ABSTRACT
The Tsc1–Tsc2 complex homologous to human tuberous sclerosis complex proteins governs amino acid uptake by regulating the expression and intracellular distribution of amino acid transporters in Schizosaccharomyces pombe. Here, we performed a genetic screening for molecules that are involved in amino acid uptake and found Arn1 (also known as Any1). Arn1 is homologous to ART1, an arrestin-related trafficking adaptor (ART) in Saccharomyces cerevisiae, and contains a conserved arrestin motif, a ubiquitination site, and two PY motifs. Overexpression of arn1+ confers canavanine resistance on cells, whereas its disruption causes hypersensitivity to canavanine. We also show that Arn1 regulates endocytosis of the Cat1 amino acid transporter. Furthermore, deletion of arn1+ suppresses a defect of amino acid uptake and the aberrant Cat1 localization in tsc2Δ. Arn1 interacts with and is ubiquitinated by the Pub1 ubiquitin ligase, which is necessary to regulate Cat1 endocytosis. Cat1 undergoes ubiquitinations on lysine residues within the N-terminus, which are mediated, in part, by Arn1 to determine Cat1 localization. Correctively, Arn1 is an ART in S. pombe and contributes to amino acid uptake through regulating Cat1 endocytosis in which Tsc2 is involved.
Keywords: Arrestin-related trafficking adaptor, ART, Arn1/Any1, Amino acid uptake, Endocytosis of transporter, Ubiquitination
INTRODUCTION
Proper regulation of distribution of plasma membrane proteins in response to environmental cues is important for securing cell growth and survival. In mammalian cells, members of the G-protein-coupled receptor family, which couple primarily to heterotrimeric G-proteins and β-arrestins, initiate a wide range of signaling events involved in several cellular processes when they bind to a diverse array of ligands, such as hormones and peptides (Musnier et al., 2010; Shukla et al., 2011). Desensitization and endocytosis of G-protein-coupled receptors following the agonist binding are mediated by β-arrestins, which function as adaptor molecules that combine E3 ubiquitin ligases with the receptors to ubiquitinate the receptors (Shenoy and Lefkowitz, 2011). β-Arrestins also mediate internalization of growth factor receptors, cytokine receptors, and channels (Lefkowitz et al., 2006).
In the budding yeast Saccharomyces cerevisiae, subcellular distribution of several plasma membrane transporters for nutrients including amino acids, nucleobases, and metals is regulated in response to environmental levels of those substrates so that their uptake is regulated. Similar to the regulation of membrane proteins in mammalian cells, ubiquitination of those transporters, which is catalyzed by Rsp5, a HECT-type E3 ubiquitin ligase in the Nedd4 family, governs their intracellular trafficking (Lauwers et al., 2010). It has recently been demonstrated that arrestin-related trafficking adaptors (ARTs) in S. cerevisiae containing structurally conserved features with the mammalian arrestin proteins interact with Rsp5 and act as adaptor molecules for the ubiquitination of the nutrient transporters by the ubiquitin ligase to regulate their intracellular trafficking. Moreover, the ARTs are themselves ubiquitinated by Rsp5 (Lin et al., 2008; Nikko et al., 2008; Nikko and Pelham, 2009; O'Donnell et al., 2010; Hatakeyama et al., 2010; Merhi and André, 2012).
In the fission yeast Schizosaccharomyces pombe, the Tsc1–Tsc2 complex is a homologous protein complex of human tuberous sclerosis complex (TSC), TSC1 and TSC2, a GTPase-activating protein for the Rheb small GTPase, which is involved in cell growth through the regulation of mechanistic target of rapamycin complex (mTORC1) signaling (Matsumoto et al., 2002; Sengupta et al., 2010). The Tsc1–Tsc2 complex and its target protein, Rhb1, the ortholog of human Rheb, engage in incorporation of amino acids and nucleobases by regulating the localization and expression of the nutrient transporters (Matsumoto et al., 2002; van Slegtenhorst et al., 2004; Urano et al., 2005; Nakase et al., 2006; Aspuria and Tamanoi, 2008; Murai et al., 2009). In addition, like the mammalian system, the Tsc1–Tsc2 complex and Rhb1 are also involved in the TORC1 signaling that plays a critical role in the switch between cell proliferation and differentiation in response to nutrient availability and controls the phosphorylation and activity of Psk1, an ortholog of p70 S6 kinase, which, in turn, regulates the phosphorylation of ribosomal protein S6 (Urano et al., 2005; Alvarez and Moreno, 2006; Uritani et al., 2006; Hayashi et al., 2007; Matsuo et al., 2007; Urano et al., 2007; Weisman et al., 2007; Nakashima et al., 2010; Nakashima et al., 2012).
Pub1 is a homolog of Rsp5 ubiquitin ligase in S. pombe and has been identified as a ubiquitin ligase that ubiquitinates and downregulates Cdc25, which is a tyrosine phosphatase for the Cdc2 cyclin-dependent kinase (Nefsky and Beach, 1996). On the other hand, Pub1 is involved in cell proliferation in media at low pH (Saleki et al., 1997). Furthermore, it also contributes to amino acid uptake via the regulation of the localization of Aat1 and Cat1, transporters for general amino acids and cationic amino acids, respectively (Karagiannis et al., 1999; Aspuria and Tamanoi, 2008; Nakase et al., 2012). Pub1 mediates ubiquitination of Aat1, which governs its subcellular distribution (Nakase et al., 2012). Intriguingly, in addition, loss of Pub1 suppresses mislocalization of Cat1 in the tsc2 disruptant (Aspuria and Tamanoi, 2008). However, the regulatory mechanism of amino acid uptake in which the Tsc1–Tsc2 complex and Pub1 are involved remains largely unclear. As the TSC molecules are not conserved in S. cerevisiae (Aspuria et al., 2007), S. pombe is an adequate model organism to gain insight into the regulation of amino acid incorporation and the amino acid transporters by the Tsc1–Tsc2 complex.
In this study, in a genetic screening using an S. pombe genomic library, we identified arn1+ (also known as any1+) encoding an ART as a molecule whose overexpression led to resistance to canavanine in wild-type cells. Arn1 was involved in the regulation of Cat1 endocytosis and acted downstream of Tsc2 in the regulation of amino acid uptake. Arn1 required the interaction with and ubiquitination by Pub1 for its function. In addition, the ubiquitinations of Cat1 determined its subcellular distribution, which may be mediated, in part, by Arn1. Collectively, these results suggest that Arn1 contributes to amino acid uptake as an ART in S. pombe through regulation of endocytosis of Cat1.
RESULTS
Identification of arn1+ that causes resistance to canavanine in a genetic screening
Canavanine, a toxic analog of arginine, is taken up in a manner similar to that of amino acid uptake that utilizes Cat1, a primary cationic amino acid transporter, and consequently causes a severe growth defect of Schizosaccharomyces pombe cells (Aspuria and Tamanoi, 2008). To gain insight into the regulation of amino acid uptake, we screened for genes using an S. pombe genomic library that suppress the growth defect on a solid medium containing canavanine when they were expressed in the high-copy-number plasmid and obtained two genomic clones, clone 1 and clone 8, from 6×104 transformants. Cells carrying either of the genomic clones showed tolerance to a high concentration of canavanine compared with cells carrying the empty vector (supplementary material Fig. S1A). Sequence analysis revealed that each clone includes distinct chromosomal fragment containing the predicted coding regions and a non-coding RNA as listed in supplementary material Table S1. Because the clone 1 transformant showed more resistance to canavanine than that of clone 8, we looked for the attributes in clone 1 that contribute to the canavanine tolerance. Two predicted open reading frames included in the genomic region of clone 1 are annotated as an ortholog of atg14+ and SPBC18H10.20c in the S. pombe database, PomBase (http://www.pombase.org) (supplementary material Table S1; Fig. 1A). We, thus, first constructed high-copy-expression plasmids in which either of the open reading frames together with its 5′ and 3′ flanking regions is integrated and expressed under its own promoter, and then evaluated the tolerance to canavanine of their transformants (Fig. 1A). Growth of cells expressing the atg14+ homolog alone on the canavanine-containing medium was inhibited as severely as the control cells transformed with the empty vector, whereas cells expressing SPBC18H10.20c were grown comparably to the clone 1 transformant. Similar results were obtained when these genes were individually overexpressed under the ectopic nmt1 promoter (Maundrell, 1993) (supplementary material Fig. S1B). These results suggest that overexpression of SPBC18H10.20c confers resistance to canavanine.
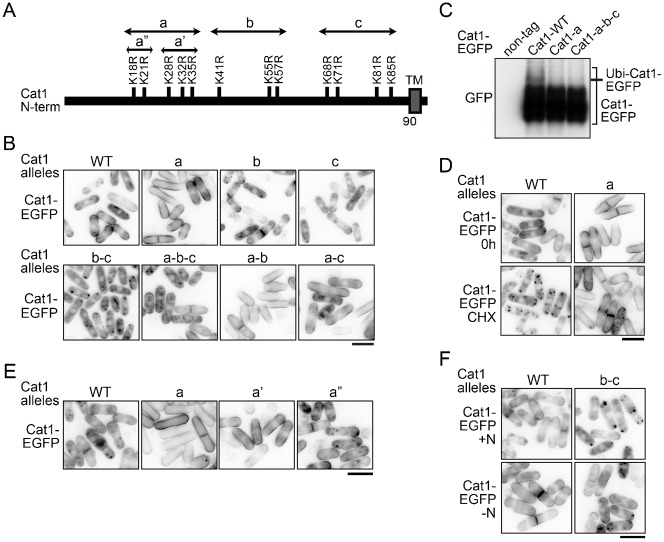
Fig. 1. Identification of arn1+ as a gene that causes resistance to canavanine in a genetic screening.
(A) The upper panel shows a schematic representation of the predicted genomic fragment in the clone 1 plasmid. The results in the lower panels are summarized on the right of the diagram (+, −). The lower panels: JUp1211 cells carrying an empty vector, the genomic clone 1 plasmid, pAL-spbc18h10.20c, or pAL-atg14+ were spotted on EMM with or without 60 µg/ml canavanine and incubated for 3 days (EMM) or 5 days (EMM with canavanine). (B) Comparison of the predicted structure of S. cerevisiae ART1, S. pombe Arn1, and SpArn2. Each homologous protein contains one arrestin fold domain (blue box), one arrestin motif (orange box), and two PY motifs (yellow boxes) in addition to a conserved lysine residue (red text with yellow–green box). (C) JUp1204 (WT) and AN0196 (arn1Δ) were spotted on EMM and EMM with canavanine as indicated and incubated as in panel A. (D) L972 (WT) and AN0195 (arn1Δ) were spotted on EMM and YES and incubated for 3 days at temperatures as indicated. (E) JUp1211 (WT) and AN0194 (arn1Δ) carrying pAL-KS (empty), pAL-arn1+, or pAL-arn2+ were spotted on EMM and EMM with canavanine as indicated and incubated for 3–4 days.
Searching the ortholog of the gene product encoded by SPBC18H10.20c using the BLAST program revealed that it exhibits a high homology to ART1, a member of the ART family in Saccharomyces cerevisiae, which includes a conserved arrestin motif within an arrestin fold domain, a putative ubiquitination site, and two PY (X1–Pro–X2–Tyr, where X1 is often a Pro) motifs (Lin et al., 2008). It has been known that the arrestin domain in human β-arrestin is required for the interaction with G-protein-coupled receptors (Gurevich et al., 1995; Vishnivetskiy et al., 2004), whereas PY motifs are important to bind to WW (Trp–Trp) domains of HECT-type E3 ubiquitin ligases in the Nedd4 family (Staub et al., 2000; Kay et al., 2000). These structural motifs and the putative modification site are highly conserved in the predicted product of SPBC18H10.20c (Fig. 1B; supplementary material Fig. S2). We therefore named SPBC18H10.20c arn1+ (arrestin 1) and carried out the following experiments. While we were preparing this manuscript, Nakase et al. have independently identified SPBC18H10.20c as a gene whose mutation suppressed a growth defect of tsc2Δ caused by a failure of leucine uptake and referred to this gene product as Any1 (Nakase et al., 2013; see Discussion). S. pombe possesses one homologous protein to Arn1, which is encoded by SPAC1F12.05, and it shares 38% identity and 57% similarity to Arn1. Similar to Arn1, the homolog is found to contain an arrestin motif, a putative ubiquitination site, and two PY motifs. Therefore, the latter was designated as Arn2 (Fig. 1B).
Deletion of arn1+ increases sensitivity to canavanine and suppresses the defect of amino acid uptake in tsc2Δ
To elucidate the function of Arn1 in amino acid uptake, we constructed a deletion mutant of arn1+ and examined its growth on the medium containing canavanine. As opposed to overexpression of arn1+, its deletion caused higher sensitivity to a low concentration of canavanine than that in the wild type (Fig. 1C). arn1Δ also showed slight cold-sensitivity of growth at 25°C on a minimal medium (EMM) but not on a rich medium (YES) (Fig. 1D).
It has been known that a tsc2 deletion mutant is resistant to a high concentration of canavanine and shows a growth defect in the minimal medium containing leucine when the mutant has a leucine auxotrophy, owing to the defect of amino acid uptake (Matsumoto et al., 2002; van Slegtenhorst et al., 2004). We therefore examined whether Arn1 engages in the Tsc2-dependent amino acid transport. Fig. 2A shows that unlike the tsc2Δ single mutant, a double null mutant of arn1+ and tsc2+ showed high sensitivity to canavanine as with arn1Δ. Furthermore, deletion of arn1+ in a tsc2Δ background suppressed a growth defect of a leucine-requiring tsc2 mutant on EMM medium containing leucine (Fig. 2B). Taken together, these results suggest that Arn1 participates in the amino acid uptake machinery in which Tsc2 is involved.
Fig. 2. Loss of Arn1 elevates canavanine sensitivity and suppresses the defect of amino acid uptake in tsc2Δ.
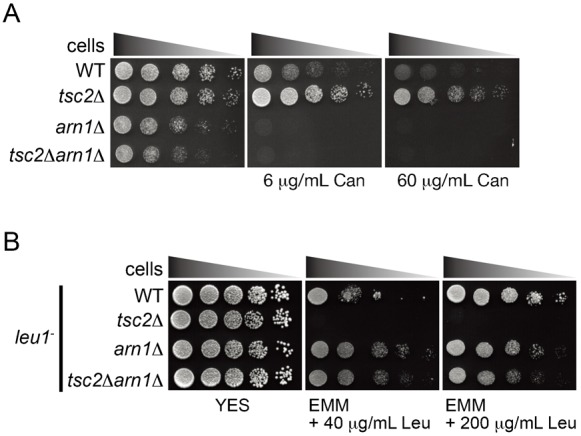
(A) L972 (WT), PJ001 (tsc2Δ), AN0196 (arn1Δ), and AN0209 (tsc2Δarn1Δ) were spotted on EMM and EMM with canavanine as indicated and incubated for 2–4 days. (B) JUp1211 (WT), AN0207 (tsc2Δ), AN0194 (arn1Δ), and AN0208 (tsc2Δarn1Δ) were spotted on YES and EMM with leucine as indicated and incubated for 3 days.
Arn1 is responsible for the endocytosis and stability of the Cat1 transporter
Tsc2 has been known to regulate the localization of the Cat1 cationic amino acid transporter (Aspuria and Tamanoi, 2008). Thus, the results above raise the possibility that Arn1 regulates the function of Cat1. Possible involvement of Arn1 in the regulation of the subcellular localization of the transporter was examined. In wild-type cells, Cat1-GFP chromosomally expressed under the control of its own promoter was predominantly seen on the plasma membrane (PM) at cell tips and the septum together with punctate cytoplasmic structures (Fig. 3A) (Aspuria and Tamanoi, 2008), whereas the fluorescent signal of Cat1-GFP at these locations was increased in arn1Δ (Fig. 3A). Consistent with the results from fluorescent microscopy, the protein level of Cat1-GFP in arn1Δ was higher than that in the wild type in western blot analysis (Fig. 3B). On the other hand, Cat1-GFP was mislocalized to and predominantly accumulated in punctate cytoplasmic structures in tsc2Δ as previously described (Fig. 3A) (Aspuria and Tamanoi, 2008). Deletion of arn1+ suppressed the mislocalization of Cat1-GFP in a tsc2Δ background, and its distribution in the tsc2Δarn1Δ mutant was clearly similar to that in arn1Δ (Fig. 3A). The increased PM localization of Cat1-GFP caused by the arn1 disruption was strongly correlated with the high canavanine sensitivity in arn1Δ and tsc2Δarn1Δ (Fig. 2A).
Fig. 3. Arn1 participates in the regulation of the subcellular localization and stability of Cat1.
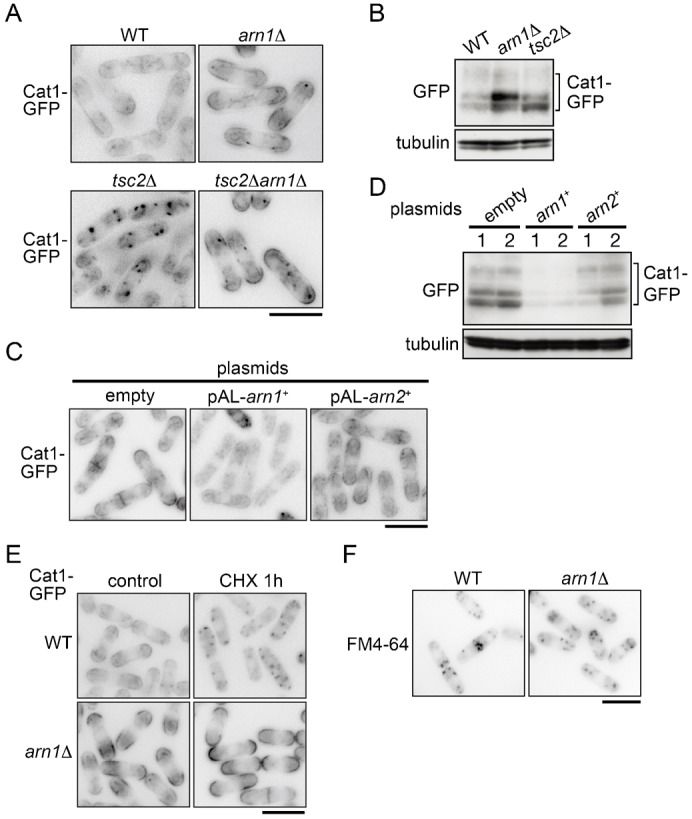
(A) PJ390 (WT), AN0223 (arn1Δ), PJ380 (tsc2Δ), and AN0224 (tsc2Δarn1Δ) were grown in EMM. (B) PJ390 (WT), AN0223 (arn1Δ), and PJ380 (tsc2Δ) were grown in EMM. Tubulin is shown as a loading control. (C) AN0276 carrying the indicated plasmids was grown in EMM. (D) Cells as in panel C were grown in EMM. (B,D) Proteins were probed with the indicated antibodies. (E) PJ390 (WT) and AN0223 (arn1Δ) were grown in EMM and then treated with 100 µg/ml cycloheximide for 1 hour. (F) PJ390 (WT) and AN0223 (arn1Δ) were grown in EMM and labeled with 50 µM FM4-64 dye on ice for 30 minutes. Five minutes after changing to a fresh medium without the dye at 30°C, the fluorescent signal was observed under a fluorescence microscope. (A,C,E,F) The fluorescence images are shown inverted for clarity. Scale bars: 10 µm.
In contrast, overexpression of arn1+ markedly decreased the fluorescent signal of Cat1-GFP (Fig. 3C). In addition, the Cat1-GFP protein in cells overexpressing arn1+ was hardly detected (Fig. 3D). This Cat1 destabilization by arn1+ overexpression was correlated with the tolerance to canavanine (Fig. 1A, E; supplementary material Fig. S1B). Taken together, these results suggest that Arn1 is involved in the regulation of the subcellular localization and the protein stability of Cat1.
These findings prompted us to examine whether Arn1 engages in the endocytosis of Cat1. It has been known that cycloheximide induces the internalization of Can1, an arginine transporter, in S. cerevisiae (Lin et al., 2008). In wild-type cells, Cat1-GFP was internalized and accumulated in punctate cytoplasmic structures within 1 hour after addition of cycloheximide, whereas Cat1-GFP in arn1Δ remained and somewhat increased on the PM and septum even after the cycloheximide addition (Fig. 3E). However, the internalization of FM4-64, a general fluorescent marker of endocytosis, in arn1Δ was comparable to that in the wild type (Fig. 3F), suggesting that Arn1 regulates specifically the endocytosis of Cat1.
Arn2 does not show functional redundancy with Arn1 in the regulation of Cat1
Arn2 is homologous to Arn1 in S. pombe as described above, and therefore we examined whether Arn2 is also involved in the regulation of Cat1. As shown in Fig. 1Ea, unlike arn1+, cells overexpressing arn2+ were not resistant to canavanine, and overexpression of arn2+ did not rescue high sensitivity to the arginine analog in arn1Δ (Fig. 1Eb). Furthermore, overexpression of arn2+ provided a little impact on the intracellular distribution and the stability of Cat1-GFP (Fig. 3C,D). These results suggest that Arn2 does not have a redundant function of Arn1 in the control of Cat1.
Cat1 distribution is regulated in response to nutrient conditions, which is partially dependent on Arn1
To next examine the effect of nutrient conditions on the distribution of Cat1, cells expressing Cat1-GFP were nitrogen-starved for 1 hour and observed. Nitrogen starvation concentrated Cat1-GFP on the PM in wild-type cells and in arn1Δ (Fig. 4A). Its starvation-induced translocation to the PM was also observed in tsc2Δ where Cat1-GFP was accumulated in punctate cytoplasmic structures in the nutrient rich medium (Fig. 4A). Furthermore, Cat1-GFP on the PM in the wild type under nitrogen starvation was mostly internalized within 15 minutes following re-addition of nitrogen sources such as glutamine, arginine, and ammonium (Fig. 4B). It is of interest that internalization of Cat1-GFP responded not only to arginine but also to glutamine and ammonium, although Cat1 is a specific transporter for basic amino acids (Aspuria and Tamanoi, 2008). In contrast, in arn1Δ, although a portion of the transporter was internalized within 15 minutes following the stimulation with nitrogen sources, the majority remained on the PM (Fig. 4B), suggesting that Arn1 is involved, but is not indispensable, in the nutrient-dependent Cat1 endocytosis.
Fig. 4. The localization of Cat1 is regulated in response to nutrient conditions, which is partially dependent on Arn1.
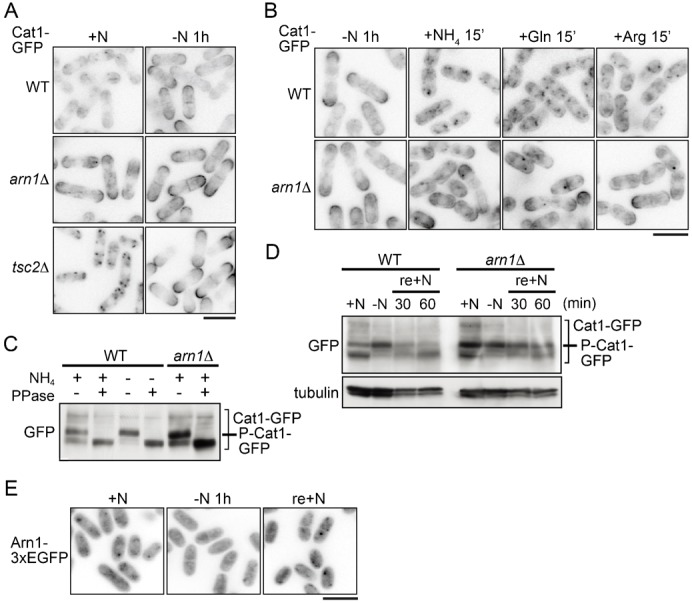
(A) PJ390 (WT), AN0223 (arn1Δ), PJ380 (tsc2Δ), and AN0224 (tsc2Δarn1Δ) were grown in EMM (+N), washed, and incubated in EMM-N for 1 hour (−N 1 h). (B) PJ390 (WT) and AN0223 (arn1Δ) were grown in EMM, washed, and incubated in EMM-N for 1 hour (−N 1 h). Cells were then replaced and incubated in EMM and EMM-N containing glutamine or arginine for 15 minutes. (C) PJ390 (WT) and AN0223 (arn1Δ) were grown in EMM. PJ390 was also subsequently incubated in EMM-N for 1 hour. Protein extracts prepared from membrane rich fractions were incubated with or without λ-phosphatase. (D) PJ390 (WT) and AN0223 (arn1Δ) were grown in EMM (+N), washed, and incubated in EMM-N for 1 hour (−N). Ammonium was subsequently added to the cultures (final concentration, 0.5%), and cells were incubated for the indicated times (re+N). (C,D) Proteins were probed with the indicated antibodies. (E) AN0352 (Arn1-3×EGFP) was grown in EMM (+N), washed, and incubated in EMM-N for 1 hour (−N 1 h). Ammonium was added to the culture, and cells were incubated for 15 minutes (re-+N). (A,B,E) The GFP images are shown inverted for clarity. Scale bars: 10 µm.
As shown in Fig. 3B,D, Cat1-GFP was detected as two major bands with minor bands showing low mobility in western blotting analysis, suggesting some posttranslational modifications of Cat1. The electrophoretic mobility shift of the two major Cat1-GFP bands was increased by nitrogen starvation; the lower one disappeared, whereas the upper one was increased (Fig. 4C,D). The λ-phosphatase assay revealed that the mobility shift was due to phosphorylation (Fig. 4C). A similar modification of Cat1 was observed when it was tagged with three tandem HA epitopes (data not shown), suggesting that Cat1 itself is phosphorylated. Fig. 4D shows that increased Cat1 phosphorylation under nitrogen starvation was promptly decreased by re-addition of ammonium. Alteration of Cat1 phosphorylation seems to be correlated with its localization, because when Cat1 was concentrated on the PM by nitrogen starvation, it was highly phosphorylated. Furthermore, Cat1-GFP in arn1Δ, which tended to be localized on the PM, showed a higher phosphorylation state than that in the wild type in the nutrient rich medium (Fig. 4D). Consistent results were obtained when cells were treated with cycloheximide. Cat1-GFP in arn1Δ following the treatment with cycloheximide, which was accumulated on the PM (Fig. 3E), was highly phosphorylated, whereas it exhibited decreased phosphorylation regardless of nutrient conditions in the wild type or tsc2Δ (supplementary material Fig. S3), which was internalized by the treatment (Fig. 3E; data not shown). Collectively, these results suggest that Cat1 is subjected to phosphorylation on the PM in a nutrient-independent manner. Alternatively, the phosphorylation of Cat1 might facilitate its transport to the PM.
To next examine whether Arn1 responds to nutrient conditions, we constructed a strain chromosomally expressing Arn1-3EGFP under its own promoter and initially tested its canavanine sensitivity to assess the function of the fusion protein. Cells expressing Arn1-3EGFP could grow comparably to the control cells in the presence of a low concentration of canavanine, which inhibits the growth of arn1Δ (data not shown), suggesting that the fusion protein, at least in part, maintains the function of Arn1. Arn1-3EGFP was distributed throughout the cell, and some were localized in punctate cytoplasmic structures in the growing condition (Fig. 4E). After 1 hour of nitrogen starvation, the fluorescent signals of Arn1-3EGFP on the cytoplasmic puncta were dispersed, and then it was accumulated in the punctate structures again within 15 minutes after refeeding ammonium (Fig. 4E). Accordingly, the localization of Arn1 was regulated in response to nutrient conditions.
Significance of the conserved motifs and ubiquitination of Arn1 for the regulation of Cat1 endocytosis
In S. cerevisiae, the arrestin and PY motifs as well as the ubiquitination of ART1 are required for the regulation of membrane protein endocytosis (Lin et al., 2008). Therefore, to address the role of the conserved motifs in Arn1 in the regulation of Cat1, we constructed mutant strains chromosomally expressing 3HA-tagged mutant Arn1 in which the corresponding residues in either the arrestin or PY motifs to those of S. cerevisiae ART1 were substituted with tryptophan or alanine (Fig. 5A; supplementary material Fig. S2) and measured their sensitivity to canavanine. As shown in Fig. 5B, either the mutation in the arrestin or PY motifs in Arn1 caused a high sensitivity to a low concentration of canavanine with similar sensitivity as its null mutant, although the Arn1 mutant proteins were expressed similarly to that of the wild type (Fig. 5E). These mutations also caused a defect of the internalization of Cat1 induced by cycloheximide (Fig. 5C), suggesting that the arrestin and PY motifs in Arn1 are important to control Cat1 endocytosis.
Fig. 5. Roles of the conserved motifs and ubiquitination of Arn1 in regulation of Cat1.
(A) Schematic diagram of the mutations in Arn1 (P116G117/WW in an arrestin motif, K263/R at a conserved ubiquitination site, and P348F350P358Y360/4A in PY motifs). (B) AN0195 (arn1Δ), AN0220 (arn1+), AN0273 (arn1PG/WW), AN0264 (arn1K263R), and AN0267 (arn1PFPY/4A) were spotted on EMM and EMM with 6 µg/ml canavanine, and incubated for 3 and 6 days, respectively. (C) AN0282 (arn1+), AN0286 (arn1PG/WW), AN0288 (arn1K263R), and AN0284 (arn1PFPY/4A) were grown in EMM and then treated with 30 µg/ml cycloheximide for 1 hour. The GFP images are shown inverted for clarity. (D) L972 (non-tag), AN0220 (Arn1WT), AN0264 (Arn1K263R), and AN0259 (Arn1WTpub1Δ) were grown in YES. Arn1-3HA was immunoprecipitated from protein extracts. (E) L972 (non-tag), AN0220 (Arn1WT), AN0273 (Arn1PG/WW), AN0264 (Arn1K263R), AN0267 (Arn1PFPY/4A), and AN0259 (Arn1WTpub1Δ) were grown in YES. (Fa) AN0293 (WT), AN0295 (K263R), and AN0297 (PFPY/4A) strains carrying pREP41-2myc-6His-pub1+ were grown in EMM. (Fb) AN0293 (Arn1-3HA) carrying an empty vector (−), pREP41-2myc-6His-pub1+ (WT), -pub1C2Δ (C2Δ), or -pub1WWΔ (WWΔ) were grown in EMM. Arn1-3HA (Fa) and MH-Pub1 (Fb) were immunoprecipitated from protein extracts. (D,E,Fa,Fb) Proteins were probed with the indicated antibodies. Scale bar: 10 µm.
Moreover, the substitution of Lys263 of Arn1 to arginine, which corresponds to the Lys486 ubiquitination site in S. cerevisiae ART1 (supplementary material Fig. S2; Fig. 5A), also led to the loss of Arn1 function (Fig. 5B,C). This result prompted us to investigate whether Arn1 is actually ubiquitinated. Fig. 5D shows that immunoprecipitated wild-type Arn1-3HA was mainly observed as three bands and that only the top band but not the others was detected with the anti-ubiquitin antibody. In addition, the electrophoretic mobility shifts and the ubiquitination of Arn1-3HA were completely lost by the K263R mutation (Fig. 5D). Taken together, these results suggest that Arn1 is subjected to ubiquitination, which is important in the regulation of Cat1 endocytosis, and that the Lys263 residue directly undergoes or is indirectly involved in its ubiquitination. To next examine the effect of mutations in the arrestin and PY motifs of Arn1 on its ubiquitination, we assessed the ubiquitination of the two Arn1-3HA mutants. Because the PY motif is required for the binding to the WW domains in the NEDD4/Rsp5 ubiquitin ligase (Staub et al., 2000; Kay et al., 2000), as expected, the defect of the PY motifs abolished the top band that corresponded to ubiquitinated Arn1-3HA (Fig. 5D,E), whereas the mutations of the arrestin motif also markedly attenuated its modification (Fig. 5E). These results suggest that the conserved and functional motifs of Arn1, especially the PY motifs, are required for its ubiquitination.
Pub1 E3 ubiquitin ligase associates with and mediates ubiquitination of Arn1
In S. pombe, Pub1 is an ortholog of the Rsp5 HECT-type ubiquitin ligase (Nefsky and Beach, 1996; Saleki et al., 1997) and is involved in trafficking of Cat1 and Aat1 (Aspuria and Tamanoi, 2008; Nakase et al., 2012). Therefore, we examined the involvement of Pub1 in Arn1 ubiquitination and found that deletion of pub1+ resulted in the defect of Arn1 ubiquitination, which was similar to that observed in the PY motif mutant (Fig. 5D,E). Moreover, as shown in Fig. 5Fa,Fb, 2myc- and 6His-tagged wild-type Pub1 was co-purified with immunoprecipitated wild-type Arn1-3HA, and reciprocally, wild-type Arn1-3HA was bound to immunoprecipitated wild-type 2myc-6His-Pub1. As expected, the PY motifs in Arn1 were necessary for the interaction with Pub1, and the defect of Arn1 ubiquitination caused by the K263R substitution markedly decreased its interaction with Pub1 (Fig. 5Fa). Pub1 contains a C2 domain at the N-terminal and three WW domains in the middle region in addition to a HECT domain at the C-terminal, a catalytic domain of the E3 ubiquitin ligase (supplementary material Fig. S4A) (Nefsky and Beach, 1996; Saleki et al., 1997). Deletion of only the WW domains in Pub1 was sufficient to abolish the interaction with Arn1, whereas deletion of the C2 domain provided little effect on this protein interaction (Fig. 5Fb). Taken together, these results suggest that Pub1 probably directly ubiquitinates Arn1 and that the PY motifs and WW domains mediate the interaction between the substrate and the enzyme as previously reported (Staub et al., 2000; Kay et al., 2000).
It is interesting to note that the middle band of Arn1-3HA was slightly reduced but sufficiently remained in the mutant of the PY motifs or in a pub1 disruptant, although it was abolished by the K263R substitution (Fig. 5D,E). On the other hand, this band was not detected with the anti-ubiquitin antibody (Fig. 5D). It has been known that in mammalian cells, arrestin-3, which regulates internalization of β2-adrenergic receptor, is modified by small ubiquitin-like modifier protein (SUMO) at a lysine residue (Wyatt et al., 2011). Therefore, to explore the possibility that Arn1 undergoes SUMOylation, Arn-3HA was expressed in cells deficient in pmt3+ encoding the sole SUMO protein in S. pombe (Tanaka et al., 1999) or in cells overexpressing 2myc-epitopes tagged Pmt3. The middle band of Arn1-3HA was not abolished by the pmt3 deletion and did not overlap with 2myc-Pmt3 (data not shown). The additional modification of Arn1, thus, needs to be determined.
Arn1 and Pub1 cooperatively regulate Cat1 function
As we reported previously (Aspuria and Tamanoi, 2008), loss of Pub1 led to canavanine hypersensitivity and to stabilization and accumulation of Cat1 to the PM (Fig. 6A; supplementary material Fig. S4B,C). These phenomena were similar to those in arn1Δ. Therefore, the genetic relationship between arn1+ and pub1+ was next examined. Fig. 6B shows that deletion of pub1+ dispersed the cytoplasmic punctate Arn1-3EGFP and slightly increased its nuclear accumulation. In addition, the pub1 deletion completely suppressed tolerance to canavanine and destabilization of Cat1-GFP caused by arn1+ overexpression (Fig. 6Ca,Da,Db). In contrast, overexpression of pub1+ did not rescue hypersensitivity of arn1Δ to canavanine (Fig. 6Cb). These results suggest that Arn1 and Pub1 cooperatively control amino acid uptake through the Cat1 regulation.
Fig. 6. Arn1 and Pub1 cooperatively work on amino acid uptake through Cat1 regulation.

(A) L972 and AN0259 (pub1Δ) were spotted on EMM and EMM with 6 µg/ml canavanine, and incubated for 4 and 5 days, respectively. (B) AN0351 (WT) and AN0399 (pub1Δ) were grown in EMM. (Ca,b) AN276 (WT, a), AN0290 (pub1Δ, a), and AN0319 (arn1Δ, b) carrying an empty or expression plasmids as indicated were spotted on EMM and EMM with 6 µg/ml canavanine, and incubated for 3 and 4 days, respectively. (Cb) Thiamine was added in media to express moderately pub1+ to avoid a growth defect caused by overexpression of pub1+. (Da,b) Strains as in panel Ca were grown in EMM. (Db) Proteins were probed with the indicated antibodies. (B,Da) The GFP images are shown inverted for clarity. Scale bars: 10 µm.
Cat1 undergoes ubiquitination on the several lysine residues in the N-terminal region, which determine its subcellular distribution
The above results raise the possibility that Arn1 acts as an adaptor molecule for Pub1 to mediate ubiquitination of Cat1 as ART1 in S. cerevisiae (Lin et al., 2008). To explore this possibility, we examined ubiquitination of Cat1 in the presence or absence of arn1+ gene. As shown in Fig. 7A, the pull-down assay using Ni2+ resin revealed that the conjugation of Cat1-GFP and 2myc- and 6His-tagged ubiquitin which was exogenously expressed was observed not only in the wild type but also in arn1Δ and pub1Δ. The Cat1-GFP protein pulled down with ubiquitin was to some extent increased in both mutants (Fig. 7A), but this seems to be due to an increased stability of Cat1 caused by loss of Arn1 or Pub1 as shown in Fig. 3B and supplementary material Fig. S4C. Furthermore, the conjugation of Cat1 with endogenous ubiquitin with or without stimulation by ammonium in arn1Δ as well as that in the wild type was also detected when Cat1-3HA was immunoprecipitated (Fig. 7B). A possible role of Arn1 in Cat1 ubiquitination is mentioned in Discussion.
Fig. 7. Ubiquitination of Cat1 in arn1Δ and pub1Δ.
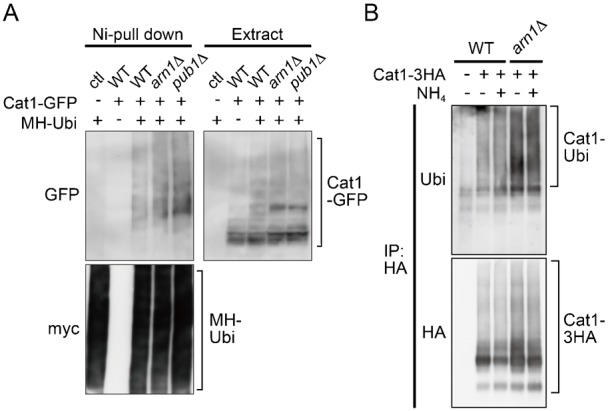
(A) JUp1211(ctl), AN0276 (WT), AN0319 (arn1Δ), and AN0290 (pub1Δ) carrying pREP273 (−) or pREP41-2myc-6His-ubiquitin (+) were grown in EMM. MH-ubiquitin was pulled down from protein extracts. (B) L968 (WT, −), AN0330 (WT, +), and AN0335 (arn1Δ) were grown in EMM, washed, and incubated in EMM-N for 45 minutes (−). Cells were further incubated for 15 minutes following the addition of ammonium (+). Cat1-3HA was immunoprecipitated from protein extracts. (A,B) Proteins were probed with the indicated antibodies.
We next attempted to determine the interaction of Arn1 with Cat1 in cells expressing Arn1-3HA and Cat1-GFP under stimulation of glutamic acid as a nitrogen source in the presence of chemical cross linkers, dithiobis (succinimidylpropionate) (DSP) or both DSP and dimethyl 3,3′-dithiobis propionimidate dihydrochloride (DTBP). However, in the conditions we employed, the interaction between Arn1 and Cat1 was not detected (data not shown).
It has been reported that the Aat1 transporter has several potential ubiquitination sites on lysine residues in the cytoplasmic N-terminal region and that those ubiquitinations appear to determine its translocation (Nakase et al., 2012). Cat1 also includes many lysine residues in the region between the N-terminal end and the first transmembrane domain (Fig. 8A). To investigate the effect of the modification of these lysine residues on the localization of Cat1, the Cat1-EGFP mutants in which the lysine residues in the N-terminal region were substituted by arginine as shown in Fig. 8A were expressed, and their localization was examined. As shown in Fig. 8B, the Cat1-a-EGFP mutant was exclusively localized on the PM, whereas either the b- or c-mutant showed little effect on the localization of Cat1-EGFP or those combinations with Cat1-a-EGFP. The combination of b- and c-mutations resulted in predominantly internalized Cat1-EGFP, whereas the all a-b-c-mutations exhibited an intermediate effect between the a- and b-c-mutations on the distribution. The ubiquitination of the Cat1-a- and -a-b-c-EGFP mutants was significantly decreased compared with that of the wild type (Fig. 8C), suggesting that Cat1 undergoes ubiquitination on the lysine residues, at least in part, in the N-terminal region. Furthermore, the Cat1-a mutation notably blocked the internalization of Cat1-EGFP by the cycloheximide treatment, similar to that observed in arn1Δ (Fig. 3E, Fig. 8D). Within the Cat1-a mutation, the substitution of the latter 3 lysine residues (Cat1-a′) was enough for its PM localization, whereas the substitution of the former 2 residues (Cat1-a″) showed little effect, which was indistinguishable from that of the wild type (Fig. 8E). On the other hand, nitrogen starvation moved Cat1-b-c-EGFP from punctate cytoplasmic structures to the PM (Fig. 8F). The distribution of Cat1-b-c-EGFP was similar to that of Cat1-GFP in tsc2Δ (compare with Fig. 4A). Taken together, these results suggest that modifications of specific lysine residues in the N-terminus of Cat1 by ubiquitin determine its localization.
Fig. 8. Determination of Cat1 distribution by its modification at the N-terminal lysine residues.
(A) Schematic representation of substitutions of lysine residues by arginine in the N-terminal of Cat1. Double-head arrows show the extent of the respective Cat1 mutants including the substitutions of lysine residues. TM denotes a transmembrane domain. (B) JUp1209 cells carrying pREP273-Cat1-WT or the mutants fused with EGFP as indicates were grown in EMM + uracil. (C) L968 (non-tag), AN0541 (Cat1-WT), AN0542(Cat1-a), and AN0543(Cat1-a-b-c) were grown in EMM. Protein extracts were prepared from membrane rich fractions, and proteins were probed with the GFP antibody. (D) JUp1209 carrying pREP273-Cat1-WT or -a-EGFP was grown in EMM + uracil (0 h) and then treated with 30 µg/ml cycloheximide for 1 hour (CHX). (E) JUp1209 carrying pREP273-Cat1-WT or the mutants as indicates were grown in EMM + uracil. (F) JUp1209 carrying pREP273-Cat1-WT- or -b-c-EGFP was grown in EMM + uracil (+N), washed, and incubated in EMM-N for 1 hour (−N). (B,D–F) The GFP images are shown inverted for clarity. Scale bars: 10 µm.
DISCUSSION
In mammalian cells, one of the major functions of β-arrestin is to act as an adaptor of E3 ubiquitin ligases, such as MDM2 and NEDD4 that mediate ligand-induced ubiquitination and internalization of G-protein-coupled receptors and the other types of membrane proteins (Lefkowitz et al., 2006; Shenoy and Lefkowitz, 2011). β-Arrestin itself undergoes ubiquitination by Mdm2 (Shenoy et al., 2001). Here, in S. pombe, we identified Arn1, a homolog of budding yeast arrestin-related trafficking adaptor (ART) 1, in a genetic screening using an S. pombe genomic library for molecules that confer resistance to canavanine, a toxic analog of arginine, when they are highly expressed in wild-type cells. This screening can isolate molecules that participate in amino acid uptake (Fig. 1). We further demonstrated that Arn1 is implicated in endocytosis of Cat1, a primary cationic amino acid transporter, in response to extracellular conditions, such as nutrients and cycloheximide treatment (Fig. 3, Fig. 4B). On the other hand, deletion of arn1+ rescues a defect of amino acid uptake and the aberrantly internalized Cat1 localization in tsc2Δ (Fig. 2). It has been known that the internalized Cat1 in tsc2Δ causes the resistance to canavanine (Aspuria and Tamanoi, 2008). The restoration of Cat1 localization in tsc2Δ by deletion of arn1+ abolished the canavanine resistance. These results suggest that Arn1 functions downstream of Tsc2 in amino acid uptake. The TSC molecules are conserved between fission yeast and mammal, whereas there are no counterparts of TSCs in S. cerevisiae (Aspuria et al., 2007). Therefore, the finding that the TSC–ART pathway contributes the regulation of membrane transporter distribution in S. pombe may provide insight into the relationship between the TSC and arrestin proteins in higher eukaryotes.
Arn1 is predicted to contain one arrestin motif and two PY motifs (Fig. 1B). These motifs in Arn1 were required for the regulation of Cat1 endocytosis (Fig. 5). In addition, Arn1 directly interacted with and was ubiquitinated probably at a conserved lysine residue (Lys263) by Pub1, an E3 ubiquitin ligase in the Nedd4 family. These actions were mediated by the association of the PY motifs in the substrate with the WW domains in the ligase. This ubiquitination in Arn1 was also required for the regulation of Cat1 endocytosis (Fig. 5). Furthermore, epistatic analyses showed that Arn1 and Pub1 cooperatively engaged in Cat1 function (Fig. 6). These findings propose Arn1 as an ART in S. pombe, and that the modulation of ART function through ubiquitination by the E3 ubiquitin ligase is evolutionarily conserved among two distantly related yeasts and mammal.
Cat1 was ubiquitinated at multiple sites on lysine residues, at least in part, in the cytoplasmic N-terminal region (Fig. 7, Fig. 8C). The Cat1-a and, especially, Cat1-a′ mutants (substitution of lysine residues from Lys18 to Lys35 and from Lys28 to Lys35 by arginine, respectively (Fig. 8A)) were exclusively localized on the PM and were deficient in their internalization from the PM induced with cycloheximide, whereas the replacement of lysine residues from Lys41 to Lys85 by arginine (b-c mutation) confined the transporter in punctate cytoplasmic structures (Fig. 8B,D). Therefore, Cat1 might undergo different types of ubiquitin-mediated regulations to determine its subcellular localization: one of them (especially ubiquitinations on Lys28–Lys35) promotes endocytosis, and the other (ubiquitinations on Lys41–Lys85) facilitates transport to the PM. On the other hand, nitrogen starvation translocated the Cat1-b-c mutant from punctate cytoplasmic structures to the PM (Fig. 8F). This observation raises the possibility that if nitrogen starvation attenuates the ubiquitinations on Lys28–Lys35, it may be enough for keeping Cat1 on the PM even though the absence of the ubiquitinations on Lys41–Lys85. Therefore, the ubiquitinations on Lys41–Lys85 in Cat1 may alternatively block the ubiquitinations of Lys28–Lys35 to prevent its endocytosis. Cat1 in tsc2Δ was accumulated in punctate structures in the cytoplasm in nutrient rich conditions, but the deletion of arn1+ or nitrogen starvation facilitated translocation of Cat1 to the PM (Fig. 3A, Fig. 4A). Tsc2 may therefore regulate ubiquitinations on Lys41–Lys85 in Cat1.
Here, we could not obtain direct evidence that Arn1 interacts with and mediates ubiquitination of Cat1. However, Arn1 carries one arrestin motif that is known to be important for the interaction with membrane proteins in mammal (Gurevich et al., 1995; Vishnivetskiy et al., 2004), and this motif was required for Cat1 endocytosis (Fig. 5C). Similarly, the arrestin motif of ART1 in S. cerevisiae is also necessary to regulate the endocytosis of its targets (Lin et al., 2008). Therefore, Arn1 possibly interacts with Cat1, but its interaction may be transient and weak or, alternatively, require other unknown protein(s). Moreover, the observation that deletion of arn1+ caused a defect of Cat1 internalization from the PM induced by cycloheximide was comparable to the endocytosis defect of the Cat1-a mutant, which was probably deficient in ubiquitination on lysine residues from Lys18 to Lys35 (Fig. 3E, Fig. 8D). Therefore, Arn1 seems to participate in Cat1 ubiquitination on lysine residues, at least from Lys18 to Lys35, especially from Lys28 to Lys35, which would contribute to Cat1 endocytosis. On the other hand, Cat1 would be ubiquitinated at several lysine residues in addition to the residues modified by Arn1 and Pub1 (Fig. 8). Therefore, as shown in Fig. 7, the defect of ubiquitination caused by loss of Arn1 or Pub1 may be eclipsed by the other multiple ubiquitinations.
As described above, Nakase et al. independently reported that Arn1/Any1 (they named SPBC18H10.20c Any1) regulates subcellular distribution of some transporters including Aat1, an S. pombe homolog of S. cerevisiae general amino acid transporter Gap1 (Nakase et al., 2010; Nakase et al., 2013). However, Cat1 was not characterized. Although several features of Arn1/Any1 showed in our and their studies are overlapping, some major points are different as summarized: 1) Arn1 and Any1 were identified from distinct screening approaches. We identified arn1+ as a gene whose high-copy expression confers canavanine resistance on wild-type cells using a S. pombe genomic library (Fig. 1), whereas any1+ was isolated in a screening for mutations that restore a growth defect of tsc2Δ owing to a failure of leucine uptake (Nakase et al., 2013). 2) We demonstrated that Arn1 is ubiquitinated probably at Lys263 by Pub1 E3 ubiquitin ligase and that its ubiquitination is important for regulation of Cat1 endocytosis (Fig. 5). This is the first report of the ubiquitination and its function of ART in S. pombe. In addition, this study presented the significance of structurally conserved features, the arrestin and PY motifs, in Arn1 function (Fig. 5B,C). 3) Nakase et al. concluded that Any1 is required to store Aat1 in the Golgi and that deletion of any1+ mislocalizes the transporter to the PM (Nakase et al., 2013). By contrast, our findings revealed that Arn1 is required for Cat1 endocytosis in response to environmental conditions. These different actions of Arn1/Any1 on the distribution of Aat1 and Cat1, however, may be dependent on the feature of the amino acid transporters. In S. cerevisiae, two homologous ARTs, Aly1 and Aly2, have been known to express distinct actions for different amino acid transporters: Aly1 and Aly2 regulate intracellular sorting of Gap1, a general amino acid transporter, from endosome to the trans-Golgi network and/or the PM, but not its endocytosis (O'Donnell et al., 2010), whereas they also contribute to endocytosis of the aspartic acid/glutamic acid transporter Dip5 (Hatakeyama et al., 2010). In S. pombe, Aat1 is a homolog of Gap1, a general amino acid transporter (Nakase et al., 2010), whereas Cat1 is a specific transporter for cationic amino acids (Aspuria and Tamanoi, 2008). Therefore, similar to Aly1 and Aly2, Arn1/Any1 may also have distinct functions for different amino acid transporters. Further work is needed to understand how Arn1/Any1 affects different amino acid transporters.
Most recently, Zhao et al. have presented an interesting aspect of the arrestin function that ARTs together with Rsp5 compose a plasma membrane quality control system, which protects cells from proteotoxic stress in S. cerevisiae (Zhao et al., 2013). In S. pombe genome, there are at least 10 predicted ART genes including arn1+ and arn2+, whose products include an arrestin motif. Among these, for example, Ste7 appears to be involved in the mating pheromone signaling that is activated following activation of the heterotrimeric G-protein triggered by the interaction between pheromone peptides and those receptors, members in the G-protein-coupled receptor family (Matsuyama et al., 2000). Further studies are needed to gain the overview of functions of ARTs in S. pombe.
MATERIALS AND METHODS
Yeast strains, growth media, and general methods
S. pombe strains used in this study are listed in supplementary material Table S2. Cells were grown at 30°C, unless otherwise indicated, in yeast extract with supplements (YES) medium and Edinburgh minimal medium (EMM) supplemented with leucine or uracil, when indicated, which were prepared as described (Sabatinos and Forsburg, 2010). EMM-N, a nitrogen free version, was employed as a starvation medium. General and molecular genetic techniques followed standard protocols (Okazaki et al., 1990; Moreno et al., 1991). Disruption of arn1+ with the ura4+ cassette and integration of 3×HA-hphMX or -kanMX cassette at the C-terminal of arn1+ and cat1+ were performed using the PCR-based direct chromosomal integration methods (Bähler et al., 1998; Sato et al., 2005).
Antibodies
Following antibodies were purchased from the commercial sources: anti-myc (9E10) and anti-α-Tubulin (B5-1-2) antibodies from Sigma (St Louis, MO); anti-HA (12CA5) and anti-GFP mouse antibodies from Roche (Mannheim, Germany); anti-ubiquitin (P4D1) from Cell Signaling Technology (Danvers, MA); horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit antibodies from Promega (Madison, WI).
Genetic screening
JUp1211 cells were transformed with an S. pombe genomic library included in a high-copy plasmid, pTN-L1 (obtained from National BioResource Project (NBRP), Osaka Japan) and incubated overnight on EMM plate. Then, the cells were transferred on EMM plate containing 60 µg/ml canavanine (Sigma) by the replica plating method and further incubated. The recovered genomic plasmid clone from the cells was introduced again in JUp1211, and the transformants were assessed canavanine sensitivity. The recovered clone was sequenced to determine the included genomic region.
Construction of expression plasmids and modified strains
To construct expression plasmids of arn1+, atg14+, and arn2+, the ORFs with approximately 600 bp of their 5′ and 3′ flanking regions were amplified by PCR using the genomic clone 1 plasmid or genomic DNA as a template and were cloned into pAL-KS vector (NBRP). To construct expression plasmids of pub1+ and its mutants, pub1+ ORF amplified by PCR using a cDNA library (NBRP) as a template was cloned into pREP41-2myc-6His vector, which was prepared from pREP41-2myc-6His-ubiquitin (kindly provided by Dr Nic Jones). Deletion of C2 and three WW domains in Pub1 was carried out by the PCR method. To construct expression plasmids of cat1+ and its point mutants, cat1+ ORF amplified by PCR using a genomic library (NBRP) as a template was cloned into pREP273-EGFP, which was generated by the replacement of multi-cloning sites together with the nmt41 promoter (Basi et al., 1993) and 3HA fragment from pSLF273 (NBRP) and the insertion of EGFP ORF in NotI and BglII sites. The mutations of Cat1 were introduced by the site-directed mutagenesis.
To construct a series of the arn1 mutant strains, arn1+ ORF and approximately 600 bp of its 5′ and 3′ flanking regions were cloned into pCR2.1. The point mutations in arn1 were performed by the site-directed mutagenesis. The mutated arn1 DNA fragments, 3HA-hphMX, and the arn1+ 3′-UTR were amplified and combined by PCR, and those fragments were integrated in the chromosomal locus of arn1+ by homologous recombination. A strain expressing Arn1-3EGFP was constructed by homologous recombination using pBS-arn1-CT-3EGFP-KanMX that the arn1-CT fragment replaced ste20-CT in the pBS-3EGFP-KanMX (kindly provided by Dr Kazuhiro Shiozaki). The sequences of all coding regions amplified by PCR were confirmed. To construct strains expressing the genomic copy of EGFP-fused Cat1-wt and its mutants (Cat1-a and -a-b-c), which are controlled under the nmt41 promoter, JUp1211 cells were transformed with linearized pREP273-EGFP carrying wild-type Cat1 or its mutants, which was digested with MluI, to integrate into the ars1+ locus by homologous recombination.
Protein preparation and phosphatase treatment
Protein extracts to assess Arn1-3HA was prepared from cells, which were boiled when harvested, as described previously (Nakashima et al., 2010). To assess Cat1 protein, cells, which were not boiled, were disrupted with glass beads in urea buffer (Nakashima et al., 2012) by vortexing twice for 5 minutes at 4°C, then mixed with 3× SDS sample buffer, and were incubated for 30 minutes at 37°C.
For the λ-phosphatase treatment of Cat1-GFP, cells were broken with glass beads in buffer A without NP-40 (Nakashima et al., 2010) by vortexing twice for 5 minutes at 4°C and were centrifuged at 14,000 g for 5 minutes at 4°C to prepare a membrane-rich fraction. The membrane-rich fraction was washed twice with buffer A without NP-40, and proteins were solubilized within urea buffer without sodium pyrophosphate, β-glycerophosphate, Na3VO4, p-nitrophenyl phosphate, and NaF for 30 minutes on ice. Glass beads and cell debris were removed by centrifugation. Supernatants including membrane proteins were diluted 7.5-fold into a reaction buffer and incubated with λ-phosphatase (400 IU; New England Biolabs, Ipswich, MA) for 30 minutes at 30°C.
Immunoprecipitation and pull-down assay
To assess the interaction between Arn1-3HA and 2myc-6His-Pub1, cells were broken in buffer A with glass beads. After centrifugation at 12,000 g for 10 minutes and subsequently at 14,000 g for 20 minutes at 4°C, supernatants were incubated with anti-HA (3F10) affinity matrix (Roche) or with anti-myc (9E10) antibody and Protein-G-Sepharose (GE Healthcare Bio-Sciences, Buckinghamshire, England) for 2 hours at 4°C. Immunoprecipitates were washed three times with buffer A without protease inhibitors. Immunoprecipitated 2myc-6His-Pub1 was eluted with 600 µg/ml myc-peptides in buffer A.
To assess ubiquitination of immunoprecipitated-Arn1-3HA or -Cat1-3HA, cells were broken in urea buffer with glass beads, and cell extracts were diluted over 9-fold into buffer A. After centrifugation, supernatants were incubated with anti-HA (3F10) affinity matrix for 2 (Arn1-3HA) or 4 (Cat1-3HA) hours at 4°C. Immunoprecipitates were washed three times with buffer A without protease inhibitors.
For pull-down experiments for 6His-tagged ubiquitin, cells were broken in urea buffer without EDTA containing 10 mM imidazole with glass beads. After centrifugation, supernatants were incubated with Ni-NTA superflow (Qiagen, Venlo, The Netherlands) for 2 hours at 4°C, and the resins were washed four times with wash buffer [50 mM Tris-HCl (pH 7.5), 300 mM NaCl, 1% Triton X-100, and 20 mM imidazole]. Proteins were eluted with 300 mM imidazole in wash buffer.
Immunoblotting
Protein extracts or immunoprecipitates were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with the indicated primary antibodies. Detection of proteins was performed using ECL plus system (GE Healthcare Bio-Sciences). When detecting ubiquitination of immunoprecipitated Cat1-3HA, a transferred membrane was dried and boiled in distilled water for 30 minutes before blocking (Swerdlow et al., 1986).
Microscopy
All cell images were captured using fluorescence microscopes (BZ-8000 or BZ-9000; Keyence, Osaka, Japan) with a Nikon Plan Apo 60× oil immersion objective lens (NA 1.40, Nikon, Tokyo, Japan). Images were adjusted for brightness and contrast, inverted using Adobe Photoshop. To assess incorporation of FM4-64 dye (Life Technologies, Carlsbad, CA), cells were labeled with FM4-64 as described (Kita et al., 2004). In brief, cells were labeled with 50 µM FM4-64 for 30 minutes on ice, and then replaced to fresh medium without the dye at 30°C. Five minutes later, cells were examined under the fluorescence microscope.
Supplementary Material
Acknowledgments
We thank Drs Masamitsu Sato, Takashi Toda, Nic Jones, Kazuhiro Shiozaki, Susan Forsburg, and the Yeast Genetic Resource Center of Japan, supported by the National BioResource Project, for S. pombe strains and plasmids. We also thank Drs Terunao Takahara, Tatsuya Maeda and Taro Nakamura for technical advice.
Footnotes
Author Contributions: A.N. performed experiments. A.N., S.K., F.T. and U.K. conceived and designed experiments. A.N., F.T. and U.K. performed data analysis and wrote the manuscript.
Competing interests: The authors have no competing interests to declare.
Funding
This work was supported by Grants-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science [grant number 23770229 to A.N.]; Hyogo Science and Technology Association [grant number 24S029 to A.N.]; and the National Institutes of Health [grant number CA41996 to F.T.].
References
- Alvarez B., Moreno S. (2006). Fission yeast Tor2 promotes cell growth and represses cell differentiation. J. Cell Sci. 119, 4475–4485 10.1242/jcs.03241 [DOI] [PubMed] [Google Scholar]
- Aspuria P. J., Tamanoi F. (2008). The Tsc/Rheb signaling pathway controls basic amino acid uptake via the Cat1 permease in fission yeast. Mol. Genet. Genomics 279, 441–450 10.1007/s00438-008-0320-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspuria P. J., Sato T., Tamanoi F. (2007). The TSC/Rheb/TOR signaling pathway in fission yeast and mammalian cells: temperature sensitive and constitutive active mutants of TOR. Cell Cycle 6, 1692–1695 10.4161/cc.6.14.4478 [DOI] [PubMed] [Google Scholar]
- Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., III, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 [DOI] [PubMed] [Google Scholar]
- Basi G., Schmid E., Maundrell K. (1993). TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123, 131–136 10.1016/0378-1119(93)90552-E [DOI] [PubMed] [Google Scholar]
- Gurevich V. V., Dion S. B., Onorato J. J., Ptasienski J., Kim C. M., Sterne-Marr R., Hosey M. M., Benovic J. L. (1995). Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic, and m2 muscarinic cholinergic receptors. J. Biol. Chem. 270, 720–731. [DOI] [PubMed] [Google Scholar]
- Hatakeyama R., Kamiya M., Takahara T., Maeda T. (2010). Endocytosis of the aspartic acid/glutamic acid transporter Dip5 is triggered by substrate-dependent recruitment of the Rsp5 ubiquitin ligase via the arrestin-like protein Aly2. Mol. Cell. Biol. 30, 5598–5607 10.1128/MCB.00464-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Hatanaka M., Nagao K., Nakaseko Y., Kanoh J., Kokubu A., Ebe M., Yanagida M. (2007). Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12, 1357–1370 10.1111/j.1365-2443.2007.01141.x [DOI] [PubMed] [Google Scholar]
- Karagiannis J., Saleki R., Young P. G. (1999). The pub1 E3 ubiquitin ligase negatively regulates leucine uptake in response to NH4+ in fission yeast. Curr. Genet. 35, 593–601 10.1007/s002940050457 [DOI] [PubMed] [Google Scholar]
- Kay B. K., Williamson M. P., Sudol M. (2000). The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14, 231–241. [PubMed] [Google Scholar]
- Kita A., Sugiura R., Shoji H., He Y., Deng L., Lu Y., Sio S. O., Takegawa K., Sakaue M., Shuntoh H. et al. (2004). Loss of Apm1, the micro1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol. Biol. Cell 15, 2920–2931 10.1091/mbc.E03-09-0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers E., Erpapazoglou Z., Haguenauer-Tsapis R., André B. (2010). The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 20, 196–204 10.1016/j.tcb.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Rajagopal K., Whalen E. J. (2006). New roles for β-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell 24, 643–652 10.1016/j.molcel.2006.11.007 [DOI] [PubMed] [Google Scholar]
- Lin C. H., MacGurn J. A., Chu T., Stefan C. J., Emr S. D. (2008). Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135, 714–725 10.1016/j.cell.2008.09.025 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Bandyopadhyay A., Kwiatkowski D. J., Maitra U., Matsumoto T. (2002). Role of the Tsc1–Tsc2 complex in signaling and transport across the cell membrane in the fission yeast Schizosaccharomyces pombe. Genetics 161, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Otsubo Y., Urano J., Tamanoi F., Yamamoto M. (2007). Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol. Cell. Biol. 27, 3154–3164 10.1128/MCB.01039-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A., Yabana N., Watanabe Y., Yamamoto M. (2000). Schizosaccharomyces pombe Ste7p is required for both promotion and withholding of the entry to meiosis. Genetics 155, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130 10.1016/0378-1119(93)90551-D [DOI] [PubMed] [Google Scholar]
- Merhi A., André B. (2012). Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol. Cell. Biol. 32, 4510–4522 10.1128/MCB.00463-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 10.1016/0076-6879(91)94059-L [DOI] [PubMed] [Google Scholar]
- Murai T., Nakase Y., Fukuda K., Chikashige Y., Tsutsumi C., Hiraoka Y., Matsumoto T. (2009). Distinctive responses to nitrogen starvation in the dominant active mutants of the fission yeast Rheb GTPase. Genetics 183, 517–527 10.1534/genetics.109.105379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musnier A., Blanchot B., Reiter E., Crépieux P. (2010). GPCR signalling to the translation machinery. Cell. Signal. 22, 707–716 10.1016/j.cellsig.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Nakase Y., Fukuda K., Chikashige Y., Tsutsumi C., Morita D., Kawamoto S., Ohnuki M., Hiraoka Y., Matsumoto T. (2006). A defect in protein farnesylation suppresses a loss of Schizosaccharomyces pombe tsc2+, a homolog of the human gene predisposing to tuberous sclerosis complex. Genetics 173, 569–578 10.1534/genetics.106.056895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase M., Tani M., Morita T., Kitamoto H. K., Kashiwazaki J., Nakamura T., Hosomi A., Tanaka N., Takegawa K. (2010). Mannosylinositol phosphorylceramide is a major sphingolipid component and is required for proper localization of plasma-membrane proteins in Schizosaccharomyces pombe. J. Cell Sci. 123, 1578–1587 10.1242/jcs.059139 [DOI] [PubMed] [Google Scholar]
- Nakase M., Nakase Y., Chardwiriyapreecha S., Kakinuma Y., Matsumoto T., Takegawa K. (2012). Intracellular trafficking and ubiquitination of the Schizosaccharomyces pombe amino acid permease Aat1p. Microbiology 158, 659–673 10.1099/mic.0.053389-0 [DOI] [PubMed] [Google Scholar]
- Nakase Y., Nakase M., Kashiwazaki J., Murai T., Otsubo Y., Mabuchi I., Yamamoto M., Takegawa K., Matsumoto T. (2013). The fission yeast β-arrestin-like protein Any1 is involved in TSC-Rheb signaling and the regulation of amino acid transporters. J. Cell Sci. 126, 3972–3981 10.1242/jcs.128355 [DOI] [PubMed] [Google Scholar]
- Nakashima A., Sato T., Tamanoi F. (2010). Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J. Cell Sci. 123, 777–786 10.1242/jcs.060319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A., Otsubo Y., Yamashita A., Sato T., Yamamoto M., Tamanoi F. (2012). Psk1, an AGC kinase family member in fission yeast, is directly phosphorylated and controlled by TORC1 and functions as S6 kinase. J. Cell Sci. 125, 5840–5849 10.1242/jcs.111146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefsky B., Beach D. (1996). Pub1 acts as an E6-AP-like protein ubiquitiin ligase in the degradation of cdc25. EMBO J. 15, 1301–1312. [PMC free article] [PubMed] [Google Scholar]
- Nikko E., Pelham H. R. (2009). Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic 10, 1856–1867 10.1111/j.1600-0854.2009.00990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikko E., Sullivan J. A., Pelham H. R. (2008). Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 9, 1216–1221 10.1038/embor.2008.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell A. F., Apffel A., Gardner R. G., Cyert M. S. (2010). Alpha-arrestins Aly1 and Aly2 regulate intracellular trafficking in response to nutrient signaling. Mol. Biol. Cell 21, 3552–3566 10.1091/mbc.E10-07-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Okazaki N., Kume K., Jinno S., Tanaka K., Okayama H. (1990). High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 18, 6485–6489 10.1093/nar/18.22.6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinos S. A., Forsburg S. L. (2010). Molecular genetics of Schizosaccharomyces pombe. Methods Enzymol. 470, 759–795 10.1016/S0076-6879(10)70032-X [DOI] [PubMed] [Google Scholar]
- Saleki R., Jia Z., Karagiannis J., Young P. G. (1997). Tolerance of low pH in Schizosaccharomyces pombe requires a functioning pub1 ubiquitin ligase. Mol. Gen. Genet. 254, 520–528 10.1007/s004380050447 [DOI] [PubMed] [Google Scholar]
- Sato M., Dhut S., Toda T. (2005). New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22, 583–591 10.1002/yea.1233 [DOI] [PubMed] [Google Scholar]
- Sengupta S., Peterson T. R., Sabatini D. M. (2010). Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 10.1016/j.molcel.2010.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S. K., Lefkowitz R. J. (2011). β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 32, 521–533 10.1016/j.tips.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S. K., McDonald P. H., Kohout T. A., Lefkowitz R. J. (2001). Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science 294, 1307–1313 10.1126/science.1063866 [DOI] [PubMed] [Google Scholar]
- Shukla A. K., Xiao K., Lefkowitz R. J. (2011). Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 36, 457–469 10.1016/j.tibs.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O., Abriel H., Plant P., Ishikawa T., Kanelis V., Saleki R., Horisberger J. D., Schild L., Rotin D. (2000). Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int. 57, 809–815 10.1046/j.1523-1755.2000.00919.x [DOI] [PubMed] [Google Scholar]
- Swerdlow P. S., Finley D., Varshavsky A. (1986). Enhancement of immunoblot sensitivity by heating of hydrated filters. Anal. Biochem. 156, 147–153 10.1016/0003-2697(86)90166-1 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nishide J., Okazaki K., Kato H., Niwa O., Nakagawa T., Matsuda H., Kawamukai M., Murakami Y. (1999). Characterization of a fission yeast SUMO-1 homologue, pmt3p, required for multiple nuclear events, including the control of telomere length and chromosome segregation. Mol. Cell. Biol. 19, 8660–8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano J., Comiso M. J., Guo L., Aspuria P. J., Deniskin R., Tabancay A. P., Jr, Kato-Stankiewicz J., Tamanoi F. (2005). Identification of novel single amino acid changes that result in hyperactivation of the unique GTPase, Rheb, in fission yeast. Mol. Microbiol. 58, 1074–1086 10.1111/j.1365-2958.2005.04877.x [DOI] [PubMed] [Google Scholar]
- Urano J., Sato T., Matsuo T., Otsubo Y., Yamamoto M., Tamanoi F. (2007). Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc. Natl. Acad. Sci. USA 104, 3514–3519 10.1073/pnas.0608510104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uritani M., Hidaka H., Hotta Y., Ueno M., Ushimaru T., Toda T. (2006). Fission yeast Tor2 links nitrogen signals to cell proliferation and acts downstream of the Rheb GTPase. Genes Cells 11, 1367–1379 10.1111/j.1365-2443.2006.01025.x [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst M., Carr E., Stoyanova R., Kruger W. D., Henske E. P. (2004). Tsc1+ and tsc2+ regulate arginine uptake and metabolism in Schizosaccharomyces pombe. J. Biol. Chem. 279, 12706–12713 10.1074/jbc.M313874200 [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy S. A., Hosey M. M., Benovic J. L., Gurevich V. V. (2004). Mapping the arrestin-receptor interface. Structural elements responsible for receptor specificity of arrestin proteins. J. Biol. Chem. 279, 1262–1268 10.1074/jbc.M308834200 [DOI] [PubMed] [Google Scholar]
- Weisman R., Roitburg I., Schonbrun M., Harari R., Kupiec M. (2007). Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 175, 1153–1162 10.1534/genetics.106.064170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt D., Malik R., Vesecky A. C., Marchese A. (2011). Small ubiquitin-like modifier modification of arrestin-3 regulates receptor trafficking. J. Biol. Chem. 286, 3884–3893 10.1074/jbc.M110.152116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., MacGurn J. A., Liu M., Emr S. (2013). The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress. eLife 2, e00459 10.7554/eLife.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.