Abstract
Rationale
The mammalian target of rapamycin complex 1 (mTORC1) inhibitor, rapamycin, has been shown to decrease atherosclerosis, even while increasing plasma LDL levels. This suggests an anti-atherogenic effect possibly mediated by modulation of inflammatory responses in atherosclerotic plaques.
Objective
To assess the role of macrophage mTORC1 in atherogenesis.
Methods and Results
We transplanted bone marrow from mice in which a key mTORC1 adaptor, Raptor, was deleted in macrophages by Cre/loxP recombination (Mac-RapKO mice) into Ldlr-/- mice and then fed them the Western-type diet (WTD). Atherosclerotic lesions from Mac-RapKO mice showed decreased infiltration of macrophages, lesion size and chemokine gene expression compared with control mice. Treatment of macrophages with minimally modified LDL (mmLDL) resulted in increased levels of chemokine mRNAs and STAT3 phosphorylation; these effects were reduced in Mac-RapKO macrophages. While wild-type and Mac-RapKO macrophages showed similar STAT3 phosphorylation on Tyr705, Mac-RapKO macrophages showed decreased STAT3 Ser727 phosphorylation in response to mmLDL treatment and decreased Ccl2 promoter binding of STAT3.
Conclusions
The results demonstrate cross-talk between nutritionally-induced mTORC1 signaling and mmLDL-mediated inflammatory signaling via combinatorial phosphorylation of STAT3 in macrophages, leading to increased STAT3 activity on the CCL2 (MCP-1)promoter with pro-atherogenic consequences.
Keywords: Atherosclerosis, mTORC1, chemokine, macrophage
Introduction
The mammalian target of rapamycin (mTOR) is a serine–threonine kinase that as part of the mTORC1 complex regulates anabolic and catabolic processes required for autophagy, RNA translation, protein synthesis, ribosome biogenesis, and cell survival.1 mTOR acts as a central regulator of cell growth and proliferation by integrating signals from nutrients, energy status, and growth factors, in part by regulating the phosphorylation of p70 S6 kinase (p70S6k) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1).1 Upstream, the TSC1/2 complex negatively regulates mTORC1 activity.2 Raptor (regulatory associated protein of mTOR) is an mTOR binding partner that also binds p70S6k and 4E-BP1 and is essential for mTOR signaling.3, 4 While whole body RaptorKO mice are embryonic lethal,5 it has been possible to probe tissue specific functions of mTOR using Tsc1flox/flox or Raptorflox/flox (Rapflox/flox) mice, with Cre recombinase expression resulting in increased or decreased mTOR activity respectively.6
Rapamycin is an immunosuppressant with potent anti-proliferative and anti-inflammatory effects that is used to prevent cardiac transplantation vasculopathy.7 However, treatment of patients with rapamycin also leads to increased plasma LDL levels.8 In mouse models mTORC1 inhibition caused downregulation of LDL receptor levels in liver and decreased the activity of lipoprotein lipase (LPL), leading to higher plasma LDL and triglyceride levels.9, 10 Using liver specific Tsc1 knockout (Li-Tsc1KO)and Raptor knockout (Li-RapKO) mice, we showed that the mechanism of increased LDL involved mTOR—mediated suppression of Pcsk9 mRNA resulting in up-regulation of LDLR protein.9 Paradoxically, the mTORC1 inhibitor, rapamycin, was reported to reduce inflammation and atherosclerosis despite increasing plasma LDL levels.8 Since LDL levels are usually a dominant factor in atherogenesis, this suggested a potent anti-atherogenic effect of mTORC1 inhibition independent of plasma LDL levels. Inflammation has an important role in atherosclerosis,11 raising the possibility that rapamycin is working through an anti-inflammatory mechanism.
In view of the central role of macrophages in atherosclerotic inflammation, we tested this hypothesis by crossing Tsc1flox/flox and Rapflox/flox mice with LysM-Cre mice, resulting in increased and decreased mTORC1 activity in macrophages, respectively; and then transplanted the bone marrow (BM) of these mice into Ldlr-/- recipients followed by WTD feeding to induce atherogenesis. While studies with the Mac-Tsc1KO mice were limited by premature death, the Mac-RapKO BM transplanted Ldlr-/- mice displayed reduced macrophage content in atherosclerotic lesions. This appeared to be related to decreased macrophage expression of pro-atherogenic chemokines. Further mechanistic studies revealed that when macrophages were treated with minimally modified LDL (mmLDL), mTORC1 activity amplified the induction of chemokines by increasing IL6 signaling.
Methods
An expanded Methods section is available in the online data supplement.
Animal and diet
Raptorflox/flox (The Jackson Laboratory, stock number 013188)or Tsc1flox/flox mice were mated with transgenic mice expressing Cre recombinase under the control of the LysM promoter (Lysm-Cre, Jackson Laboratories, stock number 004781) to generate mice with or without Raptor (Mac-RapKO) or Tsc1 (Mac-Tsc1KO) expression in myeloid cells. Raptorflox/flox (Rapflox/flox) or Tsc1flox/flox littermates without the Cre recombinase transgene were used as controls throughout the study. Tsc1flox/flox mice backcrossed to C57BL/6J for 9 generations were kindly provided by Dr David Kwiatkowski. Ldlr-/- mice (stock number 002207) were purchased from The Jackson Laboratory. Mice were fed a WTD (21% milk fat, 0.2% cholesterol; Harlan Teklad, TD88137) or chow diet (Purina Mills diet 5053). All protocols were approved by the Institutional Animal Care and Use Committee of Columbia University.
Results
Atherosclerosis is decreased in BM transplanted Mac-RapKO Ldlr-/- mice
In a preliminary study, we found that phospho-S6 ribosomal protein (phosphoS6), a downstream target of mTORC1, was increased in peritoneal macrophages from Ldlr-/- mice fed an atherogenic Western type diet (WTD) compared to chow (Online Figure IA), and also in chow-fed ob/ob mice compared to wild type mice (data not shown). To investigate the role of mTORC1 in macrophages, we crossed Rapflox/flox with LysM-Cre mice, which are known to delete targets in macrophages and neutrophils and to a lesser extent in monocytes and myeloid progenitors.12 For simplicity we refer to these mice as Mac-RapKO mice, while recognizing that the knockout is not completely macrophage-specific. In addition, we carried out selected studies in Mac-Tsc1KO mice; since TSC1 is an upstream inhibitor of mTORC1, these mice have increased mTORC1 activity. However, due to premature mortality at about 4 months of age, we were not able to carry out atherosclerosis studies in these mice. We transplanted Ldlr-/- mice with Mac-Rap-KO (Ldlr-/--Mac-RapKO) and Rapflox/flox (Ldlr-/--WT) BM. In peritoneal macrophages, mTORC1 activity reflected by phosphoS6 levels was lower in Ldlr-/--Mac-RapKO compared with Ldlr-/--WT mice (Online Figure IB). Five weeks after BM transplantation, reconstitution of the BM was >90% (Online Figure IC). The body weights, spleen weights, triglyceride levels, and cholesterol levels were similar in the two groups (Figure 1A, Online Figure ID). There was no difference in the lipoprotein cholesterol distribution (Figure 1B). Ldlr-/- mice transplanted with BM from Rapflox/flox or Mac-RapKO had similar levels of neutrophils, monocytes and Ly6Chi monocytes in peripheral blood after 10 weeks of WTD feeding (Online Figure IF). In contrast, the percentages of Ly6Chi and F4/80 positive macrophages in spleen were considerably lower in Ldlr-/--Mac-RapKO than Ldlr-/--WT mice at the 10 week timepoint (Online Figure IG).
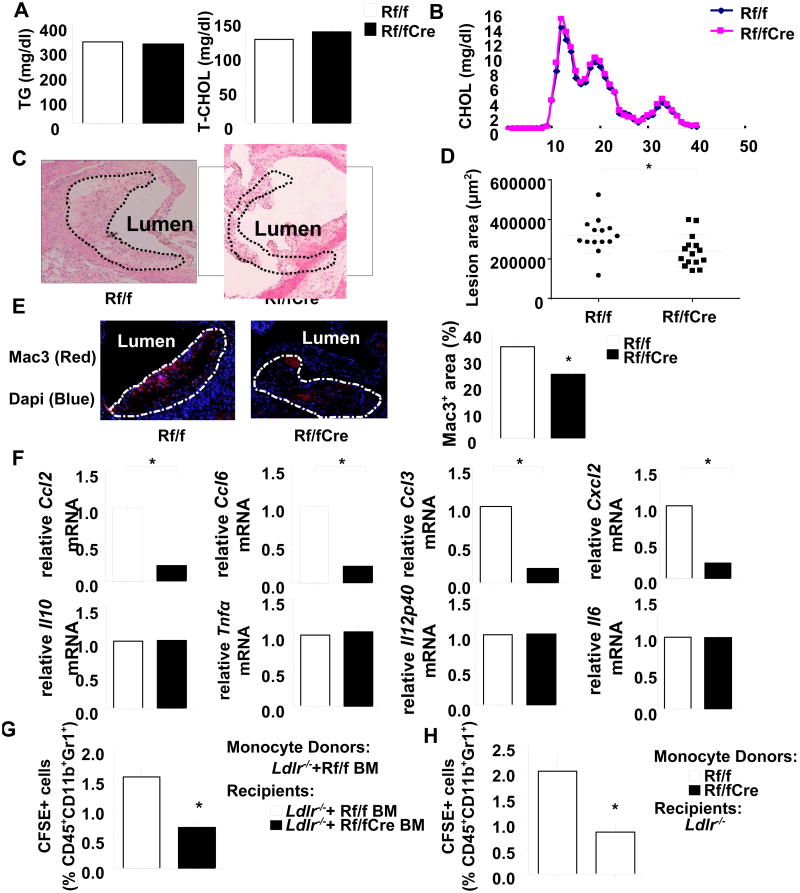
Figure 1. Plasma lipid levels and atherosclerotic lesions in the aortic root of Ldlr-/- mice transplanted with Raptorflox/flox or Mac-RapKO BM.
Mice were fed WTD for 10 weeks. A. Plasma triglycerides and total cholesterol levels were measured in Ldlr-/- mice with Raptorflox/flox (Rf/f) or Mac-RapKO (Rf/fCre) BM transplantation. N=5-8. B. Cholesterol lipoprotein distribution as determined by fast performance liquid chromatography on pooled plasma samples. N=5-8 per pool. C. H&E staining of representative aortic root sections. Atherosclerotic lesions are demarcated by the dashed lines. N=14-15. D. Quantification of lesions by morphometric analysis. E. Sections were stained with a Mac3 antibody for the presence of macrophages (left), and values represent the percentages of Mac3+ area/total lesion area (right). N=5. F. mRNA levels of the indicated targets were assayed in LCM-captured RNA obtained from atherosclerotic lesions. The data were normalized to the expression level of β-actin mRNA. N=5-7. *P<0.05. (G) Classical monocytes sorted from Ldlr-/- mice transplanted with Rapflox/flox BM were labeled with CFSE, and injected intravenously into Ldlr-/- mice that had been transplanted with BM from either Rapflox/flox or Mac-RapKO mice ; (H) monocytes from Rapflox/flox or Mac-RapKO mice were transplanted into Ldlr-/- mice. CFSE labeled cells (as a percentage of CD45+CD11b+Gr1+ cells) in the aorta were quantified by flow cytometry. N=5. *P < 0.05.
Following ten weeks of WTD feeding, mice were sacrificed and atherogenesis was assessed in the aortic root. In Ldlr-/- mice, macrophage deficiency of Raptor decreased atherosclerotic lesion area by 25% compared to the Ldlr-/--WT control group (Figure 1C, 1D). To further analyze the lesion phenotype, we examined lesion composition. Morphological analyses of the cross-sectional lesions showed no significant difference in collagen content (Online Figure IIA). In contrast, analysis of macrophage and cholesterol ester content uncovered significant reductions in mac-3+ immunostaining and oil red O staining in Mac-RapKO BM transplanted Ldlr-/- mice (Figure 1E and Online Figure IIC). The smooth muscle cell and T cell contents remained unchanged between groups as assessed by immunostaining with α-actin and CD3 antibodies, respectively (Online Figure IIB, IID). These results suggest that decreased macrophage foam cell accumulation in lesions contributes to the reduced development of atherosclerosis in Ldlr-/--Mac-RapKO mice.
Lesional macrophage accumulation is modulated by many processes such as local proliferation,13 apoptosis14 and monocyte recruitment. As shown in Online Figure IIE and IIF, Ki67-positive and TUNEL-positive macrophage staining were similar between the groups, suggesting no difference in local proliferation or apoptosis of macrophages, respectively. In addition, since reduced mTORC1 activity might lead to increased macrophage autophagy, which could contribute to decreased atherosclerosis, we stained lesions for P62, an autophagy marker. There was a 50% decrease in P62 expression, indicative of moderately increased autophagy (Online Figure IIIA, IIIB). However, Razani et al15 showed that a 3.5 fold increase of P62 in Beclin-Het autophagy deficient mice was insufficient to change atherosclerosis, while 7-9 fold increases were associated with moderately reduced lesion formation. This suggests that small changes in autophagy such as we observed might not affect atherogenesis.
To further address potential anti-atherogenic mechanisms in Mac-RapKO BM transplanted mice, we performed laser capture microscopy (LCM) in lesions and assessed the mRNA expression of pro-atherogenic chemokines and cytokines in macrophage-rich areas. Interestingly, this showed markedly decreased mRNA expression of chemokines Ccl2/Mcp-1, Ccl3/Mip1α, Ccl6, and Cxcl2 in Ldlr-/--Mac-RapKO mice compared to control, whereas there were no significant differences in Il10, Tnfα, Il12p40 and Il6 mRNA levels between the two groups (Figure 1F). All of the upregulated chemokines are involved in monocyte recruitment and have been reported to accelerate atherogenesis.16-18 Consistent with these findings, the level of CCL2 in plasma was decreased to 32% of controls in Ldlr-/--Mac-RapKO mice (Online Figure IE). These results suggested that the attenuation of the development of atherosclerosis in Mac-RapKO BM transplanted mice was at least partly due to decreased macrophage chemokine production, leading to reduced monocyte recruitment into lesions.
To assess monocyte recruitment, we performed adoptive transfer experiments using classical (Ly6Chi) monocytes sorted from BM of Ldlr-/- mice that had been transplanted with Rapflox/flox BM and fed the WTD for 8 weeks. From the donors, 1×106 monocytes were labeled with carboxy-fluorescein succinimidyl ester (CFSE) and injected intravenously into Ldlr-/- mice that had been transplanted with Rapflox/flox or Mac-RapKO BM and fed the WTD for 8 weeks. After 2 days, adoptively transferred classical monocytes were quantified in homogenates of the whole aorta recipient mice (Figure 1G). The recruitment of monocytes was significantly decreased in Ldlr-/- mice transplanted with Mac-RapKO BM compared to Rapflox/flox-transplanted controls. In addition, to study whether Raptor deficiency intrinsically affected monocyte migration in response to chemokines, we sorted classical (Ly6Chi) monocytes from BM of Rapflox/flox and Mac-RapKO mice, labeled these monocytes with CFSE and injected them intravenously into Ldlr-/- mice that had been on WTD for 8 weeks. Monocyte Raptor deficiency also significantly decreased the uptake of monocytes in the whole aorta of Ldlr-/-recipient mice (Figure 1H and Online Figure IIIC, SIIID).
Chemokine expression induced by mmLDL is regulated by mTORC1
Oxidation or other modifications of LDL are thought to have an important role in atherogenesis.19 Minimally modified LDL (mmLDL) prepared by incubating LDL with cells expressing 15-lipoxygenase, displays many pro-atherogenic properties.20 To investigate the mechanism of the regulation of chemokines by mTORC1 in macrophages, we treated BM derived macrophages (BMDM) from LysmCre, Mac-RapKO and Rapflox/flox mice with mmLDL. In LysmCre and Rapflox/flox macrophages, mmLDL treatment resulted in induction of Tnfα, Il6, Il10, Il12p40, and chemokine gene expression. In Mac-RapKO macrophages, there was a similar response of Il-6, Il10, Il12p40, and Tnfα genes (Figure 2A). However the expression of Ccl2, Ccl3, Ccl6, Ccl7, and Cxcl2 was reduced by 48%, 41%, 61%, 63%, and 63%, respectively, compared with floxed controls (Figure 2B). An additional control group consisting of macrophages from LysM-Cre mice showed no difference in responses compared to the Rapflox/flox controls. Consistent with these findings, CCL2 levels in the cell culture media were decreased by 50% (Figure 2C).
Figure 2. mmLDL mediated inflammatory gene expression in bone marrow derived macrophages (BMDMs) from LysmCre, Raptorflox/flox and Mac-RapKO mice.
A. BMDMs from LysmCre, Raptorflox/flox (Rf/f) and Mac-RapKO mice (Rf/fCre) were treated with mmLDL (50ug/ml) for 2 hours. Expression levels of Tnfα, Il6, Il10 and Il12p40 were measured by quantitative -PCR and normalized to expression levels observed without treatment (Ctrl). B. Ccl2, Ccl3, Ccl6, Ccl7 and Cxcl2 gene expression in BMDMs of LysmCre, Rf/f and Rf/fCre mice after 2 hours of mmLDL (50 μg/ml) treatment. N=3, *P<0.05 C. BMDMs were treated with mmLDL for 2 hours, washed with PBS and then incubated with fresh media for another 18 hours. CCL2 in the media was measured by ELISA and values were normalized to total cellular protein. N=3, *P<0.05, N.S., not significant
The findings in Mac-RapKO mice suggested that the anti-inflammatory effects of mTORC1 inhibition may be mediated via repression of chemokine production. To further test this hypothesis, we isolated BMDM from Mac-Tsc1KO and Tsc1flox/flox mice and measured gene expression. mmLDL increased the levels of chemokine expresson in macrophages of both genotypes but the effects were significantly more pronounced for Mac-Tsc1KO than control macrophages (Online Figure IVA). While there were trends toward higher levels of induced Tnfα and Il-6 mRNAs in the Mac-Tsc1KO mice (Online Figure IVB), these effects were less pronounced than for the chemokine genes. As expected, rapamycin treatment reversed these effects, but not for Tnfα and Il-6 (Online Figure IVA and IVB).
Mechanism of the regulation of chemokine expression by mTORC1
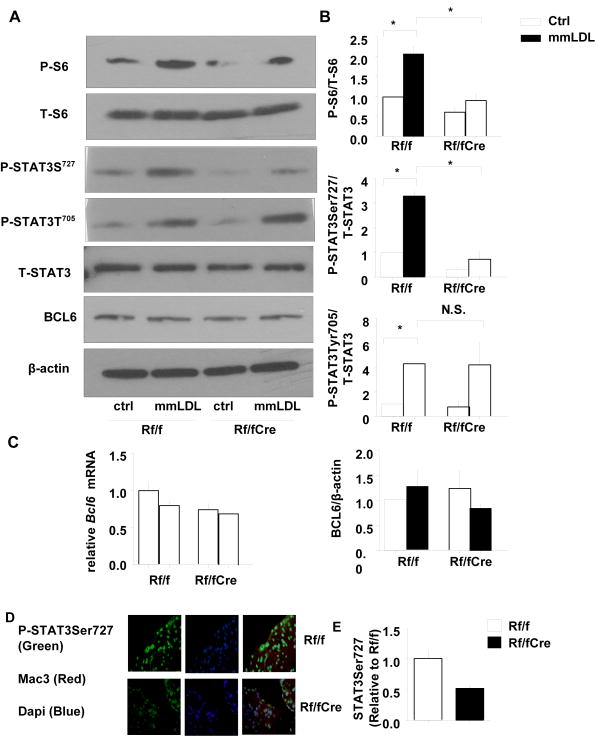
To further examine the mechanisms mediating the modification of chemokine expression by mTORC1, we explored whether the transcriptional repressor BCL6, which has a key role in regulating macrophage chemokine expression, was involved.21-24 Barish et al22 employed macrophages from Bcl6 knockout mice and showed that BCL6 containing promoter complexes constrained inflammatory cytokine and chemokine responses to mmLDL. However, the BCL6 protein and mRNA expression were not changed by macrophage Raptor deficiency (Figure 3A and 3C). Interestingly, the sequence of the BCL-6 consensus binding site overlaps that of STAT transcription factor–binding sites,25, 26 and competition between BCL-6 and STATs likely modulates innate immunological functions.26 STAT3 is phosphorylated by JAK tyrosine kinases at Tyr705 which transduces the signals of IL6. However, STAT3 also requires phosphorylation on Ser727 to achieve maximal transcriptional activity via formation of stable STAT3-STAT3-DNA complexes.27, 28 Several reports suggested that mTORC1 is responsible for STAT3 serine phosphorylation.29, 30 Here, we found that STAT3 Ser727 phosphorylation in response to mmLDL was reduced in Mac-RapKO macrophages (3.2-fold increase in controls, 2.0-fold increase in Mac-RapKO macrophages, p<0.05);as expected, S6 phosphorylation was impaired in Mac-RapKO macrophages (Figure 3A, 3B). In contrast, the phosphorylation of STAT3 at Tyr705 was similar in the two groups (Figure 3A, 3B). As shown by immunofluorescence confocal microscopy, nuclear phosphoSTAT3 Ser727 staining was increased by mmLDL and this was reduced by Raptor deficiency (Online Figure V), which is consistent with that of lesional macrophages (Figure 3C and 3D).
Figure 3. Regulation of STAT3 phosphorylation by Raptor deficiency in BMDMs.
A. Western blot analysis of S6 and STAT3 phosphorylation and BCL6 expression. β-Actin was used as internal control. P-, phosphorylated; T-, total B. Quantification of protein levels N=3, *P < 0.05. Rf/f vs. Rf/f+mmLDL, Rf/f+mmLDL vs. Rf/fCre+mmLDL, one-way ANOVA, Bonferroni post-test. N.S., not significant C. Measurement of macrophage Bcl6 mRNA by qPCR. N=3 D. Sections of the aortic root were double-stained with Mac3 and phospho-STAT3Ser727 antibodies. E. Relative fluorescence intensity for phospho-STAT3Ser727 expression (green) in the macrophage-dense areas (red) of the lesions is shown. Quantification of phospho-STAT3Ser727 in lesion macrophage area normalized to the value of Rf/f. N=5. *P < 0.05.
Opposite to Mac-RapKO macrophages, the phosphorylation of S6 induced by mmLDL was increased by 2.3-fold in Mac-Tsc1KO BMDM compared to controls (Online Figure VIA, VIB). Moreover, the phosphorylation of STAT3 at Ser727 was induced 2.2-fold in Tsc1 knockout BMDM compared to the Tsc1flox/flox group, while Tyr705 was not influenced (Online Figure VIA, VIB). Pre-incubation with rapamycin reversed the induction of both phosphoS6 and phosphoSTAT3 Ser727 (Online Figure VIA, VIB). However, the phosphorylation of STAT3 at Tyr705 was not influenced by TSC1 deficiency or rapamycin treatment (Online Figure VIA, VIB).
mTOR activation enhances the competition between STAT3 and BCL-6 on the Ccl2 promoter
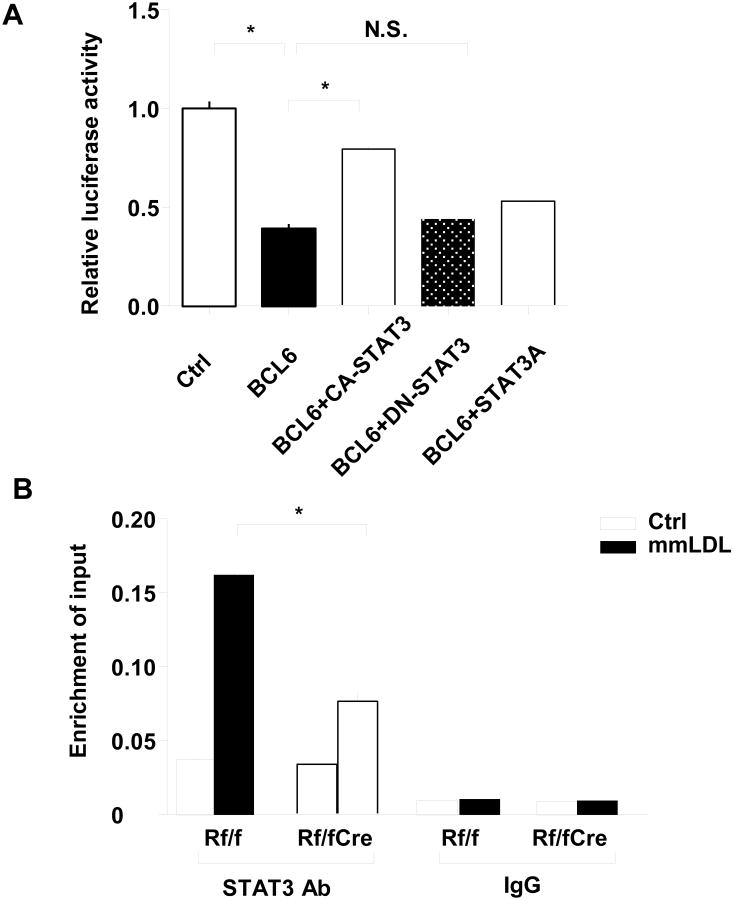
To further investigate the underlying mechanism of the regulation of chemokines by mTORC1, we co-transfected a Bcl6 expression plasmid and Ccl2 promoter-reporter plasmid into HEK- 293T cells. Compared with the empty vehicle transfection control, the luciferase activity was reduced by Bcl6 transfection. Co-transfection of wild-type STAT3 (CA-STAT3) reversed the reduction of Bcl6, while neither dominant negative (DN-STAT3) nor Ser727 mutated versions of STAT3 (STAT3A) had any effect on the decreased luciferase activity caused by Bcl6 (Figure 4A). This suggests that phosphorylation of Ser727, as mediated by mTORC1, is essential for the ability of STAT3 to reverse the inhibitory effect of BCL-6 on the chemokine promoter. We next co-transfected RAW macrophages with the Ccl2 promoter plasmid and either control or Bcl6 plasmid. Luciferase activity induced by IL6 was partly reversed by Bcl6 expression (Online Figure VIIA). Notably, quantitative chromatin-immunoprecipitation (ChIP) assays showed impaired enrichment of STAT3 protein at the Ccl2 promoter in Mac-RapKO BMDM compared with control BMDM in response to mmLDL treatment (Figure 4B). Cyclin D2 (CCND2) and Gapdh were performed as positive and negative control, respectively (Online Figure VIIB). Together, these experiments suggest that the phosphorylation of Ser 727 of STAT3 enhances its ability to counteract the effect of BCL-6 at the Ccl2 promoter.
Figure 4. Mechanism of CCl2 regulation by Raptor.
A. Ccl2 promoter inserted into a reporter system was cotransfected with CA-STAT3, DN-STAT3 or STAT3A plasmids in the presence or absence of Bcl6 plasmid in 293 cell lines. An empty vectors were used as normalization control. N=3, *P < 0.05. NS, not significant B. ChIP-qPCR analysis of STAT3 binding on the Ccl2 genomic locus in BMDMs from Rf/f or Rf/fCre mice with or without mmLDL treatment using STAT3 antibody or rabbit IgG. The fold-enrichment of the Ccl2 locus was determined by qPCR and calculated as percentage of input. Data are from three independent experiments. N=3, *P < 0.05.
The role of mTORC1 and IL6 in the induction of chemokine gene expression by mmLDL
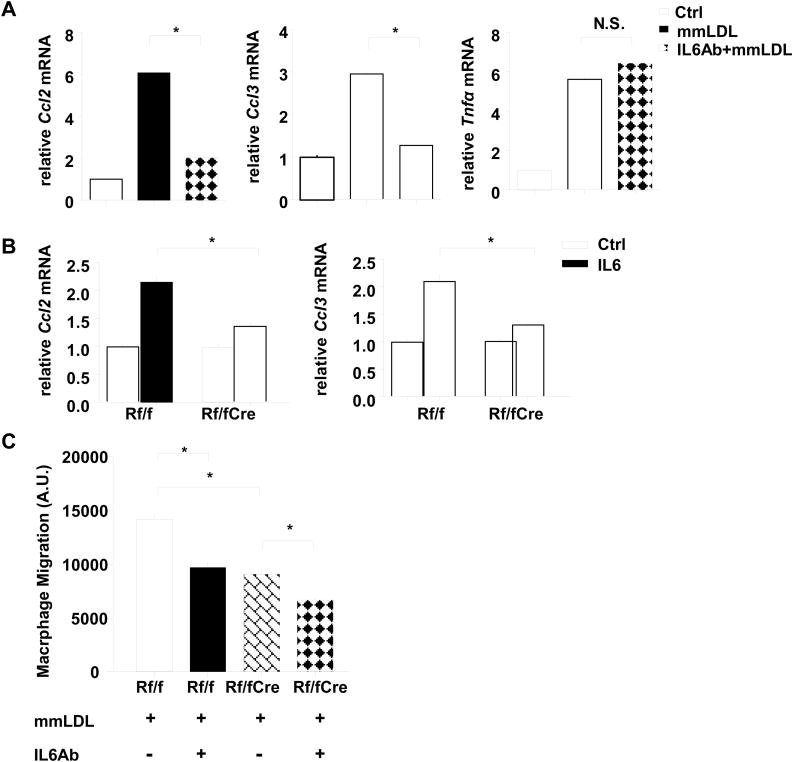
STAT3 plays a critical role in the IL6 signaling pathway. To further confirm the role of mTORC1/STAT3 and investigate the effect of IL6 in mmLDL-induced chemokine production, we employed IL6 and its neutralizing antibody. When macrophages were incubated with mmLDL in the presence of the IL6 neutralizing antibody, there was no induction of chemokine gene expression, indicating a key role of mmLDL induced IL-6 expression in chemokine production (Figure 5A, Online Figure VIIIA). Next, we asked if the effects of IL6 on chemokine gene expression could be influenced by mTORC1 deficiency. As expected, phosphorylation of STAT3 at both Ser727 and Tyr705 was induced by IL6 treatment. Different from intact phospho-STAT3Tyr705, STAT3Ser727 was reduced by Raptor deficiency (Online Figure VIIIB and VIIIC). Furthermore, unlike in control macrophages, IL-6 did not induce chemokine gene expression in Mac-RapKO cells (Figure 5B, Online Figure VIIID).
Figure 5. Chemokine expression and migratory response induced by mmLDL was blocked by IL6 neutralizing antibody in BMDMs from wild type mice.
A. Expression levels of chemokines and Tnfα were measured by quantitative RT-PCR in wild type BMDMs 2 hours after mmLDL (50 μg/ml) treatment with or without a 1 hour rat IgG against IL-6 or isotype control (0.5 μg/ml) pretreatment and normalized to Ctrl. N=3 B. BMDMs from Rf/f and Rf/fCre mice were treated with IL6 (20 ng/ml) for 2 hours. Expression levels of chemokines were measured by quantitative PCR and normalized to expression levels observed without treatment. N=3 C. Macrophage migration assay was performed in which BMDMs from Rf/f and Rf/fCre mice were added to bottom wells and pre-treated with mmLDL (50 μg/ml) and rat IgG against IL-6 or isotype control for 24 hours. The migrated macrophages were quantified by fluorescence spectroscopy. N=3, *P < 0.05. one-way ANOVA, Bonferroni post-test. N.S., not significant.
Since chemokines produced by macrophages in lesions are thought to promote monocyte-macrophage recruitment to lesions, we assessed the function of our findings on chemokine expression in a macrophage migration assay. In response to mmLDL treatment, 1.6-fold more macrophages were attracted by Rapflox/flox BMDMs in a transwell macrophage migration assay compared to Mac-RapKO BMDMs (Figure 5C and Online Figure VIIIE). Consistent with our in vivo study (Figure 1H), Raptor deficiency intrinsically reduced macrophage migration to CCL2/MCP-1 (Online Figure VIIIF). Pre-incubation with the IL6 antibody substantially reduced macrophage migration in control BMDMs, and to a lesser extent in Mac-RapKO macrophages (Figure 5C, 5D), consistent with the idea that mmLDL induced IL6, which in turn led to increased chemokine secretion in an mTORC1 dependent fashion.
Discussion
The mTOR inhibitor, rapamycin, has potent immunosuppressive effects and has been widely used to inhibit transplant rejection. Rapamycin blocks cell cycle progression and migration of T lymphocytes and thus inhibits acquired immune responses.31 Rapamycin has also been found to influence innate immune responses with suppression of chemokine production and variable effects on inflammatory gene expression.32, 33 In humans and experimental animals, rapamycin suppresses transplant vasculopathy, a specialized form of concentric atherosclerosis with prominent T-cell involvement.7, 8 There is also evidence that rapamycin decreases atherosclerosis in Apoe-/- mice,8 despite having adverse effects on VLDL/LDL cholesterol levels. We found prominent induction of mTORC1 activity in macrophages isolated from WTD-fed mice, suggesting an effect of over-nutrition to increase mTORC1 activity similar to what occurs in the liver.34 We then used a cell-specific knockout approach to demonstrate a regulatory role for macrophage mTORC1 in chemokine gene expression and atherogenesis. mTORC1 was found to amplify the effect of IL6/STAT3 on Ccl2 gene expression, uncovering a novel cross-talk between inflammatory signaling pathways induced by mmLDL and mTORC1 activation produced by over-nutrition, in macrophages of atherosclerotic lesions (Online Figure IX).
While many cellular factors could potentially contribute to the decreased macrophage content in atherosclerotic lesions of MacRapKO mice, including macrophage autophagy, proliferation or apoptosis, our studies suggested a predominant role of reduced lesional macrophage chemokine expression and monocyte recruitment into lesions. Studies using Ccr2-/- BM have suggested a predominant role of CCL2 in promoting monocyte emergence from BM;35 however, other studies in which lesional Ccl2/Mcp-1 expression is altered indicate that the gradient of CCL2 between lesion and blood may also play a role in monocyte recruitment into lesions,36 consistent with our observations. In addition to Ccl2, Ccl3/Mip1α was reduced in MacRapKO macrophages; CCL3 receptors CCR1 and CCR5 may also contribute to the recruitment of classical monocytes into atherosclerotic lesions. 16 Moreover, studies have shown that not only the expression of chemokines by an inflammation site but also by monocytes themselves has a role in the recruitment and migration of monocytes suggesting autocrine or paracrine effects of chemokines.37-40 This may explain the reduced recruitment of CFSE labeled monocytes obtained from MacRapKO donors versus Rapflox/flox controls into the aortas of WTD fed Ldlr-/- mice. Our studies suggest a dual defect involving both decreased chemokine production by plaque macrophages, as well as a cell intrinsic effect of raptor deficiency in migrating monocyte/macrophages, contributing to decreased monocyte/macrophage migration into plaques.
As reported41, 42 treatment of macrophages with mmLDL induced expression of several different chemokine genes, Ccl2, Ccl3 and Ccl7, which have all been implicated in atherogenesis.43,44, 45 In this study, we used a mildly oxidized form of LDL to study the mechanisms underlying the suppression of chemokine expression in Mac-RapKO macrophages. As shown in this study, mmLDL was found to induce Il6 gene expression and blocking antibodies revealed that the IL6 signaling pathway was required for induction of chemokines by mmLDL. As expected, IL6 treatment led to phosphorylation of STAT3 on Tyr705. This response was not affected by Raptor deficiency. Rather a second site, Ser727, was dependent on Raptor deficiency. Previous studies have shown that phosphorylation of Serine 727 on STAT3 is required for full transcriptional responses.27, 28, 46 Although IL6 production induced by mmLDL was intact in Raptor-deficient macrophages, IL6 signaling was decreased due to the reduced phosphorylation of its downstream target STAT3 at Ser727 which resulted in decreased mmLDL induced chemokine expression. Thus, the impact of macrophage mTORC1 activity was to amplify the effect of mmLDL/IL-6 on chemokine gene expression. As shown in FigS10, the binding of STAT3 to the chemokine promoter, exemplified by Ccl2, led to displacement of the transcriptional co-repressor BCL-6, leading to induction of chemokine gene expression. These findings are consistent with previous studies demonstrating a key role of BCL-6 in macrophage chemokine expression and atherosclerosis23, 47 and in mediating the effects of mmLDL on chemokine gene expression.22 Our studies show an important interface between mTORC1 signaling and the mmLDL/IL-6/STAT3 signaling axis, mediated by combinatorial STAT3 signaling and leading to an amplification of pro-atherogenic macrophage chemokine gene expression.
In this study, we have used mmLDL and focused on the modulation of IL6 signaling pathway induced chemokines secretion by mTORC1 signaling. There is increasing evidence for the involvement of IL6 in human atherosclerosis. Inflammatory markers such as C-reactive protein (CRP) have an independent predictive value for CHD incidence, and IL6 is a key inflammatory cytokine promoting CRP production.48, 49 Recent human genome wide association studies (GWAS) have shown that single nucleotide polymorphisms (SNPs) in the Il6 receptor gene are associated with coronary artery disease (CAD),50 indicating the relevance of the IL6 signaling pathway in human disease. These data suggest that our studies conducted in a muirne atherosclerosis model, and with human mmLDL in cell culture, could potentially have human relevance and suggest an anti-atherogenic role of mTORC1 inhibition in macrophages. mTOR inhibition has potential widespread applications in the treatment of human disease. In addition to transplant rejection, there may be beneficial effects on aging and cancer.51, 52 However, whole body mTORC1 inhibition may have adverse effects such as increased LDL levels.9 Our study suggests a potential role of targeted mTORC1 inhibition in macrophages in the treatment of atherosclerosis.
Supplementary Material
Novelty and Significance.
What is Known?
Global administration of the mTORC1 inhibitor, rapamycin, decreases atherosclerosis in mice.
The anti-inflammatory properties of rapamycin could be involved in its athero-protective effects, but direct evidence is lacking.
What New Information Does This Article Contribute?
Deletion of the Raptor gene in macrophages decreases mTOR activity, chemokine gene expression in plaques, and attenuates atherosclerosis.
The induction of CCL2 expression by minimally modified LDL was decreased in low mTORC1 activity macrophages due to lower STAT3 phosphorylation at Serine727, as well as increased binding with the transcription repressor Bcl6.
These findings suggest the presence of pro-atherogenic cross talk between nutritional and inflammatory signaling pathways in macrophages.
The mammalian target of rapamycin complex 1 (mTORC1) inhibitor rapamycin has been shown to decrease atherosclerosis, even while increasing plasma LDL levels. This finding suggests that inhibition of mTORC1 exerts an anti-atherogenic effect mediated by modulation of the inflammatory response in atherosclerotic plaques independent of plasma LDL cholesterol levels. We evaluated this hypothesis by investigating the contribution of macrophage mTORC1 to the development of atherosclerosis in mice. We found that macrophage Raptor deficiency decreased atherosclerosis, macrophage accumulation, and chemokine gene expression in atherosclerotic lesions independent of plasma lipid profile. In macrophages lacking both Raptor and Tsc1, the expression of chemokine genes induced by mmLDL and IL6 was modulated by mTORC1 activity. Activation of mTORC1 activity led to an increase in STAT3 phosphorylation at Ser727 site and repressed binding of BCL6 on Ccl2 promoter. These results provide direct evidence showing that the activation of macrophage mTORC1 by Western diet is pro-atherogenic in mice. Thus, targeted inhibition of mTORC1 in macrophages may be useful in the treatment of atherosclerosis.
Acknowledgments
Sources of Funding: This work was supported by NIH grant HL87123 (ART) and NIH grant HL055798 (YIM). D. Ai is supported by the National Natural Science Foundation of China [81322006] and [81370396]. Y. Zhu is supported by the Major National Basic Research Grant of China (No. 2010CB912504). M. Westerterp is supported by The Netherlands Organization of Scientific Research (NWO VENI – grant 916.11.072).
Nonstandard Abbreviations and Acronyms
- mTORC1
Mammalian target of rapamycin complex 1
- TSC1
tuberous sclerosis complex 1
- IL6
Interleukin 6
- CCL2
Chemokine (C-C motif) ligand 2
- TNFα
Tumor necrosis factor α
- p70S6k
p70 S6 kinase
- STAT3
Signal transducer and activator of transcription
- Bcl6
B cell lymphoma 6
- WTD
Western type diet
- LDLR
Low density lipoprotein receptor
- mmLDL
Minimally modified LDL
- ox-LDL
Oxidized LDL
- BMDM
Bone marrow derived macrophage
- CAD
coronary artery disease
- CFSE
carboxy-fluorescein succinimidyl ester
- 4E-BP1
eukaryotic initiation factor 4E-binding protein 1
- Raptor
regulatory associated protein of mTOR
- BM
Bone marrow
- phosphoS6
phospho-S6 ribosomal protein
- CRP
C-reactive protein
- GWAS
genome wide association studies
- SNPs
single nucleotide polymorphisms
Footnotes
Disclosures: Alan R. Tall is a consultant to Merck, Roche, Amgen, Arisaph and CSL.
References
- 1.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With tor, less is more: A key role for the conserved nutrient-sensing tor pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoki K, Li Y, Xu T, Guan KL. Rheb gtpase is a direct target of tsc2 gap activity and regulates mtor signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (tor), mediates tor action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Mtor interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mtorc components raptor, rictor, or mlst8 reveals that mtorc2 is required for signaling to akt-foxo and pkcalpha, but not s6k1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. Mtorc1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 7.Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, Edwards N, Oz M, Marks AR. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 8.Castro C, Campistol JM, Sancho D, Sanchez-Madrid F, Casals E, Andres V. Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27 kip1 -independent pathway. Atherosclerosis. 2004;172:31–38. doi: 10.1016/j.atherosclerosis.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Ai D, Chen C, Han S, Ganda A, Murphy AJ, Haeusler R, Thorp E, Accili D, Horton JD, Tall AR. Regulation of hepatic ldl receptors by mtorc1 and pcsk9 in mice. J Clin Invest. 2012;122:1262–1270. doi: 10.1172/JCI61919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 12.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of atp-binding cassette transporters a1 and g1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scull CM, Tabas I. Mechanisms of er stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2792–2797. doi: 10.1161/ATVBAHA.111.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soehnlein O, Drechsler M, Doring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gomez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy A, Gruen ML, Gutierrez DA, Surmi BK, Orr JS, Webb CD, Hasty AH. Impact of macrophage inflammatory protein-1alpha deficiency on atherosclerotic lesion formation, hepatic steatosis, and adipose tissue expansion. PLoS One. 2012;7:e31508. doi: 10.1371/journal.pone.0031508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 19.Boulanger CM, Tanner FC, Bea ML, Hahn AW, Werner A, Luscher TF. Oxidized low density lipoproteins induce mrna expression and release of endothelin from human and porcine endothelium. Circ Res. 1992;70:1191–1197. doi: 10.1161/01.res.70.6.1191. [DOI] [PubMed] [Google Scholar]
- 20.Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toney LM, Cattoretti G, Graf JA, Merghoub T, Pandolfi PP, Dalla-Favera R, Ye BH, Dent AL. Bcl-6 regulates chemokine gene transcription in macrophages. Nat Immunol. 2000;1:214–220. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- 22.Barish GD, Yu RT, Karunasiri MS, Becerra D, Kim J, Tseng TW, Tai LJ, Leblanc M, Diehl C, Cerchietti L, Miller YI, Witztum JL, Melnick AM, Dent AL, Tangirala RK, Evans RM. The bcl6-smrt/ncor cistrome represses inflammation to attenuate atherosclerosis. Cell Metab. 2012;15:554–562. doi: 10.1016/j.cmet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: Modulation by ppardelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Hatzi K, Melnick A. Lineage-specific functions of bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat Immunol. 14:380–388. doi: 10.1038/ni.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by bcl-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 26.Huang C, Hatzi K, Melnick A. Lineage-specific functions of bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat Immunol. 2013;14:380–388. doi: 10.1038/ni.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by stat1 and stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of stat-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 29.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of stat3 during cntf signaling is mediated by the rapamycin target mtor. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 30.Goncharova EA, Goncharov DA, Damera G, Tliba O, Amrani Y, Panettieri RA, Jr, Krymskaya VP. Signal transducer and activator of transcription 3 is required for abnormal proliferation and survival of tsc2-deficient cells: Relevance to pulmonary lymphangioleiomyomatosis. Mol Pharmacol. 2009;76:766–777. doi: 10.1124/mol.109.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-fkbp inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 32.Pan H, O'Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The tsc-mtor signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, Milstein S, Fitzgerald K, Murphy AJ, Woo CW, Strong A, Ginsberg HN, Tabas I, Rader DJ, Tall AR. Activation of er stress and mtorc1 suppresses hepatic sortilin-1 levels in obese mice. J Clin Invest. 2012;122:1677–1687. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor ccr2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H, Kaneko S. Mcp-1/ccr2-dependent loop for fibrogenesis in human peripheral cd14-positive monocytes. J Leukoc Biol. 2006;79:555–563. doi: 10.1189/jlb.0305127. [DOI] [PubMed] [Google Scholar]
- 38.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: Ccl2 expression by both monocytes and injured muscle is required for repair. Faseb J. 2011;25:3344–3355. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin-6/signal transducer and activator of transcription 3 (stat3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2012;288:1489–1499. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroer N, Pahne J, Walch B, Wickenhauser C, Smola S. Molecular pathobiology of human cervical high-grade lesions: Paracrine stat3 activation in tumor-instructed myeloid cells drives local mmp-9 expression. Cancer Res. 2011;71:87–97. doi: 10.1158/0008-5472.CAN-10-2193. [DOI] [PubMed] [Google Scholar]
- 41.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of nadph oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. 221p following 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 43.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jager SC, Bot I, Kraaijeveld AO, Korporaal SJ, Bot M, van Santbrink PJ, van Berkel TJ, Kuiper J, Biessen EA. Leukocyte-specific ccl3 deficiency inhibits atherosclerotic lesion development by affecting neutrophil accumulation. Arterioscler Thromb Vasc Biol. 2013;33:e75–83. doi: 10.1161/ATVBAHA.112.300857. [DOI] [PubMed] [Google Scholar]
- 45.Maddaluno M, Di Lauro M, Di Pascale A, Santamaria R, Guglielmotti A, Grassia G, Ialenti A. Monocyte chemotactic protein-3 induces human coronary smooth muscle cell proliferation. Atherosclerosis. 2011;217:113–119. doi: 10.1016/j.atherosclerosis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C. Role and regulation of stat3 phosphorylation at ser727 in melanocytes and melanoma cells. J Invest Dermatol. 2012;132:1877–1885. doi: 10.1038/jid.2012.45. [DOI] [PubMed] [Google Scholar]
- 47.Barish GD, Yu RT, Karunasiri M, Ocampo CB, Dixon J, Benner C, Dent AL, Tangirala RK, Evans RM. Bcl-6 and nf-kappab cistromes mediate opposing regulation of the innate immune response. Genes Dev. 2010;24:2760–2765. doi: 10.1101/gad.1998010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: The prime study. Arterioscler Thromb Vasc Biol. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 49.Rattazzi M, Puato M, Faggin E, Bertipaglia B, Zambon A, Pauletto P. C-reactive protein and interleukin-6 in vascular disease: Culprits or passive bystanders? J Hypertens. 2003;21:1787–1803. doi: 10.1097/00004872-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2012;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Law BK. Rapamycin: An anti-cancer immunosuppressant? Crit Rev Oncol Hematol. 2005;56:47–60. doi: 10.1016/j.critrevonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Willyard C. New findings rejuvenate age-old drug development field. Nat Med. 2013;19:520–521. doi: 10.1038/nm0513-520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.