 352 | <.0001 | |||
| PR1/PR2+ | 483 | 1.29 (1.06–1.58) | 0.0113 | ||
| Resistant disease | 306 | 1.97 (1.60–2.44) | <.0001 | ||
| KPS | |||||
| ≥ 80 | 1064 | <.0001 | |||
| < 80 | 114 | 1.87 (1.48–2.37) | <.0001 | ||
| HLA match | |||||
| HLA identical sibling | 499 | <.0001 | |||
| URD well matched | 495 | 1.30 (1.09–1.56) | 0.0043 | ||
| URD partially matched | 180 | 1.90 (1.52–2.38) | <.0001 | ||
| URD mismatched | 33 | 2.21 (1.48–3.30) | 0.0001 | ||
| Conditioning Regimen | |||||
| Nonmyeloablative | 612 | 0.03 | |||
| Reduced Intensity | 636 | 1.19 (1.02–1.39) | 0.03 | ||
Abbreviations: KPS:Karnofsky performance status; CR:complete remission; PR:partial remission; HLA:human leukocyte antigen; URD:unrelated donor; GVHD:graft versus host disease; CSA:cyclosporine A; MTX:methotrexate; Tac:tacrolimus.
compared to reference group
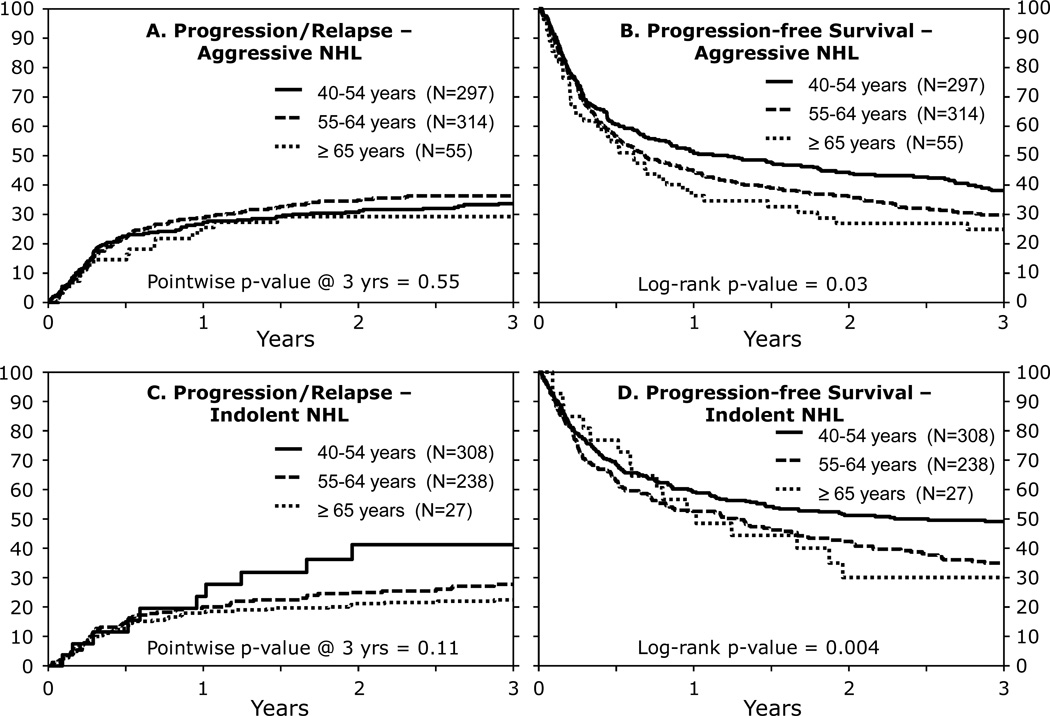
Relapse and Progression
Relapse and progression rates at 3 years were similar across all age groups and were confirmed in a separate analysis stratified by disease histology (Tables 2, 3; Figures 1A, 1C). In the younger two cohorts, relapse incidence was similar for patients receiving prior autologous HCT (both groups had 30% relapse at 3 years, p=0.88). Multivariate analysis demonstrated no significant impact of age, but the risk of progression or relapse worsened with advanced disease at HCT and by GVHD prophylaxis. T-cell depletion was associated with a significantly increased risk of progression or relapse (RR 1.52, 95% CI 1.18–1.95; p=0.001). The number of extramedullary sites involved and number of pre-transplant therapies did not influence the risks of relapse (p>0.1).
Figure 1. Three year relapse and progression-free survival based on histology.
(A) Progression/Relapse-Aggressive NHL
(B) Progression-free Survival-Aggressive NHL
(C) Progression/Relapse-Indolent NHL
(D) Progression-free Survival-Indolent NHL
Progression-Free and Overall Survival
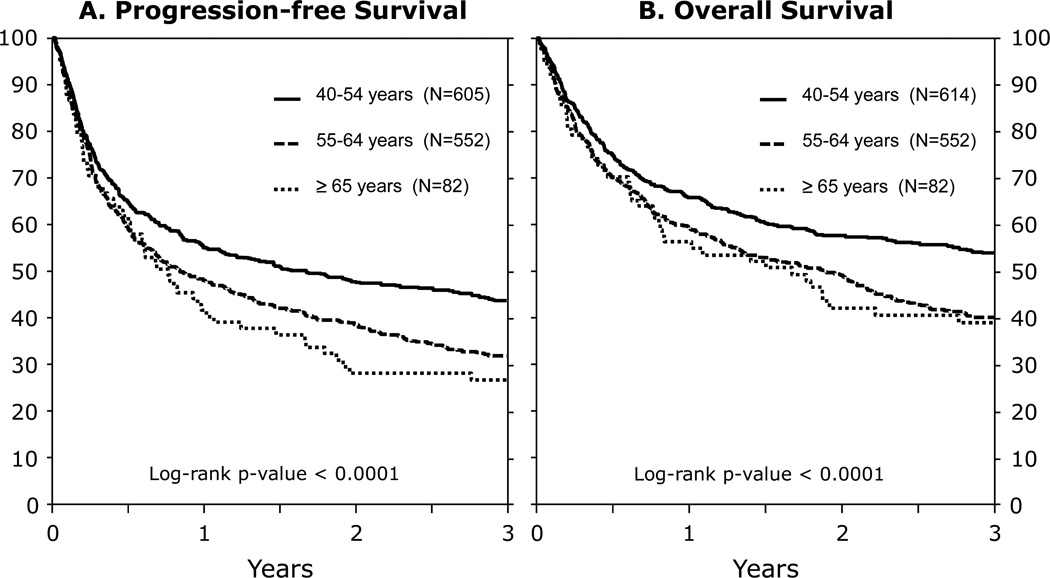
In univariate analysis, PFS at 3 years was highest in the youngest age group, but there was no difference in PFS between the two older cohorts (Table 2, Figure 2A). PFS also differed between age cohorts when patients were stratified by aggressive/indolent disease histology (Table 3, Figures 1B, 1D, 2A). Multivariate analysis (Table 4) showed that older age (≥55 and >65 years), lower KPS (<80%), disease status other than CR1/CR2, GVHD prophylaxis and greater HLA mismatch were all associated with inferior PFS.
Figure 2. Three year progression-free survival and overall survival following allogeneic HCT for NHL.
(A) Progression-free Survival
(B) Overall Survival
Three year OS also differed significantly across the age groups (Table 2, Figure 2B) though 39% of even the oldest cohort survive beyond 3 years. In the two younger cohorts, a prior autologous HCT was associated with lower 3 year OS (prior autograft 35%, 95% CI (30–41) vs. 52% (48–55), p<0.0001). In multivariate analysis, older age was associated with worse OS as was disease status at allogeneic HCT, KPS<80, HLA mismatch and use of a RIC regimen (Table 4). Following HCT, the primary causes of death were similar in the 3 age cohorts: relapse (33–37%), infection (17–21%), GVHD (14–17%), and organ failure (13–15%).
To examine the effect of histology on outcomes, we assessed the impact of five specific histologic subgroups on the incidence of relapse/progression, PFS and OS. In multivariate analysis we found no significant influence of these histologic subgroups on any outcome (all p values >0.1). Additionally, we saw no significant differences in any outcome whether patients were transplanted for a B or a T-cell NHL subtype (all p values>0.3). Neither extramedullary sites involved or number of pre-transplant therapies influence the risks of PFS or OS (p>0.1).
Discussion
We examined the impact of age in a large group of older patients receiving RIC or NMA allogeneic HCT for NHL. In this sizeable cohort, we found that only a modest number of patients over the age of 65 years received an allogeneic HCT for their disease. While age had a modest adverse effect in patients older than 55 compared to those 40–54 years, outcomes of patients age 55–64 and ≥65 years were equivalent with no significant differences in NRM, relapse, PFS or OS. Older age also did not influence the incidence of acute or chronic GHVD, major complications which might be tolerated poorly by older patients.
It is not surprising that HLA disparity, poorer performance status, T-cell depletion and advanced disease status at time of transplant adversely affected major HCT outcomes as each have been reported to have prognostic implications9,16–20. Aggressive NHL is also generally associated with worse outcomes21–22. Although histology subgroup had no significant effect on any HCT outcome in multivariate analysis, 67% of all patients in the ≥65 years and 57% in the 55–64 years age group had aggressive NHL. Small numbers of patients in the oldest age groups may have limited these analyses. The oldest cohort also rarely had prior autologous HCTs which precluded their inclusion for the effect of prior autologous HCT. In the two younger cohorts, however, we observed an adverse effect of prior autologous HCT on both NRM and OS. Other reports also demonstrate a modest negative influence of prior autologous HCT on subsequent allogeneic HCT. In two small series, Thomson et al. showed no influence of prior autologous HCT on outcomes23, while Rodriguez et al. showed that prior autologous HCT was associated with worse relapse and OS with a trend toward worse NRM24. Since autologous HCT remains the initial HCT option for most patients with NHL, the impact of prior autografting directly influences the selection of patients for allografting.
Even for patients with advanced NHL, the observed outcomes of RIC or NMA allogeneic HCT were encouraging. This is important as patients who relapse after autologous HCT have very poor median survivals reported between 3–8 months depending on histologic type of NHL25–27. In mantle cell lymphoma, a median survival of just 23 months in patients relapsed post-autologous HCT was seen with survival of only 6 months if relapse occurred within 1 year after HCT.28 In another report, relapsed/refractory diffuse large B cell lymphoma patients, 36% of patients receiving second line salvage therapy failed to respond and responses lasted only a median duration of only 4 months.29 Survival after relapse following autografting using an age-adjusted international prognostic index (IPI) showed PFS and OS rates of only 16% and 18% at 4 years even in those with chemo-sensitive disease27. In the current analysis, we observed 3 year survival of 39%, even in the oldest age group, suggesting that select older patients can benefit from allogeneic HCT and can have extended survival unattainable with other salvage approaches.
We were surprised at how few patients ≥65 years underwent allogeneic HCT even though over half of all NHL cases occur in patients ≥60 years30. Similar to observations in AML/MDS, most patients eligible for allogeneic HCT procedures are not referred and never receive such therapy31. Contemporary registry analyses from the EBMT and the CIBMTR examining relapsed high grade lymphomas emphasize the curative potential of this procedure32–33, yet recognize the rarity of its use. Thus HCT is uncommonly applied despite recent reports of improved HCT outcomes for patients in the modern era34–37.
We note the limitations of our study due to heterogeneity of pre-HCT therapies and no direct data to clarify the medical decision-making in selecting patients for allogeneic HCT. Detailed comorbidity information other than KPS was not available for this study though it may directly inform patient selection by physicians considering allogeneic HCT. Although some appropriate clinical selection bias exists in choosing only the fittest older patients for an allograft, these promising outcomes suggest that careful pre-transplantation judgments can identify patients able to tolerate this curative therapeutic approach.
Though our results suggest that outcome differences are modestly influenced by age, even the oldest patients had encouraging outcomes. Attention to disease stage, HLA disparity, and performance status prior to HCT could further improve these results by identification of NHL patients most likely to benefit from a RIC allogeneic HCT. Our analysis supports the referral of older patients with NHL for allogeneic transplantation.
Acknowledgements
Funding Support:
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
This project has been supported in part by funding from the National Marrow Donor Program and the Health Resources and Services Administration Contract No. HHSH234200637020C to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration or the National Marrow Donor Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
Contribution: DJW and BLM designed the study; DJW, BLM, KWA, HW, and TLP analyzed the data and made the figures; DJW and BLM wrote the paper; JHA, ASA, JYC, AD, COF, MH, LAH, MHJ, AAJ, MAKD, HML, AMM, RO, JP, MAP, JMR, WS, KWB, EKW and other listed co-authors from the Writing Committee provided critical review and approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Jantunen E, Sureda A. The evolving role of stem cell transplants in lymphomas. Biol Blood Marrow Transplant. 2012;18(5):660–673. doi: 10.1016/j.bbmt.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Thieblemont C, Coiffier B. Lymphoma in older patients. J Clin Oncol. 2007;25(14):1916–1923. doi: 10.1200/JCO.2006.10.5957. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Sandmaier BM, Storer BE, et al. Long term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100(13):4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 5.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(2):211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(5):538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum—Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champlin R, Khouri I, Shimoni A, et al. Harnessing graft-versus-malignancy: Non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111(1):18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 14.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118(15):4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen P, Klein J, Rosthoj S. Generalized linear models for correlated pseudo-observations, with applications to multi-state models. Biometrika. 2003;90:15–27. [Google Scholar]
- 16.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 17.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14(2):236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freytes CO, Zhang MJ, Carreras J, et al. Outcome of lower-intensity allogeneic transplantation in non-Hodgkin lymphoma after autologous transplantation failure. Biol Blood Marrow Transplant. 2012;18(8):1255–1264. doi: 10.1016/j.bbmt.2011.12.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigacci L, Puccini B, Dodero A, et al. Allogeneic hematopoietic stem cell transplantation in patients with diffuse large B cell lymphoma relapsed after autologous stem cell transplantation: a GITMO study. Ann Hematol. 2012;91(6):931–939. doi: 10.1007/s00277-011-1395-9. [DOI] [PubMed] [Google Scholar]
- 20.Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced –intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Besien K, Carreras J, Bierman PJ, et al. Unrelated donor hematopoietic cell transplantation for non-Hodgkin lymphoma: long-term outcomes. Biol Blood Marrow Transplant. 2009;15(5):554–563. doi: 10.1016/j.bbmt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale GA, Shrestha S, Le-Rademacher J, et al. Alternate donor hematopoietic cell transplantation (HCT) in non-Hodgkin lymphoma using lower intensity conditioning: a report from the CIBMTR. Biol Blood Marrow Transplant. 2012;18(7):1036–1043. doi: 10.1016/j.bbmt.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson KJ, Morris EM, Bloor A, et al. Favorable long-term survival after reducedintensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;27(3):426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12(12):1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Vose JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80(8):2142–2148. [PubMed] [Google Scholar]
- 26.Kewalramani T, Nimer SD, Zelenetz AD, et al. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;32(7):673–679. doi: 10.1038/sj.bmt.1704214. [DOI] [PubMed] [Google Scholar]
- 27.Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102(6):1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich S, Tielesch B, Rieger M, et al. Patterns and outcome of relapse after autologous stem cell transplantation for mantle cell lymphoma. Cancer. 2011;117:1901–1910. doi: 10.1002/cncr.25756. [DOI] [PubMed] [Google Scholar]
- 29.Elstrom RL, Martin P, Ostrow K, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10:192–196. doi: 10.3816/CLML.2010.n.030. [DOI] [PubMed] [Google Scholar]
- 30.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. [Accessed April 2013]. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 31.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109(4):1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 32.van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29(10):1342–1348. doi: 10.1200/JCO.2010.30.2596. [DOI] [PubMed] [Google Scholar]
- 33.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120(20):4256–4262. doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderón-Cabrera C, Márquez-Malaver FJ, de la Cruz-Vicente F, et al. Improvement over the years of long-term survival in high-risk lymphoma patients treated with hematopoietic stem cell transplantation as consolidation or salvage therapy. Transplant Proc. 2013;45(10):3665–3667. doi: 10.1016/j.transproceed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wondergem MJ, Dijkstra FS, Visser OJ, et al. Allogeneic transplantation after reduced-intensity conditioning with fludarabine-CY for both indolent and aggressive lymphoid malignancies. Bone Marrow Transplant. 2014 Jan 13; doi: 10.1038/bmt.2013.221. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Fenske TS, Zhang MJ, Carreras J, et al. Autologous or Reduced-Intensity Conditioning Allogeneic Hematopoietic Cell Transplantation for Chemotherapy-Sensitive Mantle-Cell Lymphoma: Analysis of Transplantation Timing and Modality. J Clin Oncol. 2013 Dec 16; doi: 10.1200/JCO.2013.49.2454. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]