Abstract
Current MR methods use T2* relaxation time as a surrogate measure of ligament strength. Currently, a multi-echo voxel-wise least squares fit is the gold standard to create T2* maps; however, the postprocessing is time-intensive and serves as a stopgap for clinical use. The study objective was to determine if an alternative method could improve post-processing time without sacrificing fidelity of T2* values for eventual translational use in the clinic. Using a 6 echo FLASH sequence, three different methods were used to determine intact posterior cruciate ligament (PCL) median T2*. Two of these methods utilized a voxel-wise method to establish T2* maps: 1) a current “gold standard” method using a voxel-wise 6 echo least-squares fit (6LS) and 2) a voxel-wise 2 echo point T2* determination (2MM). The third method used median ligament signal intensity and a single nonlinear least-squares fit (6LSROI) instead of a voxel-wise basis. The resulting median T2* values of the PCL and computational time were compared. The median T2* values were 42% higher using the 2MM compared to the 6LS method (p<0.0001). However, a strong correlation was found for the median T2* values between the 2MM and 6LS methods (R2= 0.80). The median T2* values were not significantly different between the 6LS and 6LSROI methods (p=0.519). Using the 2MM (which provides a regional map) and the 6LSROI (which efficiently provides the median T2* value) methods in tandem would take only minutes of postprocessing computational time compared to the 6LS method (~540 minutes), and hence would facilitate clinical application of T2* maps to predict ligament structural properties as a patient outcome measure.
Keywords: T2* relaxometry, MRI, Posterior Cruciate Ligament
INTRODUCTION
Non-invasive evaluation of a ligament’s structural properties using magnetic resonance imaging (MRI) would be valuable for quantifying tissue healing in research and clinical settings. Current methods use either signal intensity (SI) (Biercevicz et al., 2013a) or T2* relaxation time (Biercevicz et al., 2013b) as surrogate measures of ligament strength. While SI measures have been successfully used to predict ligament strength in terms of structural properties (maximum load, yield load, linear stiffness)(Biercevicz et al., 2013a; Weiler et al., 2001), these SI measures are dependent on MR scan parameters and can vary between scan sessions and manufacturers, making cross-institutional studies or development of a universal assessment standard difficult (Deoni et al., 2008).
Alternatively, T2* is an inherent tissue property, is less sensitive to scan parameters, and has been shown to be reproducible across similar strength magnets and sites (Deoni et al., 2008). However, current T2* methods involve acquiring multiple-echo sequences at high-resolution. These three-dimensional, high-resolution images are essential for accurately characterizing small structures like the posterior cruciate ligament (PCL). In a research setting, T2* maps generated from these high-resolution images are critical for identifying potential pathology and mapping regional variations as they relate to the biomechanical properties within a ligament (Biercevicz et al., 2013b). Currently, a voxel-wise multi-echo least squares fit is the gold standard to create T2* maps (Haacke et al., 1999). Multi-echo least squares fit relaxometry maps have been proven to be accurate, minimally sensitive to noise (Johnson et al., 1987) and helpful to visualize regions of interest (ROI). Unfortunately, the post-processing associated with a least-squares fit is time-intensive and thus difficult to implement clinically. While post-processing time can be reduced by decreasing total voxel number, high resolution scans are required to properly characterize small ligament structures. However, the T2* fitting function could be modified to make it time appropriate for a clinical setting without decreasing resolution. One method would be to use only two echo times (Haacke et al., 1999), allowing for the point determination of T2* using matrix math (2MM), which would be many magnitudes faster than the least squares fit algorithm (Biercevicz et al., 2013b; Dunn et al., 2004). However, two-point estimations have been shown to overestimate relaxation times (Deoni et al., 2008; Kingsley et al., 1998).
A completely different method is theoretically possible. For most relaxometry studies, a ROI is extracted from the full field of view (FOV) T2* map and simple summary statistics (such as median and quartiles) are used to summarize the ligament T2* values. These summary statistics are important as quantifiable surrogate measures of structural properties (Biercevicz et al., 2013b; Koff et al., 2013) and to characterize tissue homogeneity. However, the summary is done after the time-intensive post-processing required to generate the T2* maps. A different method would be to extract the median ligament SI for the ROI at each echo time and then use a nonlinear least-squares fit to directly calculate median T2* from the ROI median SI values (6LSROI) (Glaser et al., 2006). Despite being many magnitudes faster than the voxel-wise gold standard method, this T2* post processing approach needs to be validated for ligament tissue on a 3T magnet.
The purpose of this study was to validate two alternative methods of T2* determination (2MM and 6LSROI) in comparison to a current gold standard (6LS) by determining the differences in fidelity and post-processing time between the methods. We hypothesized that there would be a strong correlation between median T2* values for 2-echo point estimation (2MM) and voxel-wise six-echo nonlinear least squares fit gold standard (6LS). We also hypothesized that there would be no significant difference between the gold standard (6LS) and ROI (6LSROI) median T2* values for each ligament, and that there would be a strong correlation between gold standard (6LS) and 6LSROI T2* quartile values. These two alternative approaches (2MM and 6LSROI), used in tandem, would take significantly less post-processing time than the gold standard, and could be combined to offer a tool for assessing ligament structural properties.
METHODS
With IACUC approval, 12 adult sheep underwent unilateral ACL transection surgery followed by bio-enhanced ACL repair as previously described (Murray et al., 2010). After 20 weeks of healing, the animals were euthanized and the knees were harvested.
Imaging
Using the FLASH sequence, a high resolution three-dimensional data set, utilizing 6 echo times, (TR/TE/FA, 33/4.3, 7.3, 10.2, 13.1, 16.0 & 18.9/17°; FOV, 180 mm; matrix 512×512, slice thickness/gap, 0.8mm/0; avg 1; bandwidth 407) was acquired of the injured knee on a 3T scanner (Siemens Trio), immediately after limb harvest. The total scan time was 19 minutes. The intact PCL in the operative joint was then segmented from the image stack (Mimics 15.0; Materialize, Ann Arbor, MI) and three-dimensional models were created (Biercevicz et al., 2013a, 2013b) to establish ligament ROI. To create a worst-case scenario for detecting differences in the computation of T2* across the three different computational methods, the intact PCL was chosen as a standard of comparison to reduce variability in the MR variables T2* and volume. The same MR data set, ROIs, programming language (MatLab: MathWorks, Natick, MA) and computational hardware (Intel Xeon E5540 Processor, 2 Cores, 2.53 GHz, 16 GB RAM, OSCAR High-Performance Computing Cluster, Brown University) were used for all 3 post processing methods to determine T2* of the intact PCL.
Post processing: T2* determination
Gold standard: 6 echo least squares fit T2* Map (6LS)
For the gold standard T2* map, a voxel-wise nonlinear least-squares fit of voxel SI versus echo time for T2* estimation (6LS) was used. SI from all six echo times along with the SI relationship, SI (TE) = M0 e−TE/T2* + DC, where SI (TE) are the voxel specific SIs for the various echo times (TE). The three fit parameters are M0 (equilibrium magnetization), T2* and the DC offset (DC), which were used for the least squares fit estimation of T2* (Haacke et al., 1999).
Two Echo determination T2* Map (2MM)
For the first alternative method, SI from the 7.3 and 16.0 ms echo times (Biercevicz et al., 2013b) (TE) along with the SI relationship, (Haacke et al., 1999) where SI1 and SI2 are the SIs corresponding to the echo times TE1 and TE2 and where TE2 > TE1 for each voxel, was used for a 2-point estimation of T2*. The PCL voxels were extracted from both the 2MM and 6LS maps using the ligament ROIs (Figure 1). From these ligament specific maps, histograms of the voxel-wise T2* were plotted for both the 6LS and 2MM method. From each ligament’s histogram, summary statistics (median, 1st quartile and 3rd quartile) were calculated for the 6LS and 2MM methods.
Figure 1.
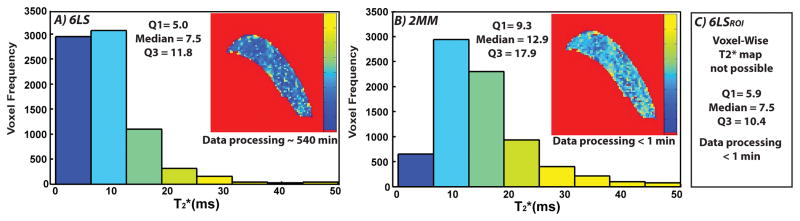
Example T2* histograms and sagittal ligament ROI maps of the intact PCL, A) determined using a voxel-wise 6 echo least squares fit (6LS) and B) determined using a voxel-wise 2 echo point determination (2MM). C) Median, Q1, Q3 T2* determined using the 6LSROI method, no histogram or map available with this method.
SI Region of Interest Median T2* (6LSROI)
For the second alternative method, the SI voxels corresponding to the ligament were extracted from all 6 echo times using the ligament ROIs. The SI summary statistics were then calculated for each ROI at each echo time. The median SI summary statistic along with the relationship SImedian (TE) = M0 e−TE/T2Median* + DC, where SImedian represents the median ROI SI for the various echo times (TE), and the three fit parameters are the ligament’s median M0, T2* and DC offset (DC), were used for a least squares fit estimation of median T2*. The same was done for the first and third quartile T2* summary statistics.
Statistics
The statistical differences of the summary statistics between the 2MM and 6LS methods, as well as between the 6LS and 6LSROI were tested using paired t-tests with Bonferroni correction. The linear relationships between the 2MM and 6LS methods and between the 6LSROI median T2* and the 6LS were tested using linear regression. Finally, as a general measure of clinical feasibility, the processing time was compared between methods.
RESULTS
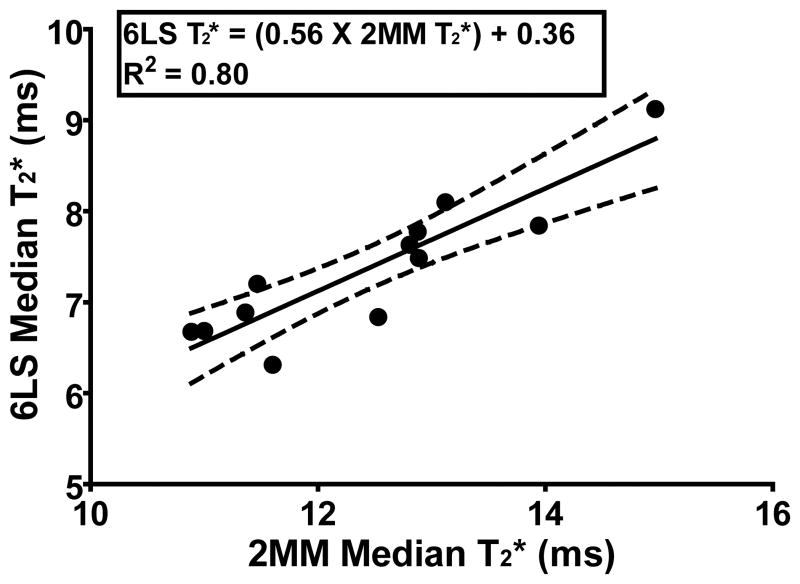
The T2* values (median, 1st quartile and 3rd quartile) were significantly higher with the 2MM method than the 6LS method (p<0.0001) (Figure 1, Table 1). The median T2* value of the 2MM method was, on average, 41.9% greater than that of the 6LS method. There was a strong linear relationship between the 2MM and 6LS median T2* values (p<0.0001) (R2= 0.80) (Figure 2, Table 1). A full FOV T2* map was not available with the 6LSROI method because the T2* values were not calculated on a voxel-wise basis. The median T2* values were not significantly different between the 6LS than the 6LSROI methods (p=0.519) (Table 1, Fig 1). The 1st and 3rd quartile T2* values were significantly different between the 6LS and the 6LSROI method (p=<0.001), and had a strong linear relationship (p<0.0001 for both) (R2= 0.88 and 0.66 respectively) (Table 1). The average processing time for the 6LS, 2MM and 6LSROI methods was ~540, < 1, < 1 minutes, respectively (Figure 1, Table 1).
Table 1.
Summary Statistics for different T2* determination methods comparison.
| Summary Stat | Relationship | Paired t-test | R2 |
|---|---|---|---|
| Median | 2MM/6LS | (P ≤ 0.001)* | 0.8 (P ≤ 0.001) |
| Median | 6LSROI/6LS | (P = 0.519) | 0.84 (P ≤ 0.001) |
| Q1 | 6LSROI/6LS | (P ≤ 0.001)* | 0.88 (P ≤ 0.001) |
| Q3 | 6LSROI/6LS | (P ≤ 0.001)* | 0.66 (P ≤ 0.001) |
Statistically significant differences
Figure 2.
Linear regression of 2MM median T2* values vs 6LS median T2* values
DISCUSSION
As expected (Deoni et al., 2008), the median and quartile T2* values for the 2MM method were significantly higher than the 6LS method, due to the natural log and exponential forms of the 2MM and 6LS methods, respectively. This indicates the absolute T2* values derived from the 2MM method will be overestimated compared to values derived using a least squares fit function. However, the highly significant correlation between the 2MM and the 6LS (gold standard) methods (Figure 2) indicates the the two methods offer similar relative insight into tissue integrtiy. This signifies that the relative distribution of T2* values in the 6LS generated map can be visualized with the 2MM map (Biercevicz et al., 2013b). This could make the 2MM T2* maps helpful for identifying potential pathology and regional variations as they relate to the biomechanical properties within a ligament. (Figure 1).
As hypothesized, there was no statistical difference between T2* median values for the 6LS and 6LSROI methods, signifying the 6LSROI method could be used to calculate a median T2* value for a ligament. While the quartile values were statistically different between the 6LS and 6LSROI methods, the strong linear relationship between them (R2=0.88 and 0.66 for Q1 and Q2, respectively) indicates that the findings seen with the gold standard 6LS method would be observed with the 6LSROI method. This strong linear relationship is critical because ROI statistics provide another tool for characterizing structural properties (Biercevicz et al., 2013a), homogeneity and potential pathology within a tissue.
At approximately 540 minutes, the magnitude of computational time for the gold standard method would be a stop gap for transitioning this method to both clinical and research settings. Seperately, the 2MM and 6LSROI methods, despite being computationally efficient (< 1 minute), have limitations. The 2MM, while having an inter-specimen relationship similar to the gold standard, overestimates T2*, while the 6LSROI method does not provide a T2* map for viewing pathology within a ligament. However, as previously mentioned, the strong linear relationship between the median T2* values for the 2MM and 6LS methods indicates that the predictable difference between their T2* values could make the 2MM method effective for viewing relative regional variations within structures (Figure 1). Therefore, a combination of the 2MM method (which provides the regional map for viewing pathology and quality control) and the 6LSROI protocol (which provides the median T2* value for quantifying tissue integrity) could provide clinics with a powerful ligament structural properties assessment tool requiring minimal computational time.
This study is limited in that the images were acquired post mortem. Future work will evaluate how this relationship changes in vivo. Additionally, the post processing times would vary due to computer hardware, processing software and code optimization. However, even with increases in computational speed or efficiency, the time for 6LS method will remain orders of magnitude longer, reducing its clinical feasibility. Furthermore, 6 echos were chosen to perform the multi-echo least-squares fit, though previous relaxometry studies can vary from 2 to 12 or more echos (Dunn et al., 2004; Wansapura et al., 1999). 6 echos was chosen as the gold standard to balance accuracy, scan time (19 minutes/specimen) and post-processing time.
Assessing T2* values of a ROI to determine ligament structural properties is very useful in a research setting (Koff et al., 2013; Williams et al., 2012). However, time intensive post processing could stop translation to clinical use. While the application of the 2MM and the 6LSROI methods are by themselves limited, using a combination of the 2MM map and the 6LSROI median T2* values (combined post processing time < 2 minutes) instead of the traditional voxel-wise 6LS method (post processing time ~540 minutes) could be a viable option to improve processing time and could be valuable clinically.
Acknowledgments
Funded by the National Institutes of Health (RO1-AR056834; RO1-AR054099; P20-GM104937) and the Lucy Lippitt Endowed Professorship. The authors wish to thank Benedikt Proffen, Brian Kelly, Emily Robbins and Scott McAllister for their assistance. All imaging was done at Brown University Magnetic Resonance Facility.
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflict of interest to report
References
- Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In Situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. The American Journal of Sports Medicine. 2013a;41:560–566. doi: 10.1177/0363546512472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biercevicz AM, Murray MM, Walsh EG, Miranda DL, Machan JT, Fleming BC. T2* MR Relaxometry and Ligament Volume Are Associated With the Structural Properties of the Healing ACL. Journal of Orthopaedic Research. 2013b doi: 10.1002/jor.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Williams SCR, Jezzard P, Suckling J, Murphy DGM, Jones DK. Standardized structural magnetic resonance imaging in multicentre studies using quantitative T1 and T2 imaging at 1.5 T. NeuroImage. 2008;40:662–671. doi: 10.1016/j.neuroimage.2007.11.052. [DOI] [PubMed] [Google Scholar]
- Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis1. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser C, Mendlik T, Dinges J, Weber J, Stahl R, Trumm C, Reiser M. Global and regional reproducibility of T2 relaxation time measurements in human patellar cartilage. Magn Reson Med. 2006;56:527–534. doi: 10.1002/mrm.21005. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Brown RW, Thompson MR, Venkatesan . Magnetic resonance imaging: physical principles and sequence design. A John Wiley and Sons; New York, NY: 1999. [Google Scholar]
- Johnson G, Ormerod IE, Barnes D, Tofts PS, MacManus D. Accuracy and precision in the measurement of relaxation times from nuclear magnetic resonance images. The British Journal of Radiology. 1987;60:143–153. doi: 10.1259/0007-1285-60-710-143. [DOI] [PubMed] [Google Scholar]
- Kingsley PB, Ogg RJ, Reddick WE, Steen RG. Correction of errors caused by imperfect inversion pulses in MR imaging measurement of T1 relaxation times. Magnetic Resonance Imaging. 1998;16:1049–1055. doi: 10.1016/s0730-725x(98)00112-x. [DOI] [PubMed] [Google Scholar]
- Koff MF, Shah P, Pownder S, Romero B, Williams R, Gilbert S, Maher S, Fortier LA, Rodeo SA, Potter HG. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthr Cartil. 2013;21:1083–1091. doi: 10.1016/j.joca.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-Bone Fixation Enhances Functional Healing of the Porcine Anterior Cruciate Ligament Using a Collagen-Platelet Composite. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26:S49–S57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball WS. NMR relaxation times in the human brain at 3.0 tesla. Journal of Magnetic Resonance Imaging. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging - A two-year study in sheep. Am J Sports Med. 2001;29:751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- Williams A, Qian Y, Golla S, Chu CR. UTE-T23 mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]