Abstract
Cardiac dysfunction is a major consequence that contributes to the high mortality of trauma-hemorrhage (TH) patients. Recent evidence suggests that innate immune and inflammatory responses mediated by Toll-like receptors (TLRs) play a critical role in the pathophysiologic mechanisms of acute organ dysfunction during TH. This study investigated the role of TLR4 in cardiac dysfunction following TH. TLR4 deficient (TLR4−/−, n=7/group) and age-matched wild type (WT, n=8/group) mice were subjected to TH that was induced by soft tissue injury and blood withdrawal from the jugular vein to a mean arterial pressure of 35 ± 5 mm Hg. Cardiac function and mean arterial pressure were measured with a Millar system before, during and after blood withdrawal. Sham surgical operated mice served as control (WT, n=9/group; TLR4−/−, n=10/group). Cardiac function in WT mice was significantly reduced following TH. However cardiac function was well preserved in TLR4−/− mice. Administration of a TLR4 antagonist (3mg/kg) to WT mice also significantly attenuated TH-induced cardiac dysfunction. Western blot showed that either TLR4−/− or TLR4 antagonist markedly attenuated TH-induced decreases in the levels of phosphorylated-Akt in myocardium. In addition, inhibition of TLR4 attenuated TH-induced myocardial NF-κB binding activity as well as lung MPO activity and TNFα production. The data indicate that TLR4 plays a central role in TH-induced cardiac dysfunction. TLR4 deficiency or TLR4 inhibition attenuated cardiac dysfunction following TH which may involve activation of the PI3K/Akt signaling and decrease of NF-κB binding activity. TLR4 antagonism may be a new and novel approach for the treatment and management of cardiac dysfunction in TH patients.
Keywords: Cardiac Function, Injury, Innate Immune, TLRs, Eritoran, NF-κB
Introduction
Trauma-hemorrhage (TH) is the most common cause of morbidity and mortality in immediate survivors of accidents, terrorist attacks, natural disasters, warfare casualties, suicide attempts and crime victims. After injury, hemorrhage remains a major cause of death and is responsible for 30% to 40% of all mortalities with half of them dying during the pre-hospital period (1). Prehospital intravenous fluid treatment has been demonstrated to be associated with higher mortality in trauma patients (2). Despite significant advances in trauma care, the outcomes for TH patients have not significantly improved (3).
Tissue damage caused by TH releases damage associated molecular patters (DAMPs) that induce inflammatory responses which contribute to organ injury and ischemia (4). Toll-like receptors (TLRs) recognize both pathogen-associated molecular patterns (PAMPs) and endogenous DAMPs. Innate immune and inflammatory responses mediated by TLRs (5, 6) contribute to multiple organ failure during TH (7). TLR4 mediated signaling predominantly induces the activation of nuclear factor–kappa B (NF-κB) which is an important transcription factor controlling inflammatory cytokine gene expression (8). NF-κB activation plays a vital pathological mechanism in cardiac dysfunction during hemorrhagic shock (9, 10). Inhibition of NF-κB activation by Calpain inhibitor I has been reported to attenuate acute organ dysfunction in hemorrhage shock (11). However, the role of TLR4 in cardiac function following TH has not been well investigated.
The phosphoinositide 3-kinases (PI3Ks) are a conserved family of signal transduction enzymes which are involved in regulating cellular proliferation and survival (12). Akt is an important mediator of the PI3K pathway which regulates cell cycle entry, growth and survival (13, 14). Recent evidences suggest that activation of PI3K/Akt is an endogenous negative feedback mechanism that prevents excessive innate immune and/or inflammatory responses (15, 16). Previous studies have reported that elevated levels of phosphorylated Akt attenuate the overproduction of inflammatory cytokines, chemokines, adhesion molecules and neutrophil infiltration following TH (17). In addition, we and others have reported that modulation of PI3K/Akt improves cardiac function in sepsis (18) and myocardial ischemic injury (19). TH has been reported to induce the decrease of cardiomyocyte Akt phosphorylation (17, 20). But we have previously reported that CpG-ODN significantly attenuates cardiac dysfunction following TH through activation of PI3K/Akt signaling (20).
In the present study we examined the role of TLR4 in cardiac dysfunction during TH. We found that TLR4 deficiency or pharmacologic antagonism of TLR4 significantly attenuates cardiac dysfunction in murine TH model. The mechanisms involve activation of PI3K/Akt signaling and decrease of NF-κB binding activity.
Materials and Methods
Animals
The murine TLR4-knockout strain (TLR4−/−, C57BL/10ScCr) and its background wild type strain (WT, C57BL/10ScSn) were purchased from Jackson Laboratory (Bar Harbor, ME). Male mice with a body weight about 30 g were used. The mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University. The experiments outlined in this article conformed to the Guide for the Care and use of Laboratory Animals published by the National Institutes of Health (NIH Publication, 8th Edition, 2011). All aspects of the animal care and experimental protocols were approved by the East Tennessee State University Committee on Animal Care.
Murine Model of Trauma-Hemorrhage
The mouse trauma-hemorrhage (TH) shock model was induced as described previously (20). Trauma was produced by soft tissue injury and hemorrhage was induced by blood withdraw from the jugular vein until the mean arterial pressure reached 35 ± 5 mm Hg (20). Briefly, male mice (~30 grams) were anesthetized with isoflurane (5.0% induction, 1.5% maintenance) and placed on a heating pad to keep body temperature around 37 °C. A 1.5 cm incision was made in the middle of the cervical skin parallel to the trachea. After the left carotid artery was exposed, a pressure catheter (Millar Instruments Inc., Houston, TX) was inserted into left carotid artery for monitoring blood pressure. The right jugular vein was cannulated with polyurethane tubing (BPU-T20, Instech Solomon, Plymouth Meeting, PA) for blood withdrawal. A 1.5 cm thoracotomy incision was performed at the left fifth intercostal space. The left ventricular (LV) apex was then punctured with a 27-gauge needle and a pressure-volume catheter (SPR-839, Millar Instruments, Inc.) was positioned in the left ventricle via the punctured apex of the heart for continuous registration of left ventricular pressure-volume loops using the PowerLab system (AD Instruments, Inc., Colorado Springs, CO) (20). After completion of the instrumentation, blood was withdrawn from the jugular vein using a Harvard syringe pump (0.82ml/10min/30g body weight) until the mean arterial pressure (AP) reached to 35 ± 5 mm Hg and was maintained at this level at the first 10 min as indicated by the time schedule. The time schedule is shown below. The arrows indicate the measurement and recording of cardiac function.

Cardiac function assessment
Cardiac function was measured before blood withdraw, 10 and 60 min after blood withdraw, respectively. The left ventricular pressure (LVP) and peak rate of pressure rise (dP/dtmax) and other hemodynamic parameters were calculated with PowerLab system software as described previously (20).
Experimental design
To determine the role of TLR4 in cardiac dysfunction following TH, TLR4 deficient and age-matched WT mice were subjected to TH. To evaluate whether inhibition of TLR4 will attenuate cardiac dysfunction during TH, WT mice were treated with a single bolus (150ul/30g body weight) of the TLR4 specific antagonist eritoran tetrasodium (E5564, Eisai Inc., 4 Corporate Drive, Andover, MA) at 0.3, 1.0, 3.0, 5.0, and 10.0 mg/kg body weight at the initiation of hemorrhage. Each dose-group included 10-12 mice. The injection of same volume vehicle serves as the placebo control. Sham surgical operated mice without blood withdrawal served as sham control. Following the final hemodynamic measurement, mice were sacrificed and tissue samples were collected and stored at −80°C for further analysis.
Western Blot
Cellular proteins were isolated from heart tissues (20), separated by SDS-polyacrylamide gel electrophoresis and transferred onto Hybond enhanced chemiluminescence (ECL) membranes (Amersham Pharmacia, Piscataway, NJ). The ECL membranes were incubated with appropriate primary antibody [anti-phospho-Akt and anti-Akt (Cell Signaling Technology, Beverly, Ma)], followed by an incubation with peroxidase-conjugated second antibodies (Cell Signaling Technology). The membranes were analyzed by the ECL system (Amersham Pharmacia). The signals were detected with the G: BOX system and quantified using GeneTools software (Syngene, Frederick, MD).
NF-κB binding activity assay
The nuclear proteins were isolated as described previously (21). NF-κB binding activities were analyzed using the LightShift Chemiluminescent EMSA Kit (Thermo Scientific) according to the suggested protocol. Briefly, Binding reaction mixtures contained 2 μl binding buffer, 15 μg nuclear proteins and 2 μl 100 fmols 3′-Biotin labeled double-stranded NF-κB consensus oligonucleotide in a 15 μl system. The reaction mixture was separated on 6% non-denaturing polyacrylamide gels and the density of the binding bands was detected by G: Box and the picture analyzed by the Gene Tools of Syngene (Frederick, MD).
Myeloperoxidase (MPO) activity assay
Neutrophil sequestration in lung tissue was measured using a MPO fluorometric Detection kit (Assay Designs Inc., Ann Arbor, MI) according to the manufacturers’ instructions (22).
ELISA
The levels of TNFα in the lung tissues were assessed by ELISA (PeproTech, Rocky Hill, NJ) according to the instructions provided by the manufacturer (22).
Statistics
Figures are displayed as group mean ± SD. Comparisons of data between groups were made using one-way analysis of variance (ANOVA) and Tukey’s procedure for multiple range tests was performed. P< 0.05 was considered to be significant.
Results
Trauma-hemorrhage induces cardiac dysfunction in WT mice but not in TLR4 deficient mice
To determine the role of TLR4 in cardiac function following TH, we induced TH in TLR4−/− and age-matched WT mice. Cardiac function was measured by using pressure-volume conductance catheter technique (20). As shown in Figure 1, TH significantly induced decreases in the left ventricular pressure (LVP) by 35.4% and peak rate of pressure rise (dP/dtmax) by 52.9% in WT mice compared with sham control. However, the values of LVP and dP/dtmax were not markedly reduced in TLR4−/− mice following TH, when compared with the TLR4−/− sham control. There was no significant difference in the values of LVP and dP/dtmax between WT sham and TLR4−/− sham groups. The data suggests that TLR4 contributes to cardiac dysfunction following TH.
Figure1. TLR4 deficiency prevents TH-induced cardiac dysfunction.
TLR4−/− and age-matched WT mice were subjected to trauma hemorrhage (TH). Sham surgical operation served as sham control. Cardiac function was measured by pressure-volume conductance catheter 60 minutes after TH. * P<0.05 compared with indicated groups. n=7-10/group.
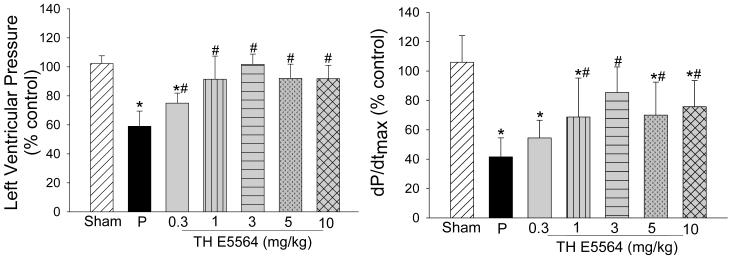
TLR4 inhibition significantly attenuated cardiac dysfunction following TH
Next, we examined the effect of TLR4 inhibition on cardiac function following TH. WT mice were treated with a specific TLR4 antagonist E5564 at 0.3, 1, 3, 5, and 10 mg/kg, respectively, immediately when hemorrhage was induced. Figure 2 shows that LVP and dP/dtmax were significantly decreased after TH in placebo-treated group compared with sham group. Administration of E5564 at 1, 3, 5 and 10 mg/kg significantly attenuated TH-induced cardiac dysfunction. Administration of E5564 at 0.3 mg/kg to TH mice improved dp/dtmax but did not reach statistical significance. There was no significant difference in the values of LVP between TH mice treated with E5564 at 1, 3, 5, and 10 mg/kg and sham control group. However there was still significant difference in dP/dtmax between E5564 dose groups and sham group except the 3 mg/kg group. Interestingly, administration of E5564 at 3 mg/kg maximally attenuated the cardiac dysfunction following TH.
Figure 2. TLR4 inhibition with E5564 attenuated TH-induced cardiac dysfunction.
WT mice were treated with E5564, a specific TLR4 antagonist, at different dose (0.3, 1, 3, 5, and 10 mg/kg) immediately induction of TH. The placebo group (P) received the same volume of vehicle (150 μl). Sham surgical operation served as sham control. Cardiac function was measured by pressure-volume conductance catheter 60 minutes after TH. * P < 0.05 compared with sham group; # P < 0.05 E5564 group compared with placebo group. n=10-12/group.
TLR4 deficiency prevented TH-induced decreases in Akt phosphorylation
We have previously reported that TLR4 deficiency resulted in a significant increase in the levels of Akt phosphorylation in the myocardium (19). Activation of PI3K/Akt signaling has been reported to protect against cardiac dysfunction following ischemic injury and septic shock (15, 18, 19). Therefore, we examined the effect of TLR4 on Akt phosphorylation in response to TH and whether TLR4 deficiency or TLR4 antagonism impact this response. As shown in Figure 3, the levels of phosphorylated Akt in WT mice were significantly reduced compared with WT sham control. In contrast, the levels of phosphorylated Akt in TLR4−/− mice were not reduced following TH, when compared with TLR4−/− sham control. The data indicate that inhibition of TLR4 activity prevents TH induced depression of myocardial Akt activity.
Figure 3. TLR4 deficiency prevents TH-induced decreases in the levels of myocardial Akt phosphorylation.
TLR4−/− and age-matched WT mice were subjected to TH. Sham surgical operation served as sham control. Hearts were harvested 60 min after TH for the isolation of cellular proteins. Phospho-Akt (p-Akt) and total Akt were measured by Western blot. *P < 0.05 compared with indicated groups. n=9-13/group.
Inhibition of TLR4 attenuated TH-induced decreases in myocardial Akt phosphorylation
We examined whether inhibition of TLR4 by E5564 will attenuate TH-induced decreases in the levels of myocardial Akt phosphorylation. WT mice were treated with E5564 at 0.3, 1, 3, 5, and 10 mg/kg immediately after induction of TH. Figure 4 shows that TH decreased the levels of phosphorylated Akt in the myocardium of placebo-treated mice, when compared with the sham group. Administration of E5564 at 0.3 and 1 mg/kg also attenuated the TH-induced decreases in myocardial Akt phosphorylation but did not reach statistical significance. However, treatment of mice with E5564 at 3, 5 and 10 mg/kg significantly attenuated TH-induced decreases in Akt phosphorylation. In fact, administration of E5564 at 3 mg/kg resulted in the highest levels of phosphorylated Akt compared with other dose of E5564. The data was also consistent with the data of cardiac function that administration of E5564 at 3 mg/kg prevented TH-induced cardiac dysfunction.
Figure 4. LR4 inhibition with E5564 attenuated TH-induced decreases in the levels of myocardial Akt phosphorylation.
WT mice were treated with E5564, a specific TLR4 antagonist, at different dose (0.3, 1, 3, 5, and 10 mg/kg) immediately following the induction of TH. The placebo group (P) received the same volume of vehicle (150ul). Sham surgical operation served as sham control. Hearts were harvested 60 min after TH for the isolation of cellular proteins. Phospho-Akt (p-Akt) and total Akt were measured by Western blot. * P < 0.05 compared with sham group; # P < 0.05 E5564 group compared with placebo group. n=6-10/group.
TH-induced NF-κB binding activity was attenuated by TLR4 inhibition
TLR4 mediated NF-κB activation contributes to organ dysfunction in TH (9, 21). Recent studies have shown that activation of PI3K/Akt signaling also negatively regulates TLR4/mediated NF-κB activation via a feedback mechanism (16). Therefore, we examined the effect of TLR4 inhibition with E5564 on myocardial NF-κB activation following TH. As shown in Figure 5, TH significantly induced myocardial NF-κB binding activity in the placebo-treated group compared with sham control. Treatment of mice with E5564 significantly attenuated TH-induced myocardial NF-κB binding activity. The levels of NF-κB binding activity in E5564 at 3 mg/kg treated TH mice were comparable with that in sham control.
Figure 5. TLR4 inhibition with E5564 attenuated TH-induced NF-κB binding activity.
WT mice were treated with E5564, a specific TLR4 antagonist, at different dose (0.3, 1, 3, 5, and 10 mg/kg) immediately following the induction of TH. The placebo group (P) received the same volume of vehicle (150ul). Sham surgical operation served as sham control. Hearts were harvested 60 min after TH for the isolation of the nuclear proteins. NF-κB binding activity was assessed by EMSA. * P < 0.05 compared with sham group; # P < 0.05 E5564 group compared with placebo group. n=6-7/group.
TLR4 Deficiency or TLR4 antagonist administration attenuates TH-induced neutrophil infiltration and TNFα production in the lung tissue
We also evaluated the effect of TLR4 deficiency or blocking TLR4 on TH-induced neutrophil infiltration and TNFα production in the lung tissue. Myeloperoxidase (MPO) activity is a marker for neutrophil infiltration into tissues (23). Figure 6A shows that TH significantly increased lung MPO activity compared with sham control. However, TLR4 deficiency markedly attenuated TH-induced increases in MPO activity in the lung tissue. Administration of TLR4 antagonist also significantly attenuated TH-increased lung MPO activity. Administration of E5564 at 3 mg/kg resulted in the maximal reduction of lung MPO activity following TH (Figure 6B).
Figure 6. TLR4 deficiency or TLR4 antagonist attenuated TH-induced neutrophil infiltration into the lung tissue.
(A) Neutrophil activity in the lung was examined by the myeloperoxidase (MPO) assay in wild type and TLR4-deficient mice subjected to TH. Sham surgical operation served as sham control. * P < .05 compared with indicated group. There were 6-10 mice in each group. (B) WT mice were treated with placebo or E5564 at different dose immediately following the induction of TH. Lung tissues were harvested 60 min after TH for preparation of cellular proteins. MPO activity was examined using a MPO kit. Sham surgical operation served as sham control. * P < 0.05 compared with sham group; # P < 0.05 E5564 group compared with placebo group. There were 7-10 mice in each group.
As expected, TH significantly increased the levels of lung TNFα, when compared with sham control (Figure 7A). In contrast, TLR4 deficiency attenuated TH-induced increases in TNFα production in the lung tissue (Figure 7A). Blocking TLR4 by administration of E5564 also significantly attenuated TH-induced increases in TNFα production in the lung tissue (Figure 7B).
Figure 7. TLR4 deficiency or TLR4 antagonist attenuated TH-induced increases in the production of TNFα in lung tissue.
(A) TNFα levels in the lung were examined by ELISA kit in wild type and TLR4-deficient mice subjected to TH. Sham surgical operation served as sham control. * P < 0.05 compared with indicated group. There were 6 mice in each group. (B) WT mice were treated with placebo or E5564 at different dose immediately following the induction of TH. Lung tissues were harvested 60 min after TH for preparation of cellular proteins. TNFα levels were measured using an ELISA kit. Sham surgical operation served as sham control. * P < 0.05 compared with sham group; # P < 0.05 E5564 group compared with placebo group. There were 6-8 mice in each group.
Discussion
The present study demonstrated that TLR4 plays an important role in cardiac dysfunction following TH. We have observed that either TLR4 deficiency or inhibition of TLR4 by E5564, a specific TLR4 antagonist, significantly attenuated TH-induced cardiac dysfunction. TLR4 deficiency and TLR4 inhibition markedly attenuated TH-induced decreases in the levels of myocardial Akt phosphorylation. In addition, inhibition of TLR4 with E5564 attenuated TH-induced myocardial NF-κB binding activity as well as lung MPO activity and TNFα production. Our data suggest that attenuation of TH-induced cardiac dysfunction by TLR4 deficiency or TLR4 inhibition involves activation of PI3K/Akt signaling. The data also indicated that pharmacologic antagonism of TLR4 may be an appropriate treatment for cardiac dysfunction in TH patients.
It is well known that TH causes myocardial depression (17, 24). However the mechanisms are unclear. Recent evidence suggests that innate immune and inflammatory responses contribute to the pathophysiologic mechanisms of multiple organ failure in TH (6). TLRs play a critical role in the induction of innate immune and inflammatory responses (4, 6). It has been reported that TLR4 activation stimulates inflammatory responses that depress cardiac contractility in septic shock and ischemia/reperfusion injury (18, 19). In addition, TLR4 is also involved in gut injury, lung injury and multiple organ failure in hemorrhage shock and TH (7, 25). Meng et al reported that nonresuscitated hemorrhagic shock significantly increased the levels of TNFα and TNFα receptor at 30 min and suppressed the cardiac function at 4 h in wild type mice. However, TLR4 deficiency abolished TNFα responses and attenuated cardiac dysfunction (10). Our results are consistent with that reported by Meng et al. The resent study demonstrated that TLR4 deficiency significantly prevented TH-induced cardiac dysfunction. Specifically, the levels of LVP and dP/dtmax following TH were significantly reduced in WT mice but not in TLR4 deficient mice. In addition, we have observed that TLR4 inhibition with E5564 significantly attenuated TH-induced cardiac dysfunction.
Eritoran tetrasodium (E5564) is a potent TLR4 antagonist with an IC50 of 1.5 nM (26). E5564 is a synthetic lipopolysaccharide that binds directly to the hydrophobic pocket of MD-2, resulting in the prevention of dimerization of TLR4 and subsequent intracellular signaling (27). Eritoran is primarily designed as a potent TLR4 antagonist for human. However, it has been well demonstrated that TLRs are conserved receptor family and present in mammalian, vertebrates and nonvertebrates (28). Eritoran has been employed in in vivo and in vitro studies using mice, guinea pigs, rats and dogs (29, 30). We observed that administration of E5564 at 1, 3, 5 and 10 mg/kg body weight significantly improved the values of LVP and dP/dtmax at 60 min after TH. Interestingly, administration of E5564 at 3 mg/kg body weight resulted in the optimum improvement of cardiac function.
Activation of PI3K/Akt signaling has been reported to negatively regulate the TLR-mediated NF-κB activation pathway (16). Recent evidence has shown that there is a cross talk between PI3K/Akt and TLR-mediated NF-κB signaling (31). Meng et al reported that TLR4 deficient mice showed the attenuation non-resuscitation hemorrhage induced cardiac dysfunction (10). These authors also showed the levels of TNFα and TNFα receptor were increased in wild type mice but not in TLR4 deficient mice following hemorrhage (10). We have observed in the present study that TH significantly decreases the levels of myocardial Akt phosphorylation in WT mice, but not in TLR4−/− mice. We have previously shown that TLR4 deficient mice showed higher levels of phosphorylated Akt in the myocardium (19). At present, we do not understand why deficiency of TLR4 increases Akt phosphorylation. However, the data suggest that increased Akt phosphorylation could be one of mechanism by which TLR4 deficiency protects against TH-induced cardiac dysfunction. We also observed that administration of the TLR4 antagonist E5564 markedly attenuated TH-induced decreases in the levels of myocardial Akt phosphorylation. It has been reported that Akt phosphorylation has anti-apoptotic properties (32, 33) and improves cardiac contractility via a calcium dependent mechanism (34). Collectively, the data suggest that maintaining Akt activity could be an important approach for the treatment and management of TH patients.
We have observed that TLR4 inhibition with E5564 significantly attenuated TH-induced myocardial NF-κB binding activity. It has been reported that activation of TLR4-mediated NF-κB contributes to organ injury after TH through induction of pro-inflammatory cytokines (5, 6, 7). Therefore, inhibition of TLR4-mediateed NF-κB activation during TH could be an important approach for attenuation of cardiac dysfunction. Indeed, we observed that the TLR4 antagonist E5564 prevented TH-induced myocardial NF-κB binding activity which positively correlated with the data of improved cardiac function. We also observed that the myocardial NF-κB binding activity inversely correlated with the levels of phosphorylated Akt in the myocardium of TH mice treated with E5564, indicating that there is a cross talk between PI3K/Akt and TLR/NF-κB signaling (16, 27).
It is well known that TLR4-mediated inflammatory responses on cardiac dysfunction in TH model involve local and systemic responses (6, 9, 10, 11, 24, 25). Indeed, the present study observed that either TLR4 deficiency or TLR4 antagonist also significantly reduces lung inflammatory responses following TH. We observed that TH-induced neutrophil infiltration into the lung, reflected by increased MPO activity, was significantly reduced in TLR4 deficient mice or TLR4 antagonist-treated mice. We also observed that the levels of TNFα in the lung tissues were significantly attenuated in TLR4 deficient mice or TLR4 antagonist treated mice, when compared with untreated TH mice.
In summary, our data showed that TLR4 contributes to cardiac dysfunction following TH. Administration of TLR4 antagonist attenuated TH-induced cardiac dysfunction. Pharmacologic antagonism of TLR4 may be a new approach for the treatment and management of TH patients.
Acknowledgments
This work was supported, in part, by AHA 09GRNT2020111 and NIH GM093878 to R.L.K.; NIH HL071837 to C.L.; NIH GM083016 to C.L. and D.L.W.; and NIH GM53522 to D.L.W.
References
- 1.Curry N, Hopewell S, Doree C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care. 2011;15:R92. doi: 10.1186/cc10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haut ER, Kalish BT, Cotton BA, Efron DT, Haider AH, Stevens KA, Kieninger AN, Cornwell EE, III, Chang DC. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a National Trauma Data Bank analysis. Ann Surg. 2011;253:371–377. doi: 10.1097/SLA.0b013e318207c24f. [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Benhamou Y, Favre J, Musette P, Renet S, Thuillez C, Richard V, Tamion F. Toll-like receptors 4 contribute to endothelial injury and inflammation in hemorrhagic shock in mice. Crit Care Med. 2009;37:1724–1728. doi: 10.1097/CCM.0b013e31819da805. [DOI] [PubMed] [Google Scholar]
- 6.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, Vallabhaneni R, Vodovotz Y, Billiar TR. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 7.McGhan LJ, Jaroszewski DE. The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury. 2012;43:129–136. doi: 10.1016/j.injury.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 9.Meldrum DR, Shenkar R, Sheridan BC, Cain BS, Abraham E, Harken AH. Hemorrhage activates myocardial NFkappaB and increases TNF-alpha in the heart. J Mol Cell Cardiol. 1997;29:2849–2854. doi: 10.1006/jmcc.1997.0506. [DOI] [PubMed] [Google Scholar]
- 10.Meng X, Ao L, Song Y, Raeburn CD, Fullerton DA, Harken AH. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-alpha receptor. Am J Physiol Regul Integr Comp Physiol. 2005;288:R600–R606. doi: 10.1152/ajpregu.00182.2004. [DOI] [PubMed] [Google Scholar]
- 11.McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S, Plevin R, Chatterjee PK, Yaqoob MM, Thiemermann C. Calpain inhibitor I reduces the activation of nuclear factor-kappaB and organ injury/dysfunction in hemorrhagic shock. FASEB J. 2001;15:171–186. doi: 10.1096/fj.99-0645com. [DOI] [PubMed] [Google Scholar]
- 12.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 13.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ. Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 15.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 16.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 17.Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Bland KI, Chaudry IH. Mechanism of salutary effects of estrogen on cardiac function following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. Crit Care Med. 2009;37:2338–2344. doi: 10.1097/CCM.0b013e3181a030ce. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Hua F, Ha T, Singh K, Lu C, Kalbfleisch J, Breuel KF, Ford T, Kao RL, Gao M, Ozment TR, Williams DL. Activation of myocardial phosphoinositide-3-kinase p110alpha ameliorates cardiac dysfunction and improves survival in polymicrobial sepsis. PLoS One. 2012;7:e44712. doi: 10.1371/journal.pone.0044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Gao M, Ha T, Kalbfleisch JH, Williams DL, Li C, Kao RL. The toll-like receptor 9 agonist, CpG-oligodeoxynucleotide 1826, ameliorates cardiac dysfunction after trauma-hemorrhage. Shock. 2012;38:146–152. doi: 10.1097/SHK.0b013e31825ce0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–H552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- 22.Gao M, THa T, Zhang X, Liu L, Wang X, Kelley J, Singh K, Kao RL, Gao X, Williams DL, Li C. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2012;40:2390–2399. doi: 10.1097/CCM.0b013e3182535aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–67. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, Rue LW, III, Bland KI, Chaudry IH. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am. J. Physiol Heart Circ. Physiol. 2004;287:H2183–H2191. doi: 10.1152/ajpheart.00624.2003. [DOI] [PubMed] [Google Scholar]
- 25.Reino DC, Palange D, Feketeova E, Bonitz RP, Xu dZ, Lu Q, Sheth SU, Pena G, Ulloa L, De MA, Feinman R, Deitch EA. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38:107–114. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czarniecki M. Small molecule modulators of toll-like receptors. J. Med. Chem. 2008;51:6621–6626. doi: 10.1021/jm800957k. [DOI] [PubMed] [Google Scholar]
- 27.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Roach J, Glusman G, Rowen L, Kaur A, Purcell M, Smith K, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102(27):9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barochia A, Solomon S, Cui X, Natanson C, Eichacker PQ. Eritoran tetrasodium (E5564) treatment for sepsis: review of preclinical and clinical studies. Expert Opin Drug Metab Toxicol. 2011;16:479–494. doi: 10.1517/17425255.2011.558190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I-270–I-274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 31.Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, Vogel SN. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]