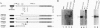Abstract
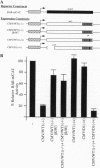
Germline loss-of-function mutations at the Wilms tumor (WT) suppressor locus WT1 are associated with a predisposition to WTs and mild genital system anomalies. In contrast, germ-line missense mutations within the WT1 gene encoding the DNA-binding domain often yield a more severe phenotype consisting of WT, sexual ambiguity, and renal nephropathy. In this report, we demonstrate that the products of mutant alleles that impair DNA recognition can antagonize WT1-mediated transcriptional repression. We demonstrate that WT1 can self-associate in vitro and in vivo and that the responsible domain maps to the amino-terminal region of the protein. Oligomers of full-length protein form less efficiently or produce less stable complexes than oligomers between truncated polypeptides and full-length protein. Our data suggest a molecular mechanism to explain how WT1 mutations may act in deregulating cellular proliferation and differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardeesy N., Zabel B., Schmitt K., Pelletier J. WT1 mutations associated with incomplete Denys-Drash syndrome define a domain predicted to behave in a dominant-negative fashion. Genomics. 1994 Jun;21(3):663–664. doi: 10.1006/geno.1994.1333. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Bruening W., Bardeesy N., Silverman B. L., Cohn R. A., Machin G. A., Aronson A. J., Housman D., Pelletier J. Germline intronic and exonic mutations in the Wilms' tumour gene (WT1) affecting urogenital development. Nat Genet. 1992 May;1(2):144–148. doi: 10.1038/ng0592-144. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Coppes M. J., Liefers G. J., Paul P., Yeger H., Williams B. R. Homozygous somatic Wt1 point mutations in sporadic unilateral Wilms tumor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1416–1419. doi: 10.1073/pnas.90.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Goodyer P., Dehbi M., Torban E., Bruening W., Pelletier J. Repression of the retinoic acid receptor-alpha gene by the Wilms' tumor suppressor gene product, wt1. Oncogene. 1995 Mar 16;10(6):1125–1129. [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Larsson S. H., Charlieu J. P., Miyagawa K., Engelkamp D., Rassoulzadegan M., Ross A., Cuzin F., van Heyningen V., Hastie N. D. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 1995 May 5;81(3):391–401. doi: 10.1016/0092-8674(95)90392-5. [DOI] [PubMed] [Google Scholar]
- Little M. H., Prosser J., Condie A., Smith P. J., Van Heyningen V., Hastie N. D. Zinc finger point mutations within the WT1 gene in Wilms tumor patients. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4791–4795. doi: 10.1073/pnas.89.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S., Park S., Bernard A., Morris J. F., Rauscher F. J., 3rd, Hill D. E., Haber D. A. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E. Genetics of Wilms' tumor. Hum Genet. 1981;57(3):231–246. doi: 10.1007/BF00278936. [DOI] [PubMed] [Google Scholar]
- Nakagama H., Heinrich G., Pelletier J., Housman D. E. Sequence and structural requirements for high-affinity DNA binding by the WT1 gene product. Mol Cell Biol. 1995 Mar;15(3):1489–1498. doi: 10.1128/mcb.15.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschwang S., Tiret A., Laurent-Puig P., Muleris M., Parc R., Thomas G. Restriction of ocular fundus lesions to a specific subgroup of APC mutations in adenomatous polyposis coli patients. Cell. 1993 Dec 3;75(5):959–968. doi: 10.1016/0092-8674(93)90539-3. [DOI] [PubMed] [Google Scholar]
- Park S., Tomlinson G., Nisen P., Haber D. A. Altered trans-activational properties of a mutated WT1 gene product in a WAGR-associated Wilms' tumor. Cancer Res. 1993 Oct 15;53(20):4757–4760. [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd The WT1 Wilms tumor gene product: a developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J. 1993 Jul;7(10):896–903. [PubMed] [Google Scholar]
- Reddy J. C., Morris J. C., Wang J., English M. A., Haber D. A., Shi Y., Licht J. D. WT1-mediated transcriptional activation is inhibited by dominant negative mutant proteins. J Biol Chem. 1995 May 5;270(18):10878–10884. doi: 10.1074/jbc.270.18.10878. [DOI] [PubMed] [Google Scholar]
- Spirio L., Olschwang S., Groden J., Robertson M., Samowitz W., Joslyn G., Gelbert L., Thliveris A., Carlson M., Otterud B. Alleles of the APC gene: an attenuated form of familial polyposis. Cell. 1993 Dec 3;75(5):951–957. doi: 10.1016/0092-8674(93)90538-2. [DOI] [PubMed] [Google Scholar]
- Varanasi R., Bardeesy N., Ghahremani M., Petruzzi M. J., Nowak N., Adam M. A., Grundy P., Shows T. B., Pelletier J. Fine structure analysis of the WT1 gene in sporadic Wilms tumors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3554–3558. doi: 10.1073/pnas.91.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Enger K. T., Deuel T. F. A second transcriptionally active DNA-binding site for the Wilms tumor gene product, WT1. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8896–8900. doi: 10.1073/pnas.90.19.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Gurrieri M., Huang J., Deuel T. F. WT1, the Wilms' tumor suppressor gene product, represses transcription through an interactive nuclear protein. Oncogene. 1995 Mar 16;10(6):1243–1247. [PubMed] [Google Scholar]