Summary
Recently, loss of beta cell-specific traits has been proposed as an early cause of beta cell failure in diabetes. However, the molecular mechanisms that underlie this loss of beta cell features remain unclear. Here, we identify an Nkx6.1-controlled gene regulatory network as essential for maintaining the functional and molecular traits of mature beta cells. Conditional Nkx6.1 inactivation in adult mice caused rapid-onset diabetes and hypoinsulinemia. Genome-wide analysis of Nkx6.1-regulated genes and functional assays further revealed a critical role for Nkx6.1 in the control of insulin biosynthesis, insulin secretion and beta cell proliferation. Over time, Nkx6.1-deficient beta cells acquired molecular characteristics of delta cells, revealing a molecular link between impaired beta cell functional properties and loss of cell identity. Given that Nkx6.1 levels are reduced in human type 2-diabetic beta cells, our study lends support to the concept that loss of beta cell features could contribute to the pathogenesis of diabetes.
Introduction
Type 2 diabetes mellitus (T2D) is characterized by reduced insulin sensitivity of insulin target tissues and impaired insulin secretion by pancreatic beta cells. Although both of these factors play a role, genetic studies suggest that the ability of beta cells to respond to metabolic stressors is the predominant factor in determining the predisposition to T2D (Muoio and Newgard, 2008).
In T2D, beta cells exhibit an impaired capacity to compensate for increased insulin demand (Cerasi and Luft, 1967), a defect that has been ascribed to both inadequate cellular capacity to secrete insulin (Hosker et al., 1989) and beta cell death (Butler et al., 2003). Among the earliest defects observed in T2D patients is a reduced ability of beta cells to secrete insulin in response to elevated blood glucose levels (Hosker et al., 1989). This impairment in glucose-stimulated insulin secretion has been attributed to defects in glucose sensing (Froguel et al., 1992), mitochondrial dysfunction (Supale et al., 2012), as well as to oxidative stress (Robertson, 2004). Thus, mounting evidence suggests that defects in multiple cellular processes can compromise beta cell function and could be a factor in T2D development. Furthermore, hyperglycemia has been shown to impair the expression of genes important for beta cell identity (Jonas et al., 1999). More recently, Talchai et al. (Talchai et al., 2012) described a loss of beta cell features, characterized by a decline in insulin production, acquisition of progenitor-like characteristics, and fate conversion into other endocrine cell types in mouse models of T2D, suggesting that loss of the differentiated beta cell state also contributes to beta cell failure in T2D. However, it is currently unknown whether the loss of beta cell functional properties, namely regulated insulin secretion, and loss of beta cell identity are linked during T2D progression. A simultaneous loss of beta cell function and identity could be explained by reduced expression of a central transcriptional regulator controlling genes involved in both processes.
Several lines of evidence suggest that the beta cell-enriched transcription factor Nkx6.1 could have a role in T2D. First, genome wide association studies suggest that variants of Nkx6.1 associate with T2D (Yokoi et al., 2006). Second, decreased Nkx6.1 expression has been shown to accompany the development of T2D in rodents and humans (Guo et al., 2013; Talchai et al., 2012). Third, in vitro studies in beta cell lines and isolated islets suggest a possible role for Nkx6.1 in the regulation of glucose-stimulated insulin secretion as well as beta cell proliferation (Schisler et al., 2008; Schisler et al., 2005). Additionally, we have recently shown that Nkx6.1 is necessary and sufficient to confer beta cell identity to differentiating endocrine precursors in the embryo (Schaffer et al., 2013), raising the possibility that Nkx6.1 could also help maintain the differentiated state of adult beta cells. Together, these findings suggest that Nkx6.1 may be a regulator of beta cell function and identity in adult animals.
To explore the role of Nkx6.1 in mature beta cells, we ablated Nkx6.1 specifically in beta cells of adult mice and identified Nkx6.1 target genes in beta cells by combining gene expression profiling and chromatin immunoprecipitation with massively parallel sequencing (ChIP-seq). We found that loss of Nkx6.1 causes rapid onset diabetes due to defects in insulin biosynthesis and secretion. The observed loss in insulin production and beta cell functional properties was later accompanied by ectopic activation of delta cell genes in beta cells. Thus, by impairing beta cell function and destabilizing beta cell identity, reduced Nkx6.1 levels, as seen in T2D, could contribute to the pathogenesis of T2D.
Results
Loss of Nkx6.1 in mature beta cells causes diabetes and reduced insulin production
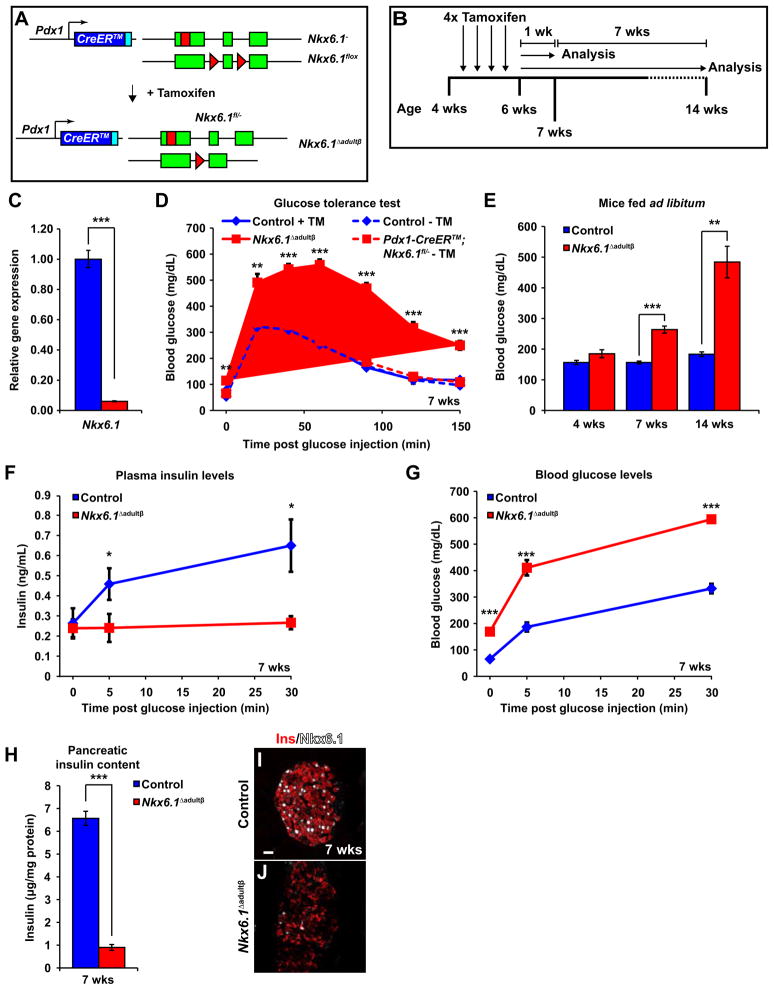
To examine Nkx6.1 function in mature beta cells in vivo, we conditionally inactivated Nkx6.1 in islet cells of adult mice by triggering recombination of an Nkx6.1flox (Nkx6.1fl) allele with the tamoxifen-inducible Pdx1-CreER™ transgene. Pdx1-CreER™;Nkx6.1fl/− and Pdx1-CreER™;Nkx6.1fl/+ mice were injected with tamoxifen between 4 and 6 weeks of age to produce Nkx6.1 Δadultβ and control mice, respectively (Figure 1A,B). Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and immunofluorescence staining for Nkx6.1 demonstrated efficient recombination of the Nkx6.1fl allele in beta cells (Figure 1C,I,J).
Figure 1. Deletion of Nkx6.1 in adult beta cells results in diabetes and loss of pancreatic insulin.
(A) Schematic of alleles and transgenes used to inactivate Nkx6.1 in adult beta cells. Rectangles, coding sequences; Triangles, loxP sites. (B) Schematic of experimental design. (C) qRT-PCR analysis of isolated islets shows a significant reduction of Nkx6.1 in Nkx6.1 Δadultβ mice (n=3). (D) Intraperitoneal glucose tolerance test reveals glucose intolerance in male Nkx6.1 Δadultβ mice compared to non-injected and tamoxifen (TM)-treated control mice at 7 wks (n=6). Solid lines, post-TM treatment; Dashed lines, without TM treatment. (E) Blood glucose measurements of mice fed ad libitum show diabetes in male Nkx6.1Δ adultβ mice (n=6). (F,G) Nkx6.1 Δadultβ mice have lower plasma insulin levels and elevated blood glucose after a glucose stimulus compared to control mice (n=6). (H) Pancreatic insulin content normalized to protein concentration is reduced in Nkx6.1 Δadultβ mice (n=6). (I,J) Immunofluorescence staining reveals almost complete absence of Nkx6.1 and reduced insulin expression in Nkx6.1 Δadultβ mice at 7 wks. Scale bar = 20 μm. Ins, insulin; Wk, week. Data are shown as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. See also Figures S1 and S2.
To determine whether Nkx6.1 deletion affects beta cell function, we conducted glucose tolerance tests and measured blood glucose levels. Glucose tolerance tests performed one week after the last tamoxifen injection revealed elevated fasting blood glucose levels and glucose intolerance in male Nkx6.1 Δadultβ mice when compared to tamoxifen-treated and non-tamoxifen-treated control mice (Figure 1D). Likewise, blood glucose levels were significantly elevated in Nkx6.1 Δadultβ mice fed ad libitum, with levels reaching near 500 mg/dL within eight weeks after the last tamoxifen injection (Figure 1E). Female Nkx6.1 Δadultβ mice also became diabetic, but the phenotype developed slightly later and was less severe than in males (Figure S1A–C). Thus, loss of Nkx6.1 in adult beta cells causes rapid development of diabetes.
To investigate whether diabetes in Nkx6.1 Δadultβ mice is caused by insulin insufficiency, we measured plasma insulin levels after glucose administration. As expected, control mice responded to a glucose bolus with a rapid increase in plasma insulin levels within 5 minutes of glucose administration (Figure 1F). By contrast, insulin levels did not increase in Nkx6.1 Δadultβ mice and blood glucose levels were significantly higher than in control mice (Figure 1F,G). A striking reduction in pancreatic insulin content in Nkx6.1 Δadultβ mice (Figure 1H) further demonstrated decreased overall pancreatic insulin production. To determine whether the reduction in pancreatic insulin levels in Nkx6.1 Δadultβ mice is a result of beta cell loss, we next examined beta cell survival and quantified beta cell mass. Nkx6.1 Δadultβ mice did not show increased beta cell apoptosis (Figure S2A–D) or reduced mass of endocrine or beta cells (Figure S2E-H), suggesting that diabetes in Nkx6.1 Δadultβ mice is caused by decreased insulin biosynthesis rather than beta cell loss. Consistent with a possible defect in the cellular production of insulin, the insulin fluorescence signal was dramatically reduced in Nkx6.1-deficient beta cells (Figure 1I,J). Notably, reduced insulin production did not appear to be accompanied by a change in endocrine cell type identity, as insulin+ cells in Nkx6.1 Δadultβ mice did not co-express other pancreatic hormones (Figure S2I-N). These data suggest that diabetes following Nkx6.1 inactivation is initially caused, at least in part, by loss of insulin production but not cell death or conversion into other endocrine cell types.
Nkx6.1 directly regulates islet transcription factors and genes involved in glucose metabolism and insulin biosynthesis
To more globally understand how loss of Nkx6.1 impacts beta cell gene expression and to identify molecular pathways immediately affected following Nkx6.1 inactivation, we next conducted transcriptional profiling of Nkx6.1 Δadultβ and control islets 3 days after completion of tamoxifen-induced Nkx6.1 ablation (Figure 2A). At this time point, Nkx6.1 Δadultβ mice were only mildly glucose intolerant and blood glucose levels of mice fed ad libitum were still below 250 mg/dL (Figure S3A,B). Comparison of gene expression profiles between Nkx6.1 Δadultβ and control islets revealed significant differences in the expression of 1455 genes (FDR <0.01 and fold change (FC) >1.5; Table S1), of which 887 were upregulated and 568 downregulated. To define the cellular processes affected by Nkx6.1 inactivation, we performed Gene Ontology (GO) analysis of the differentially expressed genes. Consistent with the diabetic phenotype, Nkx6.1-regulated genes showed association with biological processes that are critical for beta cell function, such as ion transport, regulation of secretion, oxidation reduction, insulin secretion, and hexose biosynthesis (Figure 2B). These data suggest that reduced insulin production may not be the only cause of hyperglycemia following Nkx6.1 deletion, but that simultaneous impairment of multiple processes required for proper beta cell function could contribute to the development of diabetes in Nkx6.1 Δadultβ mice.
Figure 2. Nkx6.1 regulates important beta cell genes.
(A) Schematic of experimental design for microarray analysis. (B) Gene ontology analysis of differentially expressed genes as identified by cDNA microarray analysis of Nkx6.1 Δadultβ and control islets. (C) Distribution of Nkx6.1 binding peaks from ChIP-seq analysis within the genome. (D) De novo motif analysis of Nkx6.1 binding peaks identifies a consensus Nkx6.1 binding motif. (E) Venn diagram of genes bound and regulated by Nkx6.1 in mature islets. (F,G) Analysis of Nkx6.1-occupied genes reveals Nkx6.1 target genes that are up- and down-regulated after Nkx6.1 deletion. Yellow boxes represent Nkx6.1-bound and regulated genes with known function in beta cells. TTS, transcriptional termination site. See also Figure S3 and Tables S1–S3.
To distinguish between direct transcriptional target genes of Nkx6.1 and genes indirectly affected by Nkx6.1 inactivation, we performed ChIP-seq for Nkx6.1 on primary mouse islets to identify Nkx6.1-occupied genes. We detected a total of 6771 Nkx6.1 binding regions throughout the murine genome (FDR < 0.001; Table S2), of which 4066 were near genes or intronic (Figure 2C). De novo motif analysis revealed a TAAT core motif and two flanking nucleotides on each side as the sequence motif preferentially occupied by Nkx6.1 (Figure 2D). Notably, the TAAT core of the Nkx6.1 de novo binding motif has been previously identified by in vitro EMSA analysis (Jorgensen et al., 1999). The TAAT motif is shared among many homeodomain transcription factors (Wilson et al., 1996), demonstrating binding of Nkx6.1 to the core homeodomain-binding motif.
To next determine the overlap between those genes occupied by Nkx6.1 in beta cells and those whose expression is affected by Nkx6.1 loss, we analyzed which of the 1988 Nkx6.1 binding sites within 10kb of a transcriptional start site were associated with genes significantly regulated in Nkx6.1-deficient islets. Surprisingly, only 8% of Nkx6.1-occupied genes (135/1818) were also regulated by Nkx6.1 (Figure 2E). Similarly, of the 1455 genes with statistically significant changes in expression only 9% were bound by Nkx6.1 (Figure 2E), indicating that only a fraction of genes affected by Nkx6.1 inactivation directly depends on transcriptional input by Nkx6.1. Statistical analysis using hypergeometric distribution (Bhinge et al., 2007) revealed that this overlap between Nkx6.1-bound and regulated genes was still significantly greater than randomly expected (P<0.05). Interestingly, an equal percentage of Nkx6.1-bound and regulated genes were upregulated as were downregulated (Figure 2F,G), suggesting that Nkx6.1 can act as both a transcriptional repressor and activator.
Nkx6.1 was found to directly regulate various critical beta cell genes, including genes involved in glucose uptake and metabolism (Slc2a2 (Glut2), Pcx, and G6pc2), insulin processing (Ero1lb and Slc30a8), as well as transcriptional regulators with known roles in islet development and/or beta cell function (MafA, Rfx6, Mnx1, and Tle3) (Figure 2E). These results suggest that Nkx6.1 exerts its function by transcriptionally regulating mediators of multiple beta cell processes.
The insulin secretory response is impaired after Nkx6.1 inactivation
The insulin secretory response of beta cells is regulated by the coupling of glucose metabolism to insulin secretion (Muoio and Newgard, 2008). Glycolysis results in an increase in the ATP:ADP ratio, which serves as the key trigger for closure of ATP-sensitive potassium channels (KATP channels), ultimately stimulating calcium influx and insulin secretion. Because Nkx6.1 directly regulates the glucose metabolic genes Glut2, Pcx, and G6pc2 (Table S1; Figure 3A–C), we hypothesized that glucose uptake, glycolytic flux and energy production could be impaired in Nkx6.1-deficient beta cells. Supporting that the downregulation of Glut2 is physiologically significant, we found that Nkx6.1 Δadultβ mice were resistant to streptozotocin-induced beta cell death (data not shown), which depends on Glut2-mediated uptake of streptozotocin (Schnedl et al., 1994).
Figure 3. Islets from Nkx6.1 Δadultβ mice exhibit reduced insulin secretion in vitro.
(A) qRT-PCR analysis of islets from Nkx6.1 Δadultβ and control mice at 6 wks for genes involved in glycolytic flux (n=3). (B–E′) Immunofluorescence staining of pancreata from Nkx6.1 Δadultβ and control mice at 6 wks. (F) ATP measurement in islets from Nkx6.1 Δadultβ and control mice at 6 wks. (G) qRT-PCR analysis of islets from Nkx6.1 Δadultβ and control mice at 6 wks for genes involved in insulin secretion (n=3). (H,I) Immunofluorescence staining for Ucn3 in Nkx6.1 Δadultβ and control pancreata at 6 wks. (J) Static incubation of islets from Nkx6.1 Δadultβ and control mice with 2.7mM glucose, 16.7mM glucose, 30mM KCl, or 2μM Bay K8644 for 1 hour reveals that islets from Nkx6.1 Δadultβ mice secrete less of their total insulin content per hour than control islets (n=6). Scale bar = 20 μm. Dashed lines in C and H represent islet area. Ins, insulin; p-AMPK, phospho-AMP kinase; Wk, week; Hr, hour. Data are shown as mean ± SEM. Ψ = 16.27 with an SEM of ± 2.93. Slashes in A = change in Y axis scale. *p<0.05, **p<0.01, ***p<0.001.
To investigate whether the changes in expression of glucose metabolic genes are associated with defects in energy production, we stained pancreata for phospho-AMP kinase (p-AMPK), a sensitive indicator of cellular energy depletion (low ATP:AMP ratio) (Porat et al., 2011). Nkx6.1 Δadultβ islets displayed strikingly more intense p-AMPK staining than control islets (Figure 3D–E′), indicating that loss of Nkx6.1 causes reduced glycolytic flux and energy stress. Further supporting this conclusion, intracellular ATP content was also significantly decreased in Nkx6.1Δ adultβ islets (Figure 3F). We conclude that despite increased blood glucose levels, Nkx6.1 deficiency results in energy-depleted beta cells. Since energy depletion has been shown to impair insulin secretion and cause diabetes in mice (Piston et al., 1999; Porat et al., 2011; Terauchi et al., 1995), the defect in energy production in Nkx6.1Δ adultβ mice could lead to a severely impaired insulin secretory response.
In addition to affecting ATP production, Nkx6.1 deletion also led to reduced expression of Sytl4, a vesicle-associated protein implicated in the modulation of insulin secretion (Gomi et al., 2005), as well as Ucn3 and Glp1r (Figure 3G–I), which are involved in peptide-mediated potentiation of insulin secretion (Li et al., 2007; Preitner et al., 2004). These changes in gene expression suggest that Nkx6.1 also has glucose metabolism-independent roles in insulin secretion. Notably, core components of the stimulus-secretion-coupling mechanism (e.g. Abcc8, Kcnj11, and Cacna1c) and genes encoding proteins important for vesicle docking (e.g. Pclo and Noc2) were normally expressed in Nkx6.1-deficient islets (Figure 3G).
To directly test whether the observed changes in gene expression affect insulin secretion at a functional level, we performed in vitro glucose stimulated insulin secretion (GSIS) assays on isolated islets from Nkx6.1 Δadultβ and control mice. To account for decreased insulin content of Nkx6.1-deficient beta cells, we calculated insulin secretion as a percentage of total islet insulin content. Islets from Nkx6.1 Δadultβ mice secreted less of their total insulin than control islets under conditions of basal (2.7 mM) and high (16.7 mM) glucose concentrations (Figure 3J), showing that insulin secretion is impaired after Nkx6.1 deletion. However, stimulation of secretion by glucose appeared to be unaffected by Nkx6.1 deletion, as there was a similar increase in insulin secretion in Nkx6.1 Δadultβ and control islets between low and high glucose conditions (3.75-fold increase between 2.7mM and 16.7 mM glucose in Nkx6.1 Δadultβ islets versus 3.06-fold increase in control islets). The insulin secretory pattern of Nkx6.1 Δadultβ islets is highly similar to the pattern observed in Glut2-deficient islets (Guillam et al., 2000), suggesting that loss of Glut2 in Nkx6.1 Δadultβ beta cells has a major contribution to the insulin secretory defect. Notably, additional defects downstream of KATP channel-mediated membrane depolarization also appear to exist, as insulin secretion in Nkx6.1-deficient islets was also reduced in response to membrane depolarization and calcium influx (30 mM KCl and 2μM Bay K8644, respectively; Figure 3J). Together, these results imply that impaired insulin secretion is a major contributor to the diabetic phenotype of Nkx6.1 Δadultβ mice.
Decreased beta cell proliferation in Nkx6.1 Δadultβ mice
It has been suggested that glycolytic flux and ATP production serve as a trigger for beta cell replication (Porat et al., 2011). Specifically, glucose metabolism is thought to control beta cell proliferation by regulating expression of Cyclin D2 (Ccnd2) (Salpeter et al., 2010; Salpeter et al., 2011), which is a critical regulator of beta cell mass in mice (Georgia and Bhushan, 2004; Kushner et al., 2005). Since Nkx6.1-deficient beta cells have defects in energy production, we examined whether Nkx6.1 deletion affects Ccnd2 mRNA levels. We found that Ccnd2 mRNA levels were indeed decreased in Nkx6.1 Δadultβ islets (Table S1; Figure 4A). Strikingly, Nkx6.1 inactivation specifically affected Ccnd2, while mRNA levels of other cyclins were unaffected (Table S1; Figure 4A). Immunofluorescence staining (Figure 4B–C′) and Western blot analysis (Figure 4D) further demonstrated significantly reduced Cyclin D2 protein levels in beta cells of Nkx6.1 Δadultβ mice. Similar to the phenotype of Ccnd2 null mutant mice (Georgia and Bhushan, 2004; Kushner et al., 2005), Nkx6.1-deficient beta cells exhibited a reduction in the number of beta cells expressing the proliferation marker Ki67 (Figure 4E), showing that beta cell proliferation is impaired after Nkx6.1 inactivation. Notably, Nkx6.1 did not occupy Ccnd2 regulatory sequences (Table S2), suggesting that the regulation of Ccnd2 by Nkx6.1 could be indirect.
Figure 4. Nkx6.1 maintains Cyclin D2 expression and beta cell proliferative capacity through regulation of glucose uptake.
(A) qRT-PCR analysis of islets shows a decrease in Ccnd2 expression in Nkx6.1 Δadultβ compared to control mice at 6 wks (n=3). (B–C′) Immunofluorescence staining for insulin and Cyclin D2 shows a decrease in Cyclin D2 expression in beta cells of Nkx6.1 Δadultβ mice at 6 wks. B′ and C′ are higher magnification images of B and C, respectively. White arrowheads point to Cyclin D2high cells. (D) Immunoblot analysis of whole cell islet lysates confirms reduced Cyclin D2 expression in Nkx6.1 Δadultβ mice. (E) Quantification of the percentage of insulin+ cells expressing Ki67 shows decreased beta cell proliferation in Nkx6.1 Δadultβ mice at 6 wks (n=3). (F) qRT-PCR analysis of genes with decreased expression in islets from Nkx6.1 Δadultβ mice after adenoviral infection of Nkx6.1 Δadultβ islets with Ad-CMV-Glut2 (Ad-Glut2) and control islets with Ad-CMV-β-gal (Ad-β-gal) (n=3). Ad-Glut2 restores Ccnd2 expression to levels observed in control islets infected with Ad-β-gal. (G) Quantification of the percentage of insulin+ cells expressing Ki67 after infection of Nkx6.1 Δadultβ and control islets with Ad-Glut2 or Ad-β-gal shows that Ad-Glut2 restores the number of Ki67+ beta cells in Nkx6.1 Δadultβ islets to control values (n=3). (H) qRT-PCR analysis after a three-hour treatment of Nkx6.1 Δadultβ islets with the calcium channel activator Bay K8644 and control islets treated with vehicle (n=3). (I) Quantification of the percentage of insulin+ cells expressing Ki67 after injection of Nkx6.1 Δadultβ mice with 8mg/kg Bay K8644 or control mice injected with vehicle (n=3). Stimulation of calcium influx restores Ccnd2 expression and beta cell proliferation in Nkx6.1 Δadultβ mice. (J,K) qRT-PCR analysis of islets from Nkx6.1 Δadultβ mice treated with 10 μM glucokinase activator (GKA) (J) or 100 nM insulin (K) for 3 hours and islets from control mice treated with vehicle (n=3). Scale bar = 20 μm. Wk, week. Data are shown as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Restoring glucose import reinstates Ccnd2 expression in Nkx6.1 Δadultβ islets
To explore whether limited glucose uptake capacity due to loss of Glut2 could be the main cause of reduced Ccnd2 expression in Nkx6.1-deficient islets, we investigated whether restoring Glut2-mediated glucose import could increase Ccnd2 levels after Nkx6.1 inactivation. To examine this, we reconstituted Glut2 expression in Nkx6.1 Δadultβ islets using an adenovirus containing Glut2 cDNA (Ad-Glut2), which resulted in a 25-fold increase in Glut2 mRNA levels compared to Ad-β-gal-treated control islets as well as restored Glut2 protein expression (Figure 4F; Figure S4). Glut2 reconstitution increased Ccnd2 expression to levels of control islets, while expression of other Nkx6.1-regulated genes remained significantly reduced (Figure 4F). Glut2 reconstitution in Nkx6.1 Δadultβ islets also restored the number of insulin+ cells expressing Ki67 to control values (Figure 4G), indicating that Glut2 re-expression rescues the proliferation defect. These findings demonstrate that Ccnd2 expression does not depend on direct transcriptional input from Nkx6.1, but that Nkx6.1 controls Ccnd2 and beta cell proliferation indirectly by regulating glucose import.
Consistent with the notion that glycolytic flux regulates Ccnd2 expression via the stimulus-secretion-coupling pathway (Salpeter et al., 2010; Salpeter et al., 2011), increasing calcium influx by treating islets with the L-type dependent calcium channel opener, Bay K8644, similarly restored Ccnd2 expression in Nkx6.1 Δadultβ islets (Figure 4H). Significantly, Bay K8644 administration to mice increased the number of insulin+ cells expressing Ki67 to control values (Figure 4I), providing in vivo evidence that beta cell proliferation can be rescued by stimulating calcium influx. In contrast, culture of Nkx6.1-deficient islets in the presence of an activator for the rate-limiting enzyme of glycolysis, glucokinase, or the beta cell mitogen, insulin (Paris et al., 2003), failed to restore Ccnd2 expression (Figure 4J,K; also compare to Figure 4A). These findings illustrate that the proliferative capacity of beta cells is coupled to glucose metabolism and that Nkx6.1 controls this process by regulating Glut2 expression. Because beta cells have a low turnover rate in adult mice (Teta et al., 2005), the observed reduction in beta cell proliferation did not result in decreased beta cell mass in our acute Nkx6.1 deletion model (Figure S2G,H). However, the decreased proliferative capacity could become metabolically relevant when beta cells need to undergo adaptive expansion under conditions of increased insulin demand.
Nkx6.1 Δadultβ mice have posttranscriptional defects in insulin biosynthesis
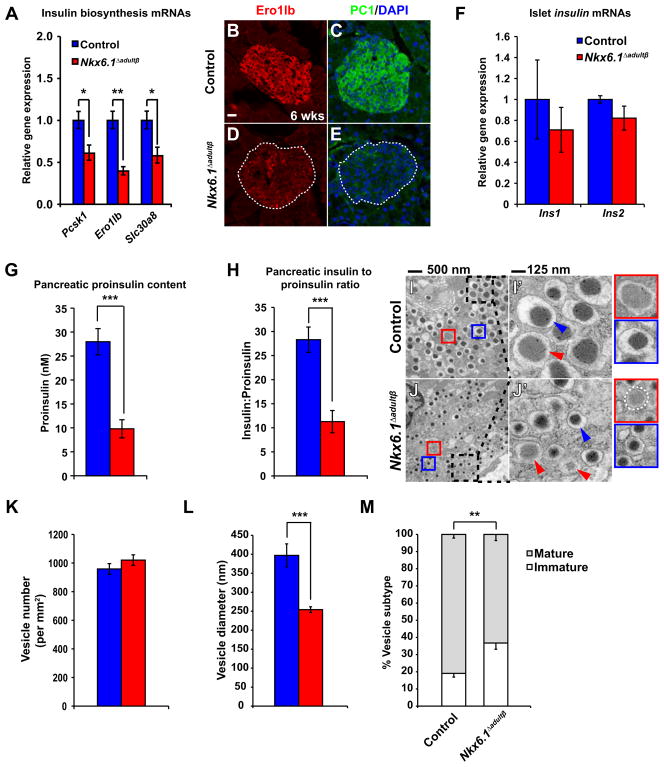
Our gene expression and ChIP-seq analysis revealed that Nkx6.1 also controlled genes required for insulin biosynthesis, which could explain the reduction in pancreatic insulin levels in Nkx6.1 Δadultβ mice. Most notably, expression of the T2D-associated zinc transporter Slc30a8, the oxidoreductase Ero1lb, and the proinsulin to insulin convertase Pcsk1 (PC1) was severely reduced in Nkx6.1-deficient beta cells (Table S1; Figure 5A–E). These proteins have established roles in insulin processing and/or maturation of insulin secretory vesicles (Bellomo et al., 2011; Zhu et al., 2002; Zito et al., 2010), implying a role for Nkx6.1 in multiple aspects of the insulin biosynthesis pathway. Notably, the finding that ins1 and ins2 mRNA levels were not significantly changed (Table S1; Figure 5F), suggests that reduced insulin production in Nkx6.1 Δadultβ mice is mainly caused by posttranscriptional defects in insulin biosynthesis. To further define which steps in insulin biosynthesis are affected by Nkx6.1 inactivation, we measured pancreatic proinsulin content and calculated the insulin to proinsulin ratio in Nkx6.1 Δadultβ islets. Compared to control mice, pancreatic proinsulin levels and the ratio of insulin to proinsulin were significantly reduced in Nkx6.1 Δadultβ mice (Figure 5G,H). While the defect in proinsulin to insulin processing was expected based on the observed decrease in Slc30a8, Ero1lb, and Pcsk1 expression, it is less clear why loss of Nkx6.1 impairs proinsulin biosynthesis.
Figure 5. Nkx6.1 is necessary for insulin biosynthesis.
(A) qRT-PCR analysis of islets reveals reduced expression of genes involved in insulin biosynthesis in Nkx6.1 Δadultβ compared to control mice at 6 wks (n=3). (B–E) Immunofluorescence staining of pancreata from Nkx6.1 Δadultβ and control mice at 6 wks. Dashed lines represent islet area. (F) qRT-PCR analysis of Nkx6.1 Δadultβ and control islets from mice at 6 wks shows no significant difference in ins1 or ins2 expression (n=3). (G) Proinsulin content normalized to protein concentration of whole pancreatic lysates (n=6). (H) The pancreatic insulin to proinsulin ratio is reduced in Nkx6.1Δ adultβ mice (n=6). (I,J) Transmission electron microscopy of pancreatic sections reveals smaller vesicle size and an increase in immature vesicles (red arrowheads) in Nkx6.1 Δadultβ compared to control mice. Dashed boxes indicate area of magnification in I′ and J′. Blue arrowheads point to vesicles containing mature insulin dense core granules. Insets framed red show a representation of a typical immature vesicle and insets framed blue a typical mature vesicle. (K–M) Quantification of vesicle numbers (K), vesicle diameter (L), and the percentage of mature and immature vesicles (M) in Nkx6.1Δ adultβ and control mice (n=10). In G–M, mice were analyzed at 7 wks. PC1, prohormone convertase 1/3; Wk, week. Data are shown as mean ± SEM for A,F,G,H and ± SD for K–M. *p<0.05, **p<0.01, ***p<0.001.
Because glucose is a direct stimulator of proinsulin translation (Wicksteed et al., 2003), we examined whether decreased Glut2 expression in Nkx6.1 Δadultβ mice limits intracellular availability of glucose and in turn reduces proinsulin production. However, restoring Glut2 expression in Nkx6.1 Δadultβ dispersed islets had no effect on proinsulin or insulin levels (Figure S5A,B), suggesting that not glucose import, but reduced expression of insulin biosynthetic enzymes limits proinsulin synthesis in Nkx6.1 Δadultβ mice.
To determine whether the defect in insulin biosynthesis affects the formation of insulin secretory vesicles, we examined secretory vesicle numbers and morphology in beta cells from Nkx6.1 Δadultβ and control mice. According to the guidelines established by Pictet et al. (Pictet et al., 1972), secretory vesicles were considered immature if they had a homogenous light gray appearance similar to the electron density of the cytoplasm or mature if the vesicles contained an electron dense granule darker than the density of the cytoplasm. Transmission electron microscopy (TEM) showed that the overall number of secretory granules was unchanged (Figure 5I–K), suggesting that loss of Nkx6.1 does not affect vesicle formation or stability. In accordance with the observed defect in insulin biosynthesis, examination of vesicle morphology revealed smaller overall vesicle size in beta cells of Nkx6.1 Δadultβ mice (Figure 5I′,J′,L). Reduced vesicle size has been similarly observed in the MODY (Ins2Akita) mouse model of impaired insulin biosynthesis (Wang et al., 1999). In addition to their reduced size, secretory granules in beta cells of Nkx6.1 Δadultβ mice exhibited a smaller halo around the dense core of mature insulin (Figure 5I′,J′; blue arrowheads); a feature reflecting reduced processing of proinsulin to insulin (Orci et al., 1984). Moreover, the proportion of immature vesicles was significantly increased in Nkx6.1-deficient beta cells (Figure 5M), representing another feature of impaired insulin processing. These findings demonstrate that the changes in the expression of insulin biosynthesis-associated genes after Nkx6.1 deletion manifest in defects in insulin processing and mature insulin secretory vesicle formation. Given previous evidence that deletion of Ero1lb and Slc30a8 in mice perturbs glucose homeostasis (Nicolson et al., 2009; Zito et al., 2010), these defects in insulin biosynthesis together with the impaired insulin secretory response are likely the predominant cause of diabetes in Nkx6.1 Δadultβ mice.
Nkx6.1 inactivation destabilizes beta cell identity
In T2D mouse models of metabolic stress, reduced beta cell insulin production is associated with a decrease in Nkx6.1, Pdx1 and NeuroD expression as well as increased expression of the pancreatic progenitor cell marker Ngn3 and the pluripotency markers Oct4, Nanog and L-Myc (Talchai et al., 2012). Because a subset of metabolically stressed beta cells eventually adopts other endocrine fates, it has been proposed that beta cell dedifferentiation followed by conversion into other endocrine cell types could cause beta cell failure in T2D (Talchai et al., 2012). While Nkx6.1 deletion did not affect the expression of Pdx1, NeuroD or pluripotency markers (Table S1; Figure 6A,C,E), we observed robust induction of Ngn3 expression in beta cells similar to what has been observed in models of metabolic stress (Table S1; Figure 6A,B,D,F). To investigate whether the upregulation of Ngn3 is associated with destabilization of beta cell identity, we examined pancreata from Nkx6.1 Δadultβ mice at 14 weeks of age (8 weeks after Nkx6.1 deletion) for co-expression of insulin with other pancreatic hormones. We did not observe co-expression of insulin with glucagon or pancreatic polypeptide in Nkx6.1 Δadultβ or control islets, but found a significant number of insulin+ cells co-expressing somatostatin in Nkx6.1 Δadultβ mice (Figure 6G–L). This finding is consistent with our previous observation of a beta-to-delta cell fate switch after Nkx6.1 ablation in embryonic beta cells (Schaffer et al., 2013) and suggests that although not immediately, Nkx6.1 inactivation in adult beta cells causes beta cells to adopt delta cell identity over time. In conjunction with our previous findings, these data strongly suggest that loss of Nkx6.1 in adult mice destabilizes beta cell identity, eventually leading to fate conversion of beta into delta cells.
Figure 6. Increased expression of the progenitor marker Ngn3 in Nkx6.1-deficient beta cells.
(A,B) qRT-PCR (A) and Western blot analysis (B) of islets from Nkx6.1Δ adultβ and control mice at 6 wks for multiple transcription factor genes (A) or Ngn3 (B) (n=3). (C–L) Immunofluorescence staining of pancreata from Nkx6.1 Δadultβ and control mice at 6 wks (C–F) and 14 wks (G–L). Dashed lines represent islet area. Insets are magnifications of selected areas. Arrowhead in (K) points to a cell co-expressing insulin (Ins) and somatostatin (Som). Scale bar = 20 μm. Gluc, glucagon; PP, pancreatic polypeptide; Wk, week. Data are shown as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Together, our analysis demonstrates that Nkx6.1 is a critical regulator of insulin biosynthesis and secretion, as well as proliferative capacity and cell identity in adult beta cells. The severe beta cell defects observed after Nkx6.1 inactivation suggest that restoring Nkx6.1 levels could be a therapeutic strategy in T2D.
Discussion
It is widely recognized that beta cell dysfunction, specifically the inability of beta cells to properly secrete insulin in response to high blood glucose levels is among the earliest clinical features during progression to T2D (Ferrannini, 2010). The ability to sense glucose levels and to couple this information to an insulin secretory response is bestowed upon beta cells by specialized transporters and enzymes. While the mechanisms that underlie glucose-mediated insulin secretion are fairly well understood, it has remained unclear which transcriptional regulators initiate and maintain the expression of genes that enable beta cells to perform their highly specialized function. In this study, we show that the transcription factor Nkx6.1 is a master regulator of genes that define the functional beta cell state; a role that is consistent with its exclusive expression in beta cells of the adult pancreas.
We show that conditional inactivation of Nkx6.1 in beta cells of adult mice results in overt diabetes within days of Nkx6.1 ablation. Loss of Nkx6.1 activity had an immediate and dramatic impact on the expression of genes that impart upon beta cells their unique ability to synthesize and release insulin in a regulated fashion. We found that genes involved in insulin biosynthesis (Slc30a8 and Ero1lb), glucose import (Glut2), and glucose metabolism (Pcx) are direct transcriptional target genes of Nkx6.1. In addition, Nkx6.1 ablation indirectly affected the expression of numerous genes important for beta cell function and interestingly, also beta cell proliferation (Figure 7). The finding that islet Ccnd2 levels and beta cell proliferation were decreased in Nkx6.1 conditional mutant mice was somewhat surprising, as several studies have shown that hyperglycemia has a stimulatory effect on beta cell Ccnd2 expression and self-renewal (Alonso et al., 2007; Bonner-Weir et al., 1989; Salpeter et al., 2011). We found that reduced availability of the beta cell mitogen insulin (Paris et al., 2003) had no apparent contribution to the reduced proliferative capacity of beta cells after Nkx6.1 ablation. Instead, our results suggest that the proliferative capacity of Nkx6.1-deficient beta cells is limited by reduced intracellular availability of glucose due to loss of Glut2 expression (Figure 7). These findings demonstrate an intricate link between the beta cell’s ability to import glucose and its proliferative capacity, which lends further support to the emerging concept that glucose metabolism plays a critical role in the regulation of beta cell proliferation (Porat et al., 2011; Salpeter et al., 2010; Salpeter et al., 2011). Combined with the finding that Nkx6.1 levels are reduced in T2D models of metabolic stress (Talchai et al., 2012), our work suggests that Nkx6.1 acts as a metabolic sensor that modulates both insulin secretion and proliferative capacity in response to metabolic stress. By preventing the proliferation of beta cells that have lost glucose responsiveness, the cell autonomous coupling of glucose import to beta cell proliferation might provide an inherent selection mechanism for healthy beta cells.
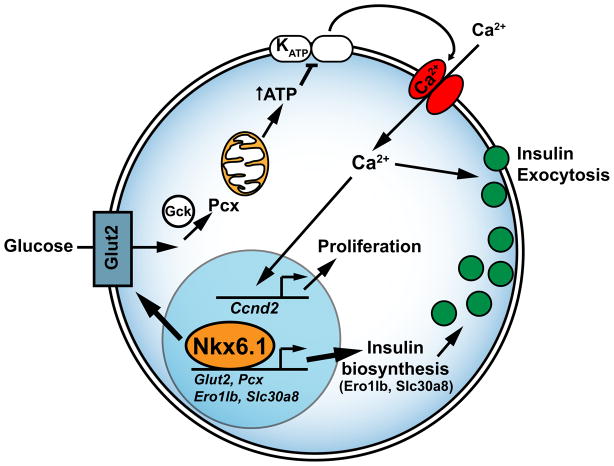
Figure 7. Nkx6.1 function in adult beta cells.
Nkx6.1 directly regulates transcription of genes encoding proteins involved in glucose uptake and metabolism (Glut2 and Pcx) and insulin biosynthesis (Ero1lb and Slc30a8). Reduced expression of these Nkx6.1 target genes affects beta cell function threefold: First, decreased glucose uptake and metabolism diminishes ATP production, leading to impaired insulin secretion via the stimulus-secretion coupling pathway. Second, by enabling glucose uptake through Glut2 regulation, Nkx6.1 indirectly controls beta cell proliferative capacity. In the absence of Nkx6.1, expression of Ccnd2, which encodes the critical beta cell mitogen Cyclin D2, is reduced and beta cell proliferation is decreased. Reconstituting Glut2 expression in Nkx6.1-deficient beta cells restores Ccnd2 levels and beta cell proliferation. Third, insulin biosynthesis is severely impaired, leading to reduced production of mature insulin and an overabundance of immature secretory vesicles. Glut2, glucose transporter 2; Gck, glucokinase; Pcx, pyruvate carboxylase; KATP, ATP-sensitive potassium channel.
Our study also reconciles previously reported, seemingly contradictory findings about the role of Nkx6.1 in beta cell proliferation. We recently reported that transgenic overexpression of Nkx6.1 in beta cells of adult mice in vivo had no positive effect on beta cell proliferation or beta cell mass (Schaffer et al., 2011). By contrast, virus-mediated expression of Nkx6.1 in cultured islets had pro-proliferative activity (Schisler et al., 2008). A possible explanation for this apparent contradiction is that in vitro culture of islets compromises Nkx6.1 expression levels. It is known that once removed from their niche and put into culture beta cells quickly lose Glut2 expression and cease to proliferate (Weinberg et al., 2007), indicating that expression of upstream Glut2 regulators, including Nkx6.1 could also be compromised. Thus, the observed pro-proliferative effect of Nkx6.1 in vitro may be explained by virally expressed Nkx6.1 restoring reduced Nkx6.1 levels and in turn also Glut2 and Ccnd2 levels in cultured islets. By contrast, increasing Nkx6.1 levels above normal in healthy beta cells in vivo appears to have no further stimulatory effect on glucose import and beta cell proliferation.
After Nkx6.1 deletion, we observed an extremely rapid decline in beta cell insulin content. The loss of insulin was not associated with beta cell death, revealing a striking similarity between Nkx6.1-deficient beta cells and “empty” beta cells observed in mouse models of T2D (Talchai et al., 2012). Furthermore, as reported under conditions of metabolic stress (Talchai et al., 2012), we found that reduced Nkx6.1 expression was also accompanied by de-repression of the endocrine progenitor cell marker Ngn3. Based on the observation that beta cells undergo fate conversion into non-beta endocrine cell types in T2D models (Talchai et al., 2012), the loss of beta cell features and gain of Ngn3 expression has been proposed to render beta cells plastic and more prone to changing their identity.
Consistent with this notion, we observed that a subset of Nkx6.1-deficient beta cells ectopically expressed somatostatin eight weeks after Nkx6.1 deletion. Previously, we have shown with lineage tracing studies that Nkx6.1 deletion in embryonic beta cells leads to a rapid beta-to-delta cell fate switch (Schaffer et al., 2013). However, conversion of beta cells into other non-beta endocrine cell types was not observed after Nkx6.1 inactivation in embryonic beta cells. Similarly, after Nkx6.1 deletion in adult beta cells, we observed co-expression of insulin exclusively with somatostatin but no other pancreatic hormones. Thus, loss of Nkx6.1 leads to selective de-repression of delta cell genes in both embryonic and adult beta cells. However, fate conversion is more complete and occurs more rapidly when Nkx6.1 is inactivated in immature beta cells. Therefore, a sequential loss of beta cell traits preceding the adoption of alternative endocrine cell fates seen after adult Nkx6.1 inactivation closely mirrors the gradual loss of functional beta cell mass observed in T2D models (Talchai et al., 2012).
Our study provides support for an evolving concept that transcription factors, such as Nkx6.1 and FoxO1 (Talchai et al., 2012), are critical for maintaining beta cells in their differentiated state. A key question that requires further exploration is whether a destabilized beta cell state is observed in humans and possibly contributes to the pathogenesis of T2D. The observation that NKX6.1 expression is decreased in beta cells from humans with T2D (Guo et al., 2013) suggests that findings in rodent models might indeed be relevant to human disease. Future studies will need to explore which aspects of the rodent phenotype are also found in humans and how loss of beta cell features relates to disease progression. Such knowledge could identify a window for therapeutic intervention, during which the functional beta cell state can be restored before beta cells convert into other endocrine cell types.
Experimental Procedures
Mouse Strains
The following mouse strains were utilized in this study: Pdx1CreER™ (Gu et al., 2002), Nkx6.1+/− (Sander et al., 2000), and Nkx6.1flox mice (Schaffer et al., 2013). All animals carrying the Nkx6.1flox allele were maintained on a mixed 129Sv/C57Bl6/J genetic background. Unless otherwise stated in the text, male mice were used for experiments. Tamoxifen (Sigma) was dissolved in corn oil at 20mg/mL and 2mg was injected subcutaneously four times over a two week period. All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of California, San Diego.
Tissue preparation and immunohistochemistry
Tissue preparation, immunofluorescence staining, TUNEL assays, and morphometry were performed as previously described (Schaffer et al., 2010; Schaffer et al., 2013). A description of the antibodies used and detailed methods are provided in the Extended Experimental Procedures.
Microscopy and image analysis
All immunofluorescent images were acquired using a Zeiss AxioOberver. Z1 microscope (Carl Zeiss, New York, NY) with the Zeiss ApoTome module and processed in Zeiss AxioVision Release 4.8 and Adobe Photoshop CS5.1. Only brightness and contrast was adjusted in images in accordance with the Journal of Cell Biology figure manipulation guidelines.
Glucose tolerance tests, GSIS assays, insulin and proinsulin measurements
Glucose tolerance tests, GSIS assays, and insulin measurements were performed as previously described (Schaffer et al., 2011). Proinsulin measurements were performed on whole pancreatic lysates using a mouse proinsulin ELISA (ALPCO). Details are described in Extended Experimental Procedures.
Islet isolation and culture
Islet isolations were performed as previously described (Schaffer et al., 2011) with Liberase TL (Roche). For incubation of islets with chemical compounds, RPMI supplemented with 2.7mM glucose and 1% BSA was used. Nkx6.1 Δadultβ islets were incubated with 100nM recombinant insulin (Sigma), 60μM +/− Bay K8644 (Sigma), or 10μM glucokinase activator (GKA) (EMD Calbiochem) and control islets were incubated with DMSO (ATCC) for three hours. For infection of islets with Ad-Glut2 and Ad-β-gal, islets were dispersed and plated before infection as described previously (Fiaschi-Taesch et al., 2009). Further details are provided in the Extended Experimental Procedures.
Microarray
RNA was isolated from islets obtained from six littermate control and six Nkx6.1 Δadultβ mice. Islets from two mice of identical genotypes were pooled to generate three independent cDNA probes per genotype for array hybridization. Labeled cDNA was hybridized to whole mouse gene expression G2519F microarrays (Agilent technologies). A detailed description of microarray analysis, qRT-PCR analysis, and a list of primer sequences can be found in Extended Experimental Procedures.
Chromatin immunoprecipitation and sequencing
Chromatin immunoprecipitation (ChIP) was performed as previously described (Schaffer et al., 2013) with rabbit anti-Nkx6.1 antiserum (1:250) on sheared chromatin obtained from ~10,000 islet equivalents (1000 cells per islet) isolated from C57BL/6J mice. ChIP-seq libraries were prepared as per Illumina’s instructions (http://www.illumina.com). Sequencing was performed on an Illumina/Solexa Genome Analyzer II in accordance with the manufacturer’s protocols. Data analysis was performed using Hypergeometric Optimization of Motif EnRichment (HOMER) (Heinz et al., 2010). Peak annotation and de novo motif analysis were performed using HOMER and venn diagrams were generated using BioVenn (Hulsen et al., 2008). See Extended Experimental Procedures for details.
Statistics
Unless otherwise stated, all values are shown as mean ± SEM; P-values were calculated using an unpaired Student’s t-test in Microsoft Excel; Hypergeometric distribution was determined using R. P<0.05 was considered significant.
Supplementary Material
Highlights.
Beta cell-specific Nkx6.1 inactivation in adult mice causes diabetes
A gene network necessary for beta cell function is regulated by Nkx6.1
Nkx6.1 controls beta cell proliferation indirectly by regulating glucose import
Nkx6.1 deficiency results in gradual loss of beta cell identity with age
Acknowledgments
We are grateful to D. Melton (Harvard University) for Pdx1-CreER™ mice, C. Newgard (Duke University) for Ad-Glut2 and Ad-β-gal adenoviruses and the following colleagues for anti-sera: Palle Serup (DanStem) for anti-Nkx6.1; C. Wright (Vanderbilt University) for anti-Pdx1; M. Huising (Salk Institute) for anti-Ucn3; D. Ron (University of Cambridge) for anti-Ero1lb; D. Steiner (University of Chicago) for anti-PC1/3; C. Kioussi (Oregon State University) for anti-GFP. We thank N. Rosenblatt for mouse husbandry; R. Xie for assistance with ChIP-seq library preparation; the University of California, San Diego BioGEM Core for microarray experiments; the University of Pennsylvania Functional Genomics Core for ChIP-seq analysis; the University of California, San Diego Electron Microscopy Core for assistance with sample preparation for electron microscopy; and members of the Sander laboratory for helpful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health (NIH) grants R01-DK068471 and U01-DK089567 to M.S.
Footnotes
Accession numbers
The GEO (http://www.ncbi.nlm.nih.gov/geo/) accession number for the microarray data set reported in this paper is GSE40470. The GEO (http://www.ncbi.nlm.nih.gov/geo/) accession number for the ChIP-seq data set reported in this paper is GSE40975.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O’Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo EA, Meur G, Rutter GA. Glucose regulates free cytosolic Zn(2)(+) concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet beta-cells. J Biol Chem. 2011;286:25778–25789. doi: 10.1074/jbc.M111.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhinge AA, Kim J, Euskirchen GM, Snyder M, Iyer VR. Mapping the chromosomal targets of STAT1 by Sequence Tag Analysis of Genomic Enrichment (STAGE) Genome Res. 2007;17:910–916. doi: 10.1101/gr.5574907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Cerasi E, Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol (Copenh) 1967;55:278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The stunned beta cell: a brief history. Cell Metab. 2010;11:349–352. doi: 10.1016/j.cmet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58:882–893. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froguel P, Vaxillaire M, Sun F, Velho G, Zouali H, Butel MO, Lesage S, Vionnet N, Clement K, Fougerousse F, et al. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356:162–164. doi: 10.1038/356162a0. [DOI] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol. 2005;171:99–109. doi: 10.1083/jcb.200505179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guillam MT, Dupraz P, Thorens B. Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes. 2000;49:1485–1491. doi: 10.2337/diabetes.49.9.1485. [DOI] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013 doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosker JP, Rudenski AS, Burnett MA, Matthews DR, Turner RC. Similar reduction of first- and second-phase B-cell responses at three different glucose levels in type II diabetes and the effect of gliclazide therapy. Metabolism. 1989;38:767–772. doi: 10.1016/0026-0495(89)90064-4. [DOI] [PubMed] [Google Scholar]
- Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Jorgensen MC, Vestergard Petersen H, Ericson J, Madsen OD, Serup P. Cloning and DNA-binding properties of the rat pancreatic beta-cell-specific factor Nkx6.1. FEBS Lett. 1999;461:287–294. doi: 10.1016/s0014-5793(99)01436-2. [DOI] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol. 2005;25:3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci U S A. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Yanaihara C, Yanaihara N, Halban P, Renold AE, Perrelet A. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol. 1984;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris M, Bernard-Kargar C, Berthault MF, Bouwens L, Ktorza A. Specific and combined effects of insulin and glucose on functional pancreatic beta-cell mass in vivo in adult rats. Endocrinology. 2003;144:2717–2727. doi: 10.1210/en.2002-221112. [DOI] [PubMed] [Google Scholar]
- Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Piston DW, Knobel SM, Postic C, Shelton KD, Magnuson MA. Adenovirus-mediated knockout of a conditional glucokinase gene in isolated pancreatic islets reveals an essential role for proximal metabolic coupling events in glucose-stimulated insulin secretion. J Biol Chem. 1999;274:1000–1004. doi: 10.1074/jbc.274.2.1000. [DOI] [PubMed] [Google Scholar]
- Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113:635–645. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development. 2010;137:3205–3213. doi: 10.1242/dev.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SJ, Klochendler A, Weinberg-Corem N, Porat S, Granot Z, Shapiro AM, Magnuson MA, Eden A, Grimsby J, Glaser B, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic beta-cells through glycolysis and calcium channels. Endocrinology. 2011;152:2589–2598. doi: 10.1210/en.2010-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, Rubenstein JL. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 2000;14:2134–2139. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Freude KK, Nelson SB, Sander M. Nkx6 Transcription Factors and Ptf1a Function as Antagonistic Lineage Determinants in Multipotent Pancreatic Progenitors. Dev Cell. 2010;18:1022–1029. doi: 10.1016/j.devcel.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Taylor BL, Benthuysen JR, Liu J, Thorel F, Yuan W, Jiao Y, Kaestner KH, Herrera PL, Magnuson MA, et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet. 2013;9:e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Yang AJ, Thorel F, Herrera PL, Sander M. Transgenic overexpression of the transcription factor Nkx6.1 in beta-cells of mice does not increase beta-cell proliferation, beta-cell mass, or improve glucose clearance. Mol Endocrinol. 2011;25:1904–1914. doi: 10.1210/me.2011-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG, et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol. 2008;28:3465–3476. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Jensen PB, Taylor DG, Becker TC, Knop FK, Takekawa S, German M, Weir GC, Lu D, Mirmira RG, et al. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc Natl Acad Sci U S A. 2005;102:7297–7302. doi: 10.1073/pnas.0502168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol Metab. 2012;23:477–487. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi Y, Sakura H, Yasuda K, Iwamoto K, Takahashi N, Ito K, Kasai H, Suzuki H, Ueda O, Kamada N, et al. Pancreatic beta-cell-specific targeted disruption of glucokinase gene. Diabetes mellitus due to defective insulin secretion to glucose. J Biol Chem. 1995;270:30253–30256. doi: 10.1074/jbc.270.51.30253. [DOI] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes. 2007;56:1299–1304. doi: 10.2337/db06-1654. [DOI] [PubMed] [Google Scholar]
- Wicksteed B, Alarcon C, Briaud I, Lingohr MK, Rhodes CJ. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet beta-cells but not regulated via a positive feedback of secreted insulin. J Biol Chem. 2003;278:42080–42090. doi: 10.1074/jbc.M303509200. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Sheng G, Jun S, Desplan C. Conservation and diversification in homeodomain-DNA interactions: a comparative genetic analysis. Proc Natl Acad Sci U S A. 1996;93:6886–6891. doi: 10.1073/pnas.93.14.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi N, Kanamori M, Horikawa Y, Takeda J, Sanke T, Furuta H, Nanjo K, Mori H, Kasuga M, Hara K, et al. Association studies of variants in the genes involved in pancreatic beta-cell function in type 2 diabetes in Japanese subjects. Diabetes. 2006;55:2379–2386. doi: 10.2337/db05-1203. [DOI] [PubMed] [Google Scholar]
- Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A. 2002;99:10299–10304. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito E, Chin KT, Blais J, Harding HP, Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol. 2010;188:821–832. doi: 10.1083/jcb.200911086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.