Abstract
We reported previously that anti-CD3 mAb treatment before HCT prevented graft versus host disease (GVHD) and preserved graft-versus-leukemia (GVL) effects in mice. These effects were associated with down-regulated donor T cell expression of tissue-specific homing and chemokine receptors, marked reduction of donor T cell migration into GVHD target tissues, and deletion of CD103+ dendritic cells (DCs) in mesenteric lymph nodes (MLN). MLN CD103+ DCs and peripheral lymph node (PLN) DCs include CCR7+ and CCR7− subsets, but the role of these DC subsets in regulating donor T cell expression of homing and chemokine receptors remain unclear. Here, we show that recipient CCR7+ but not CCR7− DCs in MLN induced donor T cell expression of gut-specific homing and chemokine receptors in a retinoid acid (RA)-dependent manner. CCR7 regulated activated DC migration from tissue to draining lymph node, but was not required for the ability of DCs to induce donor T cell expression of tissue-specific homing and chemokine receptors. Finally, anti-CD3 treatment depleted CCR7+ but not CCR7− DCs by inducing sequential expansion and apoptosis of CCR7+ DCs in MLN and PLN. Apoptosis of CCR7+ DCs was associated with DC up-regulation of Fas expression and NK cell but not T, B or dendritic cell upregulation of FasL expression in the lymph nodes. These results suggest that depletion of CCR7+ host-type DCs with subsequent inhibition of donor T cell migration into GVHD target tissues can be an effective approach in prevention of acute GVHD and preservation of GVL effects (244).
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative therapy for hematological malignancies (i.e. leukemia and lymphoma), owing to the graft versus leukemia/lymphoma (GVL) effect mediated by alloreactive T cells, but graft-versus-host disease (GVHD) mediated by the same alloreactive T cells remains as a major obstacle [1–5]. It has long been proposed that, in the pathogenesis of acute GVHD, recipient hematopoietic antigen-presenting cells (APCs) such as dendritic cells play a major role in initiating allogeneic T cell activation and induction of acute GVHD [5–10]. Critical cellular interactions occur in secondary lymphoid organs such as mesenteric lymph nodes (MLN) that function as the meeting ground between host APCs and donor T cells [11, 12]. After being activated by total body irradiation (TBI) or chemotherapy, recipient DCs migrate from tissues to draining lymph nodes (LN) where they induce donor T cell expression of tissue-specific homing and chemokine receptors [13, 14]. Activated T cells subsequently migrate to epithelial tissues such as the gut and skin to cause GVHD [15, 16].
CCR7 expressed by DCs and the CCR7 ligands CCL19 and CCL21 expressed in LNs mediate the migration of activated DCs from tissues into LNs [17], and proinflammatory cytokines such as IFN-γ augment expression of CCR7 by DCs and increase release of the CCR7 ligands in LNs to enhance this migration [18, 19]. Donor T cells are induced to express tissue-specific homing and chemokine receptors in draining LNs [13, 20], although lymphotoxin-α deficient mice lacking Peyer’s patches and lymph nodes still developed acute GVHD [21, 22]. In the MLN, T cells interact with CD103+ DCs and up-regulate expression of gut-homing receptors, including α4β7 and CCR9 [14, 23], and donor T cell expression of α4β7 has been shown to be important for development of gut GVHD [24]. In peripheral lymph nodes (PLN), T cells interact with DCs to up-regulate expression of skin-homing receptors, including E-ligand, P-ligand, CCR4 and CCR10 [23, 25, 26]. These tissue-specific homing and chemokine receptors and chemokine gradients guild T cell infiltration of GVHD target tissues [13, 27–29], and non-hematopoietic APCs in the GVHD target tissue could up-regulate MHC and mediate alloreactive T cell expansion in the tissue [30, 31].
Recent reports showed that profound depletion of host hematopoietic APCs did not prevent induction of acute GVHD [32], and recipient non-hematopoietic APCs were sufficient to induce donor T cell activation/expansion in GVHD target tissues, especially in gut tissue, and induce lethal GVHD [33]. On the other hand, a previous report indicate that retinoic acid (RA)-producing CD103+ DCs in MLN play an important role in imprinting T cell expression of α4β7 and CCR9 [14]. RA-induced donor T cell expression of gut-specific homing and chemokine receptors α4β7 and CCR9 in MLN, and blockade of RA signaling prevented donor T cell up-regulation of α4β7 and CCR9 expression and markedly reduced the severity of gut GVHD [34, 35]. The important role of α4β7 in mediating alloreactive T cell migration into gut tissues has also been demonstrated by others, in both animal models and patients [24, 36, 37].
Consistently, we observed that depletion of CD103+ DCs by anti-CD3 preconditioning prevented donor T cell expression of α4β7 and CCR9 and prevented GVHD in the gastrointestinal tract and elsewhere [38]. We have recently observed that CD103+ DCs in MLNs include both CCR7+ and CCR7− subsets. DCs in PLNs are CD103− but also include CCR7+ and CCR7− subsets. In the current studies, we attempted to determine whether these two DC subsets differ in their ability to produce RA and induce tissue-specific homing and chemokine receptors by donor T cells. We also evaluated the effects of anti-CD3 preconditioning on CCR7+ and CCR7− DC subsets, since it has been proposed that the CCR7+ subset is comprised of activated DCs that migrate from inflamed tissues into draining LNs [17].
Materials and Methods
Mice
C57BL/6 (CD45.2), congenic C57BL/6 (CD45.1) and BALB/c mice were purchased from NCI Laboratories (Frederick, MD). CCR7−/− C57BL/6 (CD45.2) mice were purchased from the Jackson Laboratory. OT-I C57BL/6 (CD45.2) mice were a gift from Dr. Hua Yu (City of Hope National Medical Center, CA). All animals were maintained in a pathogen-free room at the City of Hope Research Animal Facility. Male mice 8 to 10 weeks of age were used in the current studies. Animal use protocols were approved by the institutional review committee.
Chemicals
Retinoic acid (RA) and its receptor antagonist, LE135, were respectively purchased from Sigma (USA) and Tocris Bioscience, dissolved in DMSO (100 mM) and stored at −20° and protected from light. Ovabumin (OVA) was purchased from Sigma (USA). Collagenase D (11 088 858 001) and DNase I recombinant (04 536 282 001) were all purchased from Roche Laboratories. 3H-thymidine was purchased from Amersham (Buckinghamshire, United Kingdom).
Cell Purification
Production of anti-CD3 monoclonal antibody (mAb; 145-2C11) has been described in our previous publication [39]. To enrich dendritic cells, the MLNs and/or PLNs were harvested and digested at 37°C in RPMI 1640 medium containing 0.5 mg/ml collagenase D and 50U/ml recombinant DNase I for 10–20 minutes. Tissues were disaggregated, mononuclear cells were collected, and CD11c+ cells were enriched by magnetic antibody cell separation (MACS). Enriched CD11c+ cells were used for further separation of CCR7+ and/or CD103+ cells by FACS. CD4+ and CD8+ T cells were obtained from mouse spleens by MACS (purity>95%).
Flow cytometric analysis
The following anti-mouse mAbs were purchased from BD Biosciences Pharmingen (San Jose, CA), eBioscience (San Diego, CA), BioLegend (San Diego,CA) or R&D Systems (Minneapolis, MN): TCRβ (H57-597), CD4 (RM4-5), CD8α (53-6.7), CD11c (HL3), CD103 (M290), α4β7 (DATK32), CCR9 (CW-1.2), CD45.2 (104), CCR7(4B12), Fas/CD95 (554258), FasL/CD178 (MFL3), CD49b (DX5), CD90.2/Thy1.2 (30-H12), CD19 (eBio1D3), Annexin V (17-8007-72). Fluorescence-activated cell sorting (FACS) was performed with a 4-laser MoFlo Immunocytometry System (Dako, Glostrup, Denmark) or CyAn immunocytometry system (Dako Cytomation, Fort Collins, CO), and data were analyzed with FlowJo software (TreeStar, Ashland, OR), as described previously [40].
Quantitation of gene expression by real-time RT-PCR
Isolation of total tissue RNA and synthesis of first strand cDNA have been described previously [38]. mRNA was quantified by real-time quantitative PCR using Applied Biosystems 7300 Fast Real-Time PCR System (Applied Biosystems, Forest City, CA). The following primer sequences were obtained from previous
| RALDH2 [41]: | Forward: 5’-TGCATTCACAGGGTCTACCGA-3’, |
| Reverse: 5’-TGCCTCCAAGTTCCAGAGTT-3’; |
TUNEL assay of DC apoptosis
8 hr after treatment of BALB/c mice with anti-CD3 mAb (5µg/g i.v.), MLNs were harvested, flash-frozen and cut into 10 µm sections. Slides were then stained with TUNEL (Roche), DAPI, goat anti-mouse CCR7 (Abcam) and biotin-CD11c (Ebioscience) and imaged with the use of an LSM 510 Meta Inverted 2 Photon Confocal Microscope (Zeiss). Images were taken with a 40× water objective (Leica) and analyzed for expression of CCR7 and apoptosis using an LSM Image Browser.
Statistical analysis
Survival differences were evaluated according to the log-rank test with the use of Prism software (GraphPad, La Jolla, CA). Differences between means were evaluated according to the unpaired 2-tailed Student t test.
Results
Host CCR7+ DCs in MLNs induced alloreactive donor T cell expression of gut homing and chemokine receptors in a RA dependent-manner
Our previous report showed that preconditioning with anti-CD3 mAb 7 days before HCT prevented acute GVHD and preserved GVL effect in recipients conditioned with myeloablative TBI. Prevention of acute GVHD was associated with depletion of CD103+ DCs, reduced expression of tissue homing and chemokine receptors by donor T cells, and reduced donor T cell migration into GVHD target tissues such as gut and skin (24). Expression of CCR7 on DCs was proposed to mediate DC migration from tissue to draining LNs [17], but the role of CCR7+ DC in inducing donor T cell expression of tissue homing and chemokine receptors remains unclear. We evaluated the role of host-type CCR7+ and CCR7− DCs in inducing donor T cell expression of tissue homing and chemokine receptors; we also evaluated the impact of anti-CD3 treatment on depletion of host-type CCR7+ and CCR7− DC subsets.
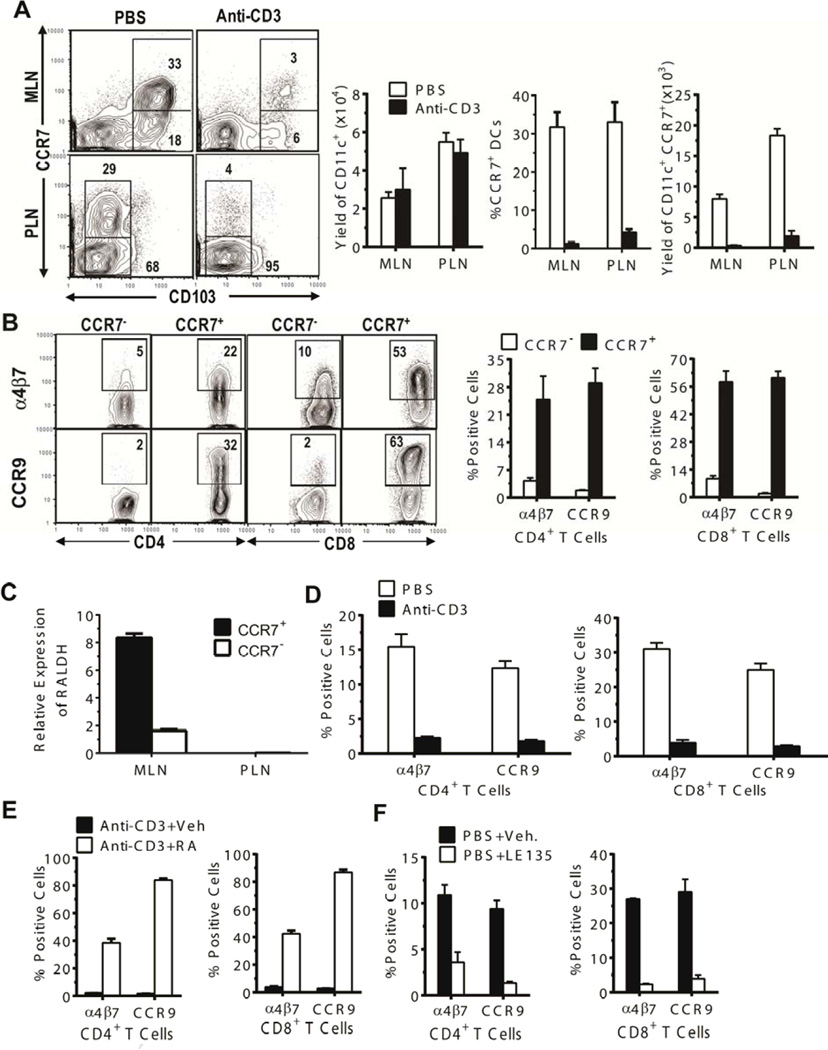
We found that host-type CD103+ DCs in MLN included CCR7+ (~30%) and CCR7− subsets (Fig. 1A). The CD103−DCs that predominate in PLN also included CCR7+ (~30%) and CCR7− subsets. Although anti-CD3 treatment did not significantly reduce the total numbers of CD11c+ DCs in MLN and PLN by 7 days after treatment, anti-CD3-treatment markedly reduced the percentage and numbers of CCR7+ DCs in both MLN and PLN (P<0.01, Fig. 1A, left panels). These results indicate that anti-CD3 treatment depletes CCR7+ but not CCR7− DCs, and that CCR7+ DCs in the draining LN may be responsible for imprinting T cell expression of tissue-specific homing and chemokine receptors.
Figure 1. CCR7+ DC subset in MLN was necessary for induction of gut-homing receptor expression by donor T cells.
(A) Mononuclear cells of MLN and PLN from BALB/c mice 7 days after treatment with PBS or anti-CD3 were stained for CD11c, CCR7, and CD103. Gated CD11c+ cells are shown as CCR7 versus CD103 staining. A representative flow cytometry pattern and the percentage and yield (mean ± SE, N=4) of CCR7+ DC subsets and total CD11c+ cell yield from 1 of 4 replicate experiments are shown. (B) CD11c+CD103+ DCs from MLN of untreated BALB/c mice were sorted into CCR7+ and CCR7− subsets. Each subset (0.05 × 106) was co-cultured with C57BL/6 T cells (0.1 × 106) for 4 days. Thereafter, cultured cells were stained with CD4, CD8, α4β7, and CCR9. Gated CD4+ or CD8+ T cells are shown as CD4 or CD8 vs α4β7 or CCR9 staining. A representative flow cytometry pattern and the percentages (mean ± SE) of α4β7+ or CCR9+ cells among CD4+ or CD8+ T cells from 1 of 3 replicate experiments are shown. (C) Total RNA was isolated from sorted CCR7+ and CCR7− DCs from MLN and PLN of untreated BALB/c mice, and RALDH expression was analyzed by real-time PCR. Relative expression levels (mean ± SE) from 3 replicate experiments are shown. (D–F) Seven days after treatment with PBS or anti-CD3, CD11c+ DCs from host-type BALB/c MLN were sorted. The sorted CD11c+ cells (0.1 × 106) from PBS- or anti-CD3-treated mice were first co-cultured with splenic CD4+ and CD8+ T cells (0.2 × 106) from donor-type C57BL/6 (D); then, the sorted CD11c+ DCs from anti-CD3-treated mice were co-cultured with the donor T cells in the presence or absence of RA (E); finally, the sorted CD11c+ DCs from PBS-treated control mice were cultured with the donor T cells in the presence or absence of RA antagonist LE135 (1 µM) (E). After culture for 4 days, expression of α4β7 and CCR9 by the cultured T cells was analyzed by flow cytometry. Percentages (mean ± SE, N = 4) of α4β7+ or CCR9+ cells among CD4+ or CD8+ T cells are shown.
To determine whether CCR7+ DCs were responsible for inducing donor T cell expression of tissue homing receptors, we decided to mechanistically focus on MLN CCR7+ DC and their capacity to imprint gut homing receptors α4β7 and CCR9. Accordingly, recipient-type BALB/c MLN CD11c+ DCs were sorted into CD103+CCR7+ and CD103+CCR7− subsets and cultured with sorted donor-type C57BL/6 splenic CD4+ and CD8+ T cells for 4 days. The percentage of α4β7+ and CCR9+ CD4+ and CD8+ T cells was approximately 5–15-fold higher in cultures with CCR7+ DCs as compared to cultures with CCR7− DCs (p < 0.001, Figure 1B). This result indicates that CD103+CCR7+ but not CD103+CCR7− DCs in MLNs induce expression of gut-homing α4β7 and CCR9 receptors by allogeneic T cells.
Production of RA by DCs in MLNs is required for induction of T cell expression of α4β7 and CCR9 [42], and the enzyme RALDH2 is needed to metabolize Vitamin A into RA [43]. We found that expression levels of RALDH mRNA were 5-fold higher in CD103+CCR7+ DCs than in CD103+CCR7− DCs from MLNs (p < 0.01), but expression of RALDH mRNA was not detected in either CCR7+ or CCR7− DCs from PLN (Figure 1C). CD11c+ DCs from MLN of mice treated with anti-CD3 had a markedly decreased ability to induce expression of α4β7 and CCR9 by allogeneic CD4+ and CD8+ T cells in an in vitro culture assay (P < 0.01, Figure 1D). Expression of α4β7 and CCR9 in this assay was restored and greatly enhanced by adding RA (10 nM) to the cultures (P< 0.01, Figure 1E). CD11c+ DCs from MLN of control mice induced expression of α4β7 and CCR9 by allogeneic CD4+ and CD8+ T cells, and expression of α4β7 and CCR9 was inhibited by adding the RA receptor antagonist LE135 (1 µM) to the cultures (P< 0.01, Figure 1F). Taken together, these results demonstrate that expression of gut homing α4β7 and CCR9 by donor T cells is induced by recipient MLN CCR7+ DCs through an RA-dependent mechanism.
Depletion of CCR7+ DCs by anti-CD3 treatment prevented tissue DC migration from intestinal lamina propria to MLN early after HCT
In the pathogenesis of GVHD, tissue damage caused by the conditioning regimen releases proinflammatory cytokines and chemokines that activate tissue DCs and upregulate their expression of CCR7, so that they migrate to draining LNs [44]. Since only CCR7+ DCs migrate from gut or skin tissues to draining LNs [45, 46], and since we observed that treatment with anti-CD3 resulted in loss of CCR7+ DCs in MLN and PLN, we tested whether treatment with anti-CD3 mAb prevented DC migration from intestinal lamina propria to MLN. For this purpose, we used an assay which measures the ability of DCs to transport orally administered ovalbumin (OVA) from the lamina propria to MLN [45].
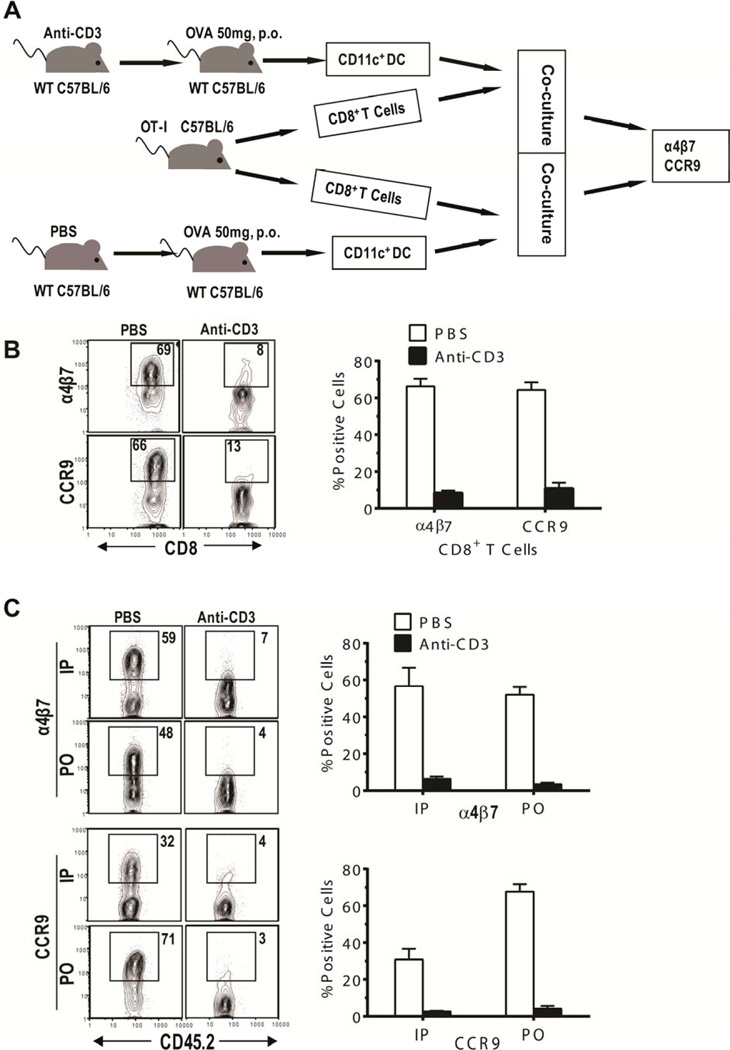
As described in Figure 2A, C57BL/6 mice were treated with control PBS or anti-CD3 mAb. The mice were given oral OVA (50mg) 7 days after treatment, and on the following day, CD11c+ DCs were isolated from MLNs and co-cultured with OVA-specific CD8+ T cells from OT-I TCR-transgenic C57BL/6 mice for 4 days. Induction of α4β7 and CCR9 expression by OVA-specific T cells was greatly reduced after stimulation with DCs from mice treated with anti-CD3 mAb as compared to those from mice treated with PBS (~10% vs 60%, p < 0.01, Figure 2B). This result indicates that few OVA-bearing DCs migrate from the lamina propria to the MLN after anti-CD3 preconditioning. These results also indicate that few CCR7+ DCs remain in the tissues after anti- CD3 treatment.
Figure 2. Preconditioning with anti-CD3 mAb inhibited CCR7+ DC migration from gut tissue to MLN.
(A) Experimental diagram: C57BL/6 mice were treated with anti-CD3 or PBS. Seven days after treatment, mice were given 50 mg OVA orally (P.O.). One day later, CD11c+ DCs (0.1× 106) sorted from MLN were cultured for 4 days with sorted CD8+ T cells (0.2 × 106) from OT-I TCR transgenic C57BL/6 mice, and expression of α4β7 and CCR9 by OT-I T cells was analyzed by flow cytometry. (B) A FACS pattern of α4β7+ and CCR9+ cells among OT-I CD8+ T cells (left) and the percentages (mean ± SE) of α4β7+ and CCR9+ cells among CD8+ T cells (right) are representative results from 1 of 3 replicate experiments. (C) CD45.1+ congenic C57BL/6 mice were treated with PBS or anti-CD3. Seven days after treatment, OT-I CD8+ T cells from CD45.2+ OT-I transgenic C57BL/6 mice were injected into the treated CD45.1+ congenic recipients. One day later, recipients were given 50 mg OVA P.O. or 5mg OVA i.p. Three days later, expression of α4β7 and CCR9 by CD45.2+ OT-I CD8+ T cells in MLN was analyzed by flow cytometry. FACS patterns (left) and the percentages (mean ± SE) of α4β7+ or CCR9+ cells among OT-I CD8+ T cells (right) are representative results from 1 of 3 replicate experiments.
We evaluated the effect of anti-CD3 treatment on migration of DC from the lamina propria to the MLN and on the subsequent interaction of DC with T cells in MLN. Accordingly, CD45.1+ C57BL/6 mice were treated with either control PBS or anti-CD3 mAb. Seven days after treatment, CD8+ T cells (3×106) from CD45.2+ OT-I TCR-transgenic C57BL/6 donors were injected into the anti-CD3-treated or control PBS-treated mice, and on the following day, the mice were given OVA by oral administration (PO) or intra-peritoneal injection (IP). In order to stimulate T cells, oral OVA must be transported from lamina propria to MLN by DCs, but IP-OVA can directly reach MLN in the absence of DC migration from lamina propria to MLN.
Three days after IP or PO administration of OVA, more than 32% of the CD45.2+ OT-I CD8+ T cells from MLNs of recipients treated with PBS expressed α4β7 and CCR9, while less than 7% of the CD45.2+ OT-I CD8+ T cells from MLNs of recipients treated with anti-CD3 mAb expressed α4β7 or CCR9 (P< 0.01, Figure 2C). IP and PO administration of OVA both induced OVA-specific T cells to express α4β7 and CCR9 in PBS-treated mice but not in anti-CD3-treated mice (Figure 2C). These results suggest that 7 days after treatment with anti-CD3 mAb, DCs could not migrate from tissue to MLN (most likely due to the lack of the CCR7+ subset), as indicated by the absence of α4β7 and CCR9 expression by OT-I T cells in MLN after oral administration of OVA. Likewise, pre-existing CCR7+ DCs in the MLN had been eliminated, as indicated by the absence of α4β7 and CCR9 expression by OT-I T cells in MLN after IP injection of OVA. Taken together, these results indicate that conditioning with anti-CD3 mAb not only depletes the preexisting CCR7+ DCs in the draining LNs, but also depletes CCR7+ DCs in the tissues, and prevents DC migration from tissues into LNs.
MLN DCs deficient in CCR7 expression were still able to induce donor T cell expression of gut-tropic homing and chemokine receptors
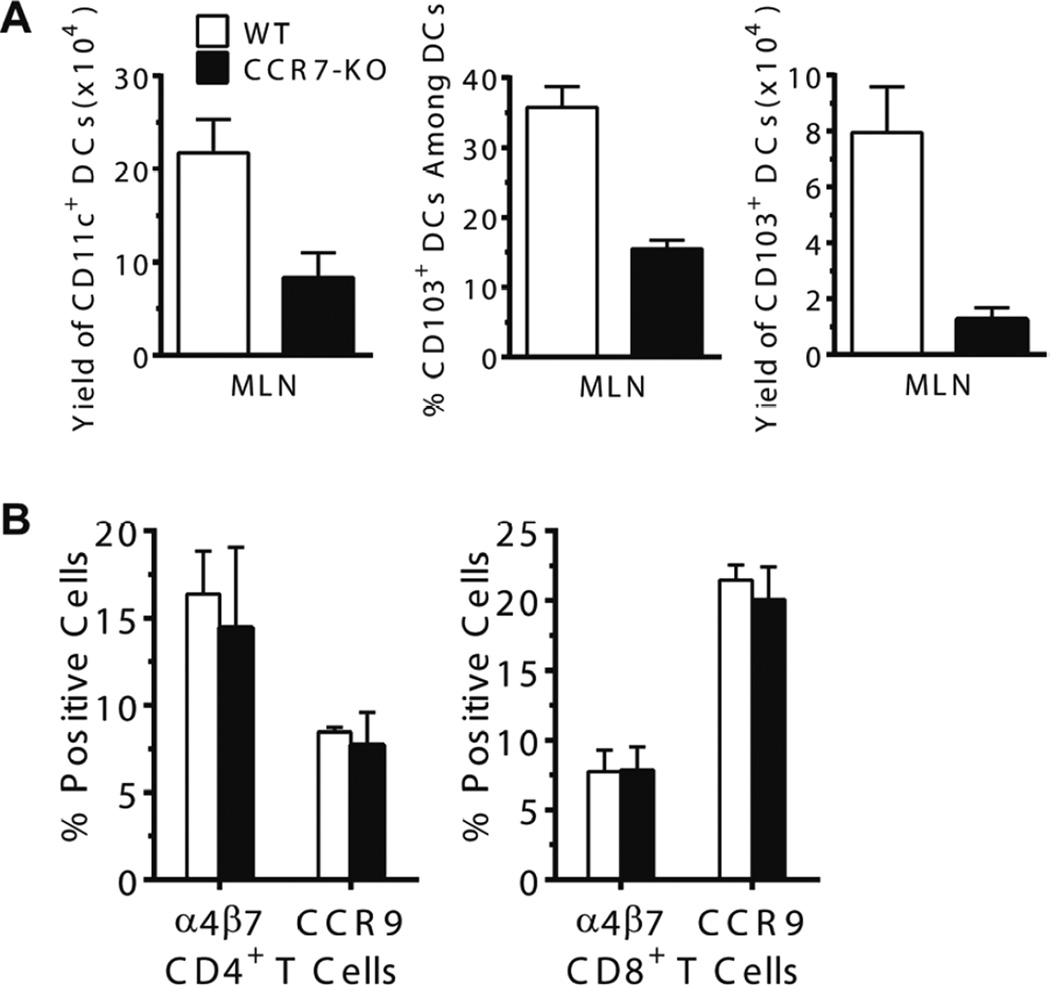
While we observed that CCR7+ DC were capable of imprinting donor T cells in an RA-dependent mechanism (Figure 1C–F), we wondered whether CCR7 itself was required for the DC function. Therefore, we tested whether DC expression of CCR7 was required for their imprinting donor T cell tissue tropism by comparing DCs from WT and CCR7−/− C57BL/6 mice. We observed that, compared with WT mice, the yield of total CD11c+ DCs in MLN of CCR7−/− mice was reduced more than 2-fold (P<0.01, Fig. 3A, left panel). The percentage and yield of CD103+ DCs of CCR7−/− mice was also markedly reduced (P<0.05, Figure 3A, middle and right panels). Sorted CD103+ DCs from MLN of both strains, however, induced donor CD4+ or CD8+ T cell expression of α4β7 and CCR9 in an in vitro culture assay, and no significant difference was observed (P>0.05, Figure 3B). The result is consistent with previous report that CCR7 plays important roles in mediating activated DC migration from tissue to draining lymph node; this result also indicates that CCR7 itself is not required for DC’s function in imprinting T cell tissue tropism.
Figure 3. Deficiency of CCR7 expression on DCs in MLN of mice did not impair DC’s ability in inducing donor T cell expression of gut-tropic homing and chemokine receptors.
(A) The MLN from wild-typed and CCR7−/− C57BL/6 mice were harvested and mononuclear cells were stained for CD11c and CD103. The mean yield of total CD11c+, the percentage and yield of CD103+ DCs among CD11chigh cells are shown (left). Each group contains 4 mice. (B) CD11c+CD103+ DCs (0.1×106) in MLN from WT and CCR7−/− mice were sorted by FACS after magnetic enrichment of CD11c+ cells and co-cultured with CD4+ or CD8+ T cells from BALB/c mice in U-bottom 96-cell plates for 4 days. Expression of α4β7 and CCR9 by the cultured T cells was analyzed by flow cytometry. Mean ± SE is shown from 4 replicate experiments.
Anti-CD3 mAb treatment induced sequential expansion and apoptosis of CCR7+ DCs in MLN and PLN
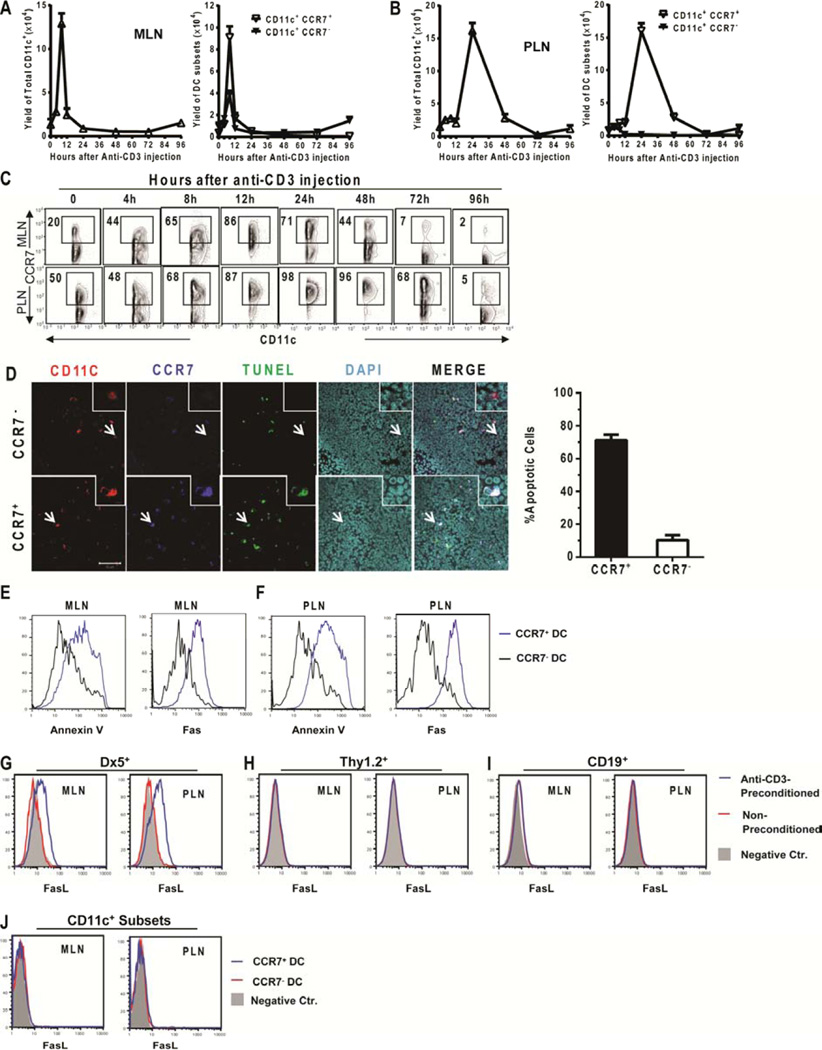
Since we observed that treatment with anti-CD3 mAb depleted CCR7+ DCs in MLN and PLN and prevented DC migration from gut tissue to MLN (Fig. 1 and 2), we tested whether this treatment induced apoptosis of CCR7+ DCs. The percentage and yield of the total CD11c+ DCs and the CCR7+ and CCR7− subsets in MLN and PLN were kinetically measured before and after injection of anti-CD3 mAb. The total numbers of CD11c+ DCs in MLN increased 10-fold at ~8 hours after antibody injection, and then rapidly decreased by 12 hours and reached a nadir at 24 – 48 hours (Figure 4A, left panel). Between 8–12 hours after antibody injection, ~90% of CD11c+ DCs became CCR7+ (Fig. 4C, upper row). By 96 hours, the total number of CD11+ DCs recovered to levels similar to those observed before the antibody injection (Figure 4A, left panel). The initial rapid increase and the subsequent decrease of total CD11c+ DCs resulted predominantly from changes in the CCR7+ DC subset, and the recovery at 96 hours came from the CCR7− (likely de novo developed) subset (Figure 4A, right panel, Figure 4C). Fluctuations in the numbers of total CD11c+ DCs as well as CCR7+ and CCR7− DC subsets in PLNs after treatment with anti-CD3 mAb were similar to those observed in MLNs, although the onset was delayed by 12–24 hours (Figure 4B and C).
Figure 4. Treatment with anti-CD3 mAb induced sequential expansion and apoptosis of CCR7+ DC in MLN and PLN.
(A and B) BALB/c mice were treated with anti-CD3 mAb. (A–C) At the indicated time-points, mononuclear cells from MLN and PLN were analyzed for the percentages and numbers of total CD11c+ DCs and the CD11c+CCR7+ and CD11c+CCR7− subsets by flow cytometry. Results are shown as the kinetic curve of the mean ± SE of 5 replicate experiments. (D) Eight hours after antibody injection, MLNs were harvested (N = 4 mice) and tissue sections were stained as follows: TUNEL indicating apoptosis (green), CD11c (red), CCR7 (blue) and DAPI (light blue). Slides were imaged at 40x magnification. Insets show 8× magnified view of image. Arrows indicate apoptotic CCR7+ DCs (lower panel) and non-apoptotic CCR7− DCs (upper panel). Apoptotic CCR7+ or CCR7− DCs were counted and shown as a percentage of CCR7+ or CCR7− DCs, respectively (N=4 mice, mean± SE). (E–F) Eight hours after antibody injection, mononuclear cells from MLN or PLN were stained for CD11c, CCR7 and Annexin V or Fas. Histograms of Annexin V or Fas staining on gated CD11chi CCR7+ cells (blue line) and CD11chi CCR7− cells (black line) are shown. Results are representative of 3 replicate experiments. (G–I) Eight hours after anti-CD3 injection, mononuclear cells from the MLN and PLN were stained for DX5, Thy1.2, CD19, or CD11c versus FasL. Gated (G) DX5+, (H) Thy1.2+, (I) CD19+, or (J) CD11c+ cells were shown in histogram of FasL. (G–I) Histograms are overlaid: with or without anti-CD3-preconditioning and negative control. Negative control is filled in grey, non-conditioned BALB/c is red, and anti-CD3-preconditoned is blue. (J) Histograms are overlaid: CCR7+ CD11c+, CCR7− CD11c+ and negative control. Negative control is filled in grey, CCR7−CD11c− is red, and CCR7+CD11c+ is blue. Results are representative of N=4 mice per group.
The rapid decrease in the number of CCR7+ DCs 8–12 hours after injection of anti-CD3 mAb in MLNs resulted from apoptosis, since the percentage of TUNEL+ DCs was 5-fold higher in the CCR7+ subset than in the CCR7− subset (P< 0.01, Figure 4D). CCR7+ DCs in MLNs and PLNs had much higher levels of Annexin V staining as compared to CCR7− DCs (Figure 4E & F). As compared to CCR7− DCs, the increased apoptosis of CCR7+ DCs was associated with higher expression of Fas (Figure 4E & F). Additionally, we found that in the MLN and PLN DX5+ NK cells, but not Thy1.2+ T, B220+ B, or CD11c+ subsets (CCR7+ or CCR7−) dendritic cells up-regulated expression of FasL (Figure 4G–J).
Discussion
We previously showed that treatment with anti-CD3 before HCT prevented GVHD while preserving strong GVL effects [38]. Prevention of GVHD was associated with down-regulated expression of tissue-specific homing and chemokine receptor by donor T cells and depletion of of CD103+ DCs in MLN [38]. In this report, we expand upon previous results and have shown: 1) CD103+ DCs in MLN included CCR7+ and CCR7− subsets, and CCR7+ DCs that produced RA were required to induce expression of gut-homing α4β7 and CCR9 by donor T cells. 2) Anti-CD3 treatment before HCT upregulated DC expression of CCR7 and Fas and induced DC apoptosis, likely through Fas/FasL signaling pathway. 3) Depletion of CCR7+ DCs prevented DC migration from tissue to draining LN and reduced donor T cell expression of tissue-specific homing and chemokine receptors.
We observed that CCR7+ but not CCR7− DCs in MLNs expressed high-levels of RALDH, an enzyme needed to convert vitamin A to RA. CCR7+ but not CCR7− DCs in MLNs were able to induce expression of gut homing α4β7 and CCR9 by alloreactive T cells in vitro through an RA-dependent mechanism. In addition, CCR7+ DCs from MLN but not from PLN showed expression of RALDH, the enzyme that catalyzes conversion of vitamin A to RA. These results indicate that only activated DCs can induce donor T cell tissue tropism, and activated DCs from different tissues have different enzyme activity that can mediate T cell tissue tropism. This result is consistent with a previous report that DCs from LN draining different GVHD target tissues induced donor T cell expression of tissue-specific homing and chemokine receptors [20].
We observed that depletion of CCR7+ DCs by anti-CD3 preconditioning resulted from sequential events of DC activation, DC up-regulation of CCR7 and Fas expression, CCR7+ DC influx into draining LNs, and finally DC apoptosis. Injection of anti-CD3 markedly increased serum levels of IL-2, IFN-γ and IL-6, which peaked ~8 hours after injection of anti-CD3 [47]. These cytokines were reported to activate DC, up-regulate DC expression of CCR7 and Fas, and augment LN release of CCR7 ligands CCL19 and CCL21 in LN [18, 19]. Consistently, we observed that administration of anti-CD3 markedly augmented MLN and PLN release of CCL19 and CCL21 (He and Zeng: unpublished data). As early as 8 hours after anti-CD3 treatment, most DCs in MLN and PLN became CCR7+, and total numbers of DC and CCR7+ DC increased by 10-fold. At 12 and 48 hours after treatment, total DC and CCR7+ DC numbers reached a nadir, most likely due to the apoptosis of CCR7+ DCs, since CCR7+ DCs express high levels of Fas. At this same time after anti-CD3 injection, DX5+ NK cells, but not Thy1.2+ T, CD19+ B, or CD11c+ dendritic cells in MLN and PLN upregulated FasL. The IL-2-activated FasL-expressing NK cells may mediate the apoptosis of the CCR7+ DCs with high level expression of Fas. While NK cells traditionally kill through use of perforin, NK cells activated in the presence of IL-2 kill via FasL/Fas pathway [48]. While the specific mechanisms by which these NK cells contribute to the apoptosis of CCR7+ cells are still under investigation, we have observed that IL-2Rγ−/− mice that are deficient in NK cells fail to deplete CCR7+ DC after anti-CD3 injection, and injection of a FCR-non-binding anti-CD3 that stimulates lower levels of IL-2 than FCR-binding anti-CD3 ([39, 49] and data not shown) only partially depletes CCR7+ DC (data not shown). Interestingly, despite upregulation of CCR7+ expression by DCs after anti-CD3 preconditioning, significant expression changes were not seen in other activation markers, such as CD40, CD80, CD86, MHCII (data not shown), although we cannot fully rule out functional differences in antigen processing/presentation after anti-CD3 conditioning. By 96 hours after anti-CD3 treatment, total DC numbers recovered to the level before treatment, but they were all CCR7−, which are most likely de novo developed DCs. Thus, anti-CD3-conditioning induces DC activation, upregulated expression of CCR7 and Fas, and apoptosis, all in CCR7+ DCs.
Anti-CD3 preconditioning and depletion of CCR7+ DCs do not interfere with activation of donor T cells in LN. First, donor T cells are infused 7 days after anti-CD3 preconditioning when serum anti-CD3 is not detectable as previously described [50]. Second, although anti-CD3 preconditioning depletes CCR7+ DCs, it does not reduce the total number of CD11c+ DCs in the MLN and PLN when donor T cells are infused. The CCR7− DCs are fully capable of stimulating allogeneic T cells in vitro and in vivo, comparable to the ability of DCs from untreated control mice [38]. Five days after HCT, donor T cell expansion in the host lympho-hematopoietic tissues is stronger in anti-CD3-preconditioned recipients as compared to the recipients without preconditioning, although the donor T cells do not migrate to GVHD target tissues such as gut and skin to cause GVHD [38]. Thus, the injected anti-CD3 had little or no direct effect on alloreactive donor T cell activation. This regimen differs from previously reported use of anti-CD3 mAb for treatment after HCT, where the main effect of the antibody is to block activation of alloreactive donor T cells in the recipient [51–53].
Depletion of CCR7+ DC subset via anti-CD3 preconditioning differs from depletion of DCs by establishing chimeras using MHC II-deficient BM or depletion of DCs by expression of DTR and injection of DT or other antibodies as described in recent publications [32, 33]. Anti-CD3-preconditioning and depletion of CCR7+ DCs (including RA-producing CCR7+ DCs in MLN) prevents GVHD by impairing donor T cell migration into GVHD target tissues. The results with anti-CD3-preconditioning are consistent with observations that blockade of RA signaling downregulated donor T cell expression of gut-specific homing and chemokine receptors and ameliorated gut GVHD [34, 35]. The results are also consistent with observations that confining donor T cells in host lymphoid tissues by administration of FY720 prevented GVHD [54]. However, depletion of host hematopoietic APCs did not prevent donor T cell migration into GVHD target tissues [33], and non-hematopoietic APCs in the target tissues upregulate MHC II and expanded donor T cells that caused GVHD.
In summary, our studies identify CCR7+ DCs in MLNs as a critical cell population required for induction of gut homing and chemokine receptors, α4β7 and CCR9 by donor T cells. Anti-CD3 preconditioning depletes this DC subset first by CCR7 up-regulation followed by induction of apoptosis. Depletion of CCR7+ DCs may explain how anti-CD3 preconditioning prevents GVHD while preserving GVL effects, as demonstrated previously [38]. Regimens targeting DC subsets that imprint donor T cell tissue tropism could be an effective approach towards controlling GVHD while sparing GVL effects.
Acknowledgments
We thank Lucy Brown and her staff at the City of Hope (COH) Flow Cytometry Facility, Sofia Loera and her staff at the COH Anatomic Pathology Laboratory, and Brian Armstrong and his staff at the Light Microscopy Digital Imaging Core for their excellent technical assistance.
This work was supported by Nesvig Lymphoma Foundation and NIH R01-AI66008 (to D. Zeng).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: W. He designed and performed research and wrote the manuscript; J. Racine, assisted on experimental design, data analysis, and writing the manuscript, H. F. Johnston performed apoptosis experiments; X. Li, N. Li, K. Cassady, C. Liu, R. Deng assisted in experiments. P. Martin advised on experimental design and critically reviewed the manuscript; S. Forman supported the research and reviewed the manuscript; D. Zeng conceptualized and designed research, wrote the manuscript, and supervised the research project.
Authors declare no conflict of interest.
References
- 1.Riddell SR. The graft-versus-leukemia effect--breaking the black box open. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:2–3. doi: 10.1016/j.bbmt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Fefer A. Graft-vs.-Tumor Responses. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoietic cell transplantation. Third Edition ed. Malden, MA: Blackwell Publishing; 2004. pp. 369–379. [Google Scholar]
- 3.Korngold R, Friedman TM. Murine Models of Graft-versus-Host Disease and Graft-versus-Tumor Effect. In: Appelbaum F, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. Bostone: Blackwell Scientic Publication; 2009. pp. 176–187. [Google Scholar]
- 4.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nature medicine. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 5.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 7.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. Journal of immunology. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Shlomchik WD, Joe G, Louboutin JP, Zhu J, Rivera A, et al. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. Journal of immunology. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik WD. Graft-versus-host disease. Nature reviews Immunology. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 10.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 11.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4:154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 13.Sackstein R. A revision of Billingham's tenets: the central role of lymphocyte migration in acute graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12:2–8. doi: 10.1016/j.bbmt.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Chakraverty R, Cote D, Buchli J, Cotter P, Hsu R, Zhao G, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 18.He T, Tang C, Xu S, Moyana T, Xiang J. Interferon gamma stimulates cellular maturation of dendritic cell line DC2.4 leading to induction of efficient cytotoxic T cell responses and antitumor immunity. Cell Mol Immunol. 2007;4:105–111. [PubMed] [Google Scholar]
- 19.Randolph GJ. Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol. 2001;13:267–274. doi: 10.1006/smim.2001.0322. [DOI] [PubMed] [Google Scholar]
- 20.Kim TD, Terwey TH, Zakrzewski JL, Suh D, Kochman AA, Chen ME, et al. Organ-derived dendritic cells have differential effects on alloreactive T cells. Blood. 2008;111:2929–2940. doi: 10.1182/blood-2007-06-096602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welniak LA, Kuprash DV, Tumanov AV, Panoskaltsis-Mortari A, Blazar BR, Sun K, et al. Peyer patches are not required for acute graft-versus-host disease after myeloablative conditioning and murine allogeneic bone marrow transplantation. Blood. 2006;107:410–412. doi: 10.1182/blood-2004-11-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, et al. Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood. 2006;107:1703–1711. doi: 10.1182/blood-2005-08-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tietz W, Allemand Y, Borges E, von Laer D, Hallmann R, Vestweber D, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. Journal of immunology. 1998;161:963–970. [PubMed] [Google Scholar]
- 26.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews Immunology. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117:3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones SC, Murphy GF, Friedman TM, Korngold R. Importance of minor histocompatibility antigen expression by nonhematopoietic tissues in a CD4+ T cell-mediated graft-versus-host disease model. The Journal of clinical investigation. 2003;112:1880–1886. doi: 10.1172/JCI19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Deng R, He W, Liu C, Wang M, Young J, et al. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. Journal of immunology. 2012;188:724–734. doi: 10.4049/jimmunol.1102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Demetris AJ, McNiff J, Matte-Martone C, Tan HS, Rothstein DM, et al. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. Journal of immunology. 2012;188:3804–3811. doi: 10.4049/jimmunol.1102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nature medicine. 2012;18:135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Dodge J, Komorowski R, Drobyski WR. A critical role for the retinoic acid signaling pathway in the pathophysiology of gastrointestinal graft-versus-host disease. Blood. 2013;121:3970–3980. doi: 10.1182/blood-2012-08-445130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoyama K, Saha A, Tolar J, Riddle MJ, Veenstra RG, Taylor PA, et al. Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration. Blood. 2013;122:2125–2134. doi: 10.1182/blood-2012-11-470252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovic A, Alpdogan O, Willis LM, Eng JM, Greenberg AS, Kappel BJ, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103:1542–1547. doi: 10.1182/blood-2003-03-0957. [DOI] [PubMed] [Google Scholar]
- 37.Chen YB, Kim HT, McDonough S, Odze RD, Yao X, Lazo-Kallanian S, et al. Up-Regulation of alpha4beta7 integrin on peripheral T cell subsets correlates with the development of acute intestinal graft-versus-host disease following allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1066–1076. doi: 10.1016/j.bbmt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, Chen Y, He W, Yi T, Zhao D, Zhang C, et al. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood. 2009;113:953–962. doi: 10.1182/blood-2008-06-165522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Racine JJ, Song X, Li X, Nair I, Liu H, et al. Mixed chimerism and growth factors augment beta cell regeneration and reverse late-stage type 1 diabetes. Science translational medicine. 2012;4:133ra59. doi: 10.1126/scitranslmed.3003835. [DOI] [PubMed] [Google Scholar]
- 40.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limana F, Bertolami C, Mangoni A, Di Carlo A, Avitabile D, Mocini D, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol. 48:609–618. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara J, Antin J, et al. The Pathophysiology of Graft-vs.-Host Disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas` Hematopoietic cell transplantation. Malden, MA: Blackwell Publishing Ltd; 2004. pp. 353–368. [Google Scholar]
- 45.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Li N, Zhao D, Kirschbaum M, Zhang C, Lin CL, Todorov I, et al. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci U S A. 2008;105:4796–4801. doi: 10.1073/pnas.0712051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley M, Zeytun A, Rafi-Janajreh A, Nagarkatti PS, Nagarkatti M. Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas− tumor cells. Blood. 1998;92:4248–4255. [PubMed] [Google Scholar]
- 49.Smith JA, Tang Q, Bluestone JA. Partial TCR signals delivered by FcR-nonbinding anti-CD3 monoclonal antibodies differentially regulate individual Th subsets. Journal of immunology. 1998;160:4841–4849. [PubMed] [Google Scholar]
- 50.Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA, et al. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. Journal of immunology. 2007;178:838–850. doi: 10.4049/jimmunol.178.2.838. [DOI] [PubMed] [Google Scholar]
- 51.Blazar BR, Jenkins MK, Taylor PA, White J, Panoskaltsis-Mortari A, Korngold R, et al. Anti-CD3 epsilon F(ab')2 fragments inhibit T cell expansion in vivo during graft-versus-host disease or the primary immune response to nominal antigen. Journal of immunology. 1997;159:5821–5833. [PubMed] [Google Scholar]
- 52.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. Anti-CD3 epsilon F(ab')2 prevents graft-versus-host disease by selectively depleting donor T cells activated by recipient alloantigens. Journal of immunology. 2001;166:5835–5839. doi: 10.4049/jimmunol.166.9.5835. [DOI] [PubMed] [Google Scholar]
- 53.Carpenter PA, Lowder J, Johnston L, Frangoul H, Khoury H, Parker P, et al. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:465–471. doi: 10.1016/j.bbmt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. The Journal of clinical investigation. 2003;111:659–669. doi: 10.1172/JCI16950. [DOI] [PMC free article] [PubMed] [Google Scholar]