Abstract
Identification of insect species is an important task in forensic entomology. For more convenient species identification, the nucleotide sequences of cytochrome c oxidase subunit I (COI) gene have been widely utilized. We analyzed full-length COI nucleotide sequences of 10 Muscidae and 6 Sarcophagidae fly species collected in Korea. After DNA extraction from collected flies, PCR amplification and automatic sequencing of the whole COI sequence were performed. Obtained sequences were analyzed for a phylogenetic tree and a distance matrix. Our data showed very low intraspecific sequence distances and species-level monophylies. However, sequence comparison with previously reported sequences revealed a few inconsistencies or paraphylies requiring further investigation. To the best of our knowledge, this study is the first report of COI nucleotide sequences from Hydrotaea occulta, Muscina angustifrons, Muscina pascuorum, Ophyra leucostoma, Sarcophaga haemorrhoidalis, Sarcophaga harpax, and Phaonia aureola.
1. Introduction
The postmortem interval (PMI) is a key piece of information that needs to be determined in the investigation of a death. In fresh bodies, early postmortem changes such as body cooling, rigidity, and lividity are used for the estimation of PMI [1]. In putrefied bodies, however, these early changes cannot be used for PMI estimation, and it is not possible to estimate PMI from the degree of putrefaction [1]. As a result, PMI estimation in putrefied bodies is one of the most difficult tasks for forensic scientists and pathologists.
Many kinds of arthropods, especially insects belonging to the orders Diptera (flies) and Coleoptera (beetles), are attracted to the bodies of dead animals. Flies, particularly blow flies (Family Calliphoridae), are typically the first to arrive and oviposit into animal carcasses [2]. In addition to blow flies, 2 other families, Muscidae (house flies and allies) and Sarcophagidae (flesh flies), are important in forensic entomology. Although house flies are not commonly attracted to putrefied meat as blow flies and flesh flies are, they are often important indicators of PMI particularly in indoor deaths [2]. When larvae or pupae in various stages of development are collected from the site of investigation and the growth rates of samples are known, an approximate time of oviposition or larviposition can be estimated [3]. Species identification is essential for determining growth rates, as these rates are species-specific [2]. Therefore, species identification is a key step in estimating the PMI from entomological evidence. The traditional species identification method is dependent on the morphological features of insects and is not easily applicable to immature samples such as eggs, larvae, and pupae [4–9]. Moreover, only a few expert taxonomists specialize in forensically important insect species, not only in Korea but also worldwide. DNA-based approaches have been developed in an effort to improve accessibility to methods of species identification. Sperling et al. developed a method to identify 3 forensically important fly species by using the mitochondrial cytochrome c oxidase subunit I (COI) gene and its flanking loci [10]. Although mitochondrial COI nucleotide sequence analysis frequently yields species-level or even genus-level paraphylies in forensically important flies, this locus is still used as the standard method of identification [11, 12]. Two previously reported studies have used the full-length DNA of the COI gene for Calliphoridae species in Korea [13, 14]. However, there has been little effort to characterize the COI haplotypes of Korean Muscidae and Sarcophagidae fly species. This study examined the full-length nucleotide sequences of the COI gene of 10 Muscidae and 6 Sarcophagidae fly species collected in Korea.
2. Materials and Methods
2.1. Sample Collection and Preparation
Fly samples were collected between 2004 and 2008 in Seoul, Guri, Pyeongtaek, and Jeju Island regions of Korea by using insect nets or traps with pork liver bait. Because fly collection was performed in private lands except for in Jeju Island, no specific permission was required in Seoul (Korea University College of Medicine), Guri (JJH's private residence), and Pyeongtaek (PWG Genetics Company). The GPS information for the collection sites in Seoul, Guri, and Pyeongtaek is 37.59,127.03, 37.58,127.11, and 37.05,126.97, respectively. For Jeju Island, we acquired permission from the Ministry of Environment of Korean Government. Pork liver bait and our collection method did not involve endangered or protected species. Species identification was performed by an expert dipterological taxonomist (Jo, TH) by using a dissecting microscope [4, 15–17]. Taxonomic information and the sample sizes of the flies analyzed are listed in Table 1. Flies were first frozen in liquid nitrogen, and the whole bodies were ground using a SKMILL-200 (Tokken, Chiba, Japan). Genomic DNA was extracted from the ground samples by using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Table 1.
Muscidae and Sarcophagidae fly species and sample sizes analyzed.
| Family | Subfamily | Tribe | Genus | Species | Sample size |
|---|---|---|---|---|---|
| Muscidae | Muscinae | Reinwardtiini | Muscina | angustifrons | 9 |
| pascuorum | 4 | ||||
| stabulans | 3 | ||||
| Azeliini | Hydrotaea | armipes = occulta | 1 | ||
| chalcogaster = Ophyra chalcogaster | 5 | ||||
| dentipes | 10 | ||||
| ignava = Ophyra leucostoma | 3 | ||||
| spinigera = Ophyra nigra | 9 | ||||
| Muscini | Musca | domestica | 5 | ||
| Phaoniinae | Phaoniini | Phaonia | aureola | 2 | |
|
| |||||
| Sarcophagidae | Sarcophaginae | Sarcophaga | haemorrhoidalis = africa | 3 | |
| peregrina | 4 | ||||
| melanura | 2 | ||||
| albiceps | 5 | ||||
| harpax | 2 | ||||
| similis | 5 | ||||
2.2. Polymerase Chain Reaction (PCR) and Automatic Sequencing
Universal primer sequences for the COI gene were taken from the literature (Table 2) [13, 14, 24–26], and PCRs were performed using a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The PCR reaction conditions consisted of an initial denaturation step at 95°C for 11 min, followed by 35 cycles at 95°C for 30 s, 50°C for 1 min, and 72°C for 1 min, and then a final elongation step at 72°C for 15 min. Each reaction mixture was prepared using 50 ng of template DNA, 2.5 μL 10× Amplitaq Gold Buffer, 0.5 U AmpliTaq Gold DNA Polymerase (Applied Biosystems, Foster City, CA, USA), 10 pmol (each) upstream and downstream primers, 62.5 nmol MgCl2, 5 nmol (each) dNTPs, and sterile distilled water to a final volume of 25 μL. After purification of the PCR products, cycle sequencing reactions were performed according to the manufacturer's instructions using a BigDye v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). The sequencing products were analyzed using an ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Assembled sequences were deposited into the NCBI GenBank database (JX861406–JX861482).
Table 2.
Universal primer sequences.
| Name | Sequence | Binding site |
|---|---|---|
| F1 | 5′-CCTTTAGAATTGCAGTCTAATGTCA-3′ | tRNA-cysteine |
| F2 | 5′-GGAGGATTTGGAAATTGATTAGTTCC-3′ | 220–245 on COI |
| F3 | 5′-CTGCTACTTTATGAGCTTTAGG-3′ | 1000–1022 on COI |
| R1 | 5′-CCTAAATTTGCTCATGTTGACA-3′ | 2–23 on COII |
| R2 | 5′-CAAGTTGTGTAAGCATC-3′ | 1327–1343 on COI |
| R3 | 5′-CCAAAGAATCAAAATAAATGTTG-3′ | 688–710 on COI |
2.3. Phylogenetic Analysis and Sequence Comparison
Phylogenetic trees were generated for 2 fly families by using the maximum likelihood method with 1,000 replicates of bootstrapping based on the Tamura-Nei model using MEGA6 software [27]. Initial trees for the heuristic search were obtained by applying the neighbor-joining method to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach. To make a root for each tree, COI sequences for Lucilia sericata (NCBI accession number EU880212), Calliphora vicina (EU880188), and Drosophila melanogaster (NC_001709) were introduced as outgroup taxa. Average intraspecific and interspecific sequence distances were calculated for sequence comparison. Sequences obtained in this study were also compared to previously announced sequence data (Table 3).
Table 3.
Reference sequences from NCBI GenBank.
| Family | Species name | NCBI accession number | Coverage on COI | Geographic region | Author | Reference |
|---|---|---|---|---|---|---|
| Muscidae | Hydrotaea cyrtoneurina | FJ025622 | 52–635 748–1454 |
Unknown | Kutty et al. | [18] |
| Hydrotaea dentipes | FJ025623 | 48–635 748–1484 |
Unknown | Kutty et al. | [18] | |
| Hydrotaea irritans | FJ025624 | 2–635 748–1484 |
Unknown | Kutty et al. | [18] | |
| Musca domestica | EU814984–EU815009* | 156–1268 | Beijing, China | Chen, Q et al. | Unpublished | |
| Musca domestica | GQ465784 | 30–1524 | Unknown | Wiegmann, BM | Unpublished | |
| Musca domestica | AY526196 | 1–1536 | Brazil | de Oliveira et al. | [19] | |
| Musca domestica | FJ153278 | 1054–1539 | Bangkok, Thailand | Preativatanyou et al. | [20] | |
| Muscina assimilis | EU627712 | 1–1536 | Unknown | Meng, J et al. | Unpublished | |
| Muscina stabulans | EF531210 | 68–659 775–1446 |
Unknown | Petersen et al. | [21] | |
| Muscina stabulans | EU627711 | 1–1536 | Unknown | Meng, J et al. | Unpublished | |
| Muscina stabulans | AJ879595 | 8–701 | Parana, Curitiba, Brazil | Schuehli, GS et al. | Unpublished | |
| Ophyra chalcogaster | EU627715 | 1–1536 | Unknown | Meng, J et al. | Unpublished | |
| Ophyra spinigera = nigra | EU627714 | 1–1536 | Unknown | Meng, J et al. | Unpublished | |
|
| ||||||
| Sarcophagidae | Sarcophaga africa | GQ223343 | 1–1539 | Unknown | Stamper, T et al. | Unpublished |
| Sarcophaga dumoga | EF405950 | 1–1534 | Malaysia | Tan et al. | [22] | |
| Sarcophaga javanica |
EF405925 EF405926 |
1–1534 | Malaysia | Tan et al. | [22] | |
| Sarcophaga peregrina | EU815029–EU815034* | 170–1277 | Beijing, China | Chen, Q et al. | Unpublished | |
| Sarcophaga peregrina |
EF405927 EF405928 |
1–1534 | Malaysia | Tan et al. | [22] | |
| Sarcophaga melanura | AY315649 | 1027–1322 | Unknown | Zehner et al. | [23] | |
| Sarcophaga melanura | HM037109 | 1049–1326 | Xining, Qinghai, China | Cai, JF et al. | Unpublished | |
| Sarcophaga melanura | HM037110 | 1049–1326 | Yinchuan, Ningxia, China | Cai, JF et al. | Unpublished | |
| Sarcophaga melanura |
HM037111 HM037112 |
1049–1326 | Shijiazhuang, Hebei, China | Cai, JF et al. | Unpublished | |
| Sarcophaga melanura | FJ746473 | 1047–1326 | Lanzhou, Gansu, China | Cai, JF et al. | Unpublished | |
| Sarcophaga albiceps |
EF405931 EF405932 |
1–1534 | Malaysia | Tan et al. | [22] | |
| Sarcophaga similis | AY879256 | 304–855 | Unknown | Song, Z et al. | Unpublished | |
| Sarcophaga dux | EF405937–EF405939 | 1–1534 | Malaysia | Tan et al. | [22] | |
3. Results
3.1. Nucleotide Sequence Distances
A pairwise percentage distance matrix of 10 Muscidae fly species is shown in Table 4. Because only 1 individual COI sequence was obtained for H. occulta, intraspecific variation was not estimated for this species. Interspecific distance was the lowest between O. chalcogaster and O. leucostoma (6.3%) and the highest between Musca domestica and Phaonia aureola (15.3%). Intraspecific distances were 0.3% or less.
Table 4.
Average pairwise percentage distances for 10 Muscidae fly species.
| De | 0.0 | |||||||||
| Oc | 7.2 | N/A | ||||||||
| Do | 11.5 | 11.4 | 0.2 | |||||||
| An | 10.1 | 9.9 | 12.2 | 0.2 | ||||||
| Pa | 12.1 | 11.8 | 14.3 | 10.2 | 0.1 | |||||
| St | 11.9 | 11.0 | 12.2 | 8.5 | 11.6 | 0.1 | ||||
| Cg | 9.1 | 8.7 | 10.9 | 11.7 | 12.8 | 12.7 | 0.0 | |||
| Le | 7.7 | 8.2 | 10.6 | 10.4 | 11.8 | 12.0 | 6.3 | 0.3 | ||
| Ni | 9.4 | 8.8 | 10.7 | 11.2 | 14.1 | 12.7 | 8.3 | 7.6 | 0.0 | |
| Au | 13.5 | 13.6 | 15.3 | 14.4 | 15.2 | 14.1 | 14.1 | 13.7 | 14.5 | 0.1 |
|
| ||||||||||
| De | Oc | Do | An | Pa | St | Cg | Le | Ni | Au | |
De = H. dentipes, Oc = H. occulta, Do = M. domestica, An = M. angustifrons, Pa = M. pascuorum, St = M. stabulans, Cg = O. chalcogaster, Le = O. leucostoma, Ni = O. nigra, Au = P. aureola, and N/A = not available.
A pairwise percentage distance matrix for the 6 Sarcophagidae fly species is shown in Table 5. Interspecific distance was the lowest between Sarcophaga similis and Sarcophaga peregrina (6.4%), whereas it was the highest between Sarcophaga haemorrhoidalis and S. peregrina (8.9%). Intraspecific distances were 0.3% or less.
Table 5.
Average pairwise percentage distances for 6 Sarcophagidae fly species.
| Hm | 0.1 | |||||
| Pg | 8.9 | 0.3 | ||||
| Me | 7.6 | 6.8 | 0.1 | |||
| Al | 8.2 | 7.3 | 6.8 | 0.0 | ||
| Ha | 7.6 | 7.7 | 7.7 | 6.5 | 0.1 | |
| Si | 6.8 | 6.4 | 6.7 | 6.5 | 6.5 | 0.1 |
|
| ||||||
| Hm | Pg | Me | Al | Ha | Si | |
Hm = S. haemorrhoidalis, Pg = S. peregrina, Me = S. melanura, Al = S. albiceps, Ha = S. harpax, and Si = S. similis.
3.2. Phylogenetic Analysis
Maximum likelihood phylogenetic trees were generated from COI nucleotide sequences of 10 Muscidae and 6 Sarcophagidae fly species. All taxa were clustered according to species and genera, without any species- or genus-level paraphyly (Figures 1 and 2). Although a few internal nodes display low bootstrap values under 50%, every bootstrap value at the species level was 100%.
Figure 1.
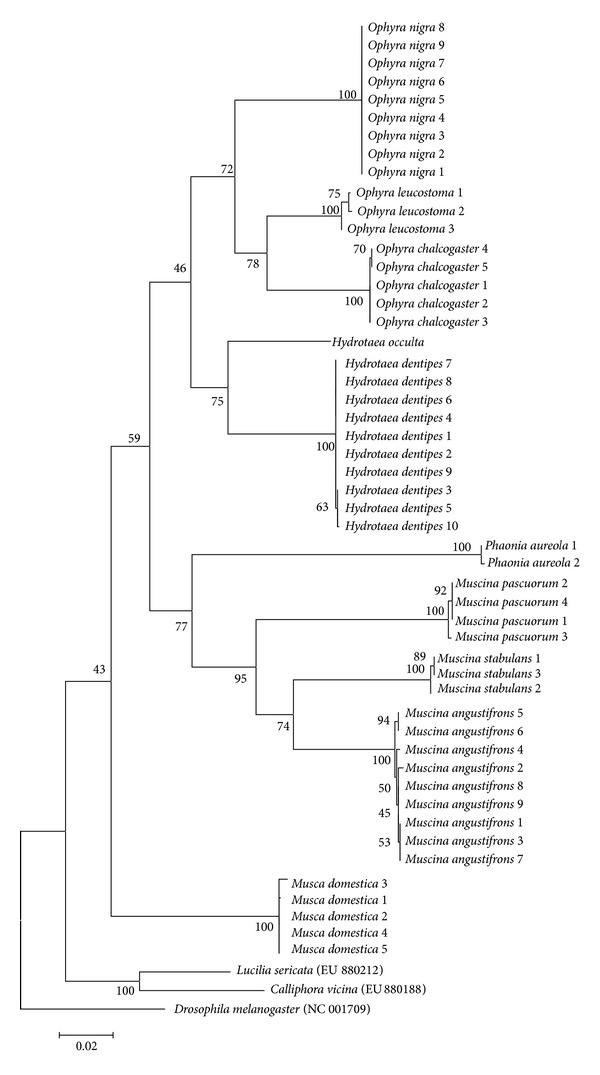
A phylogenetic tree was constructed for 10 Muscidae fly species by using the maximum likelihood method based on the Tamura-Nei model. The tree with the highest log likelihood (−8320.2383) is shown. The analysis involved 54 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 1536 positions in the final dataset. COI nucleotide sequences of Lucilia sericata (EU880212), Calliphora vicina (EU880188), and Drosophila melanogaster (NC_001709) are included as outgroup taxa.
Figure 2.
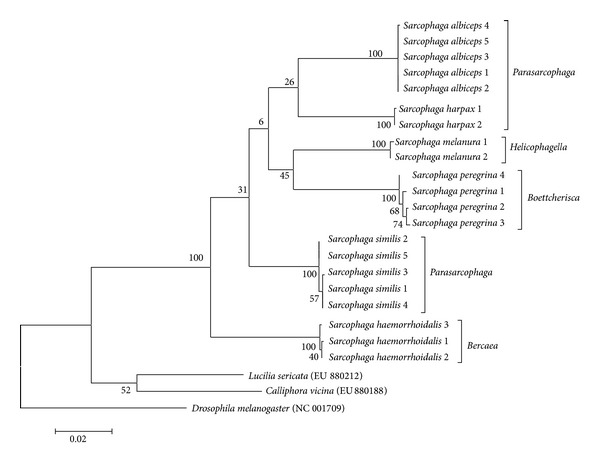
A phylogenetic tree was generated for 6 Sarcophagidae fly species by using the maximum likelihood method based on the Tamura-Nei model. The tree with the highest log likelihood (−5586.2586) is shown. The analysis involved 24 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 1536 positions in the final dataset. A COI nucleotide sequence of Lucilia sericata (EU880212) is included as an outgroup. COI nucleotide sequences of Lucilia sericata (EU880212), Calliphora vicina (EU880188), and Drosophila melanogaster (NC_001709) are included as outgroup taxa. The taxa names in the italic grouping the external nodes mean the old genera of those species.
4. Discussion
As shown in Tables 4 and 5, Korean Muscidae and Sarcophagidae fly species showed average intraspecific sequence distances of 0.0–0.3%. The phylogenetic trees did not show any species-level paraphylies (Figures 1 and 2). Although our sampling was limited to a few areas of Korea in a relatively short period, these findings suggest that Korean Muscidae and Sarcophagidae fly species are identifiable using the COI nucleotide sequences.
In this study, H. dentipes showed intraspecific sequence distances of 0–0.1% (average 0.0%). The only previous COI sequence of H. dentipes (FJ025623) in the NCBI GenBank (Table 3) showed intraspecific distances of 3.5–3.6% from the conspecific sequences in this study [18]. According to Cognato, who reported intraspecific sequence distances of 0.04–3.5% in 8 fly species, this range of intraspecific distances (3.5–3.6%) may be valid and not a result of misidentification [28]. Further sampling from other geographic regions will be required, however, to confirm the variability of COI haplotypes of H. dentipes.
Because only 1 H. occulta COI sequence was identified in this study, and there are currently no COI sequences from this species in the NCBI GenBank, it is impossible to determine the validity of this sequence. As expected, however, H. occulta formed a genus Hydrotaea clade with H. dentipes (Figure 1). Previously reported sequences from H. cyrtoneurina, H. irritans, and H. dentipes in the NCBI GenBank (Table 3) showed interspecific distances of at least 7.4% compared with the H. occulta sequence determined in this study [18].
M. domestica, the common house fly, exhibits a cosmopolitan distribution [6]. The COI gene has been widely studied in this species, and 28 COI sequences of this species from the NCBI GenBank (Table 3) are highly homologous to conspecific sequences in this study (average distance = 0.2%) [19, 20].
As reported by Shinonaga, 5 species of the genus Muscina have been identified in Japan [6]. Three of these species were analyzed in this study. Of these, M. stabulans (stable fly) is the most forensically important species, and it is more often attracted to decaying animals than are other Muscina flies [6].
All 3 Muscina flies showed very low intraspecific sequence distances (0.1–0.2%) and interspecific distances of at least 8.5%; hence, identification of Korean Muscina fly species was relatively straightforward. Compared to previously reported conspecific data, in this study, M. stabulans sequences were very similar to 2 previously reported conspecific sequences (EU627711 and AJ879595; sequence distance 0.1–0.3%) but very divergent from another reported sequence (EF531210; sequence distance 5.0–5.1%) [21]. Because only EF531210 is inconsistent with other conspecific sequences, the validity of this sequence should be reviewed by analysis of the voucher specimen and the morphological features used for identification. The M. assimilis sequence (EU627712) does not match any Muscina sequences reported in this study.
Three Ophyra species were analyzed in this study, each with low intraspecific distances and at least 6.3% interspecific distances. Therefore, identification of these 3 Korean Ophyra species is plausible. Compared to previously reported conspecific sequences, the O. nigra sequence obtained in this study was monomorphic with EU627714 (distance 0.3%), whereas O. chalcogaster showed distances of 1.2–1.3% from EU627715. Since the O. leucostoma COI gene has not previously been analyzed, conspecific comparison is not possible at this time. There are no nucleotide sequences in the NCBI GenBank database that match the O. leucostoma sequences reported in this study.
S. haemorrhoidalis showed a very low intraspecific average sequence distance (0.1%) and interspecific distances of at least 6.8% (Table 5). There are currently no other COI nucleotide sequences in the NCBI GenBank for this species name. However, a COI sequence of a synonymous species, Sarcophaga africa (GQ223343), is available [17]. Since the sequence distance between S. haemorrhoidalis and S. africa is only 0.8%, the DNA result also supports that they are conspecific.
S. peregrina sequences in this study showed a very low intraspecific average sequence distance (0.1%) and interspecific distances of at least 6.4% (Table 5). Because S. peregrina was once categorized in the old genus Boettcherisca, a phylogenetic tree was generated from S. peregrina sequences in this study and the COI sequences of old genus Boettcherisca submitted by other authors. The phylogenetic tree showed a species-level paraphyly of S. peregrina, with 2 Malaysian S. peregrina sequences, submitted by Tan et al., clustering with 2 Malaysian S. javanica sequences (Figure 3) [22]. Because these 2 Malaysian S. peregrina sequences are divergent from other conspecific sequences from Korea and China (sequence distance 2.4–3.0%), further consideration, such as a review of the voucher specimens, would be necessary.
Figure 3.
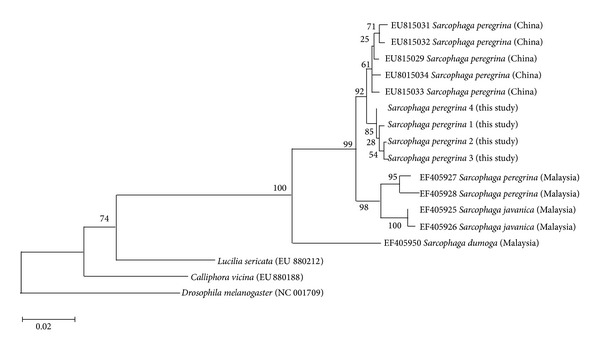
A maximum likelihood phylogenetic tree using data of the old genus Boettcherisca from this study (1–4) and the other authors' work based on the Tamura-Nei model. The tree with the highest log likelihood (−3420.3779) is shown. The analysis involved 17 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 1076 positions in the final dataset. COI nucleotide sequences of Lucilia sericata (EU880212), Calliphora vicina (EU880188), and Drosophila melanogaster (NC_001709) are included as outgroup taxa.
Sarcophaga melanura showed a very low intraspecific average sequence distance (0.1%) and interspecific distances of at least 6.5% (Table 5). Compared with the 6 short S. melanura COI sequences shown in Table 3, the S. melanura COI sequences reported in this study showed intraspecific distances of only 0.0–0.7% [23].
Three species previously classified as the old genus Parasarcophaga, that is, S. similis, Sarcophaga harpax, and Sarcophaga albiceps, showed very low intraspecific average sequence distances (0.0–0.1%) and interspecific distances of at least 6.4% (Table 5). Compared with other conspecific species in the NCBI GenBank (Table 3), S. albiceps and S. similis showed intraspecific sequence distances of only 0.3–0.7% and 0.2–0.4%, respectively [22]. Additionally, S. harpax and its known sister species S. dux are closely related with sequence distances of 1.4–1.6% [22].
In conclusion, 10 Muscidae and 6 Sarcophagidae fly species collected in Korea were identifiable using COI sequence analysis. However, a few inconsistencies with previously reported sequences require further evaluation. To our knowledge, the present study provides the first report of the COI nucleotide sequences of H. occulta, M. angustifrons, M. pascuorum, O. leucostoma, S. haemorrhoidalis, P. harpax, and P. aureola.
Acknowledgments
This study was partly funded by Korea University Research Fund K1300061. The authors would like to appreciate the assistance of PWG Genetics Company for providing their private land for field experiments.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Saukko PJ, Knight B. Knight's Forensic Pathology. London, UK: Arnold; 2004. [Google Scholar]
- 2.Byrd JH, Castner JL. Forensic Entomology: The Utility of Arthropods in Legal Investigations. Boca Raton, Fla, USA: CRC Press; 2001. [Google Scholar]
- 3.Kamal AS. Comparative study of thirteen species of sarcosaprophagous Calliphoridae and Sarcophagidae (Diptera) I. Bionomics. Annals of the Entomological Society of America. 1958;51:261–271. [Google Scholar]
- 4.Kano R, Field G, Shinonaga S. Sarcophagidae (Insecta: Diptera) Tokyo, Republic of Korea: Biogeographical Society of Japan; 1967. (141 plates (part col.), Distributor: Tokyo Electrical Engineering College Press). [Google Scholar]
- 5.Kano R, Shinonaga S. Calliphoridae (Insecta: Diptera) Tokyo, Republic of Korea: Biological [i.e. Biogeographical] Society of Japan; 1968. [Google Scholar]
- 6.Shinonaga S, Kano R. Muscidae (Insecta: Diptera) Tokyo, Republic of Korea: Academic Press of Japan; 1971. [Google Scholar]
- 7.Sukontason K, Sukontason KL, Piangjai S, et al. Identification of forensically important fly eggs using a potassium permanganate staining technique. Micron. 2004;35(5):391–395. doi: 10.1016/j.micron.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Sukontason K, Sukontason KL, Piangjai S, et al. Fine structure of the eggs of blowflies Aldrichina grahami and Chrysomya pacifica (Diptera: Calliphoridae) Biological Research. 2004;37(3):483–487. doi: 10.4067/s0716-97602004000300012. [DOI] [PubMed] [Google Scholar]
- 9.Wells JD, Byrd JH, Tantawi TI. Key to third-instar chrysomyinae (Diptera: Calliphoridae) from carrion in the continental United States. Journal of Medical Entomology. 1999;36(5):638–641. doi: 10.1093/jmedent/36.5.638. [DOI] [PubMed] [Google Scholar]
- 10.Sperling FAH, Anderson GS, Hickey DA. A DNA-based approach to the identification of insect species used for postmorten interval estimation. Journal of Forensic Sciences. 1994;39(2):418–427. [PubMed] [Google Scholar]
- 11.Wells JD, Wall R, Stevens JR. Phylogenetic analysis of forensically important Lucilia flies based on cytochrome oxidase I sequence: a cautionary tale for forensic species determination. International Journal of Legal Medicine. 2007;121(3):229–233. doi: 10.1007/s00414-006-0147-1. [DOI] [PubMed] [Google Scholar]
- 12.Stevens JR, Wall R, Wells JD. Paraphyly in Hawaiian hybrid blowfly populations and the evolutionary history of anthropophilic species. Insect Molecular Biology. 2002;11(2):141–148. doi: 10.1046/j.1365-2583.2002.00318.x. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Zhang Y, Piao H, et al. Use of cytochrome C oxidase subunit I (COI) nucleotide sequences for identification of the Korean Luciliinae Fly species (Diptera: Calliphoridae) in forensic investigations. Journal of Korean Medical Science. 2009;24(6):1058–1063. doi: 10.3346/jkms.2009.24.6.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SH, Zhang Y, Piao H, et al. Sequences of the cytochrome C oxidase subunit I (COI) gene are suitable for species identification of Korean calliphorinae flies of forensic importance (Diptera: Calliphoridae) Journal of Forensic Sciences. 2009;54(5):1131–1134. doi: 10.1111/j.1556-4029.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 15.Shinonaga S. Monograph of the Muscidae of Japan. Tokyo, Republic of Korea: Tokai Daigaku Shuppankai; 2003. [Google Scholar]
- 16.Papp L, Darvas B. Contributions to a Manual of Palaearctic Diptera: With Special Reference to Flies of Economic Importance. Budapest, Hungary: Science Herald; 1997. (Authorised Distributor: E.W. Classey). [Google Scholar]
- 17.Pape T. Catalogue of the Sarcophagidae of the World (Insecta:Diptera) Gainsville, Fla, USA: Associated Publishers; 1996. [Google Scholar]
- 18.Kutty SN, Pape T, Pont A, Wiegmann BM, Meier R. The Muscoidea (Diptera: Calyptratae) are paraphyletic: evidence from four mitochondrial and four nuclear genes. Molecular Phylogenetics and Evolution. 2008;49(2):639–652. doi: 10.1016/j.ympev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira MT, de Azeredo-Espin AML, Lessinger AC. Evolutionary and structural analysis of the cytochrome c oxidase subunit I (COI) gene from Haematobia irritans, Stomoxys calcitrans and Musca domestica (Diptera: Muscidae) mitochondrial DNA. DNA Sequence. 2005;16(2):156–160. doi: 10.1080/10425170500039901. [DOI] [PubMed] [Google Scholar]
- 20.Preativatanyou K, Sirisup N, Payungporn S, et al. Mitochondrial DNA-based identification of some forensically important blowflies in Thailand. Forensic Science International. 2010;202:97–101. doi: 10.1016/j.forsciint.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Petersen FT, Meier R, Kutty SN, Wiegmann BM. The phylogeny and evolution of host choice in the Hippoboscoidea (Diptera) as reconstructed using four molecular markers. Molecular Phylogenetics and Evolution. 2007;45(1):111–122. doi: 10.1016/j.ympev.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Tan SH, Rizman-Idid M, Mohd-Aris E, Kurahashi H, Mohamed Z. DNA-based characterisation and classification of forensically important flesh flies (Diptera: Sarcophagidae) in Malaysia. Forensic Science International. 2010;199:43–49. doi: 10.1016/j.forsciint.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Zehner R, Amendt J, Schütt S, Sauer J, Krettek R, Povolný D. Genetic identification of forensically important flesh flies (Diptera: Sarcophagidae) International Journal of Legal Medicine. 2004;118(4):245–247. doi: 10.1007/s00414-004-0445-4. [DOI] [PubMed] [Google Scholar]
- 24.Harvey ML, Dadour IR, Gaudieri S. Mitochondrial DNA cytochrome oxidase I gene: potential for distinction between immature stages of some forensically important fly species (Diptera) in western Australia. Forensic Science International. 2003;131(2-3):134–139. doi: 10.1016/s0379-0738(02)00431-0. [DOI] [PubMed] [Google Scholar]
- 25.Harvey ML, Mansell MW, Villet MH, Dadour IR. Molecular identification of some forensically important blowflies of southern Africa and Australia. Medical and Veterinary Entomology. 2003;17(4):363–369. doi: 10.1111/j.1365-2915.2003.00452.x. [DOI] [PubMed] [Google Scholar]
- 26.Saigusa K, Takamiya M, Aoki Y. Species identification of the forensically important flies in Iwate prefecture, Japan based on mitochondrial cytochrome oxidase gene subunit I (COI) sequences. Legal Medicine. 2005;7(3):175–178. doi: 10.1016/j.legalmed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics. 2008;9(4):299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cognato AI. Standard percent DNA sequence difference for insects does not predict species boundaries. Journal of Economic Entomology. 2006;99(4):1037–1045. doi: 10.1603/0022-0493-99.4.1037. [DOI] [PubMed] [Google Scholar]


