Abstract
Primary effusion lymphoma (PEL), associated with the latent infection by KSHV, constitutively expresses interferon-regulatory factor 4 (IRF4). We recently showed that IRF4 differentially regulates expression of cellular interferon-stimulated genes (ISGs) and viral genes (Forero et al., 2013). Here, using inducible IRF4 knockdown, we demonstrate that IRF4 silencing results in enhanced transcription of KSHV replication transactivator RTA. As a result viral transcription is increased leading to virus reactivation. Taken together, our results show that IRF4 helps maintain the balance between latency and KSHV reactivation in PEL cells.
Keywords: Primary effusion lymphoma, Kaposi's sarcoma-associated herpesvirus, Interferon regulatory factor 4
Introduction
The interferon regulatory factors (IRFs) are critical in the regulation of innate and adaptive immune response (Tamura et al., 2008). In contrast with other ubiquitously expressed IRFs, IRF4 is restricted to immune cells and is required for the maturation of lymphocytes (Mittrucker et al., 1997). IRF4 expression has been linked to cellular transformation, increased proliferation, and decreased apoptotic responses in diseases like multiple myeloma (Iida et al., 1997), human T-lymphotropic virus 1 (HTLV-1) infected adult T-cell leukemia (ATL) (Mamane et al., 2002; Sharma et al., 2000), Epstein–Barr Virus (EBV) transformation of B cells (Banerjee et al., 2013; Izumiya et al., 2009; Wang et al., 2011; Xu et al., 2008), and primary effusion lymphoma (PEL) (Carbone et al., 2000). Furthermore, IRF4 is involved in autoimmune diseases (reviewed in Xu et al. (2012)) and diet-induced inflammation (Eguchi et al., 2011, 2013) indicating the need to improve our understanding of IRF4 function in the context of specific disease. However, the role of IRF4 in Kaposi's sarcoma-associated herpesvirus (KSHV or human herpesvirus 8, HHV-8) maintenance and promoting PEL is yet to be clarified.
PEL is a B cell neoplasm, common amongst immunocompromised individuals (Dotti et al., 1999; Jaffe, 1996). It is characterized by a plasma cell-like phenotype and is associated with latent infection by KSHV. In PEL cells, KSHV persists as a naked episome with expression of a subset of viral genes (latency-associated genes) (Cesarman et al., 1995; Dresang et al., 2011; Sarid et al., 1998; Zhong et al., 1996). The transition from latency to lytic replication is controlled by the KSHV replication transactivator (RTA), which is necessary and sufficient to initiate lytic gene transcription, virion formation, and cell death. The role of RTA in driving lytic replication has been extensively studied and factors mediating RTA function have been well described (Guito and Lukac, 2012). However, the signaling pathways and cellular factors that control the transcriptional induction of RTA and their effects on downstream gene expression remain elusive.
We have recently reported that IRF4 can directly induce a specific subset of IFN-stimulated genes (ISGs) in a type I IFN-independent manner, and can negatively regulate KSHV RTA induction (Forero et al., 2013). Therefore, we hypothesized that downregulation of IRF4 would result in the derepression of RTA expression in PEL cells and induction of the lytic gene cascade. In this study, we have taken a reverse genetics approach to examine the effects of IRF4 on ISG induction and the maintenance of KSHV latency. Our results show that IRF4 downregulation does not result in a loss of ISG expression, but rather an increase in RTA transcription and translation accompanied by a subsequent lytic gene expression and increased virus production. These results indicate a complex interplay between RTA-responsive element (RRE) and interferon-sensitive response element (ISRE) regulated gene expression and establish IRF4 as a key regulator of KSHV reactivation.
Results
Downregulation of IRF4 leads to induction of specific ISG expression in PEL cells
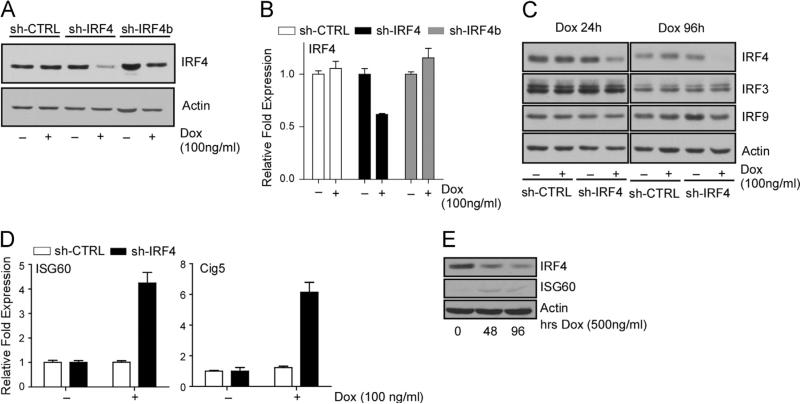
The roles of IRF4 in regulating B-cell specific transcription programs, by cooperating with various transcription factors, such as PU.1, have been well documented (Eisenbeis et al., 1995; van der Stoep et al., 2004). However, our previous results showed that IRF4 alone can directly bind to ISRE elements and is capable of upregulating ISG transcription in various cell types, including PEL cells, in the absence of PU.1 expression. This phenomenon is further influenced by the KSHV-latency associated gene v-FLIP (viral FLICE inhibitory protein) through its ability to activate NF-κB (Forero et al., 2013). To investigate whether the ISG upregulation is solely mediated through IRF4 expression, we attempted IRF4 silencing in PEL cells. Given that we were unable to obtain stable PEL cell lines constitutively expressing IRF4-targeted shRNA, we engineered BCBL-1 cells with doxycycline (Dox) inducible expression of either control or two separate IRF4-targeting shRNAs (sh-IRF4, and sh-IRF4b) using strategies previously described (Shaffer et al., 2008). Treatment of cells with Dox (100 ng/ml) resulted in appreciable reduction in IRF4 protein (Fig. 1A) and mRNA (Fig. 1B) in sh-IRF4 expressing cells, but not in scramble control (sh-CTRL) or sh-IRF4b expressing cells. To verify the specificity for IRF4 knockdown and exclude any off-target effect by shIRF4, we analyzed the expression of IRF3 and IRF9, which showed no detectable changes (Fig.1C). As we have previously shown that IRF4 acts as a positive regulator of ISRE-mediated expression of ISG60 and Cig5, we evaluated the effect of IRF4 knockdown on the expression of these genes. Unexpectedly, transcription of ISG60 and Cig5 was increased after IRF4 depletion, 4-fold and 6-fold respectively (Fig. 1D). This was accompanied by an increase in ISG60 protein synthesis after Dox treatment of BCBL-1 cells (Fig. 1E).
Fig. 1.
Downregulation of IRF4 results in the induction of ISG expression in PEL cells. (A) Loss of IRF4 protein expression in cells expressing an inducible IRF4 targeting shRNA. Whole cell lysates prepared from shRNA expressing BCBL-1 cells after 72 h treatment with 100 ng/ml Dox were immunoblotted with antibodies against IRF4 and actin. (B) Quantitative RT-PCR analysis of IRF4 mRNA levels in shRNA expressing BCBL-1 cells after 72 h treatment with 100 ng/ml dox. Samples were normalized to the housekeeping gene, RPL32, and expressed as fold change with respect to untreated cells (value 1). (C) Specificity of IRF4 targeting by the shRNA 24 h (left) and 96 h (right) after stimulation with Dox. Whole cell lysates prepared from shRNA expressing BCBL-1 cells after 72 h Dox treatment were immunoblotted with antibodies against IRF4, IRF3, IRF9 and Actin. (D) Quantitative RT-PCR analysis of ISG60 and Cig5 mRNA expression in shRNA expressing BCBL-1 cells after 72 h Dox treatment. Samples were normalized to RPL32 and expressed as fold change with respect to untreated cells (value 1). (E) Analysis of ISG60 protein induction levels in whole cell lysates prepared from sh-IRF4 expressing BCBL-1 cells after 72 h treatment with Dox.
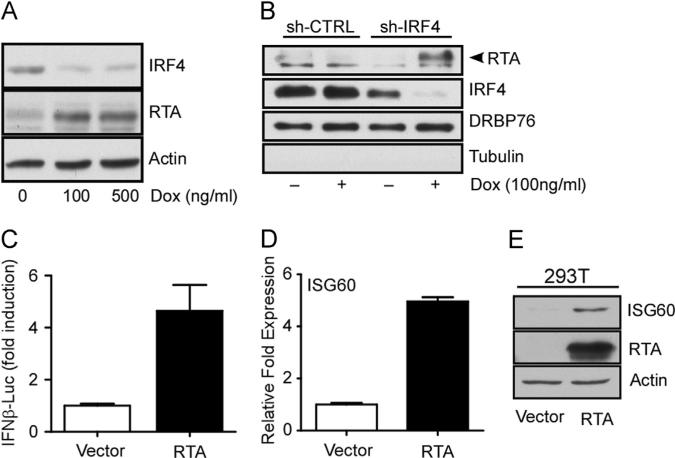
We have reported that ectopic expression of IRF4 resulted in the inhibition of TPA-stimulated RTA (encoded by ORF50) transcription in PEL cells (Forero et al., 2013). To determine whether depletion of IRF4 affects RTA expression in PEL cells, we examined RTA protein synthesis in Dox (100 ng/ml) treated BCBL-1 sh-IRF4 and observed an overall increase in RTA protein expression (Fig. 2A), and nuclear accumulation (Fig. 2B). RTA is a sequence-specific DNA binding protein that recognizes and binds to RRE containing viral gene promoters, as well as ISRE and ISRE-like sequences found in the promoter regulatory regions of cellular ISGs (Zhang et al., 2005). Indeed, ectopic expression of RTA resulted in an almost 5-fold increase in the activity of the IFNβ reporter relative to vector transfected cells (Fig. 2C). Furthermore, ectopic RTA expression resulted in a 5-fold increase in the induction of ISG60 mRNA transcription (Fig. 2D) accompanied by an increase in ISG60 protein synthesis (Fig. 2E). It is likely then that the unexpected ISG induction observed after IRF4 silencing is mediated by the upregulation of RTA (Zhang et al., 2005). Taken together, these results indicate that IRF4 is involved in maintaining a fine-tuned equilibrium between the expression levels of ISGs and RTA in PEL cells.
Fig. 2.
RTA is induced by the silencing of IRF4 and regulates ISRE mediated gene expression in PEL cells. (A) Induction of RTA protein following IRF4 knockdown. Whole cell lysates fromBCBL-1 sh-IRF4 cells after 72 h treatment with various concentrations of Dox were immunoblotted with antibodies against IRF4, RTA and Actin. (B) Nuclear accumulation of RTA expression follows loss of IRF4. Nuclear fractions were prepared from cell treated with Dox for 72 h. Fractions were resolved by SDS-PAGE and immunoblotted with antibodies against IRF4, RTA, DRBP76 (nuclear marker), and Tubulin (cytoplasmic marker). (C) Effect of RTA expression on IFNβ-promoter activity. HEK293 cells were transfected with RTA or vector control along with IFNβ125-luc and pRL-Null. Luciferase activity was measured as previously described (Forero et al., 2013). Samples were normalized to Renilla luciferase control and expressed as fold change with respect to empty vector transfected cells (value 1). (D) Quantitative RT-PCR analysis of ISG60 following ectopic expression of RTA in 293T cells. Samples were normalized to RPL32 and expressed as fold change with respect to empty vector transfected cells (value 1). (E) Analysis of ISG60 protein induction following ectopic expression of RTA in 293T cells. Samples were immunbobloted with indicated antibodies.
IRF4 inhibits KSHV lytic gene expression
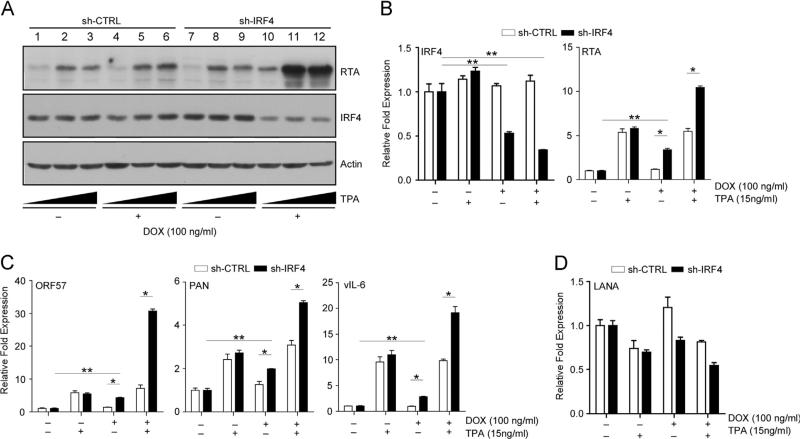
RTA plays a critical role in initiating the switch from latency to the lytic reactivation of KSHV (Guito and Lukac, 2012; Lukac et al., 1999, 1998). In order to define the mechanism of RTA protein expression followed by IRF4 depletion, we treated BCBL-1 sh-CTRL and sh-IRF4 cells with Dox for 72 h followed by stimulation with 15 ng/ml TPA for 12 h. Treatment with Dox resulted in significantly decreased IRF4 protein levels in BCBL-1 sh-IRF4 cells accompanied with an increase in RTA protein synthesis (Fig. 3A, lanes 10–12). Dox treatment did not affect IRF4 or RTA protein expression in sh-CTRL cells (lanes 4 and 1). Furthermore, co-stimulation with TPA resulted in enhanced RTA expression in sh-IRF4 cells (lanes 11 and 12) relative to Dox untreated cells (lanes 8, 9 and 5, 6) and sh-CTRL cells. It should be noted that TPA treatment had no overall effect on IRF4 expression in either sh-CTRL or sh-IRF4 BCBL-1 cells. The observed increase in protein expression was due to the transcriptional activation of RTA as determined by quantitative RT-PCR analysis (qRT-PCR). Dox treatment of sh-IRF4 cells led to an observed 3.4-fold increase in RTA mRNA compared to a 10.5-fold increase in mRNA transcription when cells were co-stimulated with Dox and TPA (15 ng/ml) (Fig. 3B). These results were concordant with our previous studies demonstrating that IRF4 acts as a repressor of RTA transcription. As seen for RTA protein synthesis, both sh-CTRL and sh-IRF4 cell lines responded equally to treatment with TPA (Fig. 3A lanes 2, 3 and 8, 9), suggesting that there were no inherent differences in the ability to respond to TPA treatment.
Fig. 3.
IRF4 silencing positively regulates RTA and RRE-mediated gene expression. (A) Analysis of RTA protein induction following Dox treatment and TPA stimulation in BCBL-1 sh-CTRL and sh-IRF4 cells. Cells were stimulated with Dox for 48 h followed by an additional treatment for 24 h with 5 or 15 ng/ml TPA. Lysates were prepared after stimulation and immunoblotted with anti-RTA, IRF4, and Actin antibodies. (B–D) qRT-PCR analysis of IRF4 and RTA mRNA levels (B); RTA target genes, ORF57, PAN and vIL-6 (C); and LANA (D) in Dox treated BCBL-1 sh-CTRL and sh-IRF4 cells. Cells were stimulated as previously described, total RNA was harvested and subjected to qRT-PCR. Samples were normalized to RPL32 and expressed as fold change with respect their respective untreated cells (value 1).
Next, to examine the consequence of IRF4 downregulation on direct RTA-target viral genes, we measured the induction of RTA-responsive genes ORF57, vIL-6, and PAN mRNA (Bu et al., 2008) following treatment of cells with Dox and TPA. Similar to the observed RTA transcript induction, loss of IRF4 resulted in a 2–4 fold increase in basal early gene transcription of the three targets examined relative to untreated cells (Fig. 3C). Furthermore, co-stimulation with Dox and TPA, resulted in a significant enhancement in ORF57, PAN, and vIL-6 transcription in sh-IRF4 expressing cells compared to that observed in co-stimulated sh-CTRL cells (Fig. 3C). To exclude the possibility that treatment with Dox leads to an overall increase in gene transcription, we measured the mRNA levels of the latency-associated nuclear antigen (LANA) following Dox and/or TPA stimulation. While treatment with TPA resulted in a comparable decrease in LANA mRNA in both sh-CTRL and sh-IRF4 cells, treatment with Dox showed a pattern of reduced LANA mRNA levels in sh-IRF4 cells, but not in sh-CTRL cells relative to non-stimulated cells. As expected, dual stimulation with Dox and TPA resulted in a decrease in LANA mRNA in both cell lines. (Fig. 3D) These results are consistent with previous observations that lytic reactivation results in reduction in LANA transcription accompanied with an induction of immediate early genes (Dillon et al., 2013). These results indicate that IRF4 downregulation in PEL cells is likely sufficient to allow entry into the viral lytic reactivation program, rather than resulting in a non-specific activation of viral gene expression.
IRF4 silencing results in KSHV reactivation and virus production
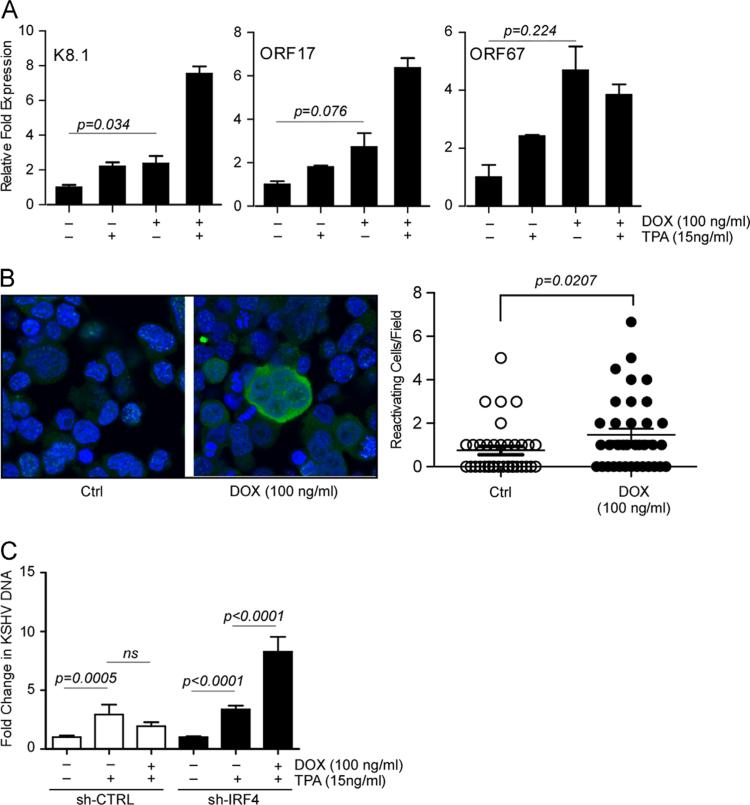
Bona fide lytic reactivation and viral replication is accompanied by an increase in virion structure and assembly associated gene expression. To determine whether this occurred in BCBL-1 sh-IRF4 cells after IRF4 silencing, we measured the transcriptional induction of the genes encoding the viral glycoprotein (K8.1), ORF17, and ORF67 by qRT-PCR, we observed higher mRNA expression of all three genes in cells treated with Dox for 72 h, relative to non-treated cells (Fig. 4A). Additional treatment of Dox stimulated cells with TPA, resulted in enhanced mRNA expression of the late genes, K8.1 and ORF17. Interestingly, while the expression of ORF67 mRNA was higher in cells treated with Dox relative to untreated or TPA-alone treated cells, co-stimulation of cells with Dox and TPA did not result in increases in mRNA levels compared to cells treated with Dox alone. We then examined the induction of KSHV viral protein expression in BCBL-1 sh-IRF4 cells stimulated with Dox for 96 h. Cells were fixed and stained using anti-KSHV sera. We observed a significant two fold increase in cytoplasmic staining in cells in Dox treated cells (Fig. 4B). To examine whether the observed upregulation of KSHV lytic genes is accompanied by an increase in viral DNA replication and virion production, we measured KSHV DNA in the supernatant of Dox and TPA-stimulated BCBL-1 sh-CTRL and sh-IRF4 cells. As expected, stimulation of cells with TPA resulted in a significant increase in viral DNA detection in both sh-CTRL and sh-IRF4 cells. However, co-stimulation with Dox and TPA did not affect viral DNA production in sh-CTRL cells, while increased viral DNA yields were observed in sh-IRF4 cells (Fig. 4C). Taken together, these results indicate that loss of IRF4 results in the induction of lytic gene expression in a portion of BCBL-1 sh-IRF4 cells and leads to an increased sensitivity to reactivation stimuli.
Fig. 4.
Loss of IRF4 results in a robust induction of KSHV lytic gene expression and viral reactivation. (A) qRT-PCR analysis of late genes K8.1, ORF17, and ORF67 in Dox treated BCBL-1 sh-CTRL and sh-IRF4 cells. Cells were stimulated as previously described, total RNA was harvested and subjected to qRT-PCR. Samples were normalized to RPL32 and expressed as fold change with respect to their respective untreated cells (value 1). (B) BCBL-1 sh-IRF4 stimulated with Dox for 96 h. Following stimulation, cells were fixed, stained with KSHV positive human sera (green), nuclei were stained with DAPI (blue), and samples imaged by confocal microscopy. Sample immunofluorescence image is shown (left). Quantification of KSHV reactivation (right). Cells positive for KSHV reactivation were manually counted from a series of ~40 fields. (C) KSHV virion production following IRF4 silencing. BCBL-1 sh-CTRL and sh-IRF4 cells were stimulated with Dox for 72 h followed by stimulation with TPA (15 ng/ml) or Dox and TPA for an additional 72 h. KSHV DNA released into the supernatant was measured by probed-based qPCR. Results are expressed as fold change with respect to their respective untreated cells (value 1).
Discussion
One important feature in the life cycle of herpesviruses is their ability to establish life-long latent infections, which acts as a mechanism for immune evasion and contributes to the pathogenesis of viral related malignancies. To understand the events that determine whether the virus will establish latency or undergo lytic replication requires an understanding the cellular signals that shape these outcomes. To date, studies have focused on understanding various stimuli for KSHV reactivation like chemical treatment of PEL cells with phorbol esters (Moore et al., 1996b) and histone deacetylase inhibitors (Lu et al., 2003), inhibition of calcium signaling (Zoeteweij et al., 2001), hypoxia (Davis et al., 2001), viral infection (Gregory et al., 2009), and BCR stimulation (Kati et al., 2013). These studies have been instrumental in identifying various kinases (such as PI3K (Kati et al., 2013), Pim-1 and Pim-3 (Cheng et al., 2009), ERK1/2 (Cohen et al., 2006), and PKC (Deutsch et al., 2004) and transcription factors (Sp1 (Ye et al., 2005), C/EBP (Wang et al., 2003), CBF1/CSL (Scholz et al., 2013; Wang and Yuan, 2007), Oct-1 (Carroll et al., 2007), HMGB-1 (Harrison and Whitehouse, 2008), and XBP-1 (Kati et al., 2013; Lai, Farrell, and Kellam, 2011)) that promote RTA induction and RTA-mediated viral gene expression. Similarly, several factors which antagonize RTA induction and RTA-mediated gene expression have also been identified. These include Tousled-like kinases (TLKs) (Dillon et al., 2013) and transcriptional repressors, K-RBP (Yang and Wood, 2007) and Hey1 (Gould et al., 2009).
Recently, we have identified IRF4 as a regulator of ISRE and RRE mediated gene expression (Forero et al., 2013). The present study extends our previous findings and confirms IRF4 as a negative regulator of RTA expression and function, contributing to the maintenance of latency in PEL cells. Previous reports have shown that latently infected PEL cells display varying levels of IRF4 expression (Arguello et al., 2003; Carbone et al., 2000; Forero et al., 2013). BCP-1 cells, which express lower IRF4 expression, show higher levels of vIL-6 mRNA accompanied by increased protein production (Moore et al., 1996a); pointing towards a possible negative regulation of lytic gene expression by IRF4. Other studies have shown that another interferon regulatory factor, IRF7, is also an inhibitor of KSHV lytic gene expression (Wang et al., 2005). Furthermore, IRF2 has also been shown to control latency of a murine gammaherpesvirus closely related to KSHV, Murine herpesvirus 68 (Mandal et al., 2011). Together, these studies highlight the contributions of IRF proteins in the balanced regulation between the gammaherpesvirus latent and lytic replication phases, which is crucial for efficient viral spread and persistence and contribute to pathogenesis and transformation.
Besides PEL, KSHV infection is associated with another B cell malignancy localized to the mantle zone of lymph nodes and the spleen, multicentric Castleman's disease (MCD). Unlike PEL, MCD is polyclonal in nature and is comprised of both KSHV− ve and KSHV+ve cells. Hassman et al. demonstrated that infection of tonsillar B cells with KSHV resulted in the establishment of latency nearly exclusively in IgMλ B cells and promoted their proliferation and differentiation (Hassman et al., 2011). Given the requirement of IRF4 expression for secondary receptor editing (Pathak et al., 2008), and its association with the plasma cell phenotype, it is possible that the initial establishment of KSHV latency in B cells is a result of KSHV infection of cells with high levels of IRF4 expression. This is followed by inhibition of RTA expression by IRF4 resulting in KSHV latency and tumorigenesis (Carbone et al., 2000). Further studies would shed new light on the role of IRF4 in restricting KSHV lytic replication after primary infection and illustrate the importance of IRF4 in the genesis of KHSV-related B cell malignancies PEL and MCD.
Materials and methods
Cells, reagents, and plasmids
HEK293 and BCBL-1-derived cells were cultured as previously described (Forero et al., 2013). Doxycycline (Dox; Clontech) stimulations were done for 24–96 h at varying doses (as indicated) and 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma Aldrich) stimulations were done with 5 or 15 ng/ml (as indicated) for 12 h. Primary antibodies used in this study: anti-IRF4 (Cell Signaling), anti-Actin and anti-Tubulin (Santa Cruz), anti- ISG60 and anti-DRBP76 (Sarkar et al., 2004), and anti-ORF50 (Toptan et al., 2013). pcDNA/ORF50 and reporter plasmids pGL3-IFNβ125 firefly lucifer-ase and pRL null have been previously described (Forero et al., 2013). Retroviral packaging plasmids pHIT60 expressing MoMuLV Gag and Pol and the ecotropic envelope-expressing plasmid pHIT/ EA6 × 3*, along with the doxycycline-inducible non-targeting shRNA (shCTRL), IRF4 targeting shRNA (shIRF4 and shIRF4b) retroviral vectors were obtained from Dr. Louis Staudt (NIH) and have been previously described (Ngo et al., 2006; Shaffer et al., 2008).
Generation of cells with inducible IRF4 knockdown
BCBL-1 cells were engineered to stably express the ecotropic retroviral receptor and the bacterial tetracycline repressor (TETR) as previously described (Ngo et al., 2006). Cells were then infected with retroviruses expressing doxycycline-inducible short-hairpins targeting IRF4 and a scramble control and placed under puromycin selection (1 ug/ml) for 7 days. Antibiotic resistant cells were then used for further assays.
Quantitative PCR analysis of cellular and KSHV gene expression
BCBL-1/sh-CTRL and BCBL-1/sh-IRF4 cells were stimulated with 100 ng/ml Dox for 72 h and/or TPA at the indicated doses for an additional 12 h. Total RNA was extracted and real-time PCR was performed as previously described (Forero et al., 2013). Primers used for KSHV target gene amplification can be found in Supplementary Table 1. Expression of mRNA was normalized to housekeeping gene RPL32 and expressed as fold change with respect to their respective mock treated cells (value 1).
Immunofluorescence microscopy
BCBL-1/sh-IRF4 cells were treated with 100 ng/ml Dox for 96 h. Cells were fixed with 4% PFA and permeabilized with 0.1% Triton-X in PBS. Cells were seeded onto poly-l-Lysine coated slides (Fisher Scientific) and left to air dry. After blocking with 10% goat serum for 1 h at 37 °C, cells were incubated with KSHV positive human sera from an individual with PEL for 1 h at 37 °C, washed and incubated with a mouse monoclonal antibody directed against human IgG (Sigma) 1 h at 37 °C. Finally, cells were incubated with mouse anti IgG–FITC-conjugated secondary antibody (Santa Cruz) for 1 h at 37 °C. Slides were fixed and mounted with Vectashield containing DAPI. Images were captured with a FV1000 Olympus confocal laser scanning microscope. A total of 36 images per condition taken from triplicate experiments were analyzed for quantification of reactivating cells.
KSHV reactivation assay
BCBL-l sh-CTRL or sh-IRF4 (2 × 106 cells) were stimulated with Dox (100 ng/ml) for 72 h followed by stimulation with TPA (15 ng/ ml) and Dox (100 ng/ml) or vehicle control for an additional 72 h (as indicated). Supernatants were clarified by centrifugation at 1200 RPM for 10 min followed by ultracentrifugation at 12,000 rpm for 3 h to isolate KSHV virions. Pellets were resuspended in 500 μl 1 × PBS and DNAse I (Ambion) treated for 30 min at 37 °C to remove any intracellular KSHV DNA. DNA was then extracted with phenol– chloroform and resuspended in 100 μl nuclease free water. KSHV DNA was quantified by probe-based quantitative PCR with Ampli-Taq Gold 360 Master Mix (Invitrogen) according to manufacturer's guidelines using primers and a probe targeting the K8 gene (Forward primer 5’–GTCTCTTGGACAAGCTCGCTGTT-3’, Reverse primer 5’–AGTGAGCATGGCAGATGTTCGT-3’; and FAM probe 5’–CG GTCTGTGAAACGGTCATTGACCTTAC-3’ (as described in Qu et al. (2011)).
Statistical analysis
Data were analyzed using two-tailed paired Student's t-test. Values were considered significant at p<0.05.
Supplementary Material
Acknowledgments
We thank Dr. Louis Staudt for providing the shRNA expression constructs and packaging plasmids, Dr. Patrick Moore and Dr. Yuan Chang for various antibodies and Dr. Carolyn Coyne and Jana Jacobs for technical assistance with confocal microscopy imaging.
Footnotes
This work was supported in part by AI082673 from National Institute of Allergy and Infectious Diseases Grant (SNS) and AIDS-related malignancy supplement from UPCI Cancer Center Support Grant. This project used the UPCI core facilities and was supported in part by award P30CA047904.
Author contribution
AF and KDM designed and performed the experiments; AF, FJJ and SNS designed experiments and wrote the manuscript.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2014.04.020.
References
- Arguello M, Sgarbanti M, Hernandez E, Mamane Y, Sharma S, Servant M, Lin R, Hiscott J. Disruption of the B-cell specific transcriptional program in HHV-8 associated primary effusion lymphoma cell lines. Oncogene. 2003;22(7):964–973. doi: 10.1038/sj.onc.1206270. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Lu J, Cai Q, Saha A, Jha HC, Dzeng RK, Robertson ES. The EBV latent antigen 3C Inhibits apoptosis through targeted regulation of interferon regulatory factors 4 and 8. PLoS Pathog. 2013;9(5):e1003314. doi: 10.1371/journal.ppat.1003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu W, Palmeri D, Krishnan R, Marin R, Aris VM, Soteropoulos P, Lukac DM. Identification of direct transcriptional targets of the Kaposi's sarcoma-associated herpesvirus Rta lytic switch protein by conditional nuclear localization. J. Virol. 2008;82(21):10709–10723. doi: 10.1128/JVI.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Cozzi MR, Capello D, Steffan A, Monini P, De Marco L, Gaidano G. Expression of MUM1/IRF4 selectively clusters with primary effusion lymphoma among lymphomatous effusions: implications for disease histogenesis and pathogenesis. Br. J. Haematol. 2000;111(1):247–257. doi: 10.1046/j.1365-2141.2000.02329.x. [DOI] [PubMed] [Google Scholar]
- Carroll KD, Khadim F, Spadavecchia S, Palmeri D, Lukac DM. Direct interactions of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50/Rta protein with the cellular protein octamer-1 and DNA are critical for specifying transactivation of a delayed-early promoter and stimulating viral reactivation. J. Virol. 2007;81(16):8451–8467. doi: 10.1128/JVI.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Cheng F, Weidner-Glunde M, Varjosalo M, Rainio EM, Lehtonen A, Schulz TF, Koskinen PJ, Taipale J, Ojala PM. KSHV reactivation from latency requires Pim-1 and Pim-3 kinases to inactivate the latency-associated nuclear antigen LANA. PLoS Pathog. 2009;5(3):e1000324. doi: 10.1371/journal.ppat.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Brodie C, Sarid R. An essential role of ERK signalling in TPA-induced reactivation of Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 2006;87(Pt 4):795–802. doi: 10.1099/vir.0.81619-0. [DOI] [PubMed] [Google Scholar]
- Davis DA, Rinderknecht AS, Zoeteweij JP, Aoki Y, Read-Connole EL, Tosato G, Blauvelt A, Yarchoan R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97(10):3244–3250. doi: 10.1182/blood.v97.10.3244. [DOI] [PubMed] [Google Scholar]
- Deutsch E, Cohen A, Kazimirsky G, Dovrat S, Rubinfeld H, Brodie C, Sarid R. Role of protein kinase C delta in reactivation of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2004;78(18):10187–10192. doi: 10.1128/JVI.78.18.10187-10192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon PJ, Gregory SM, Tamburro K, Sanders MK, Johnson GL, Raab-Traub N, Dittmer DP, Damania B. Tousled-like kinases modulate reactivation of gammaherpesviruses from latency. Cell Host Microbe. 2013;13(2):204–214. doi: 10.1016/j.chom.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti G, Fiocchi R, Motta T, Facchinetti B, Chiodini B, Borleri GM, Gavazzeni G, Barbui T, Rambaldi A. Primary effusion lymphoma after heart transplantation: a new entity associated with human herpesvirus-8. Leukemia. 1999;13(5):664–670. doi: 10.1038/sj.leu.2401390. [DOI] [PubMed] [Google Scholar]
- Dresang LR, Teuton JR, Feng H, Jacobs JM, Camp DG, 2nd, Purvine SO, Gritsenko MA, Li Z, Smith RD, Sugden B, Moore PS, Chang Y. Coupled transcriptome and proteome analysis of human lymphotropic tumor viruses: insights on the detection and discovery of viral genes. BMC Genomics. 2011;12:625. doi: 10.1186/1471-2164-12-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi J, Kong X, Tenta M, Wang X, Kang S, Rosen ED. Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes. 2013;62(10):3394–3403. doi: 10.2337/db12-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13(3):249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbeis CF, Singh H, Storb U. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 1995;9(11):1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- Forero A, Moore PS, Sarkar SN. Role of IRF4 in IFN-stimulated gene induction and maintenance of Kaposi sarcoma-associated herpesvirus latency in primary effusion lymphoma cells. J. Immunol. 2013;191(3):1476–1485. doi: 10.4049/jimmunol.1202514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F, Harrison SM, Hewitt EW, Whitehouse A. Kaposi's sarcoma-associated herpesvirus RTA promotes degradation of the Hey1 repressor protein through the ubiquitin proteasome pathway. J. Virol. 2009;83(13):6727–6738. doi: 10.1128/JVI.00351-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc. Natl. Acad. Sci. USA. 2009;106(28):11725–11730. doi: 10.1073/pnas.0905316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guito J, Lukac DM. KSHV Rta promoter specification and viral reactivation. Front. Microbiol. 2012;3:30. doi: 10.3389/fmicb.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Whitehouse A. Kaposi's sarcoma-associated herpesvirus (KSHV) Rta and cellular HMGB1 proteins synergistically transactivate the KSHV ORF50 promoter. FEBS Lett. 2008;582(20):3080–3084. doi: 10.1016/j.febslet.2008.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassman LM, Ellison TJ, Kedes DH. KSHV infects a subset of human tonsillar B cells, driving proliferation and plasmablast differentiation. J. Clin. Investig. 2011;121(2):752–768. doi: 10.1172/JCI44185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S, Rao PH, Butler M, Corradini P, Boccadoro M, Klein B, Chaganti RS, Dalla-Favera R. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat. Genet. 1997;17(2):226–230. doi: 10.1038/ng1097-226. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Izumiya C, Hsia D, Ellison TJ, Luciw PA, Kung HJ. NF-kappaB serves as a cellular sensor of Kaposi's sarcoma-associated herpesvirus latency and negatively regulates K-Rta by antagonizing the RBP-Jkappa coactivator. J. Virol. 2009;83(9):4435–4446. doi: 10.1128/JVI.01999-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe ES. Primary body cavity-based AIDS-related lymphomas. Evolution of a new disease entity. Am. J. Clin. Pathol. 1996;105(2):141–143. doi: 10.1093/ajcp/105.2.141. [DOI] [PubMed] [Google Scholar]
- Kati S, Tsao EH, Gunther T, Weidner-Glunde M, Rothamel T, Grundhoff A, Kellam P, Schulz TF. Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells. J. Virol. 2013;87(14):8004–8016. doi: 10.1128/JVI.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai IY, Farrell PJ, Kellam P. X-box binding protein 1 induces the expression of the lytic cycle transactivator of Kaposi's sarcoma-associated herpesvirus but not Epstein–Barr virus in co-infected primary effusion lymphoma. J. Gen. Virol. 2011;92(Pt 2):421–431. doi: 10.1099/vir.0.025494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. Chromatin remodeling of the Kaposi's sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J. Virol. 2003;77(21):11425–11435. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Kirshner JR, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 1999;73(11):9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Renne R, Kirshner JR, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252(2):304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Sharma S, Grandvaux N, Hernandez E, Hiscott J. IRF-4 activities in HTLV-I-induced T cell leukemogenesis. J. Interf. Cytokine Res. 2002;22(1):135–143. doi: 10.1089/107999002753452746. [DOI] [PubMed] [Google Scholar]
- Mandal P, Krueger BE, Oldenburg D, Andry KA, Beard RS, White DW, Barton ES. A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality. PLoS Pathog. 2011;7(11):e1002371. doi: 10.1371/journal.ppat.1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrucker HW, Matsuyama T, Grossman A, Kundig TM, Potter J, Shahinian A, Wakeham A, Patterson B, Ohashi PS, Mak TW. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275(5299):540–543. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996a;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, Garber R, Pellett PE, McGeoch DJ, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 1996b;70(1):549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J, Staudt LM. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441(7089):106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- Pathak S, Ma S, Trinh L, Lu R. A role for interferon regulatory factor 4 in receptor editing. Mol. Cell. Biol. 2008;28(8):2815–2824. doi: 10.1128/MCB.01946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Triulzi DJ, Rowe DT, Jenkins FJ. Detection of HHV-8 (human herpesvirus-8) genomes in induced peripheral blood mononuclear cells (PBMCs) from US blood donors. Vox Sang. 2011;100(3):267–271. doi: 10.1111/j.1423-0410.2010.01404.x. [DOI] [PubMed] [Google Scholar]
- Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 1998;72(2):1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 2004;11(11):1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- Scholz BA, Harth-Hertle ML, Malterer G, Haas J, Ellwart J, Schulz TF, Kempkes B. Abortive lytic reactivation of KSHV in CBF1/CSL deficient human B cell lines. PLoS Pathog. 2013;9(5):e1003336. doi: 10.1371/journal.ppat.1003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Emre NCT, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, Zeng Y, Chen B, Epstein J, Staudt LM. IRF4 addiction in multiple myeloma. Nature. 2008;454(7201):226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mamane Y, Grandvaux N, Bartlett J, Petropoulos L, Lin R, Hiscott J. Activation and regulation of interferon regulatory factor 4 in HTLV type 1-infected T lymphocytes. AIDS Res. Hum. Retrovir. 2000;16(16):1613–1622. doi: 10.1089/08892220050193047. [DOI] [PubMed] [Google Scholar]
- Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Ann. Rev. Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- Toptan T, Fonseca L, Kwun HJ, Chang Y, Moore PS. Complex alternative cytoplasmic protein isoforms of the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 generated through noncanonical translation initiation. J. Virol. 2013;87(5):2744–2755. doi: 10.1128/JVI.03061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stoep N, Quinten E, Marcondes Rezende M, van den Elsen PJ. E47, IRF-4, and PU.1 synergize to induce B-cell-specific activation of the class II transactivator promoter III (CIITA-PIII). Blood. 2004;104(9):2849–2857. doi: 10.1182/blood-2004-03-0790. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang J, Zhang L, Harrington W, Jr., West JT, Wood C. Modulation of human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus replication and transcription activator transactivation by interferon regulatory factor 7. J. Virol. 2005;79(4):2420–2431. doi: 10.1128/JVI.79.4.2420-2431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Toomey NL, Diaz LA, Walker G, Ramos JC, Barber GN, Ning S. Oncogenic IRFs provide a survival advantage for Epstein–Barr virus- or human T-cell leukemia virus type 1-transformed cells through induction of BIC expression. J. Virol. 2011;85(16):8328–8337. doi: 10.1128/JVI.00570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Wu FY, Yu Y, Hayward GS. CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters. J. Virol. 2003;77(17):9590–9612. doi: 10.1128/JVI.77.17.9590-9612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yuan Y. Essential role of RBP-Jkappa in activation of the K8 delayed-early promoter of Kaposi's sarcoma-associated herpesvirus by ORF50/RTA. Virology. 2007;359(1):19–27. doi: 10.1016/j.virol.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhao L, Del Valle L, Miklossy J, Zhang L. Interferon regulatory factor 4 is involved in Epstein–Barr virus-mediated transformation of human B lymphocytes. J. Virol. 2008;82(13):6251–6258. doi: 10.1128/JVI.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WD, Pan HF, Ye DQ, Xu Y. Targeting IRF4 in autoimmune diseases. Autoimmun. Rev. 2012;11(12):918–924. doi: 10.1016/j.autrev.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wood C. The transcriptional repressor K-RBP modulates RTA-mediated transactivation and lytic replication of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2007;81(12):6294–6306. doi: 10.1128/JVI.02648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Shedd D, Miller G. An Sp1 response element in the Kaposi's sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 2005;79(3):1397–1408. doi: 10.1128/JVI.79.3.1397-1408.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wood C, Xu D, Zhang L. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 replication and transcription activator regulates viral and cellular genes via interferon-stimulated response elements. J. Virol. 2005;79(9):5640–5652. doi: 10.1128/JVI.79.9.5640-5652.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA. 1996;93(13):6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeteweij JP, Moses AV, Rinderknecht AS, Davis DA, Overwijk WW, Yarchoan R, Orenstein JM, Blauvelt A. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97(8):2374–2380. doi: 10.1182/blood.v97.8.2374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.