Abstract
Background & Aim
Series studies have associated increased serum levels of ferritin with liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). We aimed to determine the accuracy with which measurements of serum ferritin determine the presence and severity of liver fibrosis, and whether combining noninvasive scoring systems with serum ferritin analysis increases the accuracy of diagnosis of advanced liver fibrosis.
Methods
We performed a retrospective analysis of data from 1014 patients with liver biopsy-confirmed NAFLD. Three cut-points of serum ferritin level, adjusted for sex, were established based on receiver operating characteristics curve analysis: 1.0 (the upper limit of normal [ULN]), 1.5-fold ULN, and 2.0-fold ULN. Three multiple logistic regression models were created to determine the association of these cutoff values with liver fibrosis, adjusting for age, sex, race, diabetes, body mass index, and level of alanine aminotransferase.
Results
A greater proportion of patients with increased serum levels of ferritin had definitive NASH and more-advanced fibrosis than patients without increased levels. In all models, serum level of ferritin was significantly associated with the presence and severity of liver fibrosis. However, for all 3 cutoff values, area under the receiver operating characteristic curve values were low (less than 0.60) for the presence of fibrosis or any stage of liver fibrosis; ferritin level identified patients with fibrosis with 16%–41% sensitivity and 70%–92% specificity. The accuracy with which noninvasive scoring systems identified patients with advanced fibrosis did not change with inclusion of serum ferritin values.
Conclusions
Although serum levels of ferritin correlate with more-severe liver fibrosis, based on adjusted multiple logistic regression analysis, serum ferritin levels alone have a low level of diagnostic accuracy for the presence or severity of liver fibrosis in patients with NAFLD.
Keywords: iron, ROC, BMI, ALT, cirrhosis
Serum ferritin is routinely measured in patients with nonalcoholic fatty liver disease (NAFLD) as part of the laboratory work-up to rule out other causes of liver disease. Ferritin levels are often elevated in patients with NAFLD with early large series [1,2] reporting increased ferritin in about a half of NAFLD patients. The relationship of serum ferritin with severity of liver disease in NAFLD has been examined in several studies. The largest series found a significant association of ferritin levels with presence and severity of nonalcoholic steatohepatitis (NASH) and liver fibrosis [3–9]. For instance, a large Italian series [8] reported a 1.67-fold greater likelihood for advanced fibrosis in patients with NAFLD with increased serum ferritin levels; similarly, in a recent American series [9], a serum ferritin level above 1.5 times the upper limit of normal was associated with a 1.66-fold higher likelihood of having advanced fibrosis.
Based on this, it has been proposed that serum ferritin levels could potentially be used to predict presence and severity of liver fibrosis in patients with NAFLD. However, the evidence associating elevated serum ferritin with severity of liver fibrosis in NAFLD comes, at the best, from the results of multiple logistic regression analyses. The accuracy of serum ferritin levels in diagnosing presence and severity of liver fibrosis has not been formally evaluated; furthermore, it remains uncertain whether adding serum ferritin levels to the several non-invasive scoring systems used routinely in predicting the severity of liver fibrosis increases the accuracy of the scoring systems. To deal with these issues we analyzed a large database of patients with well-characterized and liver biopsy-confirmed NAFLD to 1) determine the accuracy of serum ferritin levels in identifying presence and severity of liver fibrosis, and 2) to determine whether the accuracy of several non-invasive scoring systems increases in identifying advanced liver fibrosis by adding serum ferritin levels.
PATIENTS AND METHODS
This is an international, retrospective cohort study of 1,014 patients with well-characterized and liver biopsy-confirmed NAFLD. They were untreated, consecutively biopsied patients that came from one of the following four medical institutions: Newcastle University, United Kingdom, University of Sydney, Australia, University of Torino, Italy, and University of Kentucky, United States. Inclusion criteria was the diagnosis of NAFLD confirmed by liver biopsy, and the liver biopsy represents the reference standard. Liver biopsies were done regardless of the ferritin level or fibrosis scores. Exclusion criteria was a liver disease of other etiology such as alcohol-induced or drug-induced liver disease, autoimmune or viral hepatitis, and cholestatic or metabolic/genetic liver disease. These other liver diseases were excluded using specific clinical, laboratory, radiographic, and/or histological criteria. All patients had a negative history of alcohol abuse as indicated by a weekly ethanol consumption of <140 g in women, and <210 g in men. History of alcohol consumption was specifically investigated by interviewing the patients and in many cases also by interviewing close relatives during both the first and follow-up visits.
Extensive clinical and laboratory data were collected within seven days of the liver biopsy procedure. The ethnicity and race were determined based on the categories proposed by the United States Department of Health and Human Services Public Health Service [10]. Body mass index (BMI) and waist circumference were measured. Laboratory evaluation included routine liver biochemistry; complete blood count; lipid profile; fasting glucose; fasting insulin; iron studies (serum iron, serum ferritin, serum transferrin, transferrin saturation, total iron-binding capacity [TIBC]); HFE gene mutation; viral serology for hepatitis B and C infection; autoantibodies; alpha 1 antitrypsin levels and phenotype; and ceruloplasmin levels. The degree of insulin resistance was determined by the homeostatic model assessment (HOMA) [11]. Components of the metabolic syndrome [12] were recorded including central obesity, hyperglycemia or previously diagnosed type 2 diabetes, hypertriglyceridemia, hypertension, and low HDL-cholesterol. The presence of diabetes mellitus (fasting glucose ≥126 mg/dL or treatment with antidiabetic drugs), obesity (BMI ≥30 kg/m2, or ≥25 kg/m2 in Asians), and overweight (BMI 25–29.9 kg/m2, or 23–24.9 kg/m2 in Asians) was also recorded.
Serum ferritin levels were measured by enzyme-linked immunosorbent assays (ELISA) or enzyme immunoassays as recommended by the World Health Organization [13]. The upper limit of normal (ULN) for serum ferritin used for comparisons was adopted from the hemochromatosis and iron overload screening study, that is, 300 ng/mL in men and 200 ng/mL in women [14].
Four validated non-invasive scoring systems originally created to distinguish between patients with and without advanced (stage 3–4) fibrosis were calculated using the original reported formulas [15–18]. They were the NAFLD fibrosis score, formula: ; the AST/platelet ratio index, formula: ; the FIB-4 score, formula: ; the BARD score, scale 0–4: BMI ≥ 28 kg/m2 = 1 point, AST to ALT ratio ≥ 0.8 = 2 points; diabetes mellitus = 1 point. The values for the ULN for AST were set according to the International Federation of Clinical Chemistry, that is, 35 U/L for men, and 30 U/L for women. The ULN for ALT was 19 U/L in women, and 30 U/L in men [19].
Liver Histology
Liver biopsies were routinely stained with hematoxylin and eosin, Masson’s trichrome, and special stains for iron and copper. Liver biopsies were read by a single liver pathologist in each participating center who was not always blind to laboratory tests results including ferritin levels. The stage of fibrosis was scored based on a 5-point scale as proposed [20]. Briefly, stage 0 = absence of fibrosis; stage 1 = perisinusoidal or portal; stage 2 = perisinusoidal and portal/periportal; stage 3 = septal or bridging fibrosis; and stage 4 = cirrhosis. Advanced fibrosis was defined as stage 3–4 fibrosis. The grade of steatosis, inflammation and cellular ballooning were scored as proposed [20]. Presence of NASH was also recorded and categorized as definitive, borderline/suspicious, or no NASH based on pattern and distribution of liver histological lesions as proposed [21]. Semiquantitative grading (0 – 3+) of hepatic iron staining using Perls’ iron stain was recorded. To control for biopsy size, the length of the biopsy was measured with a hand ruler, and the number of portal areas on one cross-section was counted. The mean (±SD) length of the liver biopsy was 19 ± 8.5 mm (median 18 mm, interquartile range 15, 25). The number of portal areas was 11 ± 4.5 (median 10, interquartile range 7, 16).
Statistical Analysis
Baseline characteristics were compared by ferritin level status (normal vs. elevated) using a t test or ANOVA test when appropriate for continuous variables or a Chi-squared test for categorical variables. Standard non parametric tests were used to analyze variables without a normal distribution. The independent association of serum ferritin levels with increased liver fibrosis was evaluated by multiple logistic regression analysis using the forward stepwise selection method with a p value < 0.1 chosen for variable selection. First, we determined the area under the receiver operating characteristic (ROC) curves of serum ferritin to identify the most accurate cut-off of ferritin to distinguish between patients with and without advanced (stage 3–4) fibrosis. The most accurate cut-off points were ferritin from 1.0 x ULN to 1.7 x ULN. For these cut-offs, the sensitivity − (1−specificity) varied from 0.11 to 0.12. Thus, any cut-off value of ferritin from 1.0 to 1.7 x ULN would essentially provide the same results. For the purpose of simplicity for potential readers and in order to be able to compare the results of our study with prior similar studies [8,9,14] we established three multiple logistic regression models with each of the three dichotomized levels: 1.0, 1.5 and 2.0 x ULN which represent the most accurate range of ferritin cut-off points to distinguish between patients with and without increased or advanced fibrosis. The association of serum ferritin levels with liver fibrosis was adjusted by the following variables selected a priori and included in each multiple logistic regression model: age, sex, race, BMI, diabetes, ALT and site. The diagnostic accuracy of these three cut-points of serum ferritin to distinguish between patients with and without increased or advanced fibrosis was investigated by determining the area under the ROC curves, sensitivity and specificity. Subsequently, we used ROC curves analysis to determine the effect size of serum ferritin values when added to simple noninvasive scoring systems [15–18] to distinguish between patients with and without advanced liver fibrosis. Ferritin was tested in models including composite variables of score and not the score itself. This was done to determine the independent value of ferritin (i.e., the additive variable) without having fixed regression coefficients of the other variables. All tests were two-tailed and a p value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using IBM SPSS Statistics version 21, and SigmaPlot 12.3 software. The study was approved by appropriate regulatory bodies at all centers.
RESULTS
Baseline characteristics
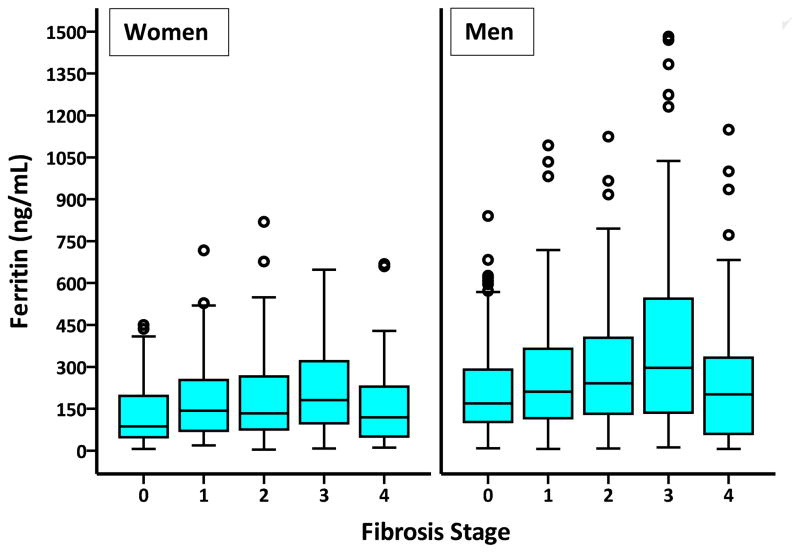
Patients were recruited from 2003 to 2011. There were no adverse events related to the reference standard (liver biopsy) or index test (ferritin measurement). The prevalence of serum ferritin >ULN was 33% [331/1,014 (189 men vs. 142 women, p = 0.8)], >1.5-fold ULN was 19% [189/1,014 (106 men vs. 83 women, p = 0.6)], and >2-fold ULN was 10% [103/1,014 (55 men vs. 48 women, p = 0.3)]. The comparison of patient with normal and elevated serum ferritin levels is described in Table 1. Patients with elevated serum ferritin levels were more likely to have the histological diagnosis of definitive NASH (45.9% vs. 34.8%, respectively, p < 0.001), and advanced (stage 3–4) liver fibrosis (33.3% vs. 23.5% respectively, p = 0.001) (Table 1; Figure 1).
Table 1.
Clinical and demographic characteristics of the patient population
| Variable | Total (n = 1,014) | Ferritin level
|
P value | |
|---|---|---|---|---|
| Normal (n = 683) | Elevated (n = 331) | |||
| Age (years) | 46.9 ± 0.4 | 46.5 ± 0.5 | 47.6 ± 0.7 | 0.2 |
| Gender | ||||
| Female | 428 | 286 (42%) | 142 (43%) | 0.8 |
| Male | 586 | 397 (58%) | 189 (57%) | |
| Ethnic group | ||||
| Hispanic | 6 | 4 (0.6%) | 2 (0.6%) | 0.97 |
| Non-Hispanic | 1,008 | 679 (99.4%) | 329 (99.4%) | |
| Race group | 0.002 | |||
| White | 929 | 641 (93.9%) | 288 (87%) | |
| Asian | 61 | 29 (4.2%) | 32 (9.7%) | |
| Black, or African American | 7 | 5 (0.7%) | 2 (0.6%) | |
| American Indian/Alaska Native | 2 | 0 (0%) | 2 (0.6%) | |
| Native Hawaiian or Other Pacific Islander | 15 | 8 (1.2%) | 7 (2.1%) | |
| Body mass index (kg/m2) | 31.3 ± 0.2 | 31.6 ± 0.2 | 30.9 ± 0.3 | 0.09 |
| BMI category | 0.2 | |||
| Normal | 111 | 76 (11.1%) | 35 (10.6%) | |
| Overweight | 333 | 229 (33.5%) | 104 (31.4%) | |
| Obese | 570 | 378 (53.3%) | 192 (58%) | |
| Waist circumference (cm) | 100.9 ± 0.5 | 102 ± 0.6 | 99 ± 0.8 | 0.02 |
| Central obesity (yes) | 599 | 431 (63%) | 168 (51%) | 0.2 |
| Diabetes (yes) | 298 | 188 (27.5%) | 110 (33.2%) | 0.07 |
| Hypertension (yes) | 363 | 231 (33.8%) | 132 (39.9%) | 0.06 |
| Hypertriglyceridemia (yes) | 542 | 361 (52.9%) | 181 (54.7%) | 0.6 |
| Hypercholesterolemia (yes) | 447 | 308 (45.1%) | 139 (42%) | 0.4 |
| Low-HDL cholesterol (yes) | 413 | 264 (38.7%) | 149 (45%) | 0.05 |
| Metabolic syndrome (yes) | 384 | 258 (37.8%) | 126 (38%) | 0.2 |
| ALT (IU/L) | 81 ± 2 | 72 ± 2 | 101 ± 4 | < 0.001 |
| AST (IU/L) | 55 ± 1 | 47 ± 1 | 70 ± 3 | < 0.001 |
| AST/ALT ratio | 0.8 ± .02 | 0.7 ± 0.02 | 0.8 ± 0.05 | 0.1 |
| Total bilirubin (mg/dL) | 0.9 ± 0.03 | 0.8 ± 0.02 | 0.9 ± 0.05 | 0.04 |
| Albumin (g/dL) | 4.4 ± 0.02 | 4.4 ± 0.02 | 4.4 ± 0.03 | 0.2 |
| Alkaline phosphatase (IU/L) | 128 ± 3 | 125 ± 3 | 134 ± 5 | 0.1 |
| GGT (IU/L) | 103 ± 5 | 100 ± 5 | 110 ± 10 | 0.3 |
| Platelet (×109) | 237 ± 3 | 240 ± 3 | 231 ± 5 | 0.1 |
| Glucose (mg/dL) | 114 ± 2 | 115 ± 3 | 210 ± 2 | 0.6 |
| Insulin (μIU/L) | 19 ± 1 | 19 ± 1 | 21 ± 2 | 0.3 |
| HOMA | 5 ± 0.2 | 4.7 ± 0.2 | 5.5 ± 0.4 | 0.05 |
| Triglycerides (mg/dL) | 194 ± 4 | 193 ± 5 | 196 ± 8 | 0.7 |
| Total Cholesterol (mg/dL) | 209 ± 2 | 210 ± 2 | 209 ± 3 | 0.7 |
| HDL-cholesterol (mg/dL) | 46 ± 0.4 | 46 ± 1 | 45 ± 1 | 0.4 |
| LDL-cholesterol (mg/dL) | 139 ± 2 | 142 ± 2 | 133 ± 3 | 0.03 |
| Iron (μg/dL) | 102 ± 2 | 95 ± 3 | 114 ± 3 | < 0.001 |
| Ferritin (ng/mL) | 252 ± 8 | 122 ± 3 | 521 ± 17 | < 0.001 |
| Transferrin (mg/dL) | 274 ± 3 | 285 ± 4 | 258 ± 4 | < 0.001 |
| TIBC (μg/dL) | 316 ± 3 | 320 ± 3 | 309 ± 4 | < 0.04 |
| Transferrin saturation (%) | 29 ± 0.5 | 26 ± 1 | 34 ± 1 | < 0.001 |
| HFE gene mutation | 704 | 0.99 | ||
| WT/WT | 492 | 300 (43.9%) | 192 (58%) | |
| C282Y/WT | 54 | 32 (4.7%) | 22 (6.6%) | |
| H63D/WT | 128 | 82 (12%) | 46 (13.9%) | |
| H63D/H63D | 20 | 12 (1.8%) | 8 (2.4%) | |
| C282Y/H63D | 10 | 6 (0.9%) | 4 (1.2%) | |
| NASH category | 0.003 | |||
| No NASH | 495 | 353 (51.7%) | 142 (42.9%) | |
| Suspicious/borderline | 129 | 92 (13.5%) | 37 (11.2%) | |
| Definitive | 390 | 238 (34.8%) | 152 (45.9%) | |
| Fibrosis stage | < 0.001 | |||
| 0 | 351 | 267 (39.1%) | 84 (25.4%) | |
| 1 | 251 | 165 (24.2%) | 86 (26%) | |
| 2 | 141 | 90 (13.2%) | 51 (15.4%) | |
| 3 | 161 | 85 (12.4%) | 76 (23%) | |
| 4 | 110 | 76 (11.1%) | 34 (10.3%) | |
Data are presented as mean ± standard error, or number (proportion)of patients with a condition. ALT, alanine aminotransferases; AST, aspartate aminotransferases; GGT, gamma-glutamyl transferase; HOMA, homeostatic model assessment; HDL, high density lipoprotein; LDL, low density lipoprotein; TIBC, total iron-binding capacity; NASH, nonalcoholic steatohepatitis.
FIGURE 1.
Boxplot illustration of the relationship of serum ferritin levels and fibrosis stage in women (p = 0.003) and men (p < 0.001).
Serum ferritin levels adjusted by sex did not differ significantly between patients who suffered from the metabolic syndrome and those who did not (p = 0.18), or among number (0 to 5) of components of the metabolic syndrome (p = 0.14). There was no a significant difference in the proportion of patients carrying one or two HFE gene mutations between patients with normal or elevated serum ferritin. Patients with elevated serum ferritin had more hepatic iron as indicated by a positive Perls’ staining (57% vs. 26% respectively, p < 0.001); and Perls’ staining intensity was significantly greater in patients with definitive NASH (p = 0.04). The amount of hepatic iron as indicated by Perls’ staining was not significantly different between patients with or without liver fibrosis (p = 0.9) or among the different stages of fibrosis (p = 0.2).
Association of serum ferritin with liver fibrosis
By multiple logistic regression the three cut-points of elevated serum ferritin levels increased the likelihood of having increased liver fibrosis, significant fibrosis, or advanced fibrosis. The odds ratio progressively increased as the serum ferritin cut-point increased for either presence of fibrosis or severity of fibrosis (Table 2).
Table 2.
Multiple logistic regression analyses of the association of ferritin levels and liver fibrosis.
| Variable | Presence of fibrosis (stage 1–4) | Severe fibrosis (stage 2–4) | Advanced fibrosis (stage 3–4) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
|
| |||||||||
| Model 1 | |||||||||
| Ferritin, normal (reference) | 1 | 1 | 1 | ||||||
| Ferritin > x ULN | 1.84 | 1.36, 2.50 | <0.001 | 1.64 | 1.22, 2.19 | 0.001 | 1.61 | 1.17, 2.18 | 0.004 |
|
| |||||||||
| Model 2 | |||||||||
| Ferritin ≤ x 1.5 ULN (reference) | 1 | 1 | 1 | ||||||
| Ferritin > x 1.5 ULN | 2.14 | 1.45, 3.15 | <0.001 | 1.95 | 1.38, 2.75 | <0.001 | 1.95 | 1.34, 2.82 | <0.001 |
|
| |||||||||
| Model 3 | |||||||||
| Ferritin ≤ x 2 ULN (reference) | 1 | 1 | 1 | ||||||
| Ferritin > x 2 ULN | 2.52 | 1.45, 4.41 | 0.001 | 2.02 | 1.30, 3.14 | 0.002 | 2.11 | 1.33, 3.34 | 0.001 |
All multiple logistic regression models include age, gender, race, diabetes, BMI, ALT and site.
Diagnostic accuracy of serum ferritin levels
The overall accuracy of serum ferritin levels to diagnose any stage or combination of stages of liver fibrosis was rather poor as indicated by an area under the ROC curves below 0.60 for any serum ferritin cut-point analyzed (Table 3). Similarly, the sensitivity of these serum ferritin cut-points was low and between 13% and 41% whereas the specificity was 70% to 95% (Table 3). Additional ROC curves were created considering serum ferritin as continuous variable and ordinal variable; the area under the ROC curves were equally poor and less than 0.60 similar to when considering ferritin as a binary variable (data not shown).
Table 3.
Diagnostic accuracy of several levels of serum ferritin in staging fibrosis (n = 1,014)
| Fibrosis stage | Ferritin > ULN (n = 331) | Ferritin > 1.5 x ULN (n = 189) | Ferritin > 2.0 x ULN (n = 103) |
|---|---|---|---|
| AUROC (95% CI) Sensitivity (%), Specificity (%) |
AUROC (95% CI) Sensitivity (%), Specificity (%) |
AUROC (95% CI) Sensitivity (%), Specificity (%) |
|
| 0 vs. 1/2/3/4 | 0.57 (0.53, 0.60) 37, 76 |
0.55 (0.52, 0.59) 22, 89 |
0.54 (0.50, 0.58) 13, 95 |
| 0/1 vs. 2/3/4 | 0.55 (0.52, 0.59) 39, 72 |
0.55 (0.52, 0.59) 25, 86 |
0.53 (0.50, 0.57) 14, 93 |
| 0/1/2 vs. 3/4 | 0.55 (0.51, 0.59) 41, 70 |
0.56 (0.52, 0.60) 27, 84 |
0.54 (0.50, 0.58) 16, 92 |
ULN, upper limit of normal; AUROC, area under the receiver operating characteristic curve.
Table 4 shows the area under the ROC curve to distinguish between patients with and without advanced (stage 3–4) fibrosis for the four noninvasive scoring systems alone and when combined with elevated serum ferritin. The accuracy of these scoring systems remained essentially the same when serum ferritin values were added. Supplemental Figure 1 illustrates the area under the ROC curves for these four scoring systems alone and the three cut-points alone of serum ferritin to distinguish between patients with and without advanced (stage 3–4) fibrosis. When serum ferritin levels were used to reclassify patients that were in the indeterminate range based on the NAFLD fibrosis score, APRI and FIB-4 score, the proportion of patients that would be correctly reclassified was pretty similar to the proportion of patients that would be erroneously reclassified (data not shown).
Table 4.
Diagnosis accuracy of simple scoring systems to differentiate between patients with and without advanced (stage 3–4) fibrosis
| Area under the ROC curve (95% confidence intervals) [p value for the comparison with the score alone) |
||||
|---|---|---|---|---|
| Score | Plus Ferritin > ULN | Plus Ferritin > 1.5 x ULN | Plus Ferritin > 2 x ULN | |
| NAFLD-FS | 0.83 (0.79, 0.86) | 0.84 (0.80, 0.88) [p = 0.64] |
0.84 (0.80, 0.87) [p = 0.63] |
0.84 (0.80, 0.87) [p = 0.68] |
| BARD | 0.72 (0.69, 0.76) | 0.75 (0.72, 0.79) [p = 0.27] |
0.76 (0.72, 0.79) [p = 0.23] |
0.74 (0.70, 0.77) [p = 0.24] |
| APRI | 0.74 (0.70, 0.78) | 0.74 (0.70, 0.76) [p = 0.99] |
0.74 (0.70, 0.78) [p = 0.99] |
0.73 (0.69, 0.77) [p = 0.92] |
| FIB-4 | 0.81 (0.78, 0.85) | 0.82 (0.78, 0.85) [p = 0.92] |
0.82 (0.78, 0.85) [p = 0.92] |
0.82 (0.78, 0.85) [p = 0.92] |
Serum ferritin levels had a similarly poor accuracy as ALT levels in the diagnosis of definitive NASH as indicated by an area under the ROC curve of 0.58 (95% CI 0.54, 0.61) and 0.60 (95% CI 0.56, 0.64), respectively (Supplemental Figure 2).
DISCUSSION
Our study shows that similar to some prior publications [3,7–9], increased serum ferritin levels are associated with the presence of liver fibrosis and with more advanced liver fibrosis in patients with NAFLD. Our study, however, extends our current knowledge on the association of ferritin and liver fibrosis, and shows that: 1) serum ferritin levels on its own lack overall accuracy to distinguish between patients with NAFLD with and without liver fibrosis and to distinguish between patients with or without severe or advanced liver fibrosis. This is indicated by an area under the ROC curves less than 0.60, and sensitivity values between 13% and 41% for any cut-point of elevated serum ferritin. 2) The overall accuracy of several non-invasive scoring systems in distinguishing between patients with and without advanced fibrosis does not significantly change by adding the serum ferritin levels. Therefore, elevated serum ferritin levels in patients with NAFLD cannot be used to accurately predict presence or severity of liver fibrosis or in making decisions regarding the need for a liver biopsy for fibrosis staging. 3) An additional important finding of our study is the relatively high specificity of increased serum ferritin to rule out the presence and severity of liver fibrosis, with specificity values between 76% and 95% to rule out presence (stage 1–4) of fibrosis, 72% to 93% to rule out significant (stage 2–4) fibrosis, and 70% to 92% to rule out presence of advanced (stage 3–4) fibrosis (Table 3).
Ferritin is an acute-phase protein that can be induced in the setting of systemic inflammation, and thus, is often elevated in conditions associated with chronic inflammation such as obesity, diabetes, and metabolic syndrome [22]. Serum ferritin levels are often elevated in the setting of chronic alcoholism and in chronic liver diseases such as hepatitis C infection and alcohol-induced liver disease [23,24]. Ferritin is the primary tissue iron-storage protein and thus its expression increases in conditions associated with iron overload resulting in increased ferritin levels in both tissue and circulation. Some evidence suggests an association between increased serum ferritin and mild iron overload, unrelated to hereditary hemochromatosis, in conditions associated to the metabolic syndrome including NAFLD [22,25,26]. The association of hyperferritinemia and increased hepatic iron has been demonstrated in studies quantifying hepatic iron though liver biopsy, radiological images, and quantitative phlebotomies [25,27–29]. However, several other series of patients with NAFLD had not found an association of serum ferritin levels with increased hepatic iron [1,2,30]; further, a recent controlled trial reported no association of iron depletion achieved by phlebotomies with improvement in liver enzymes, insulin sensitivity or amount of steatosis in patients with NAFLD [31]. Also, recent cross-sectional large series had reported increased hepatic iron accumulation associated with more advanced liver fibrosis in NAFLD [25,26], but it remains uncertain whether the increased iron deposition is a cause or a consequence of increased liver fibrosis. We found an association of increased hepatic iron accumulation and the presence of definitive NASH but not with presence and severity of fibrosis stage in our patients.
In summary, this large series reproduces prior data demonstrating a significant association of increased serum ferritin with severity of liver fibrosis based on the results of multiple logistic regression analyses. However, our study goes beyond and expands prior data demonstrating that serum ferritin values on its own cannot be used to make the diagnosis of presence or severity of liver fibrosis, although normal serum ferritin, or less elevated levels may reasonably exclude presence and severity of liver fibrosis. In addition, our study demonstrates that the accuracy of several noninvasive scoring systems to distinguish between patients with and without advanced fibrosis does not increase by adding serum ferritin levels.
Supplementary Material
Supplemental Figure 1. Receiver operating characteristic curves to distinguish between patients with and without advanced (stage 3–4) fibrosis (scores alone and serum ferritin cut-points alone).
Supplemental Figure 2. Receiver operating characteristic curves to distinguish between patients with and without definitive NASH
Acknowledgments
Funding source: This study was supported by a National Institute of Health R01 DK82426 grant (to P. Angulo), and The European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° HEALTH-F2-2009-241762 for the project FLIP (to E. Bugianesi). JG is supported by grants from the Sydney Medical Foundation and grants from the NHMRC (632630 and 1049857). These sponsors played no role in the study design or the collection, analysis, and interpretation of data.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- ALT
alanine aminotransferases
- AST
aspartate aminotransferases
- GGT
gamma-glutamyl transferase
- HOMA
homeostatic model assessment
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- BMI
body mass index
- ROC
receiver operating characteristics
- AUROC
area under the ROC
- TIBC
total iron binding capacity
Footnotes
Paul Angulo, MD Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision.
Jacob George, MD, PhD Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision.
Christopher P. Day, MD, PhD Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision.
Ester Vanni, MD, PhD Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Lee Russell Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Anna C. De la Cruz, MD Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Hammad Liaquat, MD Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Lavinia Mezzabotta, MD Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Eun Lee, MD Acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Elisabetta Bugianesi, MD, PhD Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision.
Conflict of Interest: None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 4.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–707. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Yoneda M, Nozaki Y, Endo H, Mawatari H, Iida H, Fujita K, et al. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci. 2010;55:808–814. doi: 10.1007/s10620-009-0771-y. [DOI] [PubMed] [Google Scholar]
- 6.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 7.Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011;31:730–739. doi: 10.1111/j.1478-3231.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 8.Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244–1249. doi: 10.1016/j.jhep.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.http://grants.nih.gov/grants/funding/2590/phs2590.pdf
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostatic model assessment: insulin resistance and beta-cell function from fasting glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [accessed October 5, 2013]. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. (WHO/NMH/NHD/MNM/11.2). http://www.who.int/vmnis/indicators/serum_ferritin.pdf. [Google Scholar]
- 14.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, Leiendecker-Foster C, Speechley M, Snively BM, Holup JL, Thomson E, Sholinsky P Hemochromatosis and Iron Overload Screening (HEIRS) Study Research Investigators. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 16.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 17.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 18.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. HEPATOLOGY. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA NASH Clinical Research Network (CRN) The NAS and the histopatholoic diagnosis of NASH: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozzini C, Girelli D, Olivieri O, Martinelli N, Bassi A, De Matteis G, Tenuti I, Lotto V, Pizzolo F, Corrocher R. Prevalence of body iron excess in the metabolic syndrome. Diabetes Care. 2005;28:2061–2063. doi: 10.2337/diacare.28.8.2061. [DOI] [PubMed] [Google Scholar]
- 23.Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver diseases. J Intern Med. 1994;236:315–322. doi: 10.1111/j.1365-2796.1994.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 24.Tung BY, Emond MJ, Bronner MP, Raaka SD, Cotler SJ, Kowdley KV. Hepatitis C, iron status, and disease severity: relationship with HFE mutations. Gastroenterology. 2003;124:318–326. doi: 10.1053/gast.2003.50046. [DOI] [PubMed] [Google Scholar]
- 25.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, Vanni E, Fargion S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 28.Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–939. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- 29.Valenti L, Fracanzani AL, Fargion S. Effect of iron depletion in patients with nonalcoholic fatty liver disease without carbohydrate intolerance. Gastroenterology. 2003;124:866. doi: 10.1053/gast.2003.50130. author reply 866–867. [DOI] [PubMed] [Google Scholar]
- 30.Valenti L, Dongiovanni P, Piperno A, Fracanzani AL, Maggioni M, Rametta R, Loria P, Casiraghi MA, Suigo E, Ceriani R. Alpha 1-antitrypsin mutations in NAFLD: high prevalence and association with altered iron metabolism but not with liver damage. Hepatology. 2006;44:857–864. doi: 10.1002/hep.21329. [DOI] [PubMed] [Google Scholar]
- 31.Adams LA, House MJ, St Pierre TJ, Crawford DH, Stuart KA, Ching H, Kava J, Webb M, Olynyk JK. Hepatology. doi: 10.1002/hep.27662. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Receiver operating characteristic curves to distinguish between patients with and without advanced (stage 3–4) fibrosis (scores alone and serum ferritin cut-points alone).
Supplemental Figure 2. Receiver operating characteristic curves to distinguish between patients with and without definitive NASH