Summary
Objective
To examine the influence of parent and family general and epilepsy-related stress on longitudinal generic and epilepsy-specific HRQOL for children with new-onset epilepsy, controlling for demographic characteristics, disease factors, and antiepileptic drug adherence.
Methods
This prospective, longitudinal study included 124 children with new-onset epilepsy (mean age=7.2 years, SD=2.9 years). Parents completed questionnaires on parenting stress, perceived stigma, fears and concerns, and HRQOL at 1, 13, and 25-months post-diagnosis. Adherence to antiepileptic medication was assessed using electronic monitors. A medical chart review was conducted at each visit to obtain seizure and side effect data.
Results
Higher levels of general and epilepsy-specific parent and family stress, fears and concerns, and perceived stigma negatively impacted child generic and epilepsy-specific HRQOL, above and beyond disease and demographic factors. General parenting and family stress impacted child generic and epilepsy-specific HRQOL more in the first year of disease management than at 2 years post-diagnosis. Higher fears and concerns predicted higher epilepsy-specific HRQOL at 13 months post-diagnosis, whereas 2 years post-diagnosis, higher fears and concerns predicted lower epilepsy-specific HRQOL. Several demographic (i.e., age) and disease-related variables (i.e., side effects, AED adherence) influenced child generic and epilepsy-specific HRQOL. While some findings were consistent across generic and epilepsy-specific HRQOL measures, others were unique.
Significance
Modifiable parent factors (i.e., general and disease-specific parent and family stress, perceived stigma) impact HRQOL for children with new-onset epilepsy differently over the first two years post-diagnosis. Psychosocial interventions to improve HRQOL within the first year post-diagnosis should address parenting and family stress, overall coping, and anticipatory guidance on managing epilepsy. Interventions targeting adherence, perceived stigma, and fears and concerns could improve HRQOL. Promoting parent management of stress, fears/concerns, and perceived stigma may lead to improved child HRQOL outcomes.
Keywords: psychosocial functioning, patient-reported outcome, side effects, adherence, stress, stigma
Health-related quality of life (HRQOL) is an important patient-reported outcome that has been defined in a number of ways. For example, a recent review of HRQOL measures used in the pediatric literature revealed that measures purported to assess HRQOL (i.e., defined as “a child's goals, expectations, standards or concerns about their overall health and health-related domains”) also assess components of other related constructs such as patient functioning, disability, and health and general quality of life.1 In the current manuscript, HRQOL is defined as the impact of a health condition on a patient's functioning across domains (e.g., school, emotional, social).
In pediatric epilepsy, clinical guidelines recommend that treatment goals not only consist of achieving seizure control, but also attaining good HRQOL.2, 3 Patients may experience significant psychosocial comorbidities and family stressors that impact HRQOL, particularly in school, emotional, and social domains.4, 5 These areas of functioning may not necessarily improve with medical treatment alone, even if seizure freedom is achieved. In fact, side effects from antiepileptic drugs (AEDs), the primary treatment for epilepsy, may contribute to HRQOL impairments.6 Development of effective psychosocial interventions to improve HRQOL is essential; however, the first step is to identify modifiable family factors that negatively influence child HRQOL.
Research has consistently documented poor HRQOL in children with epilepsy.3, 6-8 More recently, studies have examined biological and psychosocial predictors of HRQOL, including seizures, AED side effects, maternal depressive symptoms, and child psychological functioning.3, 6, 9, 10 This is consistent with current conceptual frameworks describing the complex interactions between biological (i.e., non-modifiable) and psychosocial (i.e., modifiable) predictors of HRQOL in children with epilepsy.11 While previously examined biological and psychosocial variables predict some variability in HRQOL outcomes in pediatric epilepsy, there are several important modifiable parent-related variables that have yet to be studied, including parenting stress, perceived stigma, and parental epilepsy-related worries and concerns.11 These parent-related variables could have immediate and concurrent impacts on HRQOL.
Parent factors are particularly important for HRQOL outcomes, especially for younger children.11, 12 While the literature indicates that caregiver adjustment (e.g., parenting stress, depressive symptoms) impacts HRQOL for children with other chronic conditions,13-15 little is known in pediatric epilepsy.9 It is also unknown how general parenting stress and stress specific to epilepsy may differentially impact child HRQOL. In addition, perceived stigma, and fears about epilepsy could impact child HRQOL outcomes given their impact on HRQOL among adult populations.16-18 Understanding the unique role of parenting factors (e.g., general parenting stress and epilepsy-specific stress), perceived stigma, and fears and concerns about epilepsy that contribute to psychosocial aspects of HRQOL above and beyond disease management factors may inform family-based interventions to improve child HRQOL.
Researchers use both generic, which allows for cross-disease comparison, and disease-specific HRQOL measures, which are more sensitive and specific, in pediatric epilepsy.19, 20 However, few studies have used both measures even though they provide unique information that is beneficial to understanding patient outcomes.19, 21 In summary, it is essential to identify modifiable, psychosocial factors impacting generic and disease-specific HRQOL over time, which would inform the development and timing of interventions for improving HRQOL outcomes.
The current prospective, longitudinal study examined modifiable, parent psychosocial factors (general and epilepsy-related stress) predicting HRQOL at three critical time points post-diagnosis (i.e., 1 month post-diagnosis, following AED titration and initial adjustment to diagnosis and treatment; 13 months post-diagnosis, treatment maintenance; 25 months post-diagnosis, earliest that AED wean could occur), after controlling for demographic characteristics, disease variables, and AED adherence. The primary aim was to identify the cross-sectional and immediate impact of parent factors (i.e., parent fears and concerns, general and epilepsy-specific parenting stress, perceived stigma) on psychosocial domains of children's generic and epilepsy-specific HRQOL. Epilepsy-specific HRQOL across other HRQOL domains were explored in secondary analyses. We hypothesized that increased parent-reported general and epilepsy-specific stress, as well as parental fears/concerns and stigma would predict poorer generic and epilepsy-specific HRQOL above and beyond demographic, disease, and adherence variables at these critical time points.
Methods
Participants and Procedure
Participants were recruited from a New Onset Seizure Clinic in a Midwestern children's hospital at epilepsy diagnosis for a two-year longitudinal study examining adherence to AEDs, predictors of AED adherence, and health outcomes. Inclusion/exclusion criteria for this prospective longitudinal study included: 1) new diagnosis of epilepsy (i.e., patient had not received a prior diagnosis of epilepsy and was diagnosed the day of recruitment); 2) aged 2-12 years; 3) no comorbid chronic illnesses requiring routine medications; 4) no significant parent-reported developmental disorders (e.g., autism, severe intellectual disability); and, 5) no prior AED treatment. A trained research assistant obtained written parental consent and assent (children 8 years and older).
At study entry, participants completed a demographics form and received an electronic monitor to assess AED adherence, a MEMS™ TrackCap. The participants were instructed to place their AED in the bottle with the MEMS™ TrackCap. Patients returned for routine clinical care one month after diagnosis and every three months thereafter (e.g., 4- and 7-months post-diagnosis) and study visits coincided with these clinic visits over the course of two years. At each study visit, the MEMS™ TrackCap was downloaded and parents completed a battery of questionnaires. Medical chart review was also conducted at each study visit to obtain disease information, and AED side effects. The study was approved by the hospital's Institutional Review Board.
Measures
Pediatric Quality of Life Inventory (PedsQL ™)
The PedsQL™ is a 23-item generic HRQOL measure designed for parents of children between 2 and 18 years of age22. Higher scores indicate better HRQOL. The Total, Emotional, Social, and School scales were included in the present analyses. Cronbach's alphas for the current sample ranged from 0.78-0.90.
Quality of Life in Childhood Epilepsy Questionnaire (QOLICE)
The QOLICE is 79-item parent proxy report of the child's HRQOL for ages 4 to 18.23 The measure assesses 15 domains of functioning and an overall HRQOL score. Higher scores indicate better HRQOL. To align with the PedsQL™ measure, the following subscales were used as primary outcomes: Energy and Fatigue, Depression, Anxiety, Social Activities, Social Interaction, and Total QOL. Attention, Memory, Language, Other Cognition, Control/Help, Self-Esteem, Behavior, and Physical Restrictions were examined as exploratory outcomes. Cronbach's alphas in the current sample ranged from 0.44-0.90.
Background Information Form
Parents completed a form regarding information about the child's age, gender, race/ethnicity, and socio-economic status. Socio-economic status was assessed using the revised Duncan score, which ranges from 15 to 97 with higher scores indicating higher socio-economic status. 24, 25
Parent Report of Psychosocial Care
The Parent Report of Psychosocial Care measures the concerns, needs for care, and satisfaction with care perceived by parents of children with new-onset seizures.26 The fears and concerns subscale was used for the present study, and assessed the extent of their concerns and fears that their child's seizures will result in a loss of intelligence, brain damage or death, that the seizures are caused by a brain tumor or that the medication used to treat the seizures will cause addiction. Higher scores indicate more fears and concerns. Cronbach's alpha for the current sample was 0.85.
Parenting Stress Index (PSI)
The PSI is a 120-item parent self-report measure that assesses the degree to which stress is related to parent functioning, the behavioral and temperamental qualities of the child, and the parent-child relationship in children ages 1 month to 12 years.27 The Total Stress Scale was used for the present study. Higher scores indicate higher levels of general stress. Cronbach's alpha for the current sample was 0.93.
Stigma Scale
Stigma was measured using a 5-item scale assessing parental perception of stigma toward the child with epilepsy.28 Higher scores signify greater perceived stigma. Cronbach's alpha for the current sample was 0.72.
Family Stress Scale-Seizure Version (FSS-Seizure)
The FSS-Seizure is a 14-item epilepsy-specific scale of parenting stress with an acceptable Cronbach's alpha (.71).29 The total score was used in the present study. Higher scores indicate higher levels of disease-related stress. Cronbach's alpha for the current sample was .86.
Seizure Occurrence
At each study visit, parents reported on the absence or presence of children's seizures since the last visit. This information was also confirmed using healthcare provider's notes in the medical record.
Pediatric Epilepsy Side Effects Questionnaire (PESQ)
The PESQ is a 19-item measure of AED side-effects in youth ages 2-18 with epilepsy.30 Items cover a broad range of neurological, behavioral, gastrointestinal, skin, and motor side effects. Total scores were used for this study and Cronbach's alpha for the current sample was 0.96.
MEMS® 6 TrackCap
The Medication Event Monitoring Systems (MEMS)™ TrackCap made by AARDEX Corporation was used to measure daily adherence to AEDs. Data from the TrackCap was downloaded at each clinic visit and documents each time the bottle was opened. For the 13-month and 25-month time points, percent adherence was calculated for the 3-month intervals before those visits (i.e., number of doses taken divided by number of doses prescribed). For example, 70 doses taken / 90 doses prescribed = .78 or 78% adherence. Three-month adherence data was not available for the time period prior to the 1-month visit.
Analytic Plan
Descriptive statistics were calculated for participant demographic characteristics and the dependent variables (i.e., HRQOL). A quadratic value for medication adherence was calculated and used to examine potential HRQOL differences at both extremes of adherence. Hierarchical linear regressions were conducted with HRQOL as the dependent variable at 1 month, 13 months, and 25 months post-diagnosis. Specifically, separate regressions were run for each PedsQL and QOLICE scale. The regressions controlled for baseline levels of HRQOL and demographic characteristics (i.e., SES, child age). For the 13- and 25-month regressions, 1- month total parenting stress, fears and concerns, stigma, and family stress were included in the next step. For all regressions, seizure occurrence, total side effects, percent medication adherence, and quadratic adherence were entered in the next step. For all regressions, the final step included total parenting stress, fears and concerns, total stigma, and family stress. Pairwise deletion was used for all regression analyses, meaning that participants contributed information to any analysis for which they had data (i.e., a participant could be dropped from a regression if they were missing relevant data, but could still be included in a different regression if they had data to contribute to that analysis). A significance level of p<.01 was used for determining statistical significance due to the multiple analyses conducted. To facilitate interpretation of results when the quadratic adherence term was significant, predictors were centered. All analyses were conducted in SPSS version 21.0.
Results
One-hundred and thirty consecutive children with new-onset epilepsy and their parents were approached for study participation. Five families declined participation due to busy schedules and lack of interest. One family provided written consent, but was later found to not meet study eligibility criterion and was thus excluded. Thus, the final sample included 124 children. Sample sizes varied by analyses due to missing data. Overall, sample retention was 64.5% (17.7% were lost to medical follow-up, 5.6% withdrew, 3.2% moved, 2.4% weaned off the prescribed AED, 2.4% did not send measures back, 2.4% did not start or discontinued the AED, and 1.6% changed healthcare providers.)
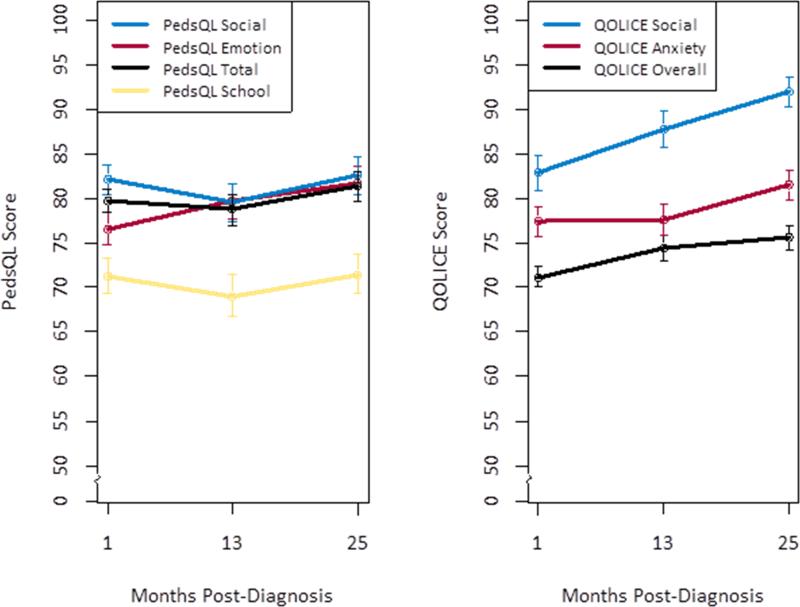
Baseline participant demographic and medical characteristics are contained in Table 1. Preliminary examination of Cronbach's alphas revealed poor internal consistency (α ≤.5) for several epilepsy-specific subscales (e.g., Social Interaction, Depression, and Energy/fatigue) and were thus excluded from analyses. Average generic and epilepsy-specific HRQOL across time is depicted in Figure 1.
Table 1.
Participant and Epilepsy-Specific Descriptive Statistics (N = 124)
| Characteristics | |
|---|---|
| Child Age (mean ± SD) | 7.2 ± 2.9 |
| Child Male (%) | 61.3 |
| Child Race (%) | |
| Caucasian | 75.0 |
| African-American | 16.9 |
| Asian | 0.8 |
| Other | 1.6 |
| Biracial | 4.8 |
| Caregiver Relation to Child (%) | |
| Mothers | 83.1 |
| Fathers | 12.9 |
| Step-mothers | 1.6 |
| Other (e.g., legal guardian) | 2.4 |
| Caregiver Marital Status (%) | |
| Married | 62.9 |
| Single | 20.2 |
| Divorced | 11.3 |
| Separated | 4.0 |
| Remarried | 0.8 |
| Widowed | 0.8 |
| Socioeconomic Status (mean ± SD)a | 52.4 ± 20.4 |
| Epilepsy Diagnosis (%) | |
| Idiopathic Localization-related | 47.6 |
| Idiopathic Generalized | 18.5 |
| Unclassified | 15.3 |
| Cryptogenic Localization-related | 8.1 |
| Cryptogenic Generalized | 4.8 |
| Symptomatic Localization-related | 4.8 |
| Symptomatic Generalized | 0.8 |
| Initial Prescribed Anti-Epileptic Drug (%) | |
| Carbamazepine | 60.5 |
| Valproic Acid | 39.5 |
Note.
Based on Duncan TSEI2 with occupations equivalent to property managers, physicians’ assistants, mail carriers, and sheriffs/law enforcement.
Figure 1.
Generic and disease-specific health-related quality of life across 2 years post-diagnosis.
PedsQL = Pediatric Quality of Life Inventory
QOLICE = Quality of Life in Childhood Epilepsy Questionnaire
Both disease factors (i.e., side effects) and parent psychosocial functioning factors (i.e., parenting stress) predicted HRQOL at 1 month post-diagnosis (Table 2). The overall variance in HRQOL accounted for by the predictors ranged from 19% to 43%. At 13 months post-diagnosis (Table 3), disease (i.e., seizure occurrence, quadratic adherence) and psychosocial factors (i.e., fears and concerns, parenting stress, stigma, and family stress) were most predictive of total epilepsy-specific HRQOL, accounting for 80% of the variance in HRQOL (F(14,47)=13.38, p<.01). Psychosocial factors (i.e., family stress, stigma) also predicted epilepsy -specific HRQOL subscales (i.e., anxiety, social activity) and the social subscale of generic HRQOL at 13 months. At 25 months post-diagnosis (Table 4), side effects were the primary predictor of generic and epilepsy-specific HRQOL, with the overall variance in HRQOL accounted for ranging from 37% to 73%. Specifically, higher levels of side effects predicted lower HRQOL.
Table 2.
Predictors of health-related quality of life at 1-month follow-up
| Analyses | Variables | b | β | Δ R2 |
|---|---|---|---|---|
| DV= PedsQL Total HRQOL | ||||
| Family Duncan | 0.05 | 0.07 | 0.06 | |
| Age in months | −0.07 | −0.16 | -- | |
| Seizure History | −2.71 | −0.09 | 0.25 | |
| Side Effects | −0.55 | −0.42** | -- | |
| Fears and Concerns | −0.40 | −0.11 | 0.12 | |
| PSI: Total Stress | −0.10 | −0.35** | -- | |
| Stigma | 1.18 | 0.06 | -- | |
| FSS: Family Stress | −0.01 | 0.17 | -- | |
| DV= PedsQL Emotional HRQOL | ||||
| Family Duncan | 0.31 | 0.03 | 0.01 | |
| Age in months | −0.02 | −0.04 | -- | |
| Seizure History | −0.33 | −0.01 | 0.10 | |
| Side Effects | −0.43 | −0.24* | -- | |
| Fears and Concerns | −0.43 | −0.09 | 0.19 | |
| PSI: Total Stress | −0.18 | −0.44** | -- | |
| Stigma | 5.23 | 0.20 | -- | |
| FSS: Family Stress | −0.23 | −0.10 | -- | |
| DV = PedsQL Social HRQOL | ||||
| Family Duncan | 0.67 | 0.74 | 0.02 | |
| Age in months | −0.03 | −0.05 | -- | |
| Seizure History | −3.33 | −0.09 | 0.07 | |
| Side Effects | −0.27 | −0.16 | -- | |
| Fears and Concerns | −0.99 | −0.22* | 0.17 | |
| PSI: Total Stress | −0.12 | −0.32** | -- | |
| Stigma | 0.64 | 0.03 | -- | |
| FSS: Family Stress | −0.18 | −0.08 | -- | |
| DV = PedsQL School HRQOL | ||||
| Family Duncan | 0.12 | 0.12 | 0.08 | |
| Age in months | −0.13 | −0.22 | -- | |
| Seizure History | −5.24 | −0.13 | 0.21 | |
| Side Effects | −0.63 | −0.34** | -- | |
| Fears and Concerns | 0.29 | 0.06 | 0.05 | |
| PSI: Total Stress | −0.07 | −0.18 | -- | |
| Stigma | 1.37 | 0.05 | -- | |
| FSS: Family Stress | −0.21 | −0.09 | -- | |
| DV = QOLICE Total HRQOL | ||||
| Family Duncan | 0.01 | 0.02 | 0.01 | |
| Age in months | −0.01 | −0.03 | -- | |
| Seizure History | −2.28 | −0.10 | 0.30 | |
| Side Effects | −0.43 | −0.43** | -- | |
| Fears and Concerns | 0.05 | −0.02 | 0.12 | |
| PSI: Total Stress | −0.06 | −0.29** | -- | |
| Stigma | −1.15 | −0.11 | -- | |
| FSS: Family Stress | −0.05 | −0.04 | -- | |
| DV = QOLICE Anxiety HRQOL | ||||
| Family Duncan | 0.10 | 0.13 | 0.13 | |
| Age in months | −0.14 | −0.29** | -- | |
| Seizure History | −1.96 | −0.06 | 0.07 | |
| Side Effects | −0.15 | −0.10 | -- | |
| Fears and Concerns | 0.45 | 0.11 | 0.11 | |
| PSI: Total Stress | 0.02 | 0.06 | -- | |
| Stigma | −4.14 | −0.20 | -- | |
| FSS: Family Stress | −0.59 | −0.30* | -- | |
| DV = QOLICE Social Activities HRQOL | ||||
| Family Duncan | −0.01 | −0.01 | 0.02 | |
| Age in months | 0.13 | 0.23* | -- | |
| Seizure History | −1.97 | −0.05 | 0.12 | |
| Side Effects | −0.56 | −0.31** | -- | |
| Fears and Concerns | −0.10 | −0.02 | 0.04 | |
| PSI: Total Stress | −0.01 | −0.04 | -- | |
| Stigma | −5.03 | −0.20 | -- | |
| FSS: Family Stress | 0.05 | 0.02 | -- | |
Note.
p<.05
p<.01
PedsQL=Pediatric Quality of Life Inventory, QOLICE=Quality of Life in Childhood Epilepsy Questionnaire, PSI=Parenting Stress Inventory, FSS=Family Stress Scale
Table 3.
Predictors of health-related quality of life at 13-month follow-up.
| Analyses | Variables | b | β | Δ R2 |
|---|---|---|---|---|
| DV= PedsQL Total HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.12 | 0.15 | 0.07 | |
| Age in months | −0.15 | −0.32** | -- | |
| Fears and Concerns | 0.17 | 0.04 | 0.17 | |
| PSI: Total Stress | 0.05 | 0.15 | -- | |
| Stigma | 0.40 | 0.02 | -- | |
| FSS: Family Stress | −0.40 | −0.20 | -- | |
| 13 months | ||||
| Seizure History | 2.88 | 0.08 | 0.19 | |
| Side Effects | −0.39 | −0.25 | -- | |
| Adherence | −0.12 | −0.21 | -- | |
| Adherence quadratic | 0.00 | 0.20 | -- | |
| Fears and concerns | −0.86 | −0.14 | 0.12 | |
| PSI: Total Stress | −0.10 | −0.29 | -- | |
| Stigma | −0.43 | −0.02 | -- | |
| FSS: Family Stress | −0.66 | −0.29 | -- | |
| DV= PedsQL Emotional HRQOL | ||||
| 1 month | ||||
| Family Duncan | −0.02 | −0.02 | 0.01 | |
| Age in months | −0.05 | −0.10 | -- | |
| Fears and Concerns | −0.51 | −0.11 | 0.12 | |
| PSI: Total Stress | 0.01 | 0.02 | -- | |
| Stigma | 4.33 | 0.18 | -- | |
| FSS: Family Stress | −0.10 | −0.04 | -- | |
| 13 months | ||||
| Seizure History | 0.90 | 0.02 | 0.17 | |
| Side Effects | −0.08 | −0.04 | -- | |
| Adherence | −0.36 | −0.60 | -- | |
| Adherence quadratic | 0.00 | 0.75 | -- | |
| Fears and concerns | −1.71 | −0.25* | 0.17 | |
| PSI: Total Stress | −0.16 | −0.38 | -- | |
| Stigma | −2.55 | −0.10 | -- | |
| FSS: Family Stress | −0.42 | −0.17 | -- | |
| DV = PedsQL Social HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.29 | 0.30* | 0.05 | |
| Age in months | −0.23 | −0.41** | -- | |
| Fears and Concerns | 0.40 | 0.08 | 0.20 | |
| PSI: Total Stress | 0.11 | 0.27 | -- | |
| Stigma | 1.84 | 0.07 | -- | |
| FSS: Family Stress | −0.50 | −0.21 | -- | |
| 13 months | ||||
| Seizure History | 7.72 | 0.17 | 0.13 | |
| Side Effects | −0.11 | −0.06 | -- | |
| Adherence | 0.29 | 0.45 | -- | |
| Adherence quadratic | −0.00 | −0.63 | 0.24 | |
| Fears and concerns | −0.22 | −0.03 | -- | |
| PSI: Total Stress | −0.16 | −0.37* | -- | |
| Stigma | −1.71 | −0.06 | -- | |
| FSS: Family Stress | −1.48 | −0.55** | -- | |
| DV = PedsQL School HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.09 | 0.08 | 0.08 | |
| Age in months | −0.25 | −0.39** | -- | |
| Fears and Concerns | −0.03 | −0.01 | 0.18 | |
| PSI: Total Stress | 0.08 | 0.19 | -- | |
| Stigma | −2.34 | −0.08 | -- | |
| FSS: Family Stress | −0.38 | −0.15 | -- | |
| 13 months | ||||
| Seizure History | −0.26 | −0.01 | 0.20 | |
| Side Effects | −0.61 | −0.30* | -- | |
| Adherence | −0.11 | −0.16 | -- | |
| Adherence quadratic | 0.00 | 0.05 | -- | |
| Fears and concerns | 0.10 | 0.01 | 0.09 | |
| PSI: Total Stress | −0.15 | −0.31 | -- | |
| Stigma | 2.28 | 0.08 | -- | |
| FSS: Family Stress | −0.80 | −0.27 | -- | |
| DV = QOLICE Total HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.03 | 0.04 | 0.01 | |
| Age in months | −0.15 | −0.39** | -- | |
| Fears and Concerns | −0.02 | −0.01 | 0.17 | |
| PSI: Total Stress | 0.10 | 0.37** | -- | |
| Stigma | 2.37 | 0.14 | -- | |
| FSS: Family Stress | 0.01 | 0.00 | -- | |
| 13 months | ||||
| Seizure History | −7.56 | −0.25** | 0.25 | |
| Side Effects | 0.11 | 0.08 | -- | |
| Adherence | 0.34 | 0.77* | -- | |
| Adherence quadratic | −0.00 | −0.90** | -- | |
| Fears and concerns | 1.31 | 0.27** | 0.37 | |
| PSI: Total Stress | −0.23 | −0.80** | -- | |
| Stigma | −4.61 | −0.25** | -- | |
| FSS: Family Stress | −0.80 | −0.43** | -- | |
| DV = QOLICE Anxiety HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.03 | 0.04 | 0.03 | |
| Age in months | −0.21 | −0.46** | -- | |
| Fears and Concerns | 0.39 | 0.10 | 0.18 | |
| PSI: Total Stress | 0.05 | 0.17 | -- | |
| Stigma | 2.81 | 0.14 | -- | |
| FSS: Family Stress | 0.09 | 0.05 | -- | |
| 13 months | ||||
| Seizure History | −3.33 | −0.09 | 0.16 | |
| Side Effects | −0.04 | −0.03 | -- | |
| Adherence | 0.43 | 0.83* | -- | |
| Adherence quadratic | −0.00 | −0.81 | -- | |
| Fears and concerns | 1.17 | 0.20 | 0.23 | |
| PSI: Total Stress | −0.23 | −0.67** | -- | |
| Stigma | −5.01 | −0.23* | -- | |
| FSS: Family Stress | −0.57 | −0.27 | -- | |
| DV = QOLICE Social Activities HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.28 | 0.31* | 0.03 | |
| Age in months | −0.13 | −0.24* | -- | |
| Fears and Concerns | −1.00 | −0.22 | 0.04 | |
| PSI: Total Stress | 0.04 | 0.11 | -- | |
| Stigma | 6.24 | 0.26* | -- | |
| FSS: Family Stress | −0.02 | −0.01 | -- | |
| 13 months | ||||
| Seizure History | −11.30 | −0.27* | 0.17 | |
| Side Effects | 0.33 | 0.19 | -- | |
| Adherence | 0.30 | 0.50 | -- | |
| Adherence quadratic | −0.00 | −0.60 | -- | |
| Fears and concerns | 0.89 | 0.13 | 0.26 | |
| PSI: Total Stress | 0.00 | 0.00 | -- | |
| Stigma | −9.73 | −0.38** | -- | |
| FSS: Family Stress | −1.45 | −0.58** | -- | |
Note.
p<.05
p<.01
PedsQL=Pediatric Quality of Life Inventory, QOLICE=Quality of Life in Childhood Epilepsy Questionnaire, PSI=Parenting Stress Inventory, FSS=Family Stress Scale
Table 4.
Predictors of health-related quality of life at 25-month follow-up.
| Analyses | Variables | b | β | Δ R2 |
|---|---|---|---|---|
| DV= PedsQL Total HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.12 | 0.16 | 0.03 | |
| Age in months | 0.03 | 0.06 | -- | |
| Fears and Concerns | 0.24 | 0.06 | 0.06 | |
| PSI: Total Stress | −0.01 | −0.03 | -- | |
| Stigma | −0.79 | −0.04 | -- | |
| FSS: Family Stress | −0.02 | −0.01 | -- | |
| 25 months | ||||
| Seizure History | 4.11 | 0.10 | 0.39 | |
| Side Effects | −1.01 | −0.59** | -- | |
| Adherence | −0.07 | −0.13 | -- | |
| Adherence quadratic | 0.00 | 0.03 | -- | |
| Fears and concerns | −0.60 | −0.11 | 0.04 | |
| PSI: Total Stress | −0.07 | −0.18 | -- | |
| Stigma | 2.32 | 0.11 | -- | |
| FSS: Family Stress | −0.13 | −0.06 | -- | |
| DV= PedsQL Emotional HRQOL | ||||
| 1 month | ||||
| Family Duncan | −0.03 | −0.04 | 0.05 | |
| Age in months | 0.09 | 0.17 | -- | |
| Fears and Concerns | 0.25 | 0.05 | 0.14 | |
| PSI: Total Stress | 0.00 | 0.01 | -- | |
| Stigma | 0.93 | 0.04 | -- | |
| FSS: Family Stress | −0.50 | −0.23 | -- | |
| 25 months | ||||
| Seizure History | 6.70 | 0.14 | 0.33 | |
| Side Effects | −0.83 | −0.41** | -- | |
| Adherence | −0.40 | −0.60 | -- | |
| Adherence quadratic | 0.00 | 0.57 | -- | |
| Fears and concerns | −3.25 | −0.51* | 0.11 | |
| PSI: Total Stress | −0.05 | −0.11 | -- | |
| Stigma | 4.63 | 0.18 | -- | |
| FSS: Family Stress | 0.18 | 0.08 | -- | |
| DV = PedsQL Social HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.17 | 0.18 | 0.02 | |
| Age in months | 0.04 | −0.07 | -- | |
| Fears and Concerns | −0.23 | −0.05 | 0.11 | |
| PSI: Total Stress | −0.05 | −0.13 | -- | |
| Stigma | 0.69 | 0.03 | -- | |
| FSS: Family Stress | 0.39 | 0.16 | -- | |
| 25 months | ||||
| Seizure History | 3.56 | 0.07 | 0.32 | |
| Side Effects | −1.16 | −0.52** | -- | |
| Adherence | −0.11 | −0.15 | -- | |
| Adherence quadratic | 0.00 | −0.07 | -- | |
| Fears and concerns | 1.18 | 0.17 | 0.05 | |
| PSI: Total Stress | −0.08 | −0.16 | -- | |
| Stigma | −1.41 | −0.05 | -- | |
| FSS: Family Stress | −0.70 | −0.26 | -- | |
| DV = PedsQL School HRQOL | ||||
| 1 month | ||||
| Family Duncan | −0.04 | −0.05 | 0.00 | |
| Age in months | −0.01 | −0.02 | -- | |
| Fears and Concerns | 0.72 | 0.15 | 0.06 | |
| PSI: Total Stress | 0.02 | 0.05 | -- | |
| Stigma | −5.91 | −0.23 | -- | |
| FSS: Family Stress | −0.26 | −0.11 | -- | |
| 25 months | ||||
| Seizure History | 11.57 | 0.23 | 0.21 | |
| Side Effects | −0.84 | −0.39* | -- | |
| Adherence | −0.15 | −0.21 | -- | |
| Adherence quadratic | 0.00 | 0.12 | -- | |
| Fears and concerns | −2.08 | −0.30 | 0.10 | |
| PSI: Total Stress | −0.09 | −0.20 | -- | |
| Stigma | 6.72 | 0.24 | -- | |
| FSS: Family Stress | 0.07 | 0.03 | -- | |
| DV = QOLICE Total HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.07 | 0.11 | 0.01 | |
| Age in months | −0.01 | −0.02 | -- | |
| Fears and Concerns | 0.30 | 0.10 | 0.11 | |
| PSI: Total Stress | −0.02 | −0.08 | -- | |
| Stigma | −4.20 | −0.26* | -- | |
| FSS: Family Stress | 0.23 | 0.15 | -- | |
| 25 months | ||||
| Seizure History | 1.90 | 0.06 | 0.42 | |
| Side Effects | −0.65 | −0.47** | -- | |
| Adherence | −0.43 | −0.94* | -- | |
| Adherence quadratic | 0.00 | 0.53 | -- | |
| Fears and concerns | −2.57 | −0.60** | 0.19 | |
| PSI: Total Stress | −0.10 | −0.36* | -- | |
| Stigma | 3.30 | 0.19 | -- | |
| FSS: Family Stress | 0.32 | 0.20 | -- | |
| DV = QOLICE Anxiety HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.00 | 0.01 | 0.01 | |
| Age in months | −0.07 | −0.16 | -- | |
| Fears and Concerns | 0.80 | 0.21 | 0.05 | |
| PSI: Total Stress | −0.01 | −0.02 | -- | |
| Stigma | −0.60 | −0.03 | -- | |
| FSS: Family Stress | 0.21 | 0.12 | -- | |
| 25 months | ||||
| Seizure History | 8.26 | 0.22 | 0.32 | |
| Side Effects | −0.84 | −0.51** | -- | |
| Adherence | 0.09 | 0.16 | -- | |
| Adherence quadratic | −0.001 | −0.19 | -- | |
| Fears and concerns | −0.42 | −0.08 | 0.05 | |
| PSI: Total Stress | −0.09 | −0.25 | -- | |
| Stigma | 0.92 | 0.04 | -- | |
| FSS: Family Stress | −0.23 | −0.11 | -- | |
| DV = QOLICE Social Activities HRQOL | ||||
| 1 month | ||||
| Family Duncan | 0.10 | 0.13 | 0.01 | |
| Age in months | −0.03 | −0.07 | ||
| Fears and Concerns | −0.29 | −0.08 | 0.05 | |
| PSI: Total Stress | −0.01 | −0.04 | -- | |
| Stigma | −0.06 | −0.003 | -- | |
| FSS: Family Stress | 0.42 | 0.23 | -- | |
| 25 months | ||||
| Seizure History | −8.40 | −0.22 | 0.45 | |
| Side Effects | −0.61 | −0.36* | -- | |
| Adherence | −0.12 | −0.21 | -- | |
| Adherence quadratic | 0.00 | 0.03 | -- | |
| Fears and concerns | −1.57 | −0.30 | 0.08 | |
| PSI: Total Stress | 0.03 | 0.10 | -- | |
| Stigma | −2.41 | −0.13 | -- | |
| FSS: Family Stress | −0.36 | −0.18 | -- | |
Note.
p<.05
p<.01
PedsQL=Pediatric Quality of Life Inventory, QOLICE=Quality of Life in Childhood Epilepsy Questionnaire, PSI=Parenting Stress Inventory, FSS=Family Stress Scale
Exploratory analyses
Exploratory analyses were conducted to examine the prediction of the following epilepsy-specific HRQOL subscales: Attention/Concentration, Memory, Language, Other Cognitive, Control/Helplessness, Self-Esteem, Behavior. Parenting stress was the only significant predictor of Behavior at 1-month and 13-months (b=−0.17, p<.001; b=−0.23, p<.001, respectively), and Attention/Concentration at 13-months (b=−0.35, p=.001). Predictors of Memory at 13-months were age and parenting stress (b=−0.30, p<.001; b=−0.38, p<.001, respectively), while greater side effects at 25-months predicted lower Memory (b=−1.54, p<.001). Significant predictors of Language at 13-months were parenting stress (b=−0.33, p=.003) and family stress (b=−1.53, p=.006), and side effects at 25-months (b=−1.30, p=.002). At 13-months, parenting stress (b=−0.31, p=.005) and family stress (b=−1.44, p=.009) were also significant predictors of Other Cognitive. Side effects were a significant predictor of 25-months Control/Helplessness (b=−0.89, p=.007). Age (b=−0.17, p<.001) and parenting stress (b=−0.29, p<.001) were significant predictors of Self-Esteem at 13 months.
Discussion
Our study identified modifiable, parent psychosocial factors that predict child HRQOL, an important patient-reported outcome, at critical time points following a diagnosis of pediatric epilepsy. Specifically, parent/family stress, fears and concerns, and perceived stigma consistently negatively impacted child HRQOL, above and beyond disease and demographic factors. Both generic and epilepsy-specific parenting and family stress were important throughout the first year of disease management when families are coping with a new epilepsy diagnosis, treatment regimen, and potential ongoing seizures; however, 2 years later, both generic and epilepsy-specific parenting and family stress had less impact on child HRQOL. This latter finding may be attributed to families/children having improved illness adjustment and potential seizure control. Our results are generally consistent with prior studies demonstrating that parental functioning influences cross-sectional and longitudinal child outcomes including HRQOL.9 For example, Ferro and colleagues (2011) found that one measure of parental functioning (i.e., maternal depressive symptoms) predicted changes in child HRQOL over time. In addition, Austin and colleagues31 demonstrated that 24 months post-diagnosis, higher parent needs for information and support predicted child internalizing problems. Our results are also consistent with prior work indicating that parent's needs for information and support related to their children's seizures are highest early on after diagnosis (e.g., 3 months), and decrease over time (e.g., up to 24 months post-diagnosis).26
In contrast, fears and concerns related to epilepsy predicted HRQOL differently across the disease course. At 13 months post-diagnosis, higher fears and concerns predicted higher HRQOL, whereas 2 years following diagnosis, higher fears and concerns predicted lower HRQOL. This finding suggests that having some level of fears and concerns about a child's illness may be adaptive. However, it is possible that parents who continue to endorse high levels of fears and concerns later in the disease course may be overprotective of their children and place excessive limits on their activities, thus leading to lower child HRQOL. As hypothesized, higher levels of perceived stigma predicted worse HRQOL, including social HRQOL. This finding suggests that interventions that address caregiver's perceptions of stigma could positively influence child social HRQOL.
Our results confirm recommendations in the literature to assess for both generic and disease-specific HRQOL.19, 20 Some HRQOL findings were consistent across the generic and epilepsy-specific measures (e.g., on both the PedsQL and QOLICE, general parenting stress predicted total HRQOL at 1 month and epilepsy-specific family stress predicted social HRQOL at 13 months). However, there were also unique findings for both generic and epilepsy-specific HRQOL. For example, general parenting stress predicted emotional and social HRQOL only on the PedsQL (i.e., generic measure) at 1 month, and general and epilepsy-specific parenting stress, fears and concerns, and stigma predicted social, anxiety, and total HRQOL only on the QOLICE (i.e., epilepsy-specific measure) at 13 months. Not surprisingly, disease-related factors, including epilepsy-related stigma, concerns, and fears were stronger predictors of the epilepsy-specific HRQOL measure. For clinicians working with pediatric patients with epilepsy and their families, multiple factors may affect the decision to administer generic or epilepsy-specific HRQOL measures, if it is not feasible to administer both. Disease-specific measures enable us to focus on areas that are particularly salient for families of children with epilepsy and could be important outcome measures for family-focused interventions targeting epilepsy-specific parenting stress, parental fear/concerns, and perceived stigma. However, if the overall goal of assessing patient-reported outcomes is to compare across different pediatric populations, then a generic instrument may be useful.
Our study results also highlight the importance of several demographic (i.e., age) and disease-related variables (i.e., side effects, AED adherence) that influence HRQOL. Consistent with prior research,6 higher side effects predicted worse HRQOL, particularly at 1 and 25-months post-diagnosis. Interestingly, side effects were more predictive of HRQOL than seizure occurrence. This underscores the importance of addressing side effects in clinic visits and through ongoing communication with families between clinic visits. A novel finding of the current study was that at 13-months post-diagnosis, there was a significant quadratic effect for AED adherence in predicting epilepsy-specific HRQOL. One potential explanation is that lower levels of AED adherence lead to increased seizure occurrence and consequently lower HRQOL, while higher levels of adherence are associated with greater treatment burden for families or ongoing seizures which lead to lower HRQOL. It is important to note that these findings do not suggest that low levels of adherence are preferred. Instead, future work could examine the factors, including AED side effects, family organizational abilities, and child behavior, that influence a family's decision to adhere to the prescribed AED. Clinically, these findings suggest that families independently make decisions about adherence; however, our role as healthcare providers is to promote the best outcomes for patients by inviting families to discuss these decisions so that they are made in an informed manner.
While several strengths of the study are notable (e.g., longitudinal design, use of objective adherence data), our study included a restricted age range for children limiting generalizability. In addition, our study used one specific definition of HRQOL; however, there are other conceptualizations of HRQOL.1 Readers should therefore proceed cautiously when comparing the current HRQOL findings with other studies using measures that use different conceptualizations of HRQOL (e.g., satisfaction with one's current physical functioning). In the current study, only parent-reported HRQOL was used and one important future direction is to expand to include child-reported HRQOL, for example with an adolescent population. Also, we examined the relationships between variables at critical time points using a cross-sectional approach and thus changes over time were not examined in our outcome measure. Future studies could examine the longitudinal prediction of HRQOL from disease and psychosocial factors at earlier time points. In addition, future work could further investigate the potentially complex relationships between the medication regimen, parent, and family factors examined in the current study. Some factors may be more proximal versus distal determinants of HRQOL outcomes. For example, perceived stigma may increase parenting stress, which then directly impacts a child's HRQOL. Identifying the temporal ordering of such factors will inform the content and timing of interventions to improve the HRQOL of children with epilepsy.
Our results have several implications for interventions aimed at promoting pediatric epilepsy patient-reported outcomes, including HRQOL. Interventions should be tailored to address specific factors most likely to impact patient-reported outcomes at critical points in the child's treatment. Within the first year post-diagnosis, psychosocial interventions may address both general and epilepsy-specific parenting and family stress and overall coping.32 Families may benefit from receiving anticipatory guidance on the challenges of managing epilepsy as a family, discussion of balancing their child's epilepsy treatment with the family's other responsibilities and activities, and assessments of other family stressors that may impact implementation of the prescribed regimen. Also, adherence-promotion interventions33 and interventions targeting perceived stigma and fears and concerns may improve HRQOL outcomes. The current results also indicate that throughout treatment, families could benefit from discussions with their health care providers about medication side effects and their impact on the child's daily functioning. In the second year post-diagnosis, addressing persistent parent fears and perceived stigma (e.g., being overly restrictive of child's activities) may encourage optimal HRQOL. In summary, parent psychosocial factors appear to impact HRQOL outcomes among children with new-onset epilepsy. Promoting parent management of stress, fears/concerns, and perceived stigma may lead to improved child HRQOL outcomes.
Supplementary Material
Acknowledgements
This research was supported by a grant from the National Institutes of Health (K23HD057333) awarded to the fourth author and a training grant from the National Institutes of Health supporting the first author (T32HD068223).
Footnotes
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Ethical Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Fayed N, de Camargo O, Kerr E, et al. Generic patient-reported outcomes in child health research: A review of conceptual content using World Health Organization definitions. Dev Med Child Neurol. 2012;54:1085–95. doi: 10.1111/j.1469-8749.2012.04393.x. [DOI] [PubMed] [Google Scholar]
- 2.Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE Treatment Guidelines: Evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094–120. doi: 10.1111/j.1528-1167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 3.Ronen GM, Streiner DL, Rosenbaum P. Health-related quality of life in childhood epilepsy: moving beyond ‘seizure control with minimal adverse effects’. Health Qual Life Outcomes. 2003;1:36. doi: 10.1186/1477-7525-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol. 2003;45:292–5. doi: 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- 5.Rodenburg R, Meijer AM, Dekovic M, et al. Family factors and psychopathology in children with epilepsy: a literature review. Epilepsy Behav. 2005;6:488–503. doi: 10.1016/j.yebeh.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Modi AC, Ingerski LM, Rausch JR, et al. Treatment factors affecting longitudinal quality of life in new onset pediatric epilepsy. J Ped Psychol. 2011;36:466–75. doi: 10.1093/jpepsy/jsq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi AC, King AS, Monahan SR, et al. Even a single seizure negatively impacts pediatric health-related quality of life. Epilepsia. 2009;50:2110–6. doi: 10.1111/j.1528-1167.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 8.Haneef Z, Grant ML, Valencia I, et al. Correlation between child and parental perceptions of health-related quality of life in epilepsy using the PedsQL.v4.0 measurement model. Epileptic Disord. 2010;12:275–82. doi: 10.1684/epd.2010.0344. [DOI] [PubMed] [Google Scholar]
- 9.Ferro MA, Avison WR, Campbell MK, et al. The impact of maternal depressive symptoms on health-related quality of life in children with epilepsy: a prospective study of family environment as mediators and moderators. Epilepsia. 2011;52:316–25. doi: 10.1111/j.1528-1167.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevanovic D, Jancic J, Lakic A. The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia. 2011;52:e75–8. doi: 10.1111/j.1528-1167.2011.03133.x. [DOI] [PubMed] [Google Scholar]
- 11.Lach LM, Ronen GM, Rosenbaum PL, et al. Health-related quality of life in youth with epilepsy: theoretical model for clinicians and researchers. Part I: the role of epilepsy and comorbidity. Qual Life Res. 2006;15:1161–71. doi: 10.1007/s11136-006-0051-7. [DOI] [PubMed] [Google Scholar]
- 12.Speechley KN, Ferro MA, Camfield CS, et al. Quality of life in children with new-onset epilepsy: a 2-year prospective cohort study. Neurology. 2012;79:1548–55. doi: 10.1212/WNL.0b013e31826e25aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzer M, Denson LA, Baldassano RN, et al. Patient and parent psychosocial factors associated with health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;52:295–9. doi: 10.1097/MPG.0b013e3181f5714e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenzuela JM, Patino AM, McCullough J, et al. Insulin pump therapy and health-related quality of life in children and adolescents with type 1 diabetes. J Ped Psychol. 2006;31:650–60. doi: 10.1093/jpepsy/jsj088. [DOI] [PubMed] [Google Scholar]
- 15.Guilfoyle SM, Zeller MH, Modi AC. Parenting stress impacts obesity-specific health-related quality of life in a pediatric obesity treatment-seeking sample. J Dev Behav Pediatr. 2010;31:17–25. doi: 10.1097/DBP.0b013e3181c73641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari P, Ram D, Haque Nizamie S, et al. Stigma and quality of life in individuals with epilepsy: A preliminary report. Epilepsy & Behavior. 2009;15:358–61. doi: 10.1016/j.yebeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin DP, Pachana NA, Mcfarland K. Stigma, seizure frequency and quality of life: the impact of epilepsy in late adulthood. Seizure. 2008;17:281–7. doi: 10.1016/j.seizure.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EK, Jones JE, Seidenberg M, et al. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004;45:544–50. doi: 10.1111/j.0013-9580.2004.47003.x. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabaz M, Cairns D, Bleasel A, et al. The health-related quality of life of childhood epilepsy syndromes. J Paediatr Child Health. 2003;39:690–6. doi: 10.1046/j.1440-1754.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 21.Solans M, Pane S, Estrada M-D, et al. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. value in health. 2008;11:742–64. doi: 10.1111/j.1524-4733.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 22.Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Sabaz M, Lawson JA, Cairns DR, Duchowny MS, Resnick TJ, Dean PM, Bye AM. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav. 2003 Dec;4(6):680–91. doi: 10.1016/j.yebeh.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Stevens G, Featherman D. A revised socioeconomic index of occupational status. Soc Sci Res. 1981;10:364–95. [Google Scholar]
- 25.Mueller C, Parcel T. Measures of socioeconomic status: alternatives and recommendations. Child Dev. 1981;52:1616–25. [Google Scholar]
- 26.Shore C, Austin J, Musick B, et al. Psychosocial care needs of parents of children with new-onset seizures. J Neurosci Nurs. 1998;30:169–74. doi: 10.1097/01376517-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Abidin RR. Parenting Stress Index: Professional Manual Odessa: Psychological Assessment Resources, Inc. 1995 [Google Scholar]
- 28.Austin JK, Macleod J, Dunn DW, et al. Measuring stigma in children with epilepsy and their parents: instrument development and testing. Epilepsy & Behavior. 2004;5:472–82. doi: 10.1016/j.yebeh.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Stevens J. Psychology. Indiana University; Bloomington: 1998. Parenting Stress, Social Support, Marital Satisfaction, and Psychological Distress in Families of Children with and without Seizure Disorders: A Contextual Approach. [Google Scholar]
- 30.Morita DA, Glauser TA, Modi AC. development and validation of the pediatric epilepsy side effects questionnaire. Neurology. 2012;79:1252–8. doi: 10.1212/WNL.0b013e3182635b87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin JK, Dunn DW, Johnson CS, et al. Behavioral issues involving children and adolescents with epilepsy and the impact of their families: recent research data. Epilepsy & Behavior. 2004;5:33–41. doi: 10.1016/j.yebeh.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Wagner JL, Smith G, Ferguson P, et al. Feasability of a pediatric cognitive-behavioral self-managemnt intervention: Coping opening and personally with epilepsy (COPE). Seizure. 2011;20:462–7. doi: 10.1016/j.seizure.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Modi AC, Guilfoyle SM, Rausch J. Preliminary feasibility, acceptability, and efficacy of an innovative adherence intervention for children With newly diagnosed epilepsy. J Pediatr Psychol. 2013 doi: 10.1093/jpepsy/jst021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.