Abstract
AIM
To characterize the prospective trajectory of cognitive development in children with new or recent onset epilepsy from baseline to 5 to 6 years after diagnosis.
METHOD
Sixty-nine children, (40 males, 29 females; age 8–18, with new or recent onset epilepsies underwent neuropsychological assessment shortly after diagnosis baseline (Wave 1), 2 years (Wave 2), and 5 to 6 years after diagnosis (Wave 3). intelligence, academic achievement, language, executive function, and psychomotor speed were evaluated.. Sixty-two children (28 males, 34 females; mean age 10y 9mo (SD 2y 2mo), range 8–18y) with typical development (comparison group) served as a comparison group at each time point. The cognitive data were examined by syndrome (localization-related epilepsy [LRE], idiopathic generalized epilepsy [IGE], comparison group). Mixed effect regression models compared trajectories among groups with respect to time since diagnosis.
RESULTS
Cognitive abnormalities exhibited by children with epilepsy in arithmetic computation, response inhibition, attention, fine motor dexterity, and psychomotor speed (all p values <0.001), are detectable at or near the time of diagnosis and largely remain stable over the ensuing 5 to 6 years without evidence of progressive worsening or recovery. This course is evident across both LRE and IGE groups, with the LRE group performing better for some outcomes (arithmetic, response inhibition, psychomotor speed) and never worse than the IGE group.
INTERPRETATION
Cognitive development in children with LRE and IGE is not characterized by progressive deterioration or lack of age-appropriate development; rather, development lags behind that of children with typical development. Cognitive abnormalities, when detected, are present near the time of diagnosis, persist over time, and require early intervention.
Community- and population-based investigations, as well as reports from tertiary care centers, show that childhood epilepsy can be associated with abnormalities in cognition, even among children with so-called ‘epilepsy-only’.1,2 These children have average intelligence, normal neuroimaging and neurological examinations, attend regular schools, and have epilepsy syndromes perceived by many clinicians to be benign and not concerning (e.g. benign epilepsy of childhood with centrotemporal spikes, childhood absence epilepsy).3–7 However, recent studies have demonstrated that abnormalities in cognition can be apparent at or near the time of epilepsy diagnosis,2,4,6 and that academic problems either antedate the first recognized seizure2,7 or are recognized soon after diagnosis.6,8 Aggregation of specific cognitive/academic abnormalities can also be observed in the families of some children with epilepsy, raising interest in potential environmental and genetic predispositions and contributions to cognitive disorders in childhood epilepsy.9–11 In addition, reports of abnormalities in quantitative brain imaging close to the time of diagnosis of epilepsy suggest that subtle neurodevelopmental brain abnormalities may underlie and contribute to these early cognitive and academic disruptions.1,12,13
What remains uncertain is the natural history of cognitive development in children with new onset epilepsies. A limited number of studies have examined cognition in children from the time of diagnosis of epilepsy onwards.2,4,8 All but one study14 report only one wave of subsequent assessment,8,15–18 involve relatively short test–retest intervals in many cases, and at times present limited cognitive assessment at each time point (e.g. IQ only).15,16 Here we report the results from a controlled serial assessment of cognition containing three waves of neuropsychological assessment up to 5 to 6 years after diagnosis. Two primary questions were addressed: (1) What are the developmental cognitive trajectories of children with new onset epilepsy compared with a healthy comparison group? (2) Are there differences in cognitive trajectories between children with localization related versus idiopathic generalized epilepsies?
METHOD
Participants
Sixty-nine children with recent onset epilepsy and 62 healthy first-degree cousin controls, aged 8 to18 years, were recruited from pediatric neurology clinics at three Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic). Children with epilepsy met the following initial inclusion criteria: a diagnosis of epilepsy within the past 12 months, no other developmental disabilities or neurological disorders, normal neurological examination, and normal neuroimaging (magnetic resonance imaging or computed tomography) results. The medical records for all patients were independently reviewed by a board-certified pediatric neurologist (before recruitment) to confirm that patients had idiopathic/genetic epilepsies and focal epilepsies of unknown etiology, to provide independent confirmation of syndrome diagnosis, and to ensure that other clinical criteria (e.g. normal neuroimaging) were met. Specific syndromes were classified using the modified diagnostic criteria of the International League Against Epilepsy Task Force on Classification and Terminology.19
Participants in the comparison group were first-degree cousins with no history of seizures, early initial precipitating injuries (e.g. febrile convulsions), other developmental or neurological disease, or history of loss of consciousness greater than 5 minutes. Participants in both groups attended regular school. Further details regarding subject selection criteria have been published previously by our group.20
Standard protocol approvals and patient consents
Approval to conduct this study was granted by the health sciences institutional review board at the University of Wisconsin School of Medicine and Public Health. Written informed consent was obtained from all legal guardians of the children and adolescents participating in the study. Written informed consent was obtained from research participants aged 18 years and over, and written informed assent was obtained from research participants aged 8 to 17 years.
Procedures
On the day of study participation, families and children gave informed consent and assent, after which the children underwent comprehensive neuropsychological testing. Parents participated in a clinical interview and completed a set of questionnaires to characterize details regarding gestation, delivery, neurodevelopmental and health histories, and seizure history of their child. Medical records pertinent to the child's epilepsy and treatment were obtained after signed release of information was obtained from the parent.
Neuropsychological assessment and analysis
All participants were administered a comprehensive test battery that included standard age-appropriate measures of intelligence, academic achievement, language, executive function, and speeded fine motor dexterity. Tests were selected not only for their pertinence to the cognitive domains of interest, but also for their broad applicable age ranges, so that the item pools were identical across the broad age range investigated here (as opposed to administering different versions of a test containing varying item pools to children across age categories), ensuring ability to directly and quantitatively characterize cognitive development. All tests are presented by domain (Table I). Children were seen for three waves of assessment: baseline (Wave 1), 2 years later (Wave 2), and 5 to 6 years after diagnosis (Wave 3).
Table I.
Neuropsychological tests, by domain, administered to epilepsy and control participants.
| Domain | Ability | Test |
|---|---|---|
| Academic achievement | Reading | Wide-Range Achievement Test – 3 (WRAT-3) (Reading)26 |
| Spelling | WRAT-3 (Spelling) | |
| Arithmetic | WRAT-3 (Arithmetic) | |
| Intelligence | Intelligence | Wechsler Abbreviated Scale of Intelligence (WASI; Full scale raw IQ)27 |
| Language | Expressive speech | Expressive Vocabulary Test (EVT)28 |
| Receptive speech | Peabody Picture Vocabulary Test-III (PPVT-III)29) | |
| Executive function | Problem solving | Delis-Kaplan Executive Function System (D-KEFS) (Confirmed Correct Sorts) |
| Response inhibitiona | D-KEFS (Color-Word Interference Test-Inhibition)30) | |
| Attentiona,b | Conners’ Continuous Performance Test – II CCPT-II (omission errors)22 | |
| Motor function | Motor dexteritya,b | Grooved Pegboard (log transformed)23 |
| Psychomotor speed | WISC-III (Digit Symbol-Coding)31 |
Reverse coded.
Log transformed for analysis.
Statistical analyses
Baseline and prospective neuropsychological data were examined as a function of syndrome (localization-related epilepsy, LRE; idiopathic generalized epilepsy, IGE; and the comparison group). The entire test battery consisted of 11 tests from which a total of 28 cognitive measures could be derived. Blinded to test results, the investigators focused the analyses on representative primary outcome measures derived from each of the 11 tests. Each of the test scores, grouped by functional domain was analyzed separately (Table I).
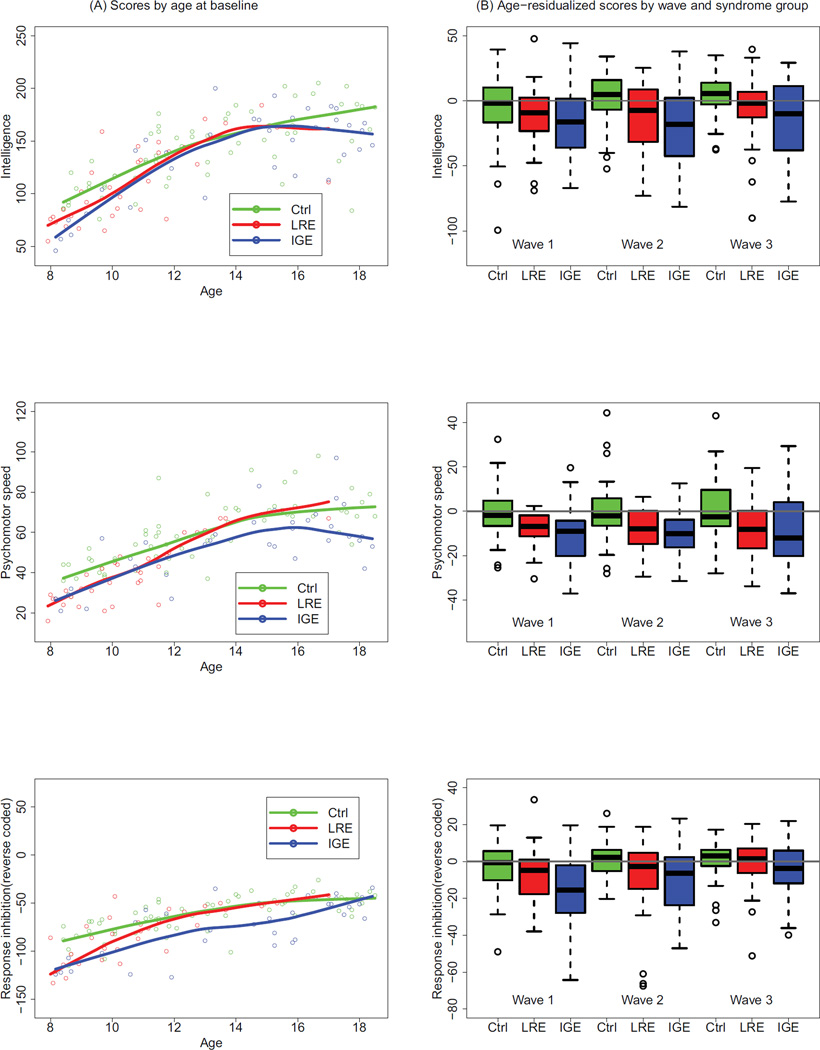
As exploratory analyses comparing LRE and IGE with the comparison group for each of 11 tests, the three groups were first compared at baseline (Wave 1) by plotting individual test scores against age, and including a flexible curve for each mean test score as a function of age.21 These plots served to present the joint distribution of test scores and age at baseline, and to display any systematic differences between the two groups across the range of ages at onset (e.g. Fig. 1a). Second, a flexible curve (not shown) for each mean test score was fitted to the comparison group test scores as a function of age, aggregating across the three waves, thus providing a reference for removal of normal age effects from test scores in the epilepsy group. Residuals from this curve were computed for each group (LRE, IGE, and comparison group) at each of the three waves of data, and the distribution of these residuals was compared between the groups via boxplots at each of three waves (e.g. Fig. 1b). The boxplot for Wave 1 (baseline) is a comparative summary among the groups, collapsed across age, of the residuals in the corresponding scatterplot versus age at baseline. The boxplots for Waves 2 and 3 (Years 2 and 5–6 years post-diagnosis) show how group differences persist or change with the number of years after diagnosis, after adjusting for trends in age under normal development (represented by the comparison group). This technique of data presentation was used because, as expected, age was a strong predictor of many of the test measures, with increasing age associated with improving test performance, reflecting normal neurodevelopmental trajectories. As such, a direct adjustment strategy was required to provide an unambiguous comparison among the groups as a function of years since baseline assessment.
Figure 1.
Intelligence, psychomotor speed, and response inhibition by age, wave, and diagnostic group. (a) Scatterplot of scores by age at baseline, with accompanying flexible curve fitted to data, for each of two epilepsy syndrome groups (LRE and IGE) and healthy comparison children. (b) Boxplots of age-residualized scores by syndrome group (LRE and IGE versus comparison group) and wave. The comparison group was used as a reference to compute residuals as explained in the text. The p values for fully adjusted comparisons are given in Table III. CG, comparison group; LRE, localization-related epilepsy; IGE: idiopathic generalized epilepsy. Boxplots follow standard statistical practice. Briefly, the top, bottom, and middle of the box represent the third and first quartiles and the median respectively. The bar at the top of the upper whisker is the largest data point that is within 1.5 times the interquartile range (IQR) from the third quartile, where the IQR is the difference between the third and first quartiles. A symmetric description applies to the bar at the bottom of the lower whisker. The data points outside the whiskers are greater than 1.5 times the IQR from the third or first quartiles, may be considered outliers, and hence are worthy of their own display. {Typesetter Change AB labels to lower case; change ‘Ctrl’ to ‘comparison group; add ‘y’ after ‘Age’ on x-axes on panel ‘a’}
Formal analyses were conducted to determine whether there were systematic differences in neuropsychological status between the major epilepsy syndrome groups as well as relative to healthy comparison group, either at baseline or over a prospective trajectory. Analyses used linear mixed effects models with a random intercept and a random slope in age to account for response (neuropsychological test score) trajectories varying from person to person. Models included an age-squared term to allow for a non-linear relationship between test scores and age, and adjusted for sex. Age at baseline and age at onset were so highly correlated (0.97) that it was not necessary or appropriate to adjust for age at onset.
In the context of this model, group effects were tested in three ways. First, in an omnibus test of group differences in overall trajectory, models included group and the interaction of group × wave and jointly tested the two terms. This assessed the null hypothesis of no group effect at any time versus the alterative of a group effect at baseline and/or a trend in group differences (i.e. differences in slope) across the three waves of assessment. Second, only if the first test rejected the null hypothesis, post-hoc tests were conducted for the group effect at each of three waves of assessment. This was accomplished by entering wave into the model as a categorical variable interacted with group, and then constructing contrasts reflecting group differences at each wave. Finally, again, only if the omnibus test rejected the null hypothesis, trend tests were conducted to determine if there were differences in slope among the groups across the three waves. These tests provide further insight into whether omnibus differences reflect relatively constant group differences over time since diagnosis, or alternatively, whether group differences grow or shrink over time.
Analyses were repeated for each of 11 test variables. Log transformation was used for non-normally distributed measures (Connors Continuous Performance Test (CCPT)-II Omissions and Grooved Pegboard), and after log-transformation, CCPT-II Omissions were reverse-coded as a measure of attention (versus inattention) and timed measures (response inhibition, motor dexterity) were reverse coded as well so that poorer performance was associated with lower scores relative to the comparison group. Graphics were prepared in R (R Core Team.: R Foundation for Statistical Computing, Vienna, Austria) and models were fitted and tested using PROC MIXED in SAS version 9.2 (SAS Institute, Cary, NC, USA). Tests are presented in terms of p values derived from F tests, accompanied by indicators of direction of effect across the three waves.
Multiple comparisons
The analyses described above involved 11 omnibus tests. To control family-wise, or overall, Type I error rate, at alpha=0.05, we used a procedure developed by Hochberg.22 This procedure is more powerful than both the Holm modification to the Bonferroni procedure or the Bonferroni procedure itself.23 Briefly, this procedure puts the 11 p values in descending order. If the first one is less than 0.05, the entire set of 11 null hypotheses is rejected. If not, we advance to the next test and compare it with alpha=0.05/2, and so on comparing with 0.05/3, 0.05/4, until one test rejects, in which case all subsequent tests (with smaller p values) reject as well. Having controlled family-wise error rate for the omnibus test, post-hoc tests are only performed when omnibus tests are significant according to this procedure. We have presented our results in terms of nominal p values, and indicate when those p values fall below the Hochberg critical value (Table III).
Table III.
Omnibus tests and post-hoc follow-up tests of longitudinal epilepsy syndrome group versus healthy control differences in 11 cognitive tests
| Omnibus | Post-hoc pa | |||||
|---|---|---|---|---|---|---|
| Response | pa | Direction | Slope | Base- line |
Year 2 | Year 5 |
| Reading | 0.4945 0.495 |
|||||
| Spelling | 0.2205 0.221 |
|||||
| Arithmetic | 0.0001 <0.001 |
CG>LRE>IGE | 0.5351 0.535 |
<0.0011 | 0.0203 0.020 |
0.008 |
| Intelligenceb | 0.0495 0.049 |
|||||
| Expressive speech | 0.0536 0.054 |
|||||
| Receptive speech | 0.5455 0.546 |
|||||
| Problem solving | 0.1454 0.145 |
|||||
| Responseb inhibition | <0.0001 <0.001 |
CG>LRE>IGEc | 0.0008 0.001 |
<0.0011 | 0.0019 0.002 |
0.294 |
| Attention | 0.0065 0.007 |
CG>LRE=IGE | 0.8975 0.898 |
0.0129 0.013 |
0.0045 0.005 |
0.006 |
| Fine motor dexterity | <0.0001 <0.001 |
CG>LRE=IGE | 0.4109 0.411 |
0.0003 <0.001 |
0.0092 0.009 |
<0.010 |
| Psychomotorb speed | <0.0001 <0.001 |
CG>LRE>IGE | 0.2076 0.208 |
<.0001 | <.0001 | <0.000 |
Details and multiple comparison adjustments are given in the text.
Critical values (not shown) for omnibus tests were based on ordered Hochberg correction for multiple comparisons across the 11 responses. Post-hoc p values not reported for responses for which omnibus test nominal p values were higher than the Hochberg critical value.
Intelligence, response inhibition and psychomotor speed are presented in Figure 1, and the remaining scales are in the online supplementary information.
For response inhibition, the contrast among groups decreased markedly over time, with LRE similar to the comparison group (CG) at year 5. (See Fig. 1, bottom right panel).
RESULTS
Research participants consisted of 131 children aged 8–18, including adolescents with recent onset epilepsy (n=69):35 with LRE (21 males, 14 females; mean age 10y 9mo, SD 2y 3mo); 34 with IGE (19 males, 15 females; mean age 14y 1mo, SD 3y 5mo); and 62 healthy first-degree cousin comparison children (28 males, 34 females; mean age 13y, SD 3y; Table II). IGE participants had later ages at onset and therefore, as result of our design, were older than LRE participants, with the comparison children falling in between; the study group had more males relative to the comparison group, and LRE participants were less likely to be on antiepileptic drugs at baseline than IGE participants. All subsequent analyses were adjusted for differences in sex and age.
Table II.
Baseline demographic and clinical characteristics of epilepsy syndrome group (LRE and IGE) and healthy comparison participants
| Variables | LRE (n=35) | IGE (n=34) | Comparison group (n=62) |
|---|---|---|---|
| Age, mean(SD), y:mo | 10:9 (2:32) | 14:1 (3:5) | 13:0 (3:0) |
| Seizure duration, mean (SD), mo | 7.7 (3.7) | 8.6 (3.6) | --- |
| Age at onset mean(SD), y | 9:98 (2:3) | 13:3 (3:6) | --- |
| Sex (male/female) | 21 /14 | 19 /15 | 28 /34 |
| Antiepileptic drugs (polytherapy, monotherapy, none) frequency) | 0/23/12 | 3/31/0 |
The localization-related epilepsy (LRE) group comprised 16 children with benign epilepsy with centrotemporal spikes; 7 with temporal lobe epilepsy, and 12 children with focal epilepsy not otherwise specified); Idiopathic generalized epilepsy (IGE) group comprised 22 children with juvenile myoclonic epilepsy, 11 with absence epilepsy and, 1 with IGE not otherwise specified.
Scatterplots and flexible curve fits for IQ, psychomotor speed, and reverse coded response inhibition (Fig. 1a panels) against age at baseline are presented as examples of outcomes for intelligence, processing speed, and executive function. Higher scores indicate better performance. For example, Figure 1a (top) shows the curve for raw IQ scores, which depicts the normal development of intellectual ability with increasing age for all three groups, as well as the lack of an interaction between group and age. The plots also show good coverage across the younger age range for all three groups; however, for the LRE group, there is weak representation in the older ages, so conclusions applied to older ages should only refer to IGE versus controls. Boxplots comparing test scores across study waves for these same measures, after flexibly removing age effects, are presented in Figure 1b, panels. Figures S1 to S3 (online supporting information) show all other test scores.. Table III lists test results in the form of p values arising from F tests for each omnibus group test comparing trajectories among groups and, in separate post-hoc tests, for those omnibus tests that were significant according to the Hochberg correction, for each wave of testing and for slope effects. As illustrated by comparing the table with the figures, the ‘Direction’ column in Table III indicates the order of groups with respect to performance.
There were significant differences by omnibus test among the three groups (LRE, IGE, and the comparison group) on tests of arithmetic, response inhibition, attention, fine motor dexterity, and psychomotor speed. These differences were significant and in the same direction comparing IGE, LRE, and the comparison group across all three waves of testing for all of these measures except response inhibition. This can be seen both by the significant wave-specific group effects and the non-significant group effects in slope. Typical response patterns are shown in Figures S1 and S2, panel B, (online supporting information). In contrast with the other outcomes, response inhibition showed strong group contrasts at baseline, but these differences declined over time, indicated by the significant slope effect and the non-significant group effect at year 5 (see Fig. S3, panel B, online supporting information). For intelligence, the omnibus test was not quite significant, but the pattern, comparison group >LRE>IGE, was fairly consistent across waves. There was no overall difference between the three groups for reading, spelling, expressive and receptive speech, and problem solving.
DISCUSSION
Our findings indicate that cognitive development in children with new onset ‘epilepsy only’ is characterized by significant differences in baseline cognitive status compared with the comparison group, the differences typically persisting in an unchanged fashion over subsequent waves of assessment up to 6-years post diagnosis. This pattern tends to be similar for children with LRE and IGE, but when differences exist, children with IGE are more adversely affected. These and other points are discussed below.
Comparing children with epilepsy (both LRE and IGE) with the comparison group the cognitive abnormalities that are present at baseline tend to be maintained, neither improving nor worsening, over the second wave (2 years after baseline) and third wave (5–6 years after baseline) of cognitive assessment. It is now well established that cognitive abnormalities can be detected at or near the time of diagnosis,2,6 even before the administration of antiepilepsy medications4, and there is presumptive evidence that cognitive and academic abnormalities may exist in some children antecedent to the first recognized seizure and diagnosis.4,7,20 Controlled investigations tracking cognitive development from the time of diagnosis onwards is limited both in number and scope. Here we were able to obtain serial assessments out to 5 to 6 years post-diagnosis, which served to facilitate the detection of subtle but significantly altered developmental trajectories, either declining or recovering to normative levels. The core finding is that, because significant interactions between group and time were rarely observed, the cognitive abnormalities identified at baseline persist in an essentially unchanged fashion over time, representing static cognitive abnormalities. Thus, the presence of childhood epilepsy clearly affects cognition early on but appears to have minimal adverse impact on subsequent cognitive neurodevelopment relative to development in a healthy population.
This same pattern is evident when examining children with LRE and IGE. Again, both broad syndrome groups differ from the comparison group at baseline, with the IGE generally doing no better, and for some tests worse, than the LRE group, at baseline and in their developmental trajectories, with identified baseline cognitive differences maintained over the subsequent waves of assessment.
Additionally, it is important to note that not all cognitive abilities are affected equally. The most vulnerable domains appear to involve motor and psychomotor speed, executive functions including attention and inhibition, and arithmetic computation skills. Some ability areas appear less affected including core language functions (word naming and comprehension) and elemental academic skills (word reading and spelling). The data suggested a weak effect for overall intelligence.
The primary clinical implications of these findings are as follows. First, even in children with uncomplicated epilepsies, many of which have been assumed to be benign in nature (e.g. benign epilepsy of childhood with centrotemporal spikes, absence), cognitive abnormalities are not only present, but are present early in the course of the disorder including at or near the time of diagnosis and treatment initiation. Second, the abnormalities that are present at that time will be largely static over the next 5 to 6 years and require attention as academic performance is likely to be affected. Finally, this finding represents a unique early window of opportunity to attempt to influence life course. Very long-term (decades) outcome studies have shown that even children with uncomplicated epilepsies may underperform in important life areas2 and early intervention should be given the opportunity to affect this course.
Study limitations include the modest number of children who were examined by broad syndrome classification (LRE, IGE). It is possible that specific syndromes of LRE and/or IGE may show unique cognitive courses. We continue to accrue research participants and plan to examine this possibility in the future. In addition, with longer follow-up it will be possible to characterize the impact of these early trajectories on important life course milestones (e.g. education, occupation, and income) and the impact, if any, of seizure remission. The clinical significance of these findings remains to be determined and efforts are under way to expand the clinical assessment of important cognitive domains (e.g. academic achievement) going forward. This investigation included only children with idiopathic/genetic epilepsies and focal epilepsies of unknown etiology—and these results pertain only to this population. It is possible that children with structural, metabolic, and other etiologies may have a different natural history. Finally, we were unable to link cognitive course to details of seizure frequency or severity realizing that such attempts are difficult given the known limitations of patient self-reported seizure frequency/severity, especially in light of the very brief and subtle nature of some of these childhood epilepsies.
Conclusions
The childhood epilepsies investigated here are characterized by early (near onset and diagnosis) cognitive abnormalities that persist in an essentially unchanged fashion over time, (up to 6 years) without evidence of progression or recovery, and preferentially affecting some abilities (motor and psychomotor speed, executive functions, some academic skills) while sparing others.
Supplementary Material
What this paper adds.
How the cognitive abnormalities that are present at epilepsy onset unfold over time remains uncertain.
Here cognitive and academic problems were seen at or near the time of diagnosis.
Abnormalities remain static over time, neither worsening nor recovering over 5 to 6 years.
ACKNOWLEDGEMENTS
All phases of this study were supported by NINDS 3RO1–44351 and by the Clinical and Translational Science Award (CTSA) program through the NIH Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The funding source was not involved in study design, data collection, analysis or manuscript preparation. We thank Raj Sheth MD and Monica Koehn MD for study participation and subject recruitment. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management.
ABBREVIATIONS
- IGE
Idiopathic generalized epilepsy
- LRE
Localization-related epilepsy
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
Supporting information
The following additional material may be found online.
Figures S1–S3: Test scores.
REFERENCES
- 1.Lin JJ, Riley JD, Hsu DA, et al. Striatal hypertrophy and its cognitive effects in newonset benign epilepsy with centrotemporal spikes. Epilepsia. 2012;53:677–685. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann BP, Jones JE, Jackson DC, Seidenberg M. Starting at the beginning: the neuropsychological status of children with new-onset epilepsies. Epileptic Disord. 2012;14:12–21. doi: 10.1684/epd.2012.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. New Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 4.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with ‘epilepsy only’--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 5.Caplan R, Siddarth P, Stahl L, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 6.Fastenau PS, Johnson CS, Perkins SM, et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526–534. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg AT, Smith SN, Frobish D, et al. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- 8.Dunn DW, Johnson CS, Perkins SM, et al. Academic problems in children with seizures: Relationships with neuropsychological functioning and family variables during the 3years after onset. Epilepsy Behav. 2010;19:455–461. doi: 10.1016/j.yebeh.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Clarke T, Strug LJ, Murphy PL, et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48:2258–2265. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AB, Kavros PM, Clarke T, Dorta NJ, Tremont G, Pal DK. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 2012;53:705–711. doi: 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012;53:301–307. doi: 10.1111/j.1528-1167.2011.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widjaja E, Kis A, Go C, Raybaud C, Snead OC, Smith ML. Abnormal white matter on diffusion tensor imaging in children with new-onset seizures. Epilepsy Res. 2013;104:105–111. doi: 10.1016/j.eplepsyres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Addis L, Lin JJ, Pal DK, Hermann B, Caplan R. Imaging and genetics of language and cognition in pediatric epilepsy. Epilepsy Behav. 2012;26:303–312. doi: 10.1016/j.yebeh.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oostrom KJ, van Teeseling H, Smeets-Schouten A, Peters AC, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behaviour in children with 'epilepsy only'. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 15.Bourgeois BF, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: a prospective study in children. Ann Neurol. 1983;14:438–444. doi: 10.1002/ana.410140407. [DOI] [PubMed] [Google Scholar]
- 16.Ellenberg JH, Hirtz DG, Nelson KB. Do seizures in children cause intellectual deterioration? New Engl J Med. 1986;314:1085–1088. doi: 10.1056/NEJM198604243141705. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell WG, Scheier LM, Baker SA. Psychosocial, behavioral, and medical outcomes in children with epilepsy: a developmental risk factor model using longitudinal data. Pediatrics. 1994;94:471–477. [PubMed] [Google Scholar]
- 18.Hermann BP, Jones JE, Sheth R, et al. Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia. 2008;49:1847–1858. doi: 10.1111/j.1528-1167.2008.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 20.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with newonset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 21.Hastie TJT, R J. Generalized Additive Models. 1ed. Chapman & Hall/CRC; 1990. [Google Scholar]
- 22.Conners CK. The Connors’ Continuous Performance Test. Toronto, Canada: Multi-Heath Systems; 1995. [Google Scholar]
- 23.Trites RL. Neuropsychological test manual. Ottawa, ON, Canada: Royal; 1977. [Google Scholar]
- 24.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1998;75:800–802. [Google Scholar]
- 25.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson GS. Wide Range Achievement Test: Manual. 3rd ed. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 27.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 28.Williams KT. Expressive Vocabulary test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 29.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd edn. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 30.Delis DC, Kaplan E, Kramer JH. Delis–Kaplan executive function system. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 31.Wechsler D. Wechsler Intelligence Scale for Children. 3rd edn. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.