Abstract
Aims
To evaluate the relationship between self-reported head injury and cognitive impairment, dementia, mortality, and Alzheimer’s (AD)-type pathological changes.
Methods
Clinical and neuropathological data from participants enrolled in a longitudinal study of aging and cognition (N=649) were analyzed to assess the chronic effects of self-reported head injury.
Results
The effect of self-reported head injury on clinical state depends on age at assessment: for a 1-year increase in age, the for transition to clinical MCI at the next visit for participants with a history of head injury is 1.21 and 1.34 for transition from MCI to dementia. Without respect to age, head injury increases the odds of mortality ( ). Head injury increases the odds of a pathological diagnosis of AD for men ( ) but not women ( ). Men with head injury have higher mean amyloid plaque counts in the neocortex and entorhinal cortex than men without.
Conclusions: Self-reported head injury is associated with earlier onset, increased risk of cognitive impairment and dementia, increased risk of mortality, and AD-type pathological changes.
Keywords: head injury, Alzheimer’s disease, neuropathology, dementia, cognition
INTRODUCTION
Although there have been many careful studies of head injury as risk factor for dementia, the literature on head injury and dementia has been limited from the standpoint that none of the longitudinal studies have used autopsy-confirmed diagnoses or examined neuropathological data. This is significant because an estimated 20–30% of cases clinically diagnosed with AD have dementia due to another cause.[1] The current study re-examines the association between self-reported head injury, risk of clinical dementia, and AD pathology using data from participants with detailed longitudinal cognitive assessments, as well as a subset of participants with textured neuropathologic data.
BACKGROUND
Autopsy-based studies of head trauma have reported that neurodegenerative pathology appears to be linked to injuries sustained many years earlier. Increases in the number of beta-amyloid precursor protein (βAPP) positive neurons were observed in cases of isolated severe head injury compared to age-matched controls.[2] Long-term increases in tau and beta-amyloid pathology, as well as increased neuroinflammatory response and white matter degradation, have been noted in patients with a history of a single head injury sustained up to 47 years before death.[3,4] Both acute and chronic axonal injuries, including accumulation of proteins associated with neurodegenerative disease, have been observed in individuals with a history of head injury.[5,6] However, the etiological links have not been solidly established in terms of how the brain trauma manifests neuropathologically.
Studies of chronic traumatic encephalopathy (CTE) have involved autopsy series.[7–9] CTE implies a history of repeated brain injury and is a progressive illness characterized by deficits in motor skills, cognition, and increased psychiatric symptoms such as depression, irritability, and aggression.[10] CTE pathology is marked by increased tau pathology, and in some cases, TAR DNA-binding protein 43 (TDP-43)[8] with or without diffuse beta-amyloid deposits.[11] Thus, CTE has both clinical and neuropathological overlap with AD, but the two are distinct diseases in terms of the severity, anatomical distribution, and other manifestations of brain pathology.
Epidemiological studies of head injury as a dementia risk have yielded conflicting evidence. Kondo and colleagues conducted a case-control study of 120 elderly Japanese matched for age and sex and evaluated multiple life-style and medical history factors as risks for AD [12]. Self-reported head injury with loss of consciousness (LOC) was associated with an increased odds of AD ( [95% CI 2.8 to 13.8]). A meta-analysis of 15 case-control studies found an overall increased odds of AD for those with a history of head injury with LOC prior to AD onset (pooled [95% CI 1.21 to 2.06]), although the odds of AD was increased for men ( [95% CI 1.47 to 3.58]) but not women ( [95% CI 0.56 to 1.47]) [13]. However, injury severity was not considered in the meta-analysis, and AD diagnosis was not autopsy-confirmed.
Results from cohort studies have also been inconsistent (see Table 1), which likely reflects differences in exposure assessment, follow-up time, loss to follow-up, study populations, and covariates selected for adjustment in calculating risk estimates. Two large prospective studies—The Rotterdam Study[14] and Adult Changes in Thought [15]—found no increased risk of dementia or AD associated with past head injury. Data from the smaller Betula study, by contrast, revealed an increased risk for participants with self-reported mild head injury and APOE-ε4.[16] Results from a Cambridge city study found no increased risk of incident dementia associated with a history of head injury in a community-dwelling population age 75 years and older after 2.4 years of follow-up. [17]
Table 1.
Summary of cohort studies of head injury and dementia
| Study | Type* | N | Effect Estimate(s) for Dementia Risk** | Exposure Assessment | Head Injury Prevalence | Limitations |
|---|---|---|---|---|---|---|
| Rotterdam Study[13] | P | 6,645 | , 95% CI: 0.4–1.9 | Self-report to study physician | 12.0% | 2 years of follow-up, inclusion of younger adults (≤ 60 years), dx not autopsy-confirmed |
| Adult Changes in Thought[14] | P | 4,225 | Age at injury <25:
, 95% CI: 0.87–1.20 Age at injury 25–54: , 95% CI: 0.78–1.38 Age at injury 55+: , 95% CI: 0.81–1.39 |
Self-report via structured interview by study personnel | 15.9% | Dx not autopsy-confirmed |
| Plassman et al. (2000) [16] | R | 1,776 | Mild injury:
95% CI: 0.18–3.29 Moderate injury: , 95% CI: 1.04–5.17 Severe injury: , 95% CI: 1.77–11.47 |
Review of military medical records between 1944–1945 | n/a | Head trauma sustained outside military service not accounted for, dx not autopsy-confirmed |
| Williams et al. (1991)[17]; Nemetz et al. (1999)[18] | R | 1,283 | , 95% CI: 0.8–1.7; median onset shortened by 8 years (p = 0.0015) | Review of Mayo Clinic medical records for cases with traumatic brain injury diagnosed between 1935–1984 | n/a | Head trauma not requiring hospital visit not captured, dx based on medical records not clinical interview, dx not autopsy-confirmed |
| Sundström et al. (2007) [15] | P | 543 | Without APOE-ε4:
(95% CI: 0.4–1.8) With APOE-ε4: (95% CI: 2.0–14.0) |
Affirmative response to questionnaire item “have you ever suffered a head injury which required medical care?” | 13.1% | Inclusion of younger adults (≤60 years), dx not autopsy-confirmed |
| WHICAP[19] | P | 271 | LOC <5 min:
, 95% CI: 0.4–7.5 LOC ≥5 min: , 95% CI: 2.3–59.8 |
Self-report via structured risk factor interview at baseline and during a one-time physician interview | 10.0% | Few participants with head injury in the sample, dx not autopsy-confirmed |
P = prospective, R = retrospective; dx = clinical diagnosis;
RR = relative risk, TR = time ratio, HR = hazard ratio, SIR = standardized incidence ratio, OR = odds ratio
Retrospective cohort studies have reported that head injury is an independent risk factor for AD or decreases time to dementia onset. Plassman et al. (2000)[18] reviewed military medical records and compared men who had been hospitalized with a closed head injury to those with an unrelated condition. All-cause dementia, and AD specifically, was associated with both moderate and severe, but not mild, injury.
A retrospective review of medical records from Olmsted County, Minnesota residents who were treated for head trauma and were over age 40 years at the time of their last medical assessment showed no increased risk of AD or all-cause dementia. [19,20] When time to onset was used as the outcome, however, persons with head trauma developed AD a median eight years earlier than expected when compared to the age-based incidence of AD in the total county population. Similarly, a prospective cohort study of Manhattan residents found that after five years of follow-up, history of head injury with LOC within the preceding 30 years was associated with earlier onset of AD, and the effect was stronger for those reporting a LOC of at least five minutes. [21]
METHODS
Subjects
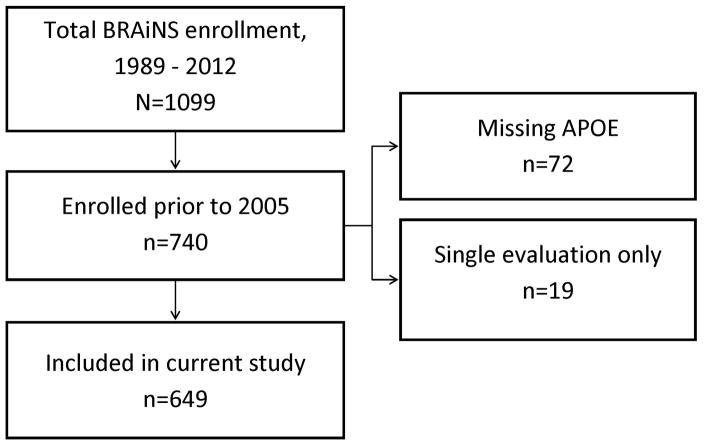
Subjects of this study are volunteers from Biologically Resilient Adults in Neurological Studies (BRAiNS) at the University of Kentucky’s Alzheimer’s Disease Center, a longitudinal cohort of approximately 1,100 individuals established in 1989 with ongoing recruitment.[22] The cohort comprises a convenience sample of older adults (age ≥ 60 years) from central Kentucky. BRAiNS exclusion criteria include prevalent neurological, psychiatric, and disabling medical disorders, as well as prevalent dementing illness (see Reference [22] for a detailed description of recruitment and study procedures). Subjects included in the current analysis (N=649) were enrolled between 1989 and 2004, evaluated at least two times, and had APOE genotyping available (Figure 1). Participants undergo annual cognitive and clinical assessments and donate their brains upon death.
Figure 1.
Flow diagram of included BRAiNS cohort participants
Participants who died and came to autopsy were included in a subset analysis. Of these, 17 cases were excluded from further analysis because quantitative neuropathology data were unavailable. An additional 15 were excluded from further analysis due to the presence of diffuse Lewy body disease, leaving 238/270 for inclusion in quantitative analyses of AD-type neuropathological burden. All enrollees were cognitively normal at study entry, and all research activities were approved by the University of Kentucky Institutional Review Board. Each participant provided written informed consent.
Statistical Analysis
Multistate Markov Chain
To test the hypothesis that self-reported history of head injury promotes transition to impaired cognition, a multistate Markov chain was fit to the data. Multistate Markov chains are attractive for modeling cognitive decline,[23–26] and they allow for the inclusion of competing risks for the outcome of interest (all-cause dementia) as participants who die or drop out before dementia onset may bias analyses.[27] Participants were retrospectively classified into states at each assessment: (1) normal cognition, (2) test-based amnestic mild cognitive impairment (aMCITB), (3) test-based mixed MCI (mMCITB), (4) clinical consensus-based MCI (MCICC), (5) dementia (all-cause), (6) drop-out without dementia, and (7) death without dementia. The classification method has been described in detail previously.[23,24] Briefly, normal cognition represents the absence of any impairments on cognitive testing as well as the absence of any clinical diagnosis of MCI or dementia; test-based MCI indicates at least one observed score of at least 1.5 standard deviations below the expected score for age based on the baseline performance of the entire normal cohort on tests of episodic memory (aMCITB) or language and executive function (mMCI TB); clinical consensus-based MCI (MCICC) indicates a diagnosis of MCI based on criteria used by the National Alzheimer’s Coordinating Center’s Uniform Data Set; [28] and dementia indicates a clinical diagnosis of dementia based on DSM-IV criteria.[29]
A multistate Markov chain with four transient states (normal cognition, aMCITB, mMCITB, and MCICC) and three absorbing states (death, dementia, and drop-out) was used. An individual may move freely among the transient states, but once an absorbing state is reached that individual’s follow-up ends for the purposes of the analysis; here records are truncated at the date of diagnosis. The model estimates the log-odds of a one-step transition between any two adjacent assessments, here called the “prior state” and the “current state,” versus remaining in or returning to a “base state.”
To account for within-subject correlation, subject-specific random effects were included in the model as described in Abner et al. (2012)[23] via PROC NLMIXED in SAS/STAT 9.3®.[30] Observed transitions are assumed to have occurred on the date of diagnosis. Predictor variables stayed in the model only if they significantly affected any one-step transition probability.
Risk Factors
History of head injury was determined from participant responses to intake interview questions, “Have you ever been knocked unconscious? If yes, how long were you unconscious, when did it happen, and how did it happen?” Participants who reported LOC of any duration, or a diagnosis of concussion without LOC, were coded as positive for history of head injury. Where head injury was reported, approximate age at injury, LOC duration (<5′, ≥5′), and external cause of the injury were recorded. Participants who described their LOC as “a few moments,” “a few minutes,” “momentarily,” or whose LOC duration was not described (n=6) were coded as <5′. Five minutes was chosen for dichotomization based on the increased risk of dementia associated with LOC longer than five minutes reported by Schofield et al. [21] Additional injury data were derived from interviews conducted longitudinally by study clinicians. Thus, head injury was treated as a time-varying factor. Two-way interactions between head injury and sex as well as age were tested for inclusion in the model. A two-way interaction between head injury and APOE-ε4 could be not evaluated due to sample size limitations.
Other risk factors of interest include age at assessment (centered at 79 years), sex, education (≤12 years, >12 years), APOE-ε4 carrier status (≥ 1 ε4 allele vs. no ε4 alleles), family history of dementia (first-degree relatives only), baseline hypertension (self-report), and baseline smoking history (never, 0–10 pack-years, 10–20 pack-years, 20+ pack-years).
Generalized Linear Regression
Multivariate analysis of covariance (MANCOVA) was used to test the hypothesis that self-reported history of head injury leads to increased AD-type pathology in the neocortex and medial temporal lobe, both of which have been identified as sites with increased βAPP-positive neurons following head injury.[2,31] MANCOVAs estimating the effect of head injury on mean diffuse (DPs) and neuritic plaque counts (NPs) as well as mean neurofibrillary tangle counts (NFTs) in the neocortex (frontal, temporal, parietal, and occipital regions) and medial temporal lobe (entorhinal cortex, amygdala, hippocampus CA1, and subiculum) were fit using PROC GLM in SAS/STAT 9.3®. Data concerning glial tangles were not available. Logistic regression was used to test the hypothesis that head injury increases the odds of AD pathology. All models included age at death and indicators for APOE-ε4 status, male sex, presence of at least mild cerebral amyloid angiopathy (CAA) (which is associated with increased amyloid plaque burden), and whether clinical dementia was observed before death. Two-way interactions between head injury and age at death, APOE-ε4, and sex were also tested. Statistical significance for all analyses was set at α=0.05.
Pathological Assessments
AD-positive pathology refers to cases with Braak stage III – VI and with moderate or severely dense neuritic amyloid plaques according to CERAD criteria.[32] Neuropathological counting metrics and methods were exactly as described previously.[33,34]
RESULTS
Clinical Data
Study participants contributed an average of 10.4 longitudinal assessments (median=10, range=2–22), with an average of 13 months between assessments (Table 2). During the study period, 386 (59.5%) participants transitioned to aMCITB at least one time, 398 (61.3%) transitioned to mMCITB at least one time, 129 (19.9%) transitioned to MCICC, 109 (16.8%) transitioned to dementia, 234 (36.1%) died without dementia, and 92 (14.2%) left the study without dementia. The overall transition structure is described in Table 3. Incident dementia required an average of 10.4±4.4 transitions to develop. The observed number of transitions required for death without dementia (9.8±4.4) or dropout without dementia (9.3±4.5) relative to incident dementia underscores the importance of accounting for competing events in modeling dementia risks.
Table 2.
Characteristics of included participants from the BRAiNS cohort; subjects enrolled in the study between 1989 and 2004
| Characteristic | All Subjects (n = 649) | Head Injury (n = 166) | No Head Injury (n =483) |
|---|---|---|---|
| Age at entry, y (mean ± SD) | 72.9 ± 7.4 | 72.8 ± 7.4 | 73.0 ± 7.4 |
| Female, % | 63.9 | 50.0 | 68.7 |
| Family history of dementia, % | 38.7 | 36.8 | 39.3 |
| At least one APOE-4 allele, % | 30.4 | 29.5 | 30.6 |
| > 12 years of education, % | 86.9 | 91.0 | 85.5 |
| History of hypertension at entry, % | 38.2 | 39.8 | 37.7 |
| Smoking history at entry, % | |||
| Never smoked | 49.2 | 43.4 | 51.1 |
| >0 – 10 pack-years | 10.3 | 9.6 | 10.6 |
| 10 – 20 pack-years | 8.3 | 9.6 | 7.9 |
| More than 20 pack-years | 32.2 | 37.4 | 30.4 |
| Number of assessments (mean ± SD) | 10.3 ± 4.7 | 10.2 ± 4.8 | 10.4 ± 4.6 |
| Time between assessments, y (mean ± SD) | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.4 |
Table 3.
One-step transition matrix (number of assessments [% of prior visit state]); total subjects = 649
| Prior state | Current state | ||||||
|---|---|---|---|---|---|---|---|
| Normal | Amnestic MCITB | Mixed MCITB | MCICC | Dementia | Dropout | Death | |
| Normal | 2634 (69.1) | 524 (13.8) | 464 (12.2) | 40 (1.1) | 15 (0.4) | 33 (0.9) | 101 (2.7) |
| Amnestic MCITB | 497 (57.6) | 172 (19.9) | 129 (15.0) | 23 (2.7) | 9 (1.0) | 13 (1.5) | 20 (2.3) |
| Mixed MCITB | 404 (30.7) | 97 (7.4) | 601 (45.7) | 66 (5.0) | 35 (2.7) | 30 (2.3) | 80 (6.2) |
| MCICC | 154 (61.4) | 50 (19.9) | 16 (6.4) | 31 (12.4) | |||
Just over one-quarter of the sample reported a head injury (166/649, 25.6%). Men (83/234, 35.5%) reported a higher proportion of head injury than women (83/415, 20.0%): , p<0.0001). APOE-ε4 carriers were equally distributed among participants with and without a reported injury, both overall ( =0.07, p=0.78) and by sex (men: , p=0.65, women: , p=1.00). Of the 166 participants reporting history of head injury, 34 transitioned to MCICC, 27 transitioned to dementia, and 72 died without transiting to dementia. The majority of reported injuries occurred prior to baseline (156/166, 94.0%). Most participants reported a single instance of head injury, but some participants reported two (n=15) or more (n=3) instances. Five of the 83 men reported that their injuries were sustained during military service, but in general veteran status was unknown. Men tended to have reported injuries resulting in LOC of at least five minutes more frequently than women (Table 4), though the difference is not statistically significant (31.3% vs. 21.7%: , p=0.16). When the reported cause of the injury (Table 4) is accounted for, however, logistic regression reveals that among participants who reported a head injury, men do have significantly increased odds of LOC of at least five minutes compared to women. This is observed whether the cases with unknown source are excluded from ( , 95% CI 1.11 to 5.87) or included in ( , 95% CI: 1.04 to 4.92) the model. The interaction between head injury and sex was not significant and was not retained (see Table 5 for final fitted model estimates). Head injury, without respect to age, was a significant risk for the onestep transition from a transient state to death without dementia ( , 95% CI 1.12 to 2.13). The interaction between head injury and age was significant for the one-step transitions from normal cognition, aMCITB, or mMCITB to MCICC (p=0.017) and the one-step transition from MCICC to dementia (p=0.0069). For a one-year increase in age, the for transition from normal cognition, aMCITB, or mMCITB to MCICC for participants with a history of head injury is 1.21 (95% CI 1.15 to 3.56) and 1.34 (95% CI 1.11 to 1.61) for transition from MCICC to dementia. Model results may also be used to estimate the expected number of one-step transitions required to reach the absorbing states.[24] In this cohort, self-reported head injury decreases the time to dementia by approximately six months.
Table 4.
Reported sources of head injury by gender and loss of consciousness (LOC)*
| Source | Men | Women | Total | |||
|---|---|---|---|---|---|---|
| LOC < 5′ | LOC ≥ 5′ | LOC < 5′ | LOC ≥ 5′ | LOC < 5′ | LOC ≥ 5′ | |
| Sports and recreation | 22 | 7 | 9 | 2 | 31 | 9 |
| Automobile accident | 9 | 10 | 22 | 8 | 31 | 18 |
| Fall | 7 | 5 | 15 | 7 | 22 | 12 |
| Interpersonal violence | 5 | 2 | 2 | 0 | 7 | 2 |
| Other blow to the head** | 8 | 2 | 7 | 0 | 15 | 2 |
| Not described | 8 | 1 | 12 | 2 | 20 | 3 |
Cell entries reflect number of unique participants reporting the source;
e.g., being struck by falling objects, striking head on ceilings or walls
Table 5.
Multistate Markov chain results for risk factors affecting transitions from transient states (T: normal cognition, aMCITB, or mMCITB)
| Parameter | Risk Comparison | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|
| T → aMCITB | ||||
| Age | 1-year difference | 1.03 | 1.02–1.04 | <0.0001 |
| Sex | Female vs. Male | 0.79 | 0.66–0.93 | 0.0056 |
| Prior = aMCITB | aMCITB vs. Normal | 1.27 | 1.03–1.58 | 0.0277 |
| Prior = mMCITB | mMCITB vs. Normal | 0.77 | 0.59–1.01 | 0.0561 |
| T → mMCITB | ||||
| Age | 1-year difference | 1.07 | 1.06–1.09 | <0.0001 |
| Education | ≤ 12 years vs. > 12 years | 1.70 | 1.38–2.10 | <0.0001 |
| Prior = aMCITB | aMCITB vs. Normal | 1.06 | 0.84–1.34 | 0.6473 |
| Prior = mMCITB | mMCITB vs. Normal | 4.78 | 3.93–5.81 | <0.0001 |
| T → MCICC | ||||
| Family History | Present vs. Absent | 1.42 | 1.11–1.83 | 0.0063 |
| APOE-ε4 | Present vs. Absent | 1.85 | 1.27–2.69 | 0.0014 |
| Age*Head Injury | 1-year difference in age when head injury is present | 1.21 | 1.15–3.56 | 0.0173 |
| Prior = aMCITB | aMCITB vs. Normal | 2.20 | 1.29–3.75 | 0.0038 |
| Prior = mMCITB | mMCITB vs. Normal | 5.86 | 3.81–9.00 | <0.0001 |
| T → Dementia | ||||
| Age | 1-year difference | 1.18 | 1.13–1.23 | <0.0001 |
| APOE-ε4 | Present vs. Absent | 2.58 | 1.52–4.38 | 0.0005 |
| Prior = aMCITB | aMCITB vs. Normal | 2.24 | 0.97–5.17 | 0.0596 |
| Prior = mMCITB | mMCITB vs. Normal | 7.65 | 4.08–14.34 | <0.0001 |
| T → Death | ||||
| Age | 1-year difference | 1.19 | 1.16–1.22 | <0.0001 |
| <1 – 10 pack-years | <1 – 10 pack-years vs. Never smoked | 1.16 | 0.68–1.96 | 0.5914 |
| 10 – 20 pack-years | 10 – 20 pack-years vs. Never smoked | 1.20 | 0.65–2.22 | 0.5566 |
| ≥20 pack-years | ≥20 pack-years vs. Never smoked | 2.05 | 1.49–2.83 | <0.001 |
| Hypertension | Present vs. Absent | 1.46 | 1.08–1.96 | 0.0133 |
| Head Injury | Present vs. Absent | 1.54 | 1.12–2.13 | 0.0089 |
| Prior = aMCITB | aMCITB vs. Normal | 0.72 | 0.43–1.19 | 0.1980 |
| Prior = mMCITB | mMCITB vs. Normal | 2.60 | 1.85–3.64 | <0.0001 |
| T → Dropout | ||||
| Age | 1-year difference | 1.06 | 1.02–1.09 | 0.0008 |
| Hypertension | Present vs. Absent | 1.90 | 1.20–3.00 | 0.0060 |
| Prior = aMCITB | aMCITB vs. Normal | 1.49 | 0.77–2.89 | 0.2324 |
| Prior = mMCITB | mMCITB vs. Normal | 1.98 | 1.77–2.22 | <0.0001 |
| MCICC → Dementia | ||||
| <1 – 10 pack-years | <1 – 10 pack-years vs. Never smoked | 0.28 | 0.08–0.94 | 0.0388 |
| 10 – 20 pack-years | 10 – 20 pack-years vs. Never smoked | 0.27 | 0.07–1.09 | 0.0654 |
| ≥20 pack-years | ≥20 pack-years vs. Never smoked | 0.31 | 0.13–0.71 | 0.0054 |
| Age*Head Injury | 1-year difference in age when head injury is present | 1.34 | 1.11–1.61 | 0.0069 |
| MCICC → Death | ||||
| Age | 1-year difference | 1.12 | 1.04–1.20 | 0.0023 |
Finally, sensitivity analyses were conducted to assess the effect of excluding participants whose head injuries occurred after baseline (n=10) and excluding participants whose head injuries occurred less than 10 years prior to their first diagnosis of clinical dementia (n=4). All three models produced a similar fit (data not shown), and conclusions did not change. Additional sensitivity analyses tested whether the effect of head injury on transition risks varied by prior state. These interactions were not significant.
Pathological Data
Among the 270 participants who died and came to autopsy, 82 (30.4%) reported a history of head injury. Fifteen of 82 (18.3%) were clinically demented at death and received neuropathological diagnoses as follows: AD (n=6), cerebrovascular disease (CVD) (n=1), AD + DLB (n=2), AD + CVD (n=2), hippocampal sclerosis (HS) + CVD (n=1), CVD + AD + HS (n=1), AD + DLB + CVD + HS (n=1), and DLB (n=1). Of the 238 participants with quantitative neuropathological data, 70 (29.4%) reported a history of head injury (Table 6).
Table 6.
Characteristics of autopsied participants from the BRAiNS cohort by gender and history of lifetime head injury (n=238)
| Men | Women | |||
|---|---|---|---|---|
| Head Injury (n=36) | No Head Injury (n=61) | Head Injury (n=34) | No Head Injury (n=107) | |
| Age at death, y* | 84.4±6.9 | 86.9±7.0 | 86.0±8.0 | 87.1±7.5 |
| Final MMSE* | 26.4±4.5 | 25.8±4.3 | 27.5±3.5 | 24.4±7.7 |
| Education, y* | 16.2±2.6 | 16.7±2.8 | 15.3±2.3 | 15.7±2.0 |
| APOE-ε4+ (%) | 22.2 | 39.3 | 20.6 | 28.0 |
| CAA+ (%) | 63.9 | 54.1 | 48.4 | 52.3 |
| AD (%) | 27.8 | 26.2 | 14.7 | 34.6 |
| Braak Stage (%) | ||||
| 0 – II | 38.9 | 52.5 | 64.7 | 46.7 |
| III – IV | 44.4 | 34.4 | 26.5 | 30.8 |
| V – VI | 16.7 | 13.1 | 8.8 | 22.4 |
| CERAD Rating (%) | ||||
| No | 36.1 | 49.2 | 52.9 | 43.0 |
| Possible AD | 11.1 | 11.5 | 11.8 | 8.4 |
| Probable AD | 33.3 | 26.2 | 20.6 | 24.3 |
| Definite AD | 19.4 | 13.1 | 14.7 | 24.3 |
Mean ± SD
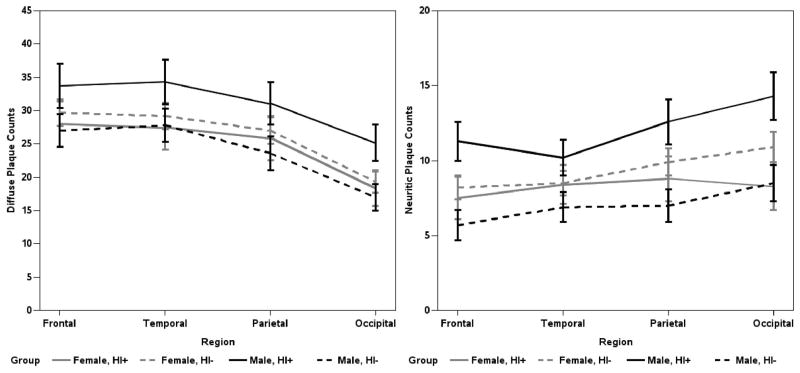
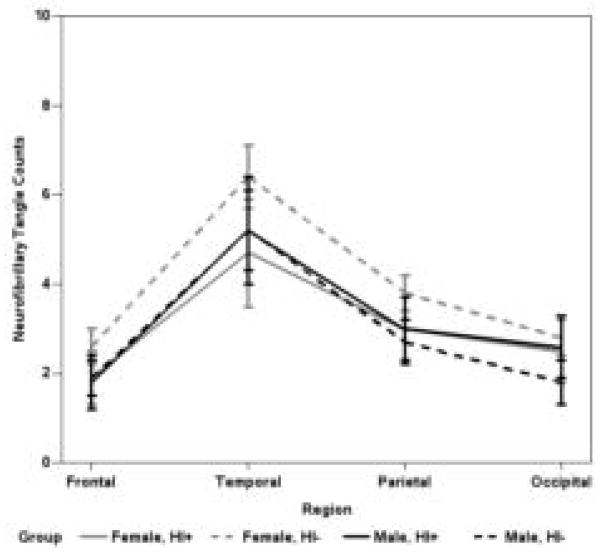
Men with a history of head injury have higher mean parietal and occipital DPs, and more NPs in all neocortical areas, than men without (p<0.05; Figure 2). Women with a history of head injury do not have significantly higher neocortical DPs or NPs than women (or men) without head injury (Figures 2). Additional analyses were performed to determine if age at injury or source of injury mitigated the observed head injury by sex interaction; the conclusions did not change (data not shown). Mean neocortical NFTs were not associated with head injury history (Figure 3).
Figure 2.
Estimated mean number of diffuse and neuritic neocortical plaque counts by region; whiskers are SEM (n = 238). The final MANCOVA models included main effects for age at death, clinical dementia status, APOE-ε4, CAA, and an interaction term and main effects for head injury and male gender.
Figure 3.
Estimated mean number of NFTs by neocortical region; whiskers are SEM (n = 238). The final MANCOVA models included main effects for age at death, clinical dementia status, APOE ε4, cerebral amyloid angiopathy, and an interaction term and main effects for head injury (HI) and male gender.
Pathological burden in the medial temporal structures, except for the entorhinal cortex, was not significantly increased by history of head injury. Men who reported a history of head injury had significantly increased DPs (20.6±2.3 vs. 13.4±1.8, p=0.0072) and NPs (3.8±0.7 vs. 1.6±0.6, p=0.013) in the entorhinal cortex compared to men who did not. NFTs in the entorhinal cortex were unaffected (data not shown).
When the analysis is restricted to the 39 cases (31 without head injury, 8 with head injury) who received a clinical diagnosis of dementia, evaluation of the sex by head injury interaction is not adequately powered. No statistically significant differences were observed between dementia cases with and without head injury after controlling for APOE-ε4, sex, age at death, and CAA. Further restricting the analysis to men with dementia (5 with head injury, 10 without) reveals that history of head injury is marginally significantly associated with increased diffuse plaques in the frontal (47.7±6.2 vs. 34.0±4.3, p = 0.097) and occipital (35.0±5.7 vs. 21.7±4.0, p = 0.081) lobes and neuritic plaques in the occipital lobe (23.8±4.7 vs. 11.6±3.3, p = 0.055). While mean NFTs, unlike neocortical DPs and NPs, were not elevated among men with history of head injury, the odds of having AD-positive pathology is again significantly increased for men with a history of head injury ( , 95% CI 1.03 to 2.09) but not women ( , 95% CI 0.83 to 1.68). This apparent conflict may result from the fact that while 61.1% of men with head injury have a Braak stage of at least III compared to 47.5% of men without head injury. However, even among men with Braak III or higher, mean neocortical NFTs are not associated with a history of head injury (p>0.5).
DISCUSSION
Clinical and neuropathological data from participants in a longitudinal study of aging and cognition (N=649) were analyzed to assess the effects of self-reported head injury. Our results support prior work identifying increased risk of cognitive impairment[35], earlier onset of dementia,[20,21] as well as increased risk of mortality with head injury.[15] Although the six-month reduction in time to dementia onset we report is much less than the eight years in the Olmstead County study,[20] differences in study design, head injury case definition, study population, and clinical diagnosis could account for the discrepancy. We also found an unexpected correlation between head injury, gender, and increased AD neuropathologic changes.
The effect of head injury on dementia risk may be modified not only by age at injury and severity of injury (or injuries) but also by other factors including gender and APOE-ε4 status.[36] We note that while a single injury of sufficient severity can increase the risk of manifesting clinical dementia, it does not follow necessarily that these events lead to the specific features of AD. In experimental studies (i.e., animal studies), however, evidence of long-term neurodegeneration was observed after a single head injury, and accelerated beta-amyloid peptide deposition and cognitive impairment was observed after repeated mild head injury.[37] Thus, animal models have provided hypothesized mechanistic links between head injury and AD and autopsy-based case series of head injury have also suggested a link between head injury and AD-promoting and AD-like pathology.[3,37–39]
Detailed neuropathological data were available on over one-third of the study participants. History of self-reported head injury was associated with increased levels of amyloid plaque deposition in the neocortex and entorhinal cortex, as well as increased odds of AD pathology for men but not women, which supports prior clinical studies. Fleminger and colleagues (2003) posited that women may be protected from the deleterious effects of head injury by the presence of female sex hormones.[13] Indeed, the women who came to autopsy in the current study were more likely to report two instances of LOC (6/34 vs. 1/36, p=0.027, Fisher’s Exact test) and were more likely to have sustained a head injury after age 55 (16/34 vs. 7/36, p=0.030, Fisher’s Exact test). If AD pathology was more affected by more recent or frequent LOC, it should follow that the women and not the men would have elevated plaque counts in association with exposure to head injury.
An alternative explanation is that male gender may be a proxy for severity, repetition, poor reporting, and particular mechanisms (e.g., contact sports) of brain injury. Men in our study tended to report injuries that led to LOC ≥ 5 minutes more often than women, which suggests that their injuries may have been more severe overall. Plassman and colleagues (2000)[18] found increased risk of both all-cause dementia and AD for veterans with moderate and severe injuries but not mild injuries, and Schofield and colleagues (1997)[21] found an increased risk of AD for participants who reported head injuries with LOC ≥5 minutes but not <5 minutes. Of the 36 autopsied men with a history of head injury, 13 (36%) reported sports and recreation as source of the injury, nine of which were identified as football or boxing. It may be that while the participant reported only one or two instances of head injury where LOC occurred, multiple instances of injury were actually experienced without LOC. Chronic effects of head injury may be due to lifetime cumulative exposure rather than an acute single event.[40] Recent data reveal an increased risk of AD for retired professional American football players, who are assumed to have been exposed to repeated blows to the head, relative to the general US population ( , 95% CI 1.55 to 7.95).[41]
We note that reported head trauma is usually quite remote from study enrollment, in some cases occurring in infancy; however, these injuries could still affect the brain chronically.[3] In addition to the influence of recall bias implicit to the study design, participants in the current study are highly educated compared to their peers, with 65.7% having at least a Bachelor’s degree vs. 24.5% of those over age 60 years nationally.[42] If, as Moretti and colleagues (2012) suggest, cognitive reserve is the best predictor of cognitive outcome and decline following head injury, [35] our participants may have been less vulnerable to its effects.
As for the neuropathology, head injury severity occurs along a spectrum, and coding such injuries dichotomously obscures relevant information.[43] Recovery (and wound repair capacity of an individual) may also be graded on a continuum, and all these factors may influence the long-term sequelae of the injury.[43] Future studies of head injury as a risk for dementia and AD should consider collecting detailed data on severity, anatomical location, post-traumatic amnesia, and receipt of treatment if possible. Furthermore, it must be emphasized that the neuropathological data reported here are descriptive rather than experimental. For example, it is possible that imposing a matched case-control design on the autopsied cases would yield different results.
Despite the limitations inherent in the data, multiple studies have found that even a single reported occurrence of head injury is associated with increased risk of incident dementia years or decades later. Although such knowledge may not be useful to those individuals for whom head injury is not a modifiable risk factor (i.e., having already sustained one), it does underscore the necessity of taking proper precautions for those involved in professions and recreational activities where head injury is common.
Acknowledgments
Funding
This work was supported by grants to the University of Kentucky’s Sanders-Brown Center on Aging from the National Institute on Aging (grant numbers R01 AG038651, R01 AG019241, and P30 AG028383), as well as a grant from the National Center for Advancing Translational Sciences to the University of Kentucky’s Center for Clinical and Translational Science (grant number UL1TR000117). The NIA did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the paper; or in the decision to submit the article for publication. The authors submit this manuscript independent of funding sources.
We thank the participants and their families, as well as the University of Kentucky Alzheimer’s Disease Center staff members.
References
- 1.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012 Apr;71(4):266–73. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenzie JE, Gentleman SM, Roberts GW, Graham DI, Royston MC. Increased number of βAPP-immunoreactive neurones in the entorhinal cortex after head injury. NeuroReport. 1994;6:161–4. doi: 10.1097/00001756-199412300-00041. [DOI] [PubMed] [Google Scholar]
- 3.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathology. 2011;22(2):142–9. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X-H, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid B plaques despite persistent accumulation of amyloid β in axons of long-term survivors of traumatic brain injury. Brain Pathology. 2009;19:214–23. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uryu K, Chen X-H, Martinez D, Browne KD, Johnson VE, Graham DI, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Experimental Neurology. 2007;208:185–92. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsellis JAN, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psych Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 8.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2012 doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geddes JF, Vowles GH, Nicoll JAR, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 10.Forstl H, Haass C, Hemmer B, Meyer B, Halle M. Boxing--acute complications and late sequelae. Dtsch Arztebl Int. 2010;107(47):835–9. doi: 10.3238/arztebl.2010.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saing T, Dick M, Nelson PT, Kim RC, Cribbs DH, Head E. Frontal cortex neuropathology in dementia pugilistica. J Neurotrauma. 2012;29:1054–70. doi: 10.1089/neu.2011.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondo K, Niino M, Shido K. A case-control study of Alzheimer’s disease in Japan – significance of lifestyles. Dement Geriatr Cogn Disord. 1994;5:314–326. doi: 10.1159/000106741. [DOI] [PubMed] [Google Scholar]
- 13.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s diease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–62. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta KM, Ott A, Kalmijn S, Slotter A, van Duijn CM, Hofman A, et al. Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology. 1999;53(9):1959–62. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 15.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry. 2012;0:1–6. doi: 10.1136/jnnp-2012-303938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundstrm A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr. 2007;19(1):159–65. doi: 10.1017/S1041610206003498. [DOI] [PubMed] [Google Scholar]
- 17.Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, O’Connor DW, Paykel ES. Vascular risks and incident dementia: results from a cohort study of the very old. Dement Geriatr Cogn Disord. 1998;9:175–180. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- 18.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 19.Williams DB, Annegers JF, Kokmen E, O’Brien PC, Kurland LT. Brain injury and neurologic sequelae: A cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41(10):1554–7. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 20.Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, et al. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. American Journal of Epidemiology. 1999;149(1):32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- 21.Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, et al. Alzheimer’s disease incidence after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–24. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C, et al. University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Curr Alzheimer Res. 2012;9(6):724–33. doi: 10.2174/156720512801322591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abner EL, Kryscio RJ, Cooper GE, Fardo DW, Jicha GA, Mendiondo MS, et al. Mild cognitive impairment: statistical models of transition using longitudinal clinical data. Int J Alzheimers Dis. 2012;2012:291920. doi: 10.1155/2012/291920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006 Mar 28;66(6):828–32. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 25.Salazar JC, Schmitt FA, Yu L, Mendiondo MM, Kryscio RJ. Shared random effects analysis of multi-state Markov models: application to a longitudinal study of transitions to dementia. Statistics in medicine. 2007;26(3):568–80. doi: 10.1002/sim.2437. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Griffith WS, Tyas SL, Snowdon DA, Kryscio RJ. A nonstationary Markov transition model for computing the relative risk of dementia before death. Stat Med. 2010 Mar 15;29(6):639–48. doi: 10.1002/sim.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy TE, Han L, Allore HG, Peduzzi PN, Gill TM, Lin H. Treatment of death in the analysis of longitudinal studies of gerontological outcomes. J Gerontol A Biol Sci Med Sci. 2011;6(1):109–14. doi: 10.1093/gerona/glq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris J, Weintraub S, Chui HC, Cummings J, DeCaril C, Ferris S, et al. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 29.American Psyciatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psyciatric Association; 1994. [Google Scholar]
- 30.SAS Institute Inc. SAS/STAT® 9.3 Procedures Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 31.Gentleman SM, Graham DI, Roberts GW. Molecular pathology of head trauma: altered βAPP metabolism and the aetiology of Alzheimer’s disease. The Neurobiology of Ischaemic Brain Damage, Progress in Brain Research. 1993;96:237–46. doi: 10.1016/s0079-6123(08)63270-7. [DOI] [PubMed] [Google Scholar]
- 32.Mirra SS, Heyman A, McKeel DW, Sumi SM, Crain BR, Brownlee LM The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 33.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer Disease Center autopsy cohort: Neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66(12):1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in age cognitively normal individuals. J Neuropathol Exp Neurol. 1999;58(4):376–88. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurology. 2012;11:1103–12. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- 36.Jellinger K. Head injury and dementia. Current Opinion in Neurology. 2004;17:719–23. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Uryu K, Laurer H, TM, Practico D, Martinez D, Leight S, et al. Repetitive mild brain trauma accelerates AB deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22(2):446–54. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Experimental Neurology. 2012 doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-B pathology: A link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–70. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dashnaw ML, Petraglia AL, Bailes JE. An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg Focus. 2012;33(6):E5. doi: 10.3171/2012.10.FOCUS12284. [DOI] [PubMed] [Google Scholar]
- 41.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–74. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.United States Census Bureau. American Community Survey. 2011. [Google Scholar]
- 43.Iverson G. Mild traumatic brain injury & risk for Alzheimer’s disease. International Brain Injury Association. 2006:4. [Google Scholar]