Abstract
This article provides a review of the role of aliskiren, a direct renin inhibitor, in pediatric hypertension and kidney diseases. Among the many mechanisms involved in regulating BP, the renin-angiotensin-aldosterone system (RAAS) plays a major role. Additionally, the RAAS has been identified as a contributing factor to cardiovascular and renal diseases for more than three decades. The potential benefits of inhibiting the RAAS by aliskiren alone or in combination with other RAAS blockers (ACEIs, ARBs) seem theoretically promising, but one should exercise caution in children, especially in those with significant chronic kidney disease until there is more evidence regarding the safety and efficacy of this new drug in the pediatric population from the ongoing clinical trials.
Keywords: Aliskiren, Renin, Angiotensin, Hypertension, Blood pressure, Hyperkalemia
Introduction
Hypertension (HTN) is a worldwide health problem associated with an increased risk for mortality and morbidity from cardiovascular and renal disease [1, 2]. Pediatric HTN remains one of the strongest predictors of adult HTN [3], which significantly increases the cardiovascular mortality risk in adults [4, 5]. Over the past decade, the prevalence of hypertension in the pediatric population has increased in correlation to the rise in childhood overweight and obesity [4, 6, 7]. Although the exact prevalence and incidence of pediatric hypertension is unknown, one study estimated the prevalence to be 4.5% after 3 separate screenings were conducted on a group of > 4000 children aged 10 to 19 years [8].
Background: Hypertension, Prehypertension and Staging
Hypertension is the sustained level of BP that over time leads to a variety of adverse effects on target organs such as the heart (left ventricular hypertrophy), the brain and central nervous system, and the kidneys. Defined statistically, hypertension is when BPs fall above the 95th percentile for age, gender and stature on at least three occasions. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents emphasizes better early detection and control of hypertension in children and recommends BP screening in children above 3 years of age who are seen in a medical setting and in younger children under special circumstances that increase the risk for HTN [9, 10]. This statistical definition of hypertension is one that is based on normative distribution of causal office BPs in healthy children and is stratified by age, gender and stature [9].
The blood pressure is measured in the office setting by non-invasive techniques such as auscultatory and oscillometric methods. Although the auscultatory method is the recommended one for measuring BP, the oscillometric technique may be used due to its ease of performance. However, the BP measurement should to be repeated by the auscultatory method if it is elevated by oscillometry.[9] The current practice of clinic-based hypertension management leads to undertreatment for some patients and overtreatment for others.[11] Even with proper techniques, BP control is misclassified for more than 25% of patients when a single office visit measurement is used.[12] Some patients exhibit “white-coat hypertension (WCH)” with elevated BP levels in the medical office but not in other settings, whereas others have “masked hypertension” with elevated BP outside the clinical setting but normal in a medical office.
24-hour ambulatory blood pressure monitoring (ABPM) is a useful tool in evaluating children with concerns for hypertension, and it is the only available method to reliably identify WCH and masked HTN in children.[13] Using 24-hour ambulatory BP monitoring as a criterion standard, an average of 6 BP readings taken at different clinic visits are needed to classify BP control with 80% accuracy.[14] This many in-person visits are impractical for most patients. It is clear from different recent studies that bringing hypertension care out of the office and into patients' homes works.[11, 15] Nonetheless, widespread adoption of home BP monitoring supported by team care has not occurred in the United States and it is not likely to occur spontaneously [11]. For home BP monitoring to become part of routine practice, major changes to the current system of reimbursement and performance measurement will be needed.
Hypertension in children is classified by the National High BP Education Program on the basis of child's blood pressure percentile into normal (< 90th percentile), prehypertension (90-94th percentile), stage 1 hypertension (>95th percentile), or stage 2 hypertension (>99th percentile plus 5). Primary hypertension, defined by the lack of an underlying causative disorder, is frequently found in children with obesity or a family history of hypertension or cardiovascular disease. The worldwide childhood obesity epidemic has had a profound impact on the frequency of hypertension and other obesity-related conditions with the result that primary hypertension should now be viewed as one of the most common health conditions in the young [16]. The secondary hypertension is more commonly seen in children than in adults. The majority of the secondary hypertension in children is caused by renal or renovascular dysfunction [9].
In children with prehypertension, actual pharmacologic treatment is controversial. For children with stage 1 primary hypertension, the primary treatment to decrease blood pressure is therapeutic lifestyle/behavior changes, unless there is an evidence of target organ damage such as left ventricular hypertrophy [8]. Therapeutic lifestyle changes include keeping healthy weight, regular physical activity, and dietary modification. Pharmacologic treatment is indicated in secondary HTN, primary stage 2 HTN or if the lifestyle measures prove inadequate [8]. Current recommendations for pharmacological management of hypertension in pediatric patients include the use of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), β-adrenergic blockers, calcium channel blockers, or diuretics. Although diuretics and β-blockers have documented safety and efficacy in pediatric patients with hypertension, ACEIs and ARBs are recommended for initial therapy in pediatric patients with concomitant diabetes and microalbuminuria or proteinuria or chronic kidney disease [4, 10, 17]. RAAS blockade is also recommended by the European Society of Hypertension as the first line treatment of children with primary hypertension associated with obesity/metabolic syndrome as ACEIs and ARBs might induce reduction of insulin resistance and subsequent changes in the lipid profile and in glucose levels [10]. Although the number of available antihypertensive medications is ever growing, selecting the most appropriate agent and effectively treating high blood pressure remains a challenge. The purpose of this article was to provide a review of the literature on the role of aliskiren, a direct renin inhibitor, in pediatric hypertension and kidney diseases. Among the many mechanisms involved in regulating BP, the renin-angiotensin-aldosterone system (RAAS) is one of the major players. The potential benefits of inhibiting the RAAS, a contributing factor to cardiovascular and renal diseases, have been known for more than three decades [18] and a recent review in this Journal by Silva and Flynn underscores the complexity of this system that is still being elucidated [19]. Four groups of RAAS blockers have been developed which are direct renin inhibitors (DRI), angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) and aldosterone antagonists.
The RAAS and the role of Aliskiren
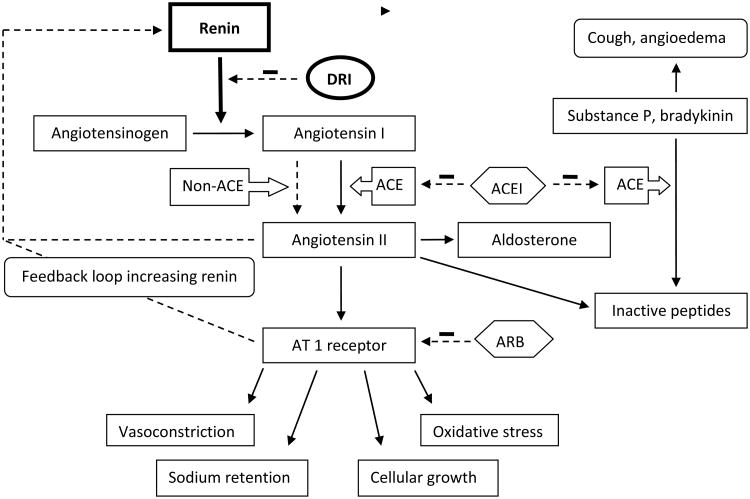
The renin-angiotensin-aldosterone system (RAAS) has an important role in the regulation of arterial blood pressure (BP) and volume and electrolytes homeostasis [20](Figure. 1). Renin, a proteinase enzyme, is secreted by the juxtaglomerular cells of the kidney in response to a decrease in circulating volume and blood pressure. It cleaves the substrate angiotensinogen to form the inactive decapeptide angiotensin I (Ang I), and it is the initial and rate limiting step in the RAAS cascade [21]. Ang I is then converted to the active octapeptide, Ang II, by the angiotensin converting enzyme (ACE). Ang II interacts with cellular receptors to induce vascular constriction and release of catecholamines from the adrenal medulla and prejunctional nerve endings. It also promotes aldosterone secretion and sodium reabsorption. In addition, Ang II inhibits renin release, providing negative feedback to the system [18, 22]. Ang II increases vascular resistance and BP at various levels (e.g. vasculature, sympathetic nervous system, cortex and medulla of the adrenal gland).
Figure 1.
The renin-angiotensin-aldosterone system. Abbreviations: DRI-direct renin inhibitor, ACE-angiotensin converting enzyme, ACEI-angiotensin converting enzyme inhibitor, AT 1 receptor-angiotensin II type 1 receptor, ARB-angiotensin receptor blocker. Modified from (22); used with permission.
The RAAS may be blocked at various levels along its pathway (Figure. 1). Angiotensin II receptor blockers (ARBs) act on the RAAS by inhibiting the interaction between Ang II and the angiotensin II type 1 receptor (AT1 receptor). ACE inhibitors block the conversion of Ang I to Ang II, and also inhibit the degradation of bradykinin. However, up to 45% of the adult congestive heart failure patients have elevated Ang II levels despite the long-term us of an ACEI [23]. This might reflect insufficient suppression of ACE due to an inadequate dose of ACEI. However, in optimum ACEI dosing, the most obvious explanation is the existence of alternative enzymes for the formation of ang II [24]. Non-ACE mediated pathways (e.g., tissue chymase mediated ang II formation) may become activated when ACE activity is reduced by an ACEI. There are also data suggesting that 30 – 40% of Ang II formation influencing the kidney in the healthy human during RAAS activation is formed via renin-dependent, but ACE-independent pathways [25]. Moreover, short and long-term ACE inhibition results in an accumulation of renin and angiotensin I known as ACE escape which was also demonstrated in the ESCAPE trial in children with chronic kidney disease and this might overcome the ability of an ACEI to effectively suppress ACE activity [26, 27]. Similar mechanism has been discovered for ARBs, the “aldosterone escape”, which leads to normal or even higher than pretreatment levels of aldosterone [28]. Direct renin inhibitors block the RAAS at an earlier stage in the cascade than ACE inhibitors and ARBs, and prevent the formation of both Ang I and Ang II by both ACE and non-ACE pathways [22]. This provides the rationale for the use of renin inhibitors for the treatment of hypertension, cardiovascular and kidney diseases. Animal studies revealed that aliskiren has favorable metabolic profile in patient with metabolic syndrome by improving glucose tolerance and insulin sensitivity [29, 30].
Previous attempts to develop renin inhibitors were limited by poor bioavailability, low renin specificity, and high production costs [31]. Aliskiren is the first in this new class of antihypertensives known as direct renin inhibitors to receive approval from the United States Food and Drug Administration (FDA) on March 6, 2007 for the treatment of hypertension in the adult population. Orally administered aliskiren displays linear pharmacokinetics over the dose range 75-600 mg in the healthy adult volunteers. Aliskiren is excreted primarily unchanged in the feces, with metabolism and renal excretion playing only a minor role, and shows only moderate protein binding (44-51%) [32]. Mean half-life for aliskiren in the healthy adult volunteers is 40 hours.
The safety and efficacy of aliskiren has been well defined through preclinical pharmacological safety studies[33] as well as phase 2 and 3 clinical studies in adults, involving more than 12,000 adult patients with hypertension [34]. Oh and colleagues [35], in a randomized placebo-controlled study involving 672 adults with mild to moderate hypertension, demonstrated that all doses of aliskiren in monotherapy (150, 300, or 600 mg once daily for 8 weeks) gave greater reductions in mean sitting systolic blood pressure and mean sitting diastolic blood pressure by -13.0/-10.3 mm Hg (150 mg), -14.7/-11.1 (300 mg), and -15.8/-12.5 mmHg compared with placebo -3.8/-4.9 mmHg. It was also found from this study that there was no rebound elevation in blood pressure after treatment withdrawal.
Aliskiren with varying doses (37.5, 75, 150, 300 mg once daily) was compared with losartan (100 mg once daily) by Stanton and colleagues [36] in a 4-week randomized double-blind study with 236 mild to moderate hypertensive adults and showed dose-dependent reductions in daytime ambulatory systolic pressures of -0.4 mmHg (37.5 mg), -5.3 mmHg (75 mg), -8.0 mmHg (150 mg) and -11.0 mmHg (300 mg). The change in daytime systolic pressure with 100 mg losartan was not significantly different from the changes seen with 75, 150 and 300 mg aliskiren. All doses of aliskiren also led to significant dose-dependent decreases of PRA between -55 % and -83%, whereas PRA increased by 110% with losartan.
In a randomized, multicenter, double blind, placebo controlled trial [37], 652 adult patients were randomized to receive double-blind treatment with once-daily oral doses of aliskiren (150, 300, or 600 mg), irbesartan 150 mg, or placebo. The study showed that the anti-hypertensive effect of aliskiren 150 mg/day was comparable to that of irbesartan (150 mg/day). However, the treatment with aliskiren 300 and 600 mg daily lowered mean sitting diastolic BP significantly more than irbesartan 150 mg daily. Aliskiren showed safety and tolerability comparable to those of placebo and irbesartan.
Adverse events associated with RAAS inhibition
ACE inhibition and ARBs
ACE-inhibitors and ARB alone or in combination therapy have been associated with the development of hyperkalemia, worsening renal function and hypotension due to the effects of RAAS blockade [38, 39]. Patients with additional risk factors for hyperkalemia such as diabetes and chronic kidney disease are at higher risk. However, these are the patients who are most often in dire need of target organ protection by blocking the deleterious effects of angiotensin II [40]. The other common side effects (dry cough and angioedema) are thought to be related to the inhibition of bradykinin metabolism. As the ARBs do not block the ACE pathway, their use seems to lead to fewer adverse effects related to bradykinin accumulation as compared to ACE inhibitors.
Direct renin inhibition
Direct renin inhibitors in theory seem to share same adverse effects as other RAAS blockers. However in a pooled analysis with more than 7000 adult patients in five placebo-controlled trials, the overall incidence of adverse events associated with 6-8 weeks of aliskiren monotherapy (75-600 mg once daily) was similar to that of placebo [34]. The safety and tolerability were affirmed with another pooled studies in adult women with 1527 patients in five placebo-controlled trials and found that adverse effect profile was same in both groups [41]. The most common adverse events thought to be related to aliskiren treatment were headache, diarrhea, and fatigue. Combination therapy with aliskiren 150 mg or 300 mg once daily and another antihypertensive agent, including an ACE inhibitors or an ARB, did not greatly affect the incidence or type of adverse events associated with respective monotherapy [34]. The rate of cough for patients with hypertension and diabetes who were treated with ramipril alone was 4.7 %, but it was 1.5% for those who treated with the combination of aliskiren and ramipril. The occurrence of hyperkalemia was similar in placebo (0.6%) and patients with aliskiren with different dosing groups (0.6-1%) except group with 600 mg who did not exhibit hyperkalemia. The rate of hyperkalemia was similar in patients with aliskiren monotherapy, placebo, and combination of aliskiren with HCTZ, valsartan, or amlodipine. However, the rate of hyperkalemia was higher, although transient when aliskiren was combined with ramipril (5.5%) than in those treated with ramipril alone (2.6%). Similarly, there were no notable changes in kidney function assessed by serum creatinine concentration or blood urea nitrogen in patients with combination of aliskiren with HCTZ, amlodipine or valsartan compared with the respective monotherapy.
The pharmacokinetics, safety and tolerability of the aliskiren were also assessed in adult patients with varying degrees of renal [42] or hepatic impairment [43]) and compared with those parameters in healthy subjects. The results of these studies concluded that the adjustment of the aliskiren is not required in patients with renal or hepatic impairment; however, according to the package insert, it is recommended to exercise caution in patients treated with aliskiren who have greater than moderate renal dysfunction, a history of dialysis therapy, nephrotic syndrome, or renovascular hypertension due to the paucity of data in these patients and the potential for other agents affecting the RAAS to increase serum creatinine and blood urea nitrogen levels.
RAAS inhibition has also proven benefit in reducing proteinuria [44], one of the most important risk factors for the progression of chronic kidney disease (CKD) and cardiovascular events [45]. The RAAS blockade with double therapy has been reported to be more effective than monotherapy [46]. The data on proteinuria reduction in adult CKD patients with aliskiren treatment are promising [47]. Patients with hypertension and diabetes, with and without microalbuminuria, have gained renoprotective benefits through inhibition of the RAAS by treatment with ACE inhibitors or ARBs [48]. According to the AVOID study[49], researchers found that treatment with 300 mg of aliskiren daily, as compared with placebo, reduced the mean urinary albumin-to-creatinine ratio by 20%, with a reduction of 50% or more in 24.7% of the patients who received aliskiren as compared with 12.5% of those who received placebo. Although dual RAAS blockade theoretically/physiologically seems to be a promising therapy in hypertension and chronic kidney disease, no data from large adult trials substantiate such a benefit [50]. The results of ALTITIUDE study in adults clearly show that dual blockade with aliskiren and an ACE inhibitor or ARB has no demonstrable clinical advantage and may be harmful in type 2 diabetes with chronic kidney disease [50]. Like ALTITUDE, other studies in adults have found that dual RAAS blockade causes such adverse events as hypotension, hyperkalemia and worsening of renal function [51, 52]. As a result, in December 2011, Novartis had to halt a clinical trial of the aliskiren after discovering increased incidence of nonfatal stroke, renal complications, hyperkalemia, and hypotension in patients with diabetes and renal impairment (ALTITUDE Trial).
There is paucity of data regarding the safety, efficacy and pharmacokinetics (PK) of aliskiren in the pediatric population and only few studies in the literature, according to our knowledge, have discussed these in children. According to one prospective, 8-day, randomized, multi-dose study across 5 countries, using once daily dose of aliskiren at 2 mg/kg and 6 mg/kg was well tolerated in pediatric patients with hypertension [17]. The PK parameters of aliskiren in pediatric patients aged 6-17 years are consistent with those observed in the adult population. The clinically meaningful BP reductions were observed with both doses of aliskiren and in 6-11 years the overall mean reduction from baseline to the end of treatment was -6.1/-0.8 mm Hg and in 12- to 17 year age group was -7.7/-5.6 mm Hg. Only 3 patients (7.7%) had adverse events (AEs) that were suspected to be related to the study drug (nausea in 2 patients, headache in 1 patient, and diarrhea in 1 patient). There were no AEs or abnormal lab values for potassium, creatinine or blood urea nitrogen leading to discontinuation from the study. Aliskiren treatment also resulted in substantial decrease in PRA.
Few case series have also retrospectively reviewed the safety and efficacy of the aliskiren when used in combination with other RAAS blockers. In one case series, aliskiren was used in combination with losartan ± ACEIs, in 4 patients (5-18 years) with varying degree of chronic kidney diseases and proteinuria [53]. The authors retrospectively reviewed the medical records of these patients and found that aliskiren was added to losartan ± ACEI (two patients were also on ACEI so triple RAAS blockade) for the refractory proteinuria after insufficient response with ARBs ± ACEIs. Although there was a remarkable reduction in proteinuria (at least > 45%), significant side effects in 3 out of 4 patients were noted, and 2 of the patients had the aliskiren discontinued due to hyperkalemia and worsening of renal function, and one patient had dose change due to symptomatic hypotension. One patient had creatinine returned to baseline after discontinuation of aliskiren. However the other patient with moderate CKD (GFR 32 mL/min/1.72m2) had acute worsening of renal function and required hemodialysis 3.5 weeks later. The other case series is an e-mail survey of the PEDHTN and PEDNEPH list which identified 10 patients with off-label use of aliskiren [54]. It was prescribed for hypertension and/or proteinuria. Hyperkalemia, angioedema and hypotension were the major side effects. These limited data suggest that aliskiren should be used with caution, especially when using in combination with other blockers of RAAS axis.
Pediatric Trial
There are currently ongoing clinical trials being done by Novartis that include a prospective double-blinded, randomized placebo-controlled trial of aliskiren in the pediatric population with mild to moderate hypertension (CSPP100A2365). This trial is designed to assess efficacy and safety of aliskiren in the pediatric population. The other trials are a multicenter, double-blind, randomized, 52 week extension study to evaluate the long term safety, tolerability and efficacy of aliskiren compared to enalapril in pediatric hypertensive patients 6-17 years of age (CSPP100A2365E1), and a multicenter, 52 to 104 week extension study to evaluate the long-term growth and development of pediatric hypertensive patients 6-17 years of age treated previously with aliskiren (CSPP100A2365E2).
Conclusion
Although data from the adult studies regarding safety and efficacy of the aliskiren seems very promising, caution should be exercised with its use, especially in combination with other RAAS blockers until we have further data regarding its pharmacokinetics, safety and efficacy in the pediatric population from ongoing clinical trials.
Questions (answers are provided following the reference list)
- The mechanism of action of aliskiren to improve BP control includes:
- Direct action on peripheral vasculature
- Centrally-mediated vasodilatation
- Decreased formation of Angiotensin I and Angiotensin II
- Effects on calcium channels
- Side effects that may be anticipated in the use of aliskiren include:
- Cough
- Angioedema
- Elevated transaminases
- Hyperkalemia
- The true statement about pediatric hypertension is:
- Aliskiren has the safest antihypertensive medication side effect profile published to date.
- Pediatric hypertension is generally defined statistically as a sustained level of BP that falls consistently >95th percentile for age, gender and stature.
- All pediatric patients diagnosed with hypertension must be managed with antihypertensive medications as soon as the diagnosis is confirmed.
- Children will likely outgrow hypertension by the time they finish puberty.
- Before the development of aliskiren, reasons that limited availability of compounds that act as a direct renin inhibitors included all but:
- Serious side effect profiles.
- Poor bioavailability.
- High production costs.
- Low renin specificity.
- A true statement about renin is:
- It is secreted by the liver when there is inflammation.
- It cleaves the substrate angiotensinogen to form Angiotensin I.
- It is produced by the kidney only in Chronic Kidney Disease (CKD) Stage 4 or higher.
- It is produced by osteoblasts.
- The antihypertensive classes that may influence the RAAS include all but:
- Angiotensin-converting enzyme inhibitors
- Angiotensin receptor blockers
- Beta blockers
- Centrally acting agents
- Aldosterone inhibitors
Answers:
C
D
B
A
B
D
References
- 1.Erlingsdottir A, Indridason OS, Thorvaldsson O, Edvardsson VO. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323–8. doi: 10.1007/s00467-009-1350-3. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. Erratum in: Lancet. 2003 361:1060. [DOI] [PubMed] [Google Scholar]
- 6.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291:2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- 8.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113(3 Pt 1):475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 9.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 10.Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, Kuznetsova T, Laurent S, Mancia G, Morales-Olivas F, Rascher W, Redon J, Schaefer F, Seeman T, Stergiou G, Wühl E, Zanchetti A Sociedad Europea de Hipertensión. An Pediatr (Barc) Vol. 73. Spanish: 2010. Management of high blood pressure in children and adolescents: Recommendations of the European Society of hypertension; pp. 51pp. e1–28. [DOI] [PubMed] [Google Scholar]
- 11.Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40–41. doi: 10.1001/jama.2013.6550. [DOI] [PubMed] [Google Scholar]
- 12.Fishman PA, Anderson ML, Cook AJ, Ralston JD, Catz SL, Carlson J, Larson EB, Green BB. Accuracy of blood pressure measurements reported in an electronic medical record during routine primary care visits. J Clin Hypertens (Greenwich) 2011;13:821–828. doi: 10.1111/j.1751-7176.2011.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhuri A. Pediatric ambulatory blood pressure monitoring: diagnosis of hypertension. Pediatr Nephrol. 2013;28:995–999. doi: 10.1007/s00467-013-2470-3. [DOI] [PubMed] [Google Scholar]
- 14.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med. 2011;154:781–788. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 15.Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, Kerby TJ, Klotzle KJ, Maciosek MV, Michels RD, O'Connor PJ, Pritchard RA, Sekenski JL, Sperl-Hillen JM, Trower NK. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr Nephrol. 2013;28:1059–1066. doi: 10.1007/s00467-012-2344-0. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan JE, Keefe D, Zhou Y, Satlin L, Fang H, Yan JH. Pharmacokinetics, safety profile, and efficacy of aliskiren in pediatric patients with hypertension. Clin Pediatr (Phila) 2013;52:599–607. doi: 10.1177/0009922813483875. [DOI] [PubMed] [Google Scholar]
- 18.Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going? J Hypertens. 2006;24:243–156. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- 19.Simões E, Silva AC, Flynn JT. The renin-angiotensin-aldosterone system in 2011: role in hypertension and chronic kidney disease. Pediatr Nephrol. 2012;27:1835–1845. doi: 10.1007/s00467-011-2002-y. [DOI] [PubMed] [Google Scholar]
- 20.Skeggs LT, Dorer FE, Kahn JR, Lentz KE, Levine M. The biochemistry of the renin-angiotensin system and its role in hypertension. Am J Med. 1976;60:737–748. doi: 10.1016/0002-9343(76)90888-3. [DOI] [PubMed] [Google Scholar]
- 21.Skeggs LT, Jr, Kahn JR, Lentz K, Shumway NP. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957;106:439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segall L, Covic A, Goldsmith DJ. Direct renin inhibitors: the dawn of a new era, or just a variation on a theme? Nephrol Dial Transplant. 2007;22:2435–2439. doi: 10.1093/ndt/gfm363. [DOI] [PubMed] [Google Scholar]
- 23.van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, van Gilst WH, Voors AA. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367–372. doi: 10.1016/j.ijcard.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, Ideishi M, Arakawa K. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–1405. doi: 10.1161/01.hyp.33.6.1399. [DOI] [PubMed] [Google Scholar]
- 25.Hollenberg NK. Pharmacologic interruption of the renin-angiotensin system and the kidney: differential responses to angiotensin-converting enzyme and renin inhibition. J Am Soc Nephrol. 1999;10(11):S239–S242. [PubMed] [Google Scholar]
- 26.ESCAPE Trial Group. Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 27.Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis A. Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution? Expert Opin Pharmacother. 2007;8:529–535. doi: 10.1517/14656566.8.5.529. [DOI] [PubMed] [Google Scholar]
- 28.McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, Tsuyuki RT, White M, Rouleau J, Latini R, Maggioni A, Young J, Pogue J The RESOLVD Pilot Study Investigators. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. Circulation. 1999;100:1056–1064. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- 29.Marchionne EM, Diamond-Stanic MK, Prasonnarong M, Henriksen EJ. Chronic renin inhibition with aliskiren improves glucose tolerance, insulin sensitivity, and skeletal muscle glucose transport activity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R137–142. doi: 10.1152/ajpregu.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou CL, Lai YH, Lin TY, Lee TJ, Fang TC. Aliskiren prevents and ameliorates metabolic syndrome in fructose-fed rats. Arch Med Sci. 2011;7:882–888. doi: 10.5114/aoms.2011.25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao C, Vaidyanathan S, Yeh CM, Maboudian M, Armin Dieterich H. Aliskiren exhibits similar pharmacokinetics in healthy volunteers and patients with type 2 diabetes mellitus. Clin Pharmacokinet. 2006;45:1125–1134. doi: 10.2165/00003088-200645110-00006. [DOI] [PubMed] [Google Scholar]
- 33.Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia. 2007;50:2398–2404. doi: 10.1007/s00125-007-0795-9. [DOI] [PubMed] [Google Scholar]
- 34.Weir MR, Bush C, Anderson DR, Zhang J, Keefe D, Satlin A. Antihypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren in patients with hypertension: a pooled analysis. J Am Soc Hypertens. 2007;1:264–277. doi: 10.1016/j.jash.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Oh BH. Aliskiren, the first in a new class of direct renin inhibitors for hypertension: present and future perspectives. Expert Opin Pharmacother. 2007;8:2839–2849. doi: 10.1517/14656566.8.16.2839. [DOI] [PubMed] [Google Scholar]
- 36.Stanton A, Jensen C, Nussberger J, O'Brien E. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42:1137–1143. doi: 10.1161/01.HYP.0000101688.17370.87. [DOI] [PubMed] [Google Scholar]
- 37.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 38.Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007;167:1930–1936. doi: 10.1001/archinte.167.18.1930. [DOI] [PubMed] [Google Scholar]
- 39.Düsing R, Sellers F. ACE inhibitors, angiotensin receptor blockers and direct renin inhibitors in combination: a review of their role after the ONTARGET trial. Curr Med Res Opin. 2009;25:2287–2301. doi: 10.1185/03007990903152045. [DOI] [PubMed] [Google Scholar]
- 40.Rastogi A, Rashid M, Wright RF. Reducing cardiorenal risk through combination therapy with a direct renin inhibitor. J Clin Hypertens. 2011;13:848–855. doi: 10.1111/j.1751-7176.2011.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gradman AH, Weir MR, Wright M, Bush CA, Keefe DL. Efficacy, safety and tolerability of aliskiren, a direct renin inhibitor, in women with hypertension: a pooled analysis of eight studies. J Hum Hypertens. 2010;24:721–729. doi: 10.1038/jhh.2010.11. [DOI] [PubMed] [Google Scholar]
- 42.Vaidyanathan S, Bigler H, Yeh C, Bizot MN, Dieterich HA, Howard D, Dole WP. Pharmacokinetics of the oral direct renin inhibitor aliskiren alone and in combination with irbesartan in renal impairment. Clin Pharmacokinet. 2007;46:661–675. doi: 10.2165/00003088-200746080-00003. [DOI] [PubMed] [Google Scholar]
- 43.Vaidyanathan S, Warren V, Yeh C, Bizot MN, Dieterich HA, Dole WP. Pharmacokinetics, safety, and tolerability of the oral Renin inhibitor aliskiren in patients with hepatic impairment. J Clin Pharmacol. 2007;47:192–200. doi: 10.1177/0091270006294404. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharjee R, Filler G. Additive antiproteinuric effect of ACE inhibitor and losartan in IgA nephropathy. Pediatr Nephrol. 2002;17:302–304. doi: 10.1007/s00467-002-0829-y. [DOI] [PubMed] [Google Scholar]
- 45.Toto RD. Aldosterone blockade in chronic kidney disease: can it improve outcome? Curr Opin Nephrol Hypertens. 2010;19:444–449. doi: 10.1097/MNH.0b013e32833ce6d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobsen P, Andersen S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol. 2003;14:992–999. doi: 10.1097/01.asn.0000054495.96193.bf. [DOI] [PubMed] [Google Scholar]
- 47.Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CD, Schalkwijk C, Danser AH, Boomsma F, Frandsen E, Parving HH. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care. 2009;32:1873–1879. doi: 10.2337/dc09-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 49.Riche DM, Minor DS, Holdiness AS, East HE. An issue of dependence: implications from the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) trial. J Clin Hypertens. 2009;11:89–93. doi: 10.1111/j.1751-7176.2008.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Leeuw PW. ACP Journal Club. Aliskiren increased adverse events in patients with diabetes and kidney disease who were receiving ACE inhibitors or ARBs. Ann Intern Med. 2013;158:JC7. doi: 10.7326/0003-4819-158-6-201303190-02007. [DOI] [PubMed] [Google Scholar]
- 51.Harel Z, Gilbert C, Wald R, Bell C, Perl J, Juurlink D, Beyene J, Shah PS. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ. 2012;344:e42. doi: 10.1136/bmj.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 53.Kelland EE, McAuley LM, Filler G. Are we ready to use aliskiren in children? Pediatr Nephrol. 2011;26:473–477. doi: 10.1007/s00467-010-1702-z. [DOI] [PubMed] [Google Scholar]
- 54.Flynn J. Not ready for prime time: aliskiren for treatment of hypertension or proteinuria in children. Pediatr Nephrol. 2011;26:491–492. doi: 10.1007/s00467-010-1726-4. [DOI] [PubMed] [Google Scholar]