Abstract
Objective
Vascular calcification is highly correlated with cardiovascular disease morbidity and mortality. Osteoprotegerin (OPG) is a secreted decoy receptor for Receptor Activator of NF-kappaB Ligand (RANKL). Inactivation of OPG in apolipoprotein E deficient mice (ApoE−/−) increases lesion size and calcification. The mechanism(s) by which OPG is athero-protective and anti-calcific have not been entirely determined. We investigated whether OPG deficient vascular smooth muscle cells (VSMCs) are more susceptible to mineralization and whether RANKL mediates this process.
Results
Lesion free aortas from 12 week old ApoE−/−OPG−/− mice had spotty calcification, an appearance of osteochondrogenic factors, and a decrease of smooth muscle markers when compared to ApoE−/−OPG+/+ aortas. In osteogenic conditions, VSMCs isolated from ApoE−/−OPG−/− (KO-VSMC) mice deposited more calcium than VSMCs isolated from ApoE−/−OPG+/+ (WT-VSMC) mice. Gene expression and biochemical analysis indicated accelerated osteochondrogenic differentiation. Ablation of RANKL signaling in KO-VSMCs rescued the accelerated calcification. While WT-VSMCs did not respond to RANKL treatment, KO-VSMCs responded with enhanced calcification and the upregulation of osteochondrogenic genes. RANKL strongly induced IL-6, which partially mediated RANKL-dependent calcification and gene expression in KO-VSMCs.
Conclusions
OPG inhibits vascular calcification by regulating the pro-calcific effects of RANKL on VSMCs and is thus a possible target for therapeutic intervention.
Keywords: OPG, RANKL, IL-6, smooth muscle cells, vascular calcification, osteochondrogenic differentiation
Introduction
Vascular calcification, a type of ectopic soft tissue mineralization, increases the risk of cardiovascular mortality. Arterial wall mineralization is estimated to be present in the vast majority of patients affected by cardiovascular disease (CVD)[1]. Patients with end stage renal disease generally have extensive medial and intimal calcification that is associated with a highly significant increase in cardiovascular mortality as compared to the general population[2]. Type II diabetics are an additional population affected by elevated vascular calcification[3]. Given the dramatic rise in diabetes and metabolic syndrome in the US, increased frequencies of vascular calcification and CVD is to be expected in the coming decade.
In the aorta, calcification promotes congestive heart failure by compromising vessel compliance and elasticity, while in coronary and carotid arteries, calcium deposits may cause atherosclerotic plaque instability[1]. In human lesions, the process leading to vascular calcification appears to follow a pathway analogous to endochondral bone formation; however, in mouse lesions, vascular calcification is accompanied by the appearance of cartilaginous metaplasia that subsequently mineralizes[4, 5]. In vitro and in vivo mechanistic studies indicate that vascular calcification is a highly regulated process involving VSMCs. Inorganic phosphate, bone morphogenetic protein (BMP), and oxidative stress signaling have emerged as key regulators of osteochondrogenic trans-differentiation of VSMCs. Up-regulation of the osteochondrogenic transcription factor Runx2 and other osteochondrogenic markers, and down-regulation of SMC lineage markers appear to be key processes in vascular cell dependent mineralization[3, 6, 7].
OPG is a member of the TNF-receptor superfamily and acts as a soluble receptor for RANKL and TRAIL. Functional studies in vitro, as well as in vivo, indicate that OPG is a major regulator of bone remodeling by blocking RANKL binding to its own cell surface receptor RANK, thus, inhibiting RANKL-dependent osteoclast formation[8]. RANKL/RANK also modulates adaptive immunity[9, 10].
RANKL and OPG are present in atherosclerotic lesions in mice and humans, however their roles in mediating vascular disease are not fully understood[5, 11, 12]. We previously reported that OPG deficiency in mice led to increased atherosclerosis, vascular calcification, and osteoporosis that correlated with elevated circulating RANKL[13]. In addition, we have recently reported that in ApoE−/− mice vessel wall-derived OPG protects from atherosclerosis and vascular calcification[14]. Further, treatment of LDLR–/– mice with recombinant OPG reduced vascular calcification[15]. OPG is expressed by the endothelium in early human atherosclerotic lesions, in SMCs of fibrous cap and fibrocalcific lesions, and lines bone structures in advanced plaques. RANKL is mainly found in the extracellular matrix surrounding vascular calcium deposits[12]. Rattazzi et al. first reported expression of OPG and RANKL in lesions in the innominate arteries of ApoE−/− mice[5]. In vitro, OPG and RANKL are expressed by VSMCs and endothelial cells and are regulated by inflammatory cytokines [16, 17]. Further, several epidemiologic studies have linked OPG to the severity of CVD[18, 19]. These findings point to an active role for OPG in the maintenance of cardiovascular homeostasis.
The increase in lesion size and vascular calcification occurs when the opg gene is inactivated in ApoE−/− mice either in the vessel wall or in the hematopoietic compartment, strongly supports the notion that OPG is an athero-protective molecule[13, 14]. However, the mechanisms by which OPG is athero-protective and anti-calcific are not known. For example, it is still unknown whether the absence of OPG in SMCs affects their response to calcification conditions and to RANKL. Thus, in this study we tested the hypothesis that OPG-deficient VSMCs mineralize more than wild type VSMCs and that this is dependent on unopposed RANKL signaling.
Material and Methods
Animals
ApoE−/−OPG−/− mice generation was described in Bennett et al.[13]. Animals were maintained in a specific pathogen-free environment and fed a normal chow diet. All protocols are in compliance with the NIH Guideline for the Care and Use of Laboratory Animals and were approved by the University of Washington Institutional Animal Care and Use Committee.
Tissue preparation, Histochemical and Immunohistochemical staining
Innominate arteries from 12 week old ApoE−/−OPG−/− and ApoE−/−OPG+/+ mice were fixed in formalin and embedded in paraffin. Five micron thick sections were used for histochemical and immunohistochemical analyses. Alizarin Red S or Von Kossa staining was used to detect calcium deposition. The following antibodies were employed: anti goat SMα-actin (Sigma, St Louis, MO), goat anti SM22α (Abcam, Cambridge, MA), rabbit anti phosphorylated ERK (CellSignaling, Danvers, MA), goat anti Runx2 (R&D Systems, Minneapolis, MN), mouse anti RANKL (Imgenex, San Diego, CA), goat anti Osteopontin (R&D Systems, Minneapolis, MN), rabbit anti cleaved caspase-3 (CellSignaling, Danvers, MA), control rabbit, goat and mouse IgG (Invitrogen, Carlsbad, CA), biotin conjugated rabbit anti-goat IgG (Pierce Thermoscientific, Rockford, IL), biotin conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA), biotin conjugated rabbit anti-mouse IgG (Invitrogen, Carlsbad, CA). Sections were counterstained with haematoxylin (Ricca Chemical Company, Arlington, TX) or Methyl Green (Sigma).
VSMC isolation and characterization
6-10 week old wild type, OPG−/−, ApoE−/−OPG−/− and ApoE−/−OPG+/+ aortas were incubated in an enzyme mix solution containing 2 mg/ml BSA (Sigma), 1 mg/ml collagenase CLS-1 (Worthington), 0.375 mg/ml soybean trypsin inhibitor (Worthington), 0.125 mg/ml elastase type III (Sigma) for 5 min at 37°C. The adventitia was then removed, the endothelium stripped and the media cut into small pieces that were dispersed in a mixture of 0.6 mg/ml collagenase CLS-2 (Worthington) and 0.25 mg/ml elastase type III (Sigma) in culture medium containing FBS and incubated at 37°C for 1h. The cell suspension was centrifuged and re-suspended in DMEM culture medium (Invitrogen) containing 100 U/ml penicillin, 100 mg/ml streptomycin, and 20% FBS. The cells were split when confluent and cultured in growth media with 10% FBS after passage 3. The cells used for the experiments were primary cultures and subcultures of 3-9 passages.
Cells at passage 1 were plated on glass chamber slides (Lab-Tek, Rockford, IL) and used for cell characterization by immunofluorescence. Cells were stained with antibodies against SMα-actin (Sigma), SM22α (Abcam), desmin (Dako, Carpinteria, CA) and CD31 (BD Bioscience, San Jose, CA). Alexa fluor 594 goat anti-mouse, alexa fluor 594 goat anti-rabbit, and alexa fluor 594 rabbit anti-goat (Invitrogen) were used as secondary antibodies.
Retroviral transduction of OPG
OPG cDNA (a gift from Dr. Ed Clark, University of Washington) was cloned into the pBMN-IGFP retroviral vector and retroviral particles generated as described by Rice et al.[20]. The pBMN-I-GFP retroviral particles were used as control. After infection, the cells were sorted by FACS for GFP.
RNA Silencing
To degrade complementary messenger RNA, siRNAs for RANKL (Ambion Silencer Select Pre-designed s75266, Carlsbad, CA) were utilized. The SMCs were transfected with the Amaxa Basic Nucleofector kit for primary mammalian SMCs (Lonza, Switzerland) following the manufacturer's protocol. Gene knock-down was confirmed by verifying mRNA levels by qPCR.
Calcification Assay
VSMCs were plated at a density of 7000 cell/cm2 in 6 well plates and cultured in control medium (CM = DMEM/3%FBS) or in osteogenic medium (OM = DMEM/3% FBS and 2.6mM phosphate). The media was changed every 2 days. The cells were harvested after 2 and 4 days for RNA, and the calcium assay was performed after 7 days. Recombinant mouse RANKL (R&D Systems, Minneapolis, MN) was used at 100 ng/ml and anti IL-6 antibody (eBioscience, San Diego, CA) or rat IgG1k control were added to the culture media at 0.1 μg/ml.
Calcium assay
Cell cultures were rinsed with PBS 0.6 mol/L HCl at 4°C for 24h. Calcium released from the cells was determined colorimetrically by the o-cresolphthalein complexone method as described previously (TECO calcium diagnostic kit, Anaheim, CA)[21]. Calcium was normalized to cellular protein and expressed as μg/mg protein.
Alkaline phosphatase activity
For determination of cellular ALP activity, cells were washed 3 times with PBS and assayed for ALP activity using the ALP Colorimetric Assay Kit (Biovision, Milpitas, CA) according to the manufacturer's protocol. ALP activity was normalized to total cellular protein concentration and expressed as Units/mg protein.
Real time Quantitative PCR (qPCR)
Total RNA was isolated from the aortas of 12 week old ApoE−/− OPG−/− or ApoE−/− OPG+/+ mice following removal of the adventitia and endothelium and from the cultured VSMCs using RNeasy kit (Qiagen, Germantown, MD). cDNA synthesis was performed using RevertAid First Strand cDNA synthesis kit (Fermentas, Rockford, IL). mRNA levels were quantified by Taqman real-time PCR and the ABI Prism 7500 (Applied Biosystem, Carlsbad, CA TaqMan®). The following Gene Expression Assays (Invitrogen) were used:
Spp1 (OPN) Mm00436767_m1;
MGP Mm00485009_m1;
BMP2 Mm01340178_m1;
Runx2 Mm00501584_m1;
IL-6 Mm00446190_m1;
SMα-actin Mm01546133_m1;
SM22α Mm00441660_m1;
APL Mm00475834_m1.
Quantification of gene expression was calculated by the standard curve method as described in Invitrogen manual (http://www.icmb.utexas.edu/core/DNA/Information_Sheets/Real-time%20PCR/Guide_to_Relative_Quantitation.pdf ). Briefly, 1μg cDNA from calvaria or VSMCs was diluted to 1, 0.5 and 0.25 to create an appropriate standard curve for each different Taqman primers-probe set. The curves were then used to quantitate the mRNA amount for each gene from the treated groups. Data were normalized to the 18S rRNA.
Reverse Transcriptase PCR
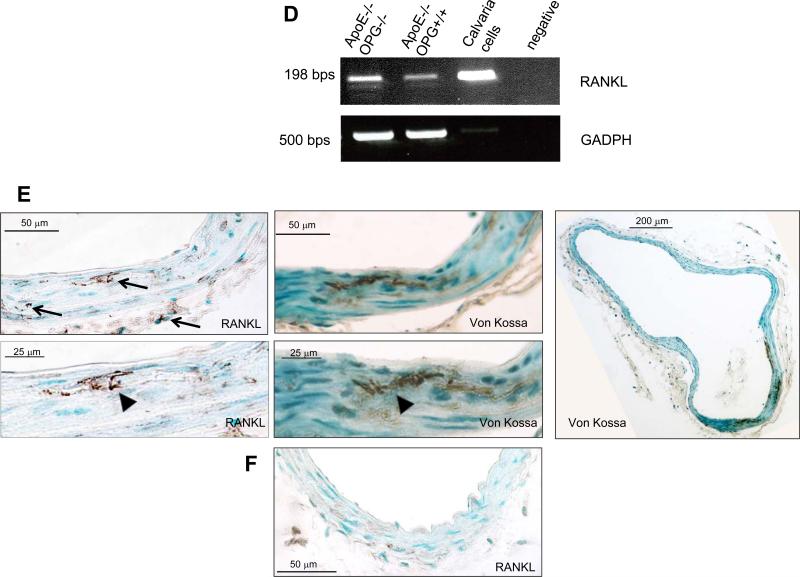
cDNA from tissue and cells lysates was also utilized in reverse transcriptase PCR using GoTaq DNA polymerase (Promega, Madison, WI). cDNA from calvaria cells served as a positive control. The PCR primers for mouse RANK were: forward 5’-AGATGTGGTCTGCAGCTCTTCCAT-3’, and reverse 5’-ACACACTTCTTGCTGACTGGAGGT-3’, amplifying a 278-bp fragment. Primers for RANKL were forward 5’-CGCTCTGTTCCTGTACTTTCGAGCG-3’ and reverse 5’-TCGTGCTCCCTCCTTTCATCACAGGTT-3’, amplifying a 198-bp fragment. Primers for GAPDH were: forward 5’-CAAGGTCATCCATGACAACTTTG-3’, and reverse 5’-GTCCACCACCCTGTTGCTGTAG-3’, amplifying a 496-bp fragment.
Western blot analysis
Lysates were produced from VSMC cultures and diluted with Laemmli buffer (62.5mM TRIS-HCl pH 6.8, 25% (w/v) Glycerol, 2% (w/v) SDS and 0.01% (w/v) Bromophenol blue). Protein concentrations were determined using a Micro BCA Protein Assay (Pierce Thermoscientific). Equal quantities of protein were loaded into each well of a Mini-PROTEAN TGX gel (BIO-RAD) and SDS-PAGE was performed using the mini-PROTEAN tetra system (BIO-RAD). Sample proteins were then transferred to an Immuno-blot PVDF membrane (BIO-RAD) and detected using protein specific antibodies, horseradish peroxidase-conjugated antibodies and Pierce ECL Western Blotting Substrate (Pierce Thermoscientific).
The following antibodies were utilized: anti Phospho-p44/42 MAPK (ERK1/2) 1:2000 (Cell signaling), anti p44/42 MAPK (ERK1/2) 1:1000 (Cell signaling), anti phospho-STAT-3 1:2000 (Millipore), anti beta Actin as loading control 1:1000 (Abcam), anti SMα-actin 1:1000 (Dako), anti RANKL (R&D Systems), anti Rabbit IgG Peroxidase Conjugate (Sigma), anti goat IgG HRP (Santa Cruz Technologies, Santa Cruz, CA), anti mouse IgG peroxidase (Sigma).
ELISA assay
Conditioned media from the treated VSMCs were collected for measurement of OPG (ELISA kit from R&D System) and IL-6 (kit from eBioscience) according to the manufacturer's instructions.
Luciferase reporter assay
To study the activity of the transcription factor Runx2, a Dual-Luciferase reporter assay system (Promega, Rockford, IL) was used. Briefly, VSMCs were transiently transfected using the liposome-mediated method (Lipofectamine 2000 Invitrogen) with p6OSE2 containing the firefly luciferase gene downstream of Runx2 response elements [22]. Promoter-less pGL4.10 vector served as a background control. The plasmid pRL containing the Renilla luciferase reporter gene was co-transfected to normalize for transfection efficiency. We find the transfection with Lipofectamine 2000 routinely yield 5-10% transfected cells. The cells were lysed and luciferase activity was measured 72h after transfection according to the manufacturer's protocol.
Statistical analysis
Data are expressed as mean±SE. Significant differences between groups was determined by the Student's t test or ANOVA. Data were considered statistically significant at a p value <0.05.
Results
Altered expression of calcification regulatory factors in ApoE−/− OPG−/− mouse aortas
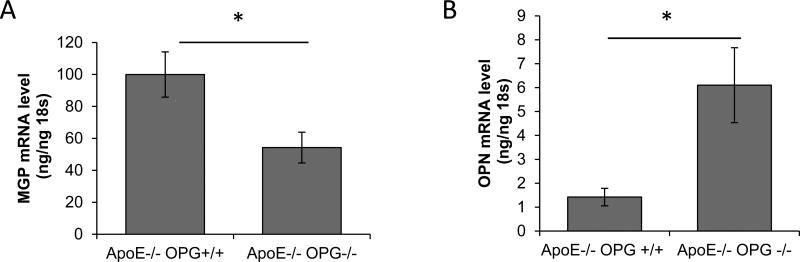
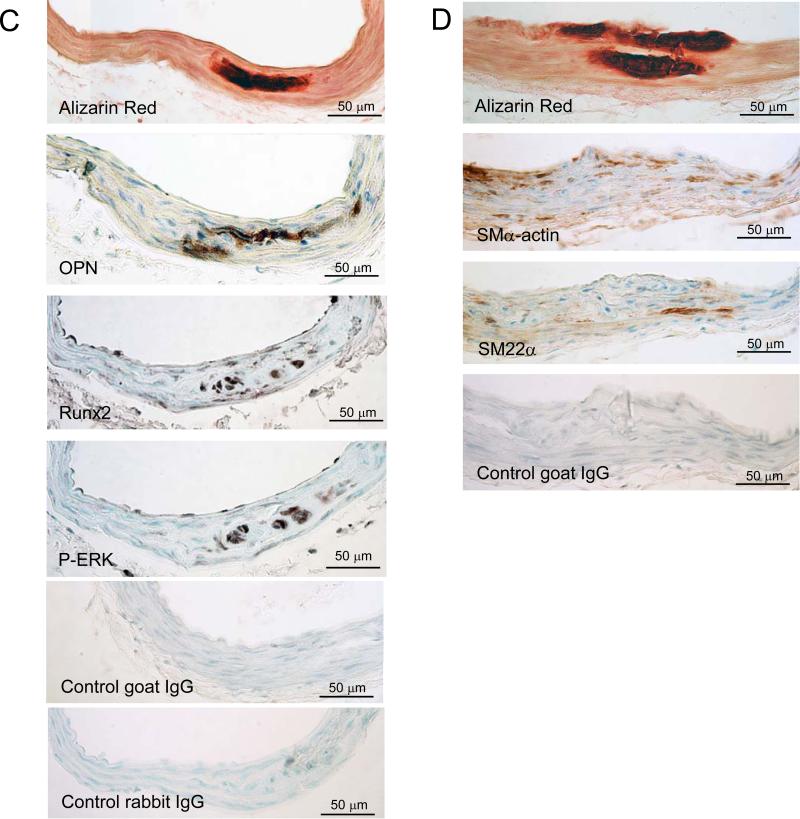
We have previously shown that ApoE−/−OPG−/− mice present more vascular calcification than ApoE−/−OPG+/+ at 40 and 60 weeks of age[13]. Here we asked whether calcification related genes were dysregulated in young, 12 week old, ApoE−/−OPG−/− mouse aortas as compared to age-matched ApoE−/−OPG+/+. We performed qPCR expression analysis on whole mouse aortas isolated from young non-diseased mice and found decreased MGP expression and increased OPN expression in the aortas from ApoE−/−OPG−/− mice compared to ApoE−/−OPG+/+ (Figure 1A and 1B). We also found sporadic medial vascular calcification in ApoE−/−OPG−/− but not in ApoE−/−OPG+/+ by Alizarin Red S staining (two out of six ApoE−/−OPG−/− had calcification, none of six ApoE−/−OPG+/+ had calcification, Figure 1C and 1D, and supplemental figure I), however statistical significance was not reached at this early age as previously shown [13]. Histological staining showed expression of OPN, Runx2, and phosphorylated ERK associated with early medial calcification in ApoE−/−OPG−/− but not in ApoE−/−OPG+/+ (Figure 1C, and supplemental figure I). Further, in the areas of early calcification SMα-actin and SM22α had disappeared (Figure 1D). There was no sign of cell death in these regions as determined by active caspase3 staining (Supplemental figure I). These data suggest that in the absence of OPG, unabated OPG-ligands signaling may lead to accelerated osteochondrogenic conversion.
Figure 1.
Altered expression of calcification regulatory factors in mouse aortas. (A-B) qPCR of RNA from aortic tissue (ascending, arch, thoracic) isolated from ApoE−/−OPG−/− mice (n=12) and ApoE−/−OPG+/+ (n=6) at 12 weeks of age. (A) MGP, (B) OPN *p<0.05. (C and D) Immunohistochemistry of ApoE−/−OPG−/− innominate artery serial sections for phosphorylated ERK, Runx2, OPN, SM22α SMα-actin, control goat rabbit and mouse IgG, and for Alizarin Red.
KO-VSMCs have enhanced calcification in response to osteogenic conditions
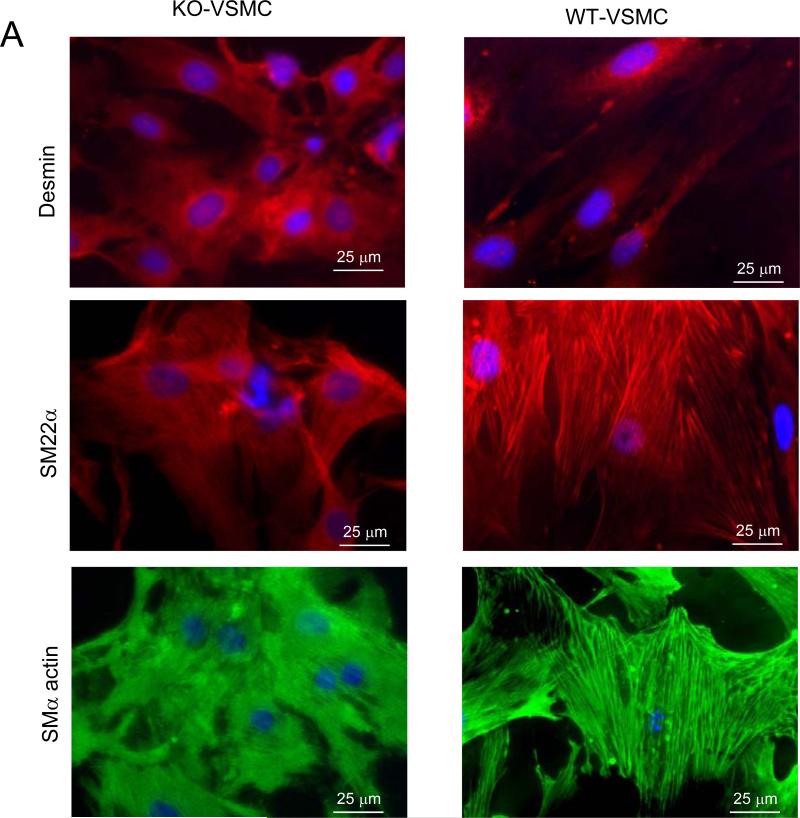
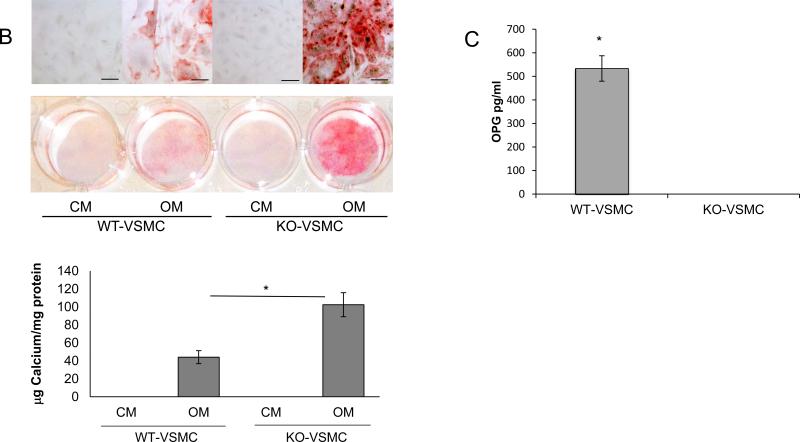
We then reasoned that VSMCs isolated from ApoE−/−OPG−/− (KO-VSMCs) and ApoE−/−OPG+/+ (WT-VSMCs) may show accelerated mineralization in response to osteogenic medium (OM). Indeed, OPG expression has been shown to decrease in response to osteogenic conditions in VSMCs[23], findings that we have confirmed in our WT-VSMCs (not shown). We found that freshly isolated (passage 1) KO-VSMC expressed the smooth muscle cell markers desmin, SMα-actin, and SM22α as expected and similarly to ApoE−/−OPG+/+ VSMCs (Figure 2A), however when cultured in OM for 7 days, KO-VSMCs showed increased calcification and increased alkaline phosphatase (APL) activity as compared to WT-VSMCs (Figure 2B and supplemental figure II). WT-VSMCs secreted very high levels of OPG (figure 2C). Taken together these data suggest that unabated signaling initiated by OPG-ligands may drive the increased calcification observed in KO-VSMCs. Similarly we found that OPG−/− VSMCs calcified more when compared to OPG+/+ VSMCs, thus, indicating that expression of the ApoE gene does not affect VSMC calcification (Supplemental figure III).
Figure 2.
Characterization of freshly isolated KO-VSMCs and WT-VSMCs. (A) Immunofluorescence for Desmin, SM22α, SMα-actin in KO- and WT-VSMCs one passage after isolation. (B) Calcification assay with WT- and KO-VSMCs. Cells were cultured in control medium (CM) and osteogenic medium(OM) for 7 days. Calcium deposition was measured as described in the methods. *p<0.05. Alizarin Red S staining was also performed directly in the plate wells (low, bottom, and high, top, magnifications) scale bar=50 μm. (C) ELISA for OPG on supernatant of WT- and KO-VSMCs. *p<0.05.
KO-VSMCs have an enhanced osteochondrogenic phenotype
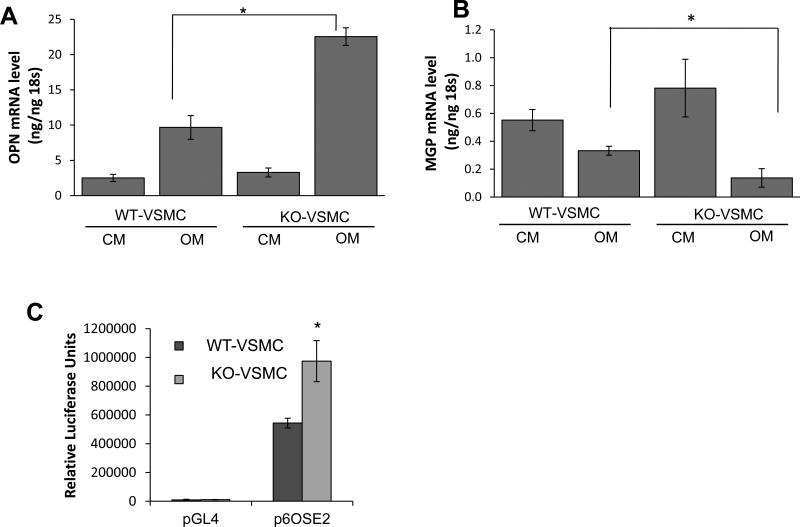
We further asked whether KO-VSMCs have altered expression of osteochondrogenic genes similarly to what was observed in vivo in the aortas. By using qPCR we found that in OM OPN was more strongly induced in KO-VSMCs than in WT-VSMCs, and that MGP was repressed more in KO-VSMCs compared to WT-VSMCs (Figure 3A and 3B). Further, KO-VSMCs expressed lower levels of SMα-actin as compared to the WT-VSMCs when cultured for 4 days in control medium (CM) and OM. (Figure 3E). Similarly, we found that Runx2 promoter activity was higher in the KO-VSMCs than WT-VSMCs (Figure 3C), which correlated with elevated ERK phosphorylation in the KO-VSMCs cultured in CM and in OM (Figure 3D). These data may suggest that lack of OPG may affect the expression of SMα-actin and activation of the ERK-Runx2 pathway even in control conditions [22, 24]. Treatment with OM did not appear to reduce SMα-actin further in KO-VSMCs. Further, caspase dependent cell death did not mediate the increased mineralization of KO-VSMCs as determined by incubation with the caspase inhibitor zVAD (Supplemental figure IV). Taken together these data suggest that the increased KO-VSMCs calcification is likely regulated by accelerated osteochondrogenic transition rather than increased cell death.
Figure 3.
Differential expression of regulatory factors in KO-VSMCs versus WT-VSMCs. (A-B) Gene expression analysis between WT- and KO-VSMCs after 4 days treatment with CM and OM (A) showed higher level of OPN and (B) lower level of MGP in KO-VSMCs compared to WT-VSMCs in OM. Data are collected from 3 independent experiments. *p<0.05. (C) Dual luciferase reporter assay indicating that Runx2 activity is higher in KO-VSMCs compared to control WT-VSMC. Cells were transduced with p6OSE2 and pGL4 as describe in the methods and cultured 72 hours before harvesting for Luciferase measurement. *<0.05 versus p6OSE2-WT-VSMC. (D) Western blot for phosphorylated-ERK (P-ERK, top panel) and ERK (bottom panel) showing increased ERK activity in KO-VSMCs and increased ERK activity in OM regardless of cell genotype. (E) KO-VSMCs express less SMα-actin than WT-VSMCs. (F) beta-actin was used as loading control.
Inhibition of RANKL signaling in KO-VSMCs rescues the increased calcification
We then reasoned that re-expression of OPG may rescue the calcification phenotype and gene expression observed in KO-VSMCs. We found that transgenic KO-VSMCs (TGKO-VSMC) in which OPG was retrovirally re-introduced calcified significantly less than vector control KOVSMCs (GPFKO-VSMCs) which were retrovirally infected with the control vector expressing GFP. (Figure 4A). As expected, TGKO-VSMCs produced OPG (Supplemental figure V A). Further, TGKO-VSMCs expressed lower levels of OPN, and higher levels of MGP mRNA than GPFKOVSMCs, again suggesting that unabated OPG ligands may be responsible for the accelerated osteochondrogenic differentiation (Supplemental figure V B and C).
Figure 4.
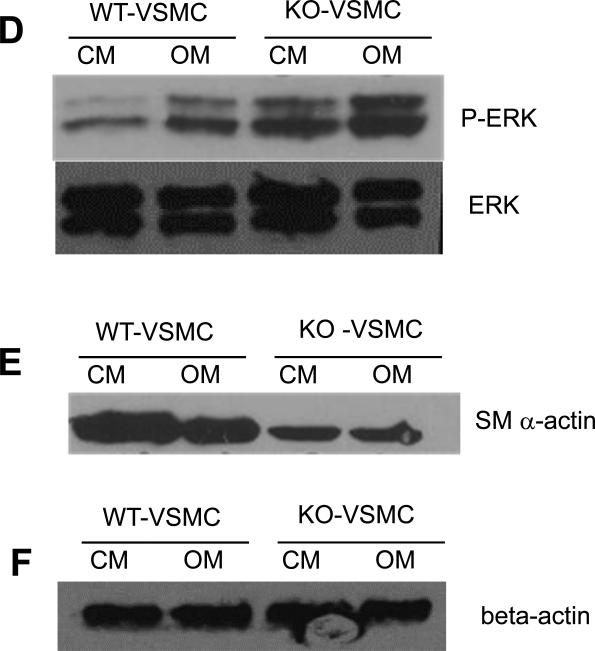
RANKL signaling inhibition in KO-VSMCs rescues increased calcification. (A) Calcification assay with TGKO-VSMCs, transgenic KO-VSMCs, in which OPG was retrovirally reintroduced. TGKO-VSMCs calcified and more than vector control KO-VSMCs (GPFKO-VSMCs), which were retrovirally infected with the control vector expressing GFP. TGKO-VSMCs and GFPKO-VSMCs were treated with OM for 7 days. *p<0.05. (B) RT-PCR expression of RANK and RANKL in KO-VSMCs and positive control calvaria cells. (C) Calcification assay with scrambled transduced (Scramble) or RANK siRNA transduced (RANK siRNA) KO-VSMCs. Cells were treated with CM or OM or 7 days. *p<0.05.
We further hypothesized that RANKL could be responsible for the accelerated calcification of KO-VSMCs. We first confirmed that RANKL and RANK were expressed by VSMCs qualitatively by RT-PCR (Figure 4B), then, we asked whether blocking RANKL signaling by down-regulating the receptor RANK with siRNA would rescue the calcification phenotype. RANK siRNA transduced KO-VSMCs deposited less calcium when compared with scrambled siRNA (Figure 4C). As a control we verified that siRNA reduced RANK expression, as shown in Supplemental figure VI. These data strongly implicate RANKL and RANK signaling in the enhanced calcification phenotype of KO-VSMCs.
RANKL treatment increases calcification in KO-VSMCs
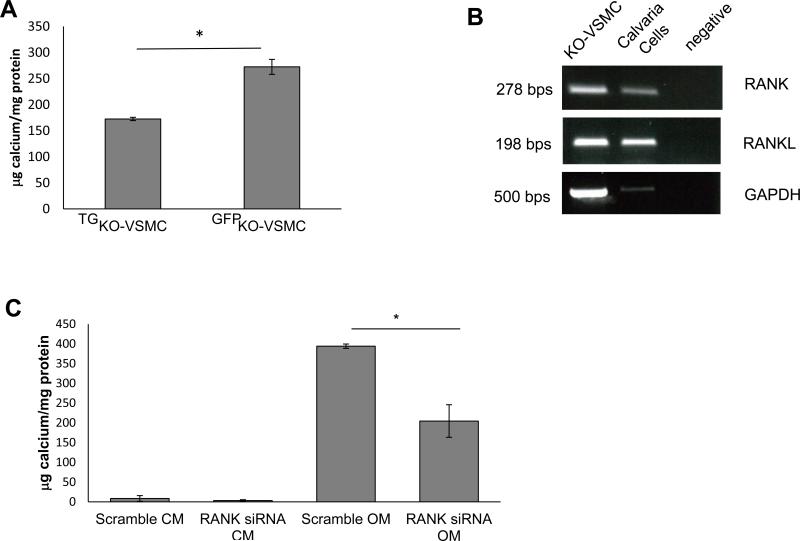
We then asked whether addition of exogenous RANKL in a dose-dependent manner would affect VSMC calcification. We found that RANKL dose-dependently enhanced KO-VSMC OM-dependent calcification (Figure 5A), however, RANKL had no effect on WT-VSMCs calcification (Figure 5B). In KO-VSMCs, RANKL was able to induce activation as well as increase expression of Runx2 (Figure 5C and Supplemental Figure VII). Further, in KO-VSMCs RANKL also up-regulated OPN, BMP2, and ALP expression, and down-regulated MGP, SMα-actin, and SM22α expression (Supplemental Figure VII).
Figure 5.
RANKL enhance calcification of KO-VSMCs but not WT-VSMCs. KO-VSMCs (A) and WT-VSMCs (B) were treated with RANKL at 50, 100, 200 ng/ml for 7 days. *p<0.05 RANKL all concentrations versus vehicle. (C) Dual luciferase reporter assay showing Runx2 activity increased in KO-VSMCs after treatment with RANKL for 72 hours. Cells were transduced with p6OSE2 and pGL4 as described in the methods and cultured with Vehicle or 100 ng/ml of RANKL before harvesting for Luciferase measurement.*p<0.05 p6OSE RANKL versus p6OSE2. (D) RT-PCR for RANKL expression in freshly isolated aortas from 12 week old ApoE−/−OPG−/−and ApoE−/−OPG+/+ mice. Calvaria cells were used as positive control. (E) Staining for RANKL and Von Kossa of 12 week old ApoE−/−OPG−/− innominate artery. Left top panel, wide view showing RANKL expression in medial cells closely associated with areas of calcification, in medial cells further from the calcified area, and in few adventitial cells (arrows). Left bottom panel shows higher magnification of RANKL staining associated with calcified area (arrowhead). Middle top panel, wide view showing VonKossa staining in the medial layer. Middle bottom panel, higher magnification VonKossa staining. Arrowhead, indicate VonKossa staining intimately associated with RANKL positive area. Right panel, wide whole vessel view of VonKossa staining. (F) RANKL staining on 12 week old ApoE−/−OPG+/+ innominate artery.
These findings suggest that unopposed RANKL may accelerate osteochondrogenic differentiation in vitro as well in vivo. Indeed, we found that RANKL was expressed in ApoE−/−aortas regardless of genotype by RT-PCR. Further, RANKL protein associated with calcified areas in young ApoE−/−OPG−/− mice innominate arteries, and it was also expressed in cells surrounding the mineral (Figure 5D, qualitative RT-PCR, and E, immunohistochemistry). However, RANKL staining in ApoE−/−OPG+/+ innominate arteries appeared weaker that in ApoE−/−OPG+/+ arteries (Figure 5F).
IL-6 partially mediates RANKL enhancement of KO-VSMC calcification
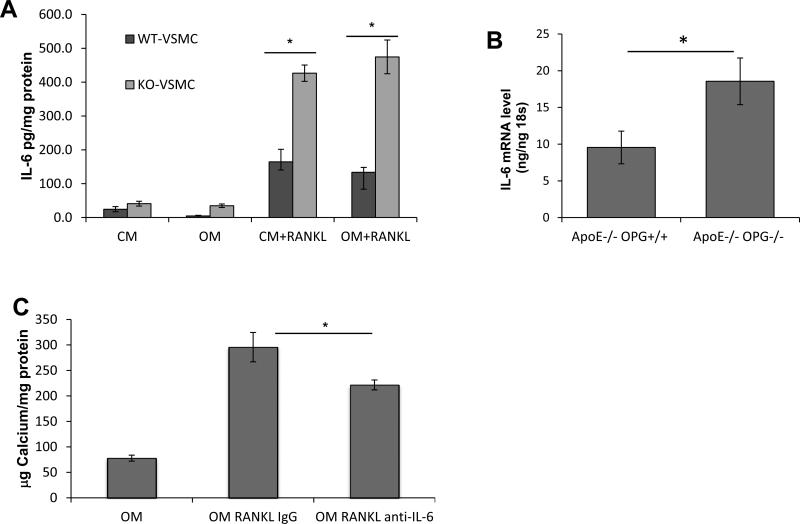
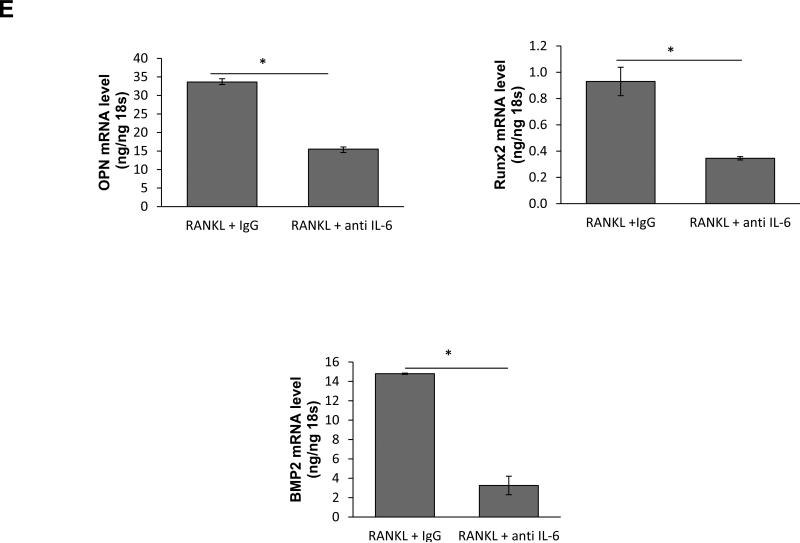
We have recently shown that RANKL up-regulates expression of IL-6 and TNF-α in macrophages[25]. In this study, we determined that RANKL also induced IL-6 in KO-VSMCs and to a lesser extent in WT-VSMCs as measured by ELISA independent of osteogenic conditions (Figure 6A). Similarly, IL-6 was up-regulated in ApoE−/−OPG−/− aortas compared to ApoE−/−OPG+/+ (Figure 6B) and downregulated in TGKO-VSMCs (not shown). IL-6 has recently been shown to be a promoter of vascular calcification[26]. Using neutralizing antibodies to IL-6 in combination with RANKL treatment, we observed that a portion of the RANKL enhanced calcification was dependent on IL-6 (Figure 6C). IL-6 by itself was a weak inducer of KO-VSMC calcification (Supplemental figure VIII). We further found that inhibition of IL-6 reversed the regulation of a portion of the RANKL-dependent osteochondrogenic genes such as OPN, Runx2, BMP2, but not MGP (Figure 6D and not shown). Finally, we find that IL-6 is a strong inducer of RANKL, RANK, and OPG suggesting a possible forward loop (Supplemental figure VIII).
Figure 6.
RANKL induces up-regulation of IL-6. (A) ELISA with cell supernatant from WT- and KO-VSMCs treated with RANKL in CM and OM conditions *p<0.05 KO-VSMC RANKL versus WT-VSMC RANKL. (B) qPCR for IL-6 expression in aortas freshly isolated from 12 week old ApoE−/−OPG−/− and ApoE−/−OPG+/+ mice. (C) Calcification assay with KO-VSMCs cultured in OM, treated RANKL and isotype control antibody (OM RANKL IgG), and treated RANKL and anti-IL-6 neutralizing antibody (OM RANKL anti-IL-6) for 7 days. *p<0.05 OM RANKL IgG versus OM RANKL anti-IL-6. (D) qPCR for OPN, Runx2 and BMP2 of KO-VSMCs treated with RANKL or RANKL and anti-IL-6 antibody for four days. *p<0.05.
Discussion
Vascular calcification, a type of ectopic soft tissue mineralization, increases the risk of cardiovascular mortality[1]. We have previously shown in ApoE−/− mice lacking OPG that vascular calcification was increased [13]. In addition, we have recently shown that vessel wall-derived OPG protects ApoE−/− mice from the development of vascular calcification[14]. In this report, we sought to determine whether the lack of OPG specifically in VSMCs facilitated their conversion to a phenotype that is more prone to mineralization. We observed that KO-VSMCs deposited more calcium than WT-VSMCs, and expressed osteochondrogenic markers in a RANKL-dependent manner when cultured in osteogenic conditions. Taken together these data strongly suggest that OPG inhibits VSMC calcification by neutralizing the pro-mineralizing effects of RANKL.
There is increasing clinical evidence suggesting that OPG and RANKL may play an important role in CVD[18, 27]. Several studies have shown that baseline serum RANKL is a highly significant predictor of CVD risk (including ischemic stroke, transient ischemic attack, myocardial infarction, and vascular death)[28-30]. Further, there is a correlation between plasma RANKL and elevated circulating phosphate levels seen in chronic kidney disease patients, a population that is affected by extensive medial and intimal calcification and significant increases in cardiovascular mortality when compared to the general population[31]. Clinical evidence also links serum OPG levels to CVD. The results of several studies have suggested that OPG is an additional prognostic biomarker of CVD and mortality in high-risk populations along with age, diabetes, markers of systemic inflammation, chronic infection, and smoking[18, 32-34]. It is being debated whether elevated serum OPG is causative or a compensatory protective response to elevated levels of RANKL in people with CVD.
In animal models OPG appears to be a vascular protective factor. As noted, we have shown that lack of OPG in ApoE−/− mice results in larger lesions and increased mineralization of the vasculature, and with a very recent study we have determined that either vessel wall- or bone marrow-derived OPG protected again atherosclerosis and vascular calcification in ApoE−/− mice[13, 14]. Further, administration of OPG to fat fed athero-prone LDLR-/- mice leads to less mineral accumulation in the vasculature[15]. With this study we also show that, in absence of OPG, microcalcification accompanied by expression of osteochondrogenic factors and disappearance of smooth muscle markers occurs in the medial layer of lesion free young ApoE−/−OPG−/− mice (Figure 1) suggesting that lack OPG and hypercholesterolemia induce VSMC osteochondrogenic differentiation. Further, in vitro KO-VSMCs appeared to express similar levels of smooth muscle markers when freshly isolated, however, with time in culture we observed downregulation of SMα actin, enhanced ERK phosphorylation, and enhanced Runx2 activity suggesting that KO-VSMCs were undergoing a phenotypic change even before the induction of mineralization. Whether unabated RANKL is responsible for these observations is a critical question. As reported by many, RANKL is expressed in the vessel wall[5, 11, 12, 35]. Accordingly, we have found that RANKL was associated with medial calcium deposits and VSMCs in young ApoE−/−OPG−/− mice (Figure 5E). In vivo inhibition of RANKL with an anti-human RANKL antibody (denosumab) in a transgenic mouse model challenged with prednisolone appeared to inhibit vascular calcification[36]. These latter findings strongly suggest a role for RANKL in vascular calcification. However, in vitro data with isolated SMCs have not always supported this hypothesis. A number of studies suggest that RANKL is a positive regulator of SMC mineralization but others dispute these findings[35, 37-40]. Correspondingly, we found that RANKL did not increase WT-VSMCs calcification in response to osteogenic conditions (of note WT-VSMCs secreted very high levels of OPG, Figure 2). However, we found that KO-VSMCs deposited more calcium than WT-VSMCs, which correlated with elevated osteochondrogenic genes (Figure 3). We further found that inhibition of RANKL signaling by down-regulation of its cell surface receptor RANK led to the rescue of mineralization in KOVSMCs as did OPG re-expression, which importantly also led to osteochondrogenic phenotype normalization (Figure 4 and Supplemental figure V). In addition, RANKL accelerated osteochondrogenic phenotypic transition and was a potent inducer of calcification in KO-VSMCs (Figure 5 and Supplemental figure VII). These latter observations are in agreement with and expand an observation by Panizo et al. and Osako et al.[35, 37]. Taken together these results suggest that RANKL is an inducer of BMP type factors that in turn activate Runx2 and osteochondrogenic genes. At the same time RANKL is an inhibitor of MGP expression that is a potent BMP inhibitor and aids in matrix vesicle clearance[3, 26, 41, 42]. Interestingly, even if RANKL induced gene changes and Runx2 activation, it was not sufficient to induce VSMC mineralization in control conditions suggesting that mineralizing permissive conditions, that were mimicked in vitro by the osteogenic medium, are necessary for RANKL to act as an enhancer of VSMC calcification.
We also show that RANKL is a potent inducer of IL-6 in VSMCs (Figure 6). We have recently found that IL-6 had no effect on WT-VSMCs calcification but did up-regulate OPN and down-regulated MGP expression[25]. However, in KO-VSMCs IL-6 weakly induced mineralization and partially mediated RANKL induction of mineralization and expression of OPN, Runx2 and BMP2, but not MGP (Supplemental figure VIII, Figure 6 and not shown). Yao et al. have shown that IL-6 modulates vascular calcification by affecting the ability of MGP to bind and inactivate BMP2[26]. To our knowledge, this is the first report that establishes IL-6 as a direct regulator of VSMC mineralization and mineralization genes. IL-6 is a CVD biomarker and a strong predictor of coronary artery disease and sudden cardiovascular death, however the mechanism of this cytokine modulation of CVD and vascular calcification is still controversial[43-45]. Some experimental studies suggest that IL-6 is pro-atherogenic while others indicate that physiological levels of IL-6 are necessary to maintain the vascular inflammatory response in check[45-50]. While vascular calcification was not evaluated in any of these studies, it is well established clinically that IL-6 is an independent predictor of cardiovascular events in chronic kidney disease patients who are affected by extensive vascular calcification[51].
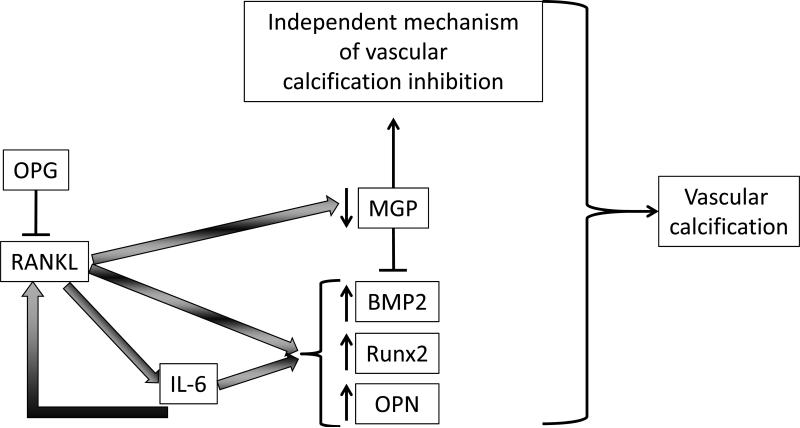
In conclusion, we have provided evidence that in the setting of OPG deficiency and in osteogenic environment RANKL accelerated VSMC calcification. Furthermore, we have presented evidence that RANKL accelerated the osteochondrogenic conversion of VSMCs partially via the induction of IL-6. Thus, we propose a model whereby unopposed RANKL in an IL-6 independent and dependent manner up-regulates a subset of osteochondrogenic genes (BMP2, Runx2, and OPN), and in an IL-6-independent manner, down-regulates MGP. In addition, the observed induction of RANKL, RANK, and OPG by IL-6 in VSMCs may indicate a feed forward loop further propagating VSMC mineralization. The combination of these effects leads to increased vascular calcification in mineralizing permissive conditions (Figure 7). Given the epidemiological data in humans correlating the OPG/RANKL system with CVD and vascular calcification, targeting the RANKL/RANK system in VSMC may be a therapeutic approach for reducing vascular calcification burden in CVD.
Figure 7.
Proposed model for RANKL-mediated vascular calcification. In absence of OPG, RANKL in an IL-6 dependent and independent manner up-regulates a subset of osteochondrogenic genes (BMP2, Runx2, and OPN) and in an IL-6-independent manner and down-regulates MGP. The combination of these effects leads to increased vascular calcification. Further, IL-6 induces RANKL, and OPG possibly suggesting a feed-forward process.
Supplementary Material
Acknowledgments
None
This work is supported by funding from NIH R01HL093469-01 and R01DK094434-01
References
- 1.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moe SM, Reslerova M, Ketteler M, O'neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (ckd). Kidney Int. 2005;67:2295–2304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 3.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: A review and perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abedin M, Tintut Y, Demer LL. Vascular calcification: Mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 5.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of apoe-deficient mice: Potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 6.Lau WL, Pai A, Moe SM, Giachelli CM. Direct effects of phosphate on vascular cell function. Adv Chronic Kidney Dis. 2011;18:105–112. doi: 10.1053/j.ackd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: Emerging role of the sodium-dependent phosphate co-transporter pit-1. Thromb Haemost. 2010;104:464–470. doi: 10.1160/TH09-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 9.Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, Pascual V, Hood LE, Clark EA. Opg/fdcr-1, a tnf receptor family member, is expressed in lymphoid cells and is up-regulated by ligating cd40. J Immunol. 1998;161:6113–6121. [PubMed] [Google Scholar]
- 10.Yun TJ, Tallquist MD, Aicher A, Rafferty KL, Marshall AJ, Moon JJ, Ewings ME, Mohaupt M, Herring SW, Clark EA. Osteoprotegerin, a crucial regulator of bone metabolism, also regulates b cell development and function. J Immunol. 2001;166:1482–1491. doi: 10.4049/jimmunol.166.3.1482. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg WJ, Yndestad A, Øie E, Smith C, Ueland T, Ovchinnikova O, Robertson AK, Müller F, Semb AG, Scholz H, Andreassen AK, Gullestad L, Damås JK, Frøland SS, Hansson GK, Halvorsen B, Aukrust P. Enhanced t-cell expression of rank ligand in acute coronary syndrome: Possible role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2006;26:857–863. doi: 10.1161/01.ATV.0000204334.48195.6a. [DOI] [PubMed] [Google Scholar]
- 12.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 13.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older apoe−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 14.Callegari A, Coons M, Ricks JL, Yang HL, Gross TS, Huber P, Rosenfeld ME, Scatena M. Bone marrow- or vessel wall-derived osteoprotegerin is sufficient to reduce atherosclerotic lesion size and vascular calcification. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.301755. [DOI] [PubMed] [Google Scholar]
- 15.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. Osteoprotegerin is an alpha vbeta 3-induced, nf-kappa b-dependent survival factor for endothelial cells. J Biol Chem. 2000;275:20959–20962. doi: 10.1074/jbc.C000290200. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer LC. Pathophysiology of rank ligand (rankl) and osteoprotegerin (opg). Ann Endocrinol (Paris) 2006;67:139–141. doi: 10.1016/s0003-4266(06)72569-0. [DOI] [PubMed] [Google Scholar]
- 18.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 19.Jono S, Otsuki S, Higashikuni Y, Shioi A, Mori K, Hara K, Hashimoto H, Ikari Y. Serum osteoprotegerin levels and long-term prognosis in subjects with stable coronary artery disease. J Thromb Haemost. 2010;8:1170–1175. doi: 10.1111/j.1538-7836.2010.03833.x. [DOI] [PubMed] [Google Scholar]
- 20.Rice J, Courter DL, Giachelli CM, Scatena M. Molecular mediators of alphavbeta3-induced endothelial cell survival. J Vasc Res. 2006;43:422–436. doi: 10.1159/000094884. [DOI] [PubMed] [Google Scholar]
- 21.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 22.Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT. Identification and functional characterization of erk/mapk phosphorylation sites in the runx2 transcription factor. J Biol Chem. 2009;284:32533–32543. doi: 10.1074/jbc.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem. 2010;110:935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deuell KA, Callegari A, Giachelli CM, Rosenfeld ME, Scatena M. Rankl enhances macrophage paracrine pro-calcific activity in high phosphate-treated smooth muscle cells: Dependence on il-6 and tnf-α. J Vasc Res. 2012;49:510–521. doi: 10.1159/000341216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Y, Watson AD, Ji S, Boström KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix gla protein. Circ Res. 2009;105:575–584. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Bartolo BA, Schoppet M, Mattar MZ, Rachner TD, Shanahan CM, Kavurma MM. Calcium and osteoprotegerin regulate igf1r expression to inhibit vascular calcification. Cardiovasc Res. 2011;91:537–545. doi: 10.1093/cvr/cvr084. [DOI] [PubMed] [Google Scholar]
- 28.Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J. Soluble receptor activator of nuclear factor-kappa b ligand and risk for cardiovascular disease. Circulation. 2007;116:385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen L, Hansen JB, Brox J, Mathiesen E, Vik A, Jacobsen BK. Serum osteoprotegerin levels are related to height loss: The tromsø study. Eur J Epidemiol. 2011;26:305–312. doi: 10.1007/s10654-011-9555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueland T, Yndestad A, Øie E, Florholmen G, Halvorsen B, Frøland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P. Dysregulated osteoprotegerin/rank ligand/rank axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 31.Fahrleitner-Pammer A, Dobnig H, Dimai HP, Holzer H, Benesch T, Borchhardt K, Cejka D, Haas M. The effect of rankl and opg on bone mineral density in pre-dialysis chronic renal failure. Clin Nephrol. 2009;71:652–659. doi: 10.5414/cnp71652. [DOI] [PubMed] [Google Scholar]
- 32.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 33.Hosbond SE, Poulsen TS, Diederichsen AC, Nybo M, Rasmussen LM, Mickley H. Osteoprotegerin as a marker of atherosclerosis: A systematic update. Scand Cardiovasc J. 2012;46:203–211. doi: 10.3109/14017431.2012.685491. [DOI] [PubMed] [Google Scholar]
- 34.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 35.Osako MK, Nakagami H, Koibuchi N, Shimizu H, Nakagami F, Koriyama H, Shimamura M, Miyake T, Rakugi H, Morishita R. Estrogen inhibits vascular calcification via vascular rankl system: Common mechanism of osteoporosis and vascular calcification. Circ Res. 2010;107:466–475. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 36.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC. Inhibition of receptor activator of nf-kappab ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175:473–478. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, López-Ongil S, Coll B, Fernandez E, Valdivielso JM. Rankl increases vascular smooth muscle cell calcification through a rank-bmp4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 38.Tseng W, Graham LS, Geng Y, Reddy A, Lu J, Effros RB, Demer L, Tintut Y. Pka-induced receptor activator of nf-kappab ligand (rankl) expression in vascular cells mediates osteoclastogenesis but not matrix calcification. J Biol Chem. 2010;285:29925–29931. doi: 10.1074/jbc.M110.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y. Runx2-upregulated receptor activator of nuclear factor κb ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olesen M, Skov V, Mechta M, Mumm BH, Rasmussen LM. No influence of opg and its ligands, rankl and trail, on proliferation and regulation of the calcification process in primary human vascular smooth muscle cells. Mol Cell Endocrinol. 2012;362:149–156. doi: 10.1016/j.mce.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix gla protein-deficient mice: Evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 43.Murabito JM, Keyes MJ, Guo CY, Keaney JF, Vasan RS, D'Agostino RB, Benjamin EJ. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The framingham offspring study. Atherosclerosis. 2009;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh artery study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 45.Empana JP, Jouven X, Canouï-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in european middle-aged men: The prime study. Arterioscler Thromb Vasc Biol. 2010;30:2047–2052. doi: 10.1161/ATVBAHA.110.208785. [DOI] [PubMed] [Google Scholar]
- 46.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 47.Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, Wollert KC, Seegert D, Rose-John S, Tietge UJ, Schieffer B, Grote K. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 48.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein e-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 49.Madan M, Bishayi B, Hoge M, Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an apoe heterozygote murine model. Atherosclerosis. 2008;197:504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJ, Drexler H. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 51.Okamura DM, Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol. 2009;24:2309–2319. doi: 10.1007/s00467-009-1199-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.