Abstract
Aim. To assess through a systematic review and meta-analysis of the literature the prognostic implication of sentinel lymph node mapping in Merkel cell carcinoma (MCC). Materials and Methods. PubMed and SCOPUS databases were searched by using “Merkel AND sentinel” as keywords. All studies with prognostic information regarding SLN mapping in cN0 MCC patients were included. Hazard ratio (HR) for overall survival (OS) and disease free survival (DFS) was used as effect size. Results. SLN biopsy predicted better DFS and OS as compared to the nodal observation in cN0 MCC patients (pooled HR for DFS: 1.61 (95% CI: 1.05–2.46), P = 0.028; pooled HR for OS: 1.08 (95% CI: 0.55–2.10), P = 0.8). Pathologically negative SLN (SLN−) patients had better OS (pooled HR: 4.42 (95% CI: 1.82–10.7), P = 0.0009) and DFS (pooled HR: 2.58 (95% CI: 1.78–3.73)) as compared to SLN+ patients. Conclusion. SLN mapping can provide strong prognostic information regarding OS and DFS in cN0 MCC patients. More importantly, SLN mapping can improve DFS and possibly OS in cN0 MCC patients as compared to nodal observation. As MCC is a rare tumor, large multicenter prospective studies are still needed to validate the survival benefit of SLN mapping.
1. Introduction
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine malignancy with high propensity for regional lymph node spread and recurrence [1]. Its origin is believed to be the primitive epidermal stem cells capable of epithelial or neuroendocrine differentiation [2]. This tumor is aggressive with high mortality and poor prognosis. However, the clinical course of MCC can be more aggressive in males, elderly patients, large tumors, and immunocompromised individuals [3, 4]. One of the most important prognostic factors in clinically node negative (cN0) MCC patients is the presence of occult regional lymph nodal involvement [2, 4–6].
Sentinel lymph node (SLN) mapping is an important and accurate technique for regional lymph node staging in many solid tumors [7–9]. By identifying the first lymph node in a nodal basin that receives the lymph flow from a solid tumor, SLN biopsy allows for careful pathologic evaluation of one or a few SLNs (instead of the whole regional basin) that are most likely to be pathologically involved.
There are several studies which reported the prognostic importance of positive SLN in MCC. It is reported that patients with positive SLN have 3 times higher relapse rate and 2 times higher disease specific mortality as compared to negative SLN patients [10, 11]. However, the literature is heterogeneous in this regard and several authors did not find statistical significant association between positive SLN pathology and recurrence or survival [4].
More importantly, a number of studies reported that patients with regional lymph node metastasis have better survival and lower recurrence when treated with lymphadenectomy or regional radiation therapy [12, 13]. It seems that early detection of regional nodal involvement in MCC patients by SLN biopsy can improve survival due to the early start of more aggressive treatment plans in the disease course [14]. However, other studies showed no survival benefit by SLN mapping in MCC and no consensus has been reached in this regard [15].
In the current study, we aimed to assess through a systematic review and meta-analysis of the literature the prognostic implication of sentinel lymph node (SLN) pathological status in patients with MCC evaluating whether SLN mapping may improve survival in cN0 MCC patients.
2. Material and Methods
2.1. Search Strategy
We followed the PRISMA guidelines for performing the current systematic review and meta-analysis (http://www.prisma-statement.org). We searched PubMed and SCOPUS databases using the following search algorithm: “merkel AND sentinel.” The literature search was performed by two authors independently and the last search was done on January 2014 without language or time limit. The reference lists of the relevant studies were reviewed for possible missing citations.
2.2. Inclusion Criteria
Studies which met one of the following criteria were included in the systematic review:
evaluation of prognostic value of pathologic involvement of SLN for determination of overall survival (OS) or disease free survival (DFS) in cN0 MCC patients;
evaluation of the prognostic value of SLN mapping in cN0 MCC patients for improvement of OS or DFS.
In addition, we collected individual patient data from the reported cases in the literature with enough prognostic information (at least pathological involvement of the SLN and time to events such as death or recurrence should be reported) in order to perform an individual patient meta-analysis. Letters to the editor and review articles were excluded. Studies without enough prognostic information were also excluded. Two authors reviewed independently the retrieved articles. All discrepancies were resolved by the third author opinion. Possible duplicate publications were discussed and only the most recent reports were considered.
2.3. Data Abstraction
Two authors independently performed the data abstraction, and data on authors, affiliations, publication date, SLN mapping method, patient characteristics, and quality of the included studies were retrieved. The main effect size we used in the current analysis was hazard ratio (HR) of DFS and/or OS which were either extracted directly from the included studies or estimated from survival curves as recommended by Parmar et al. [20]. We reported pooled values with 95% confidence intervals (95% CI). Oxford center for evidence-based medicine checklist for prognostic studies was used to assess the quality of the included studies [21].
2.4. Statistical Analyses
Dersimonian and Laird method (random effects model) was used to pool the HR among the studies [22]. The pooled results were expressed graphically by forest plots. Cochrane Q test was used for heterogeneity evaluation (P < 0.05 was considered statistically significant). The inconsistency (I 2) index was used to quantify the heterogeneity among the studies.
For publication bias evaluation, funnel plots and Egger's regression intercept were used [23]. All statistical analyses were performed by using Comprehensive Meta-analysis (version 2, Biostat Inc., USA) and SPSS (version 11.5, SPSS Inc., USA).
3. Results
Figure 1 shows the PRISMA flowchart of the study. Four studies (1417 patients) provided HR of DFS and/or OS for SLN mapping as compared to other regional treatments in cN0 MCC patients [3, 14, 17, 18]. One of the included studies compared SLN mapping with all other treatments including clinical observation and lymph node dissection [3]. The other three compared the SLN mapping with observation in cN0 MCC patients.
Figure 1.
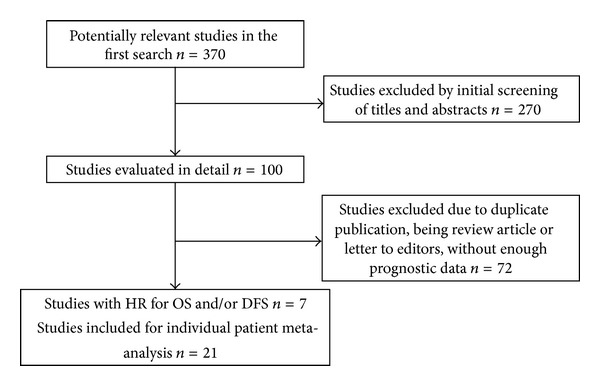
PRISMA flowchart of the study.
Three studies (883 patients) provided HR of DFS and/or OS for pathologic condition of harvested SLN [15, 16, 19].
Three studies had prognostic information based on the Surveillance, Epidemiology, and End Results (SEER) database [4, 14, 16]. One of them had duplicate information and it was excluded [4]; therefore two articles were included in the systematic review [14, 16].
The characteristics of the included studies and quality assessment are shown in Table 1.
Table 1.
Characteristics of the included studies.
| First author | Publication year | SLN mapping method | Total number of patients/mean age (year)/mean tumor size (cm)/male gender (%)/head and neck location (%)/number of patients underwent regional lymph node dissection in SLN+/SLN− patients (% of total patients) | Inclusion of patients in the study at a common point of the disease course | Duration of follow- up | Method of outcome evaluation (death or recurrence)/Blind outcome evaluation to SLN results | Adjustment for important confounding variables | Variable used for prognostication/major findings |
|---|---|---|---|---|---|---|---|---|
| Tarantola [3] | 2013 | n/a | 114 (34 with SLN mapping, 80 with other regional treatments)/70.1/1.37/70/46.3/n-a/n-a | Yes, all were included at clinical stages x and II of disease | 3.3 years (mean) | Death of all causes/n-a | Yes | SLN mapping versus other nodal treatments/OS in the patients underwent SLN mapping was higher, HR: 1.04 [0.51–2.15], P = 0.91 |
|
| ||||||||
| Kachare [14] | 2014 | n/a | 1193 (474 with SLN mapping, 719 with nodal observation)/75.9/n-a/58.8/n-a/104 (21.9%)/n-a | Yes, all were included at clinical stages I and II of disease | Median of 21 months (0–83 months) | Death of disease/n-a | Yes | SLN mapping versus nodal observation, in addition prognostic significance of SLN pathological status was evaluated/DFS was higher in patients underwent SLN mapping, HR: 1.43 [1.01–2.05], P = 0.04 DFS was also higher in SLN+ as compared to SLN− patients. This part of study was duplicate of Fritsch et al. [16] study and excluded form final analysis |
|
| ||||||||
| Bajetta [17] | 2009 | n/a | 63 (21 with SLN mapping and 42 with nodal observation)/n-a/n-a/45/18/8 (38%)/0 (0%) | Yes, all were included at clinical stages I and II of disease | Median of 65 months | Death of disease/n-a | Yes | SLN mapping versus nodal observation/operative nodal staging with SLN biopsy, HR 3.44 [1.17–10]; P = 0.023) predicted better DFS |
|
| ||||||||
| Sattler [18] | 2013 | n/a | 47 (19 with SLN mapping and 27 with nodal observation)/70.32/n-a/26.3/31.6/n-a/n-a | Yes, all were included at clinical stages I and II of disease | Median of 20 months (2–234 months) | Death of disease, and death of all causes/n-a | Yes | SLN mapping versus nodal observation/SLN m aping predicted better DFS (HR 1.38 [0.45–4.20]) and OS 1.39 [0.25–7.76]) as compared to nodal observation. HR was calculated from the survival curves according to Parmar method |
|
| ||||||||
| Fields [15] | 2011 | Combined radiotracer and blue dye | 153 (45 SLN+, and 108 SLN−)/69/n-a/59/21.5/45 (29.4%)/0 (0%) | Yes, all were included at clinical stages I and II of disease | Median of 41 months | Death of disease/n-a | Yes | SLN pathologic status/SLN− status predicted better OS (HR: 1.86 [0.22–15.49], P = 0.56), and DFS (HR: 1.69 [0.37–7.65], P = 0.49) HR was calculated from the survival curves according to Parmar method |
|
| ||||||||
| Kouzmina [19] | 2013 | Radiotracer in all, blue dye in 16 | 28 (9 SLN+ and 19 SLN−)/n-a/n-a/39.3/39.3/8 (28.5%)/0 (0%) | Yes, all were included at clinical stages I and II of disease | Mean of 3.6 years | Death of all causes/n-a | No | SLN pathologic status/SLN− status predicted better OS (HR: 4.82 [0.79–29.34], P = 0.08) HR was calculated from the survival curve according to Parmar method |
|
| ||||||||
| Fritsch [16] | 2014 | n/a | 721 (186 SLN+ and 535 SLN−)/n-a/n-a/61.7/24/n-a/n-a 12 patients were excluded from survival analysis due to less than 1 months follow-up |
Yes, all were included at clinical stages I and II of disease | Median of 34 months | Death of disease/n-a | Yes | SLN pathologic status/SLN− status predicted better DFS in head and neck (HR: 2.22 [0.84–5.86], P = 0.1), and other parts of the body (HR: 3.01 [1.77–5.11], P = 0.00005) The second P value was calculated by CMA software |
|
| ||||||||
| Cases in the literature with enough prognostic data | During the period of 1996–2013 | Radiotracer and/or blue dye | 172 (65 SLN+, and 107 SLN−)/68.6/1.84/45.3/40.1/30 (17.4%)/5 (2.9%) | Yes, all were included at clinical stages I and II of disease | Mean of 27.5 months (1–120 months) | Death of disease or death of all causes/n-a | Yes (refer to Table 2) | SLN pathologic status/SLN− status predicted better DFS in head and neck (HR: 4.09 [1.41–11.88], P = 0.009), and other parts of the body (HR: 1.65 [0.7–3.9], P = 0.15) SLN− status predicted better OS in head and neck (HR: 14.06 [1.63–121.28], P = 0.01), and other parts of the body (HR: 3.79 [0.96–14.6], P = 0.055). All analyses were performed by SPSS version 11.5 |
Twenty-one studies including 172 patients had prognostic information regarding pathological condition of SLN [10, 24–43]. Three studies had duplicate cases and were excluded [44–46]. We used cox regression model to analyze these cases and HR of OS and DFS for cN0 MCC patients with pathologically involved SLN as compared to pathologically noninvolved nodes were 6.13 (95% CI: 1.97–19.07) and 2.25 (95% CI: 1.16–4.33), respectively. Detailed survival analyses of these 172 patients are shown in Table 2.
Table 2.
Detailed survival analysis of the cases included in the individual patient analysis (n = 172).
| Factor | Number of patients | HR for OS [95% CI] | P value | HR for DFS [95% CI] | P value |
|---|---|---|---|---|---|
| Age | Mean 68.58 | 1.039 [0.98–1.09] | 0.13 | 1.003 [0.97–1.03] | 0.83 |
| Tumor size | Mean 1.85 | 0.59 [0.23–1.5] | 0.27 | 1.017 [0.65–1.53] | 0.93 |
| Gender | 0.212 | 0.55 | |||
| Male | 78 | 1.86 [0.69–5.02] | 1.23 [0.62–2.44] | ||
| Female | 83 | Referent | Referent | ||
| Regional nodal treatment | |||||
| None | 100 | 0.17 [0.04–0.72] | 0.16 | 0.28 [0.1–0.78] | 0.015 |
| Nodal dissection | 23 | 0.78 [0.18–3.31] | 0.74 | 0.68 [0.22–2.14] | 0.51 |
| Radiotherapy | 37 | 0.66 [0.11–3.99] | 0.65 | 0.37 [0.1–1.29] | 0.12 |
| Both | 12 | Referent | Referent | Referent | Referent |
| Tumor location | |||||
| Limbs | 95 | 0.95 [0.33–2.71] | 0.93 | 0.73 [0.37–1.46] | 0.38 |
| Trunk | 8 | 2.08 [0.24–17.52] | 0.46 | 1.95 [0.56–6.77] | 0.29 |
| Head and neck | 69 | Referent | Referent | Referent | Referent |
| SLN status | 0.002 | 0.015 | |||
| Positive | 65 | 6.13 [1.97–19.07] | 2.25 [1.16–4.33] | ||
| Negative | 107 | Referent | Referent |
3.1. Prognostic Importance of SLN Mapping versus Other Nodal Treatment Strategies
Quantitative synthesis is shown in Figure 2. Operative nodal staging with SLN biopsy predicted better DFS as compared to the nodal observation in cN0 MCC patients (pooled HR: 1.61 (95% CI: 1.05–2.46), P = 0.028, Cochrane Q value = 2.36, P = 0.3, I 2 = 15.3%). Nodal staging with SLN biopsy also predicted a slightly better OS, although the pooled HR (1.08 (95% CI: 0.55–2.10), Cochrane Q value = 0.093, P = 0.76, I 2 = 0%) was not statistically significant (P = 0.8).
Figure 2.
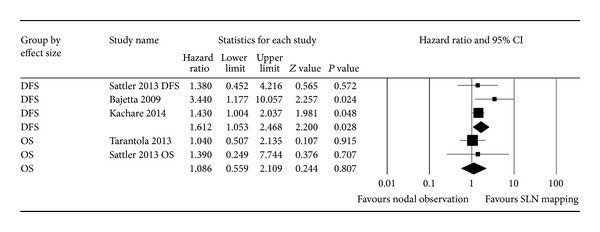
Forest plot of the hazard ratio (HR) of disease free survival (DFS) and overall survival (OS) for operative staging with SLN mapping versus nodal observation.
3.2. Prognostic Importance of SLN Pathologic Status for Prediction of DFS and OS
Quantitative synthesis is shown in Figure 3. Pathologically negative SLN (SLN−) patients had better OS (pooled HR: 4.42 (95% CI: 1.82–10.7), P = 0.0009, Cochrane Q value = 1.8, P = 0.61, I 2 = 0%) and DFS (pooled HR: 2.58 (95% CI: 1.78–3.73), P = 0.000001, Cochrane Q value = 2.47, P = 0.64, I 2 = 0%) as compared to SLN+ patients.
Figure 3.
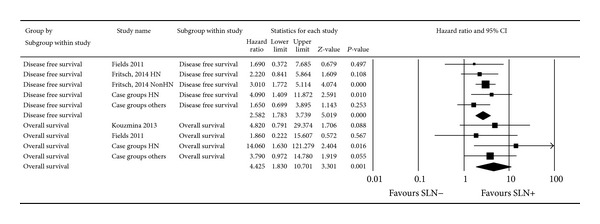
Forest plot of the hazard ratio (HR) of disease free survival (DFS) and overall survival (OS) for pathological SLN status.
Funnel plots of OS and DFS meta-analyses are shown in Figure 4. Egger's regression intercepts for DFS and OS meta-analyses were −0.79 (P = 0.52) and 0.82 (P = 0.76), respectively.
Figure 4.
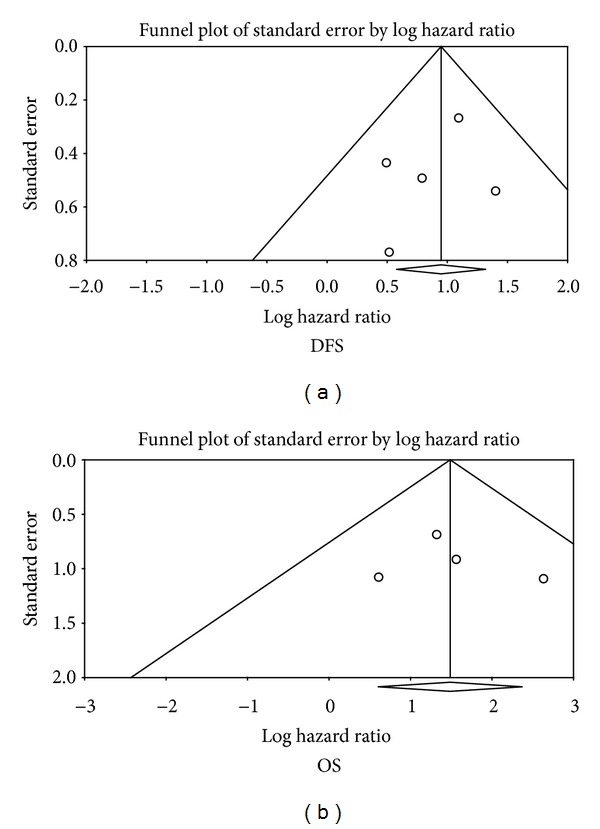
Funnel plots of meta-analyses of pathological SLN status for DFS and OS.
Subgroup analysis regarding location of MCC did not show any difference between head and neck and other parts of the body regarding prognostic importance of SLN pathologic status (pooled HR of DFS for head and neck and other parts of the body were 2.92 (95% CI: 1.42–6), P = 0.003, and 2.54 (95% CI: 1.62–4), P = 0.000048, resp.).
4. Discussion
SLN mapping is an integral part of treatment in melanoma and breast cancer patients [47–49]. SLN mapping has been used to evaluate MCC for a long time and prognostic significance of this technique has been assessed in several reports. Recently, data of SEER database have been published regarding prognostic importance of SLN mapping in MCC, increasing our understanding in this regard.
In the current study, we reviewed the medical literature for two purposes: (1) to determine the prognostic importance of SLN status in MCC and (2) to assess the prognostic impact of SLN mapping versus other nodal treatments (mainly nodal observation) in MCC.
4.1. Prognostic Significance of SLN+ Status in MCC
MCC is a rare aggressive cutaneous tumor. Accurate staging of MCC patients is highly important for proper treatment planning. Radiological examinations such as PET imaging and CT have been used for this purpose with various results [50].
Positive lymph node disease at presentation is a strong indicator of poor outcome and reduces the 5-year survival rate to less than 50% [11].
Although medical literature is rich regarding SLN mapping in MCC [27, 33], studies with true survival analysis for the importance of SLN mapping are scarce. Overall, 3 studies had appropriate survival analyses and they were included in the current systematic review. We also gathered data of 172 cases from the medical literature and performed an individual patient meta-analysis.
Our meta-analysis showed that SLN status is a strong predictor of OS and DFS in MCC patients (pooled HR of 4.42 and 2.58, resp.). In other words, SLN+ patients may suffer MCC- related death and recurrence 4.42 and 2.58 times more frequently per unit time than SLN− patients. It is worth mentioning that, only in the study of Fritsch et al. [16], SLN status was a statistically significant predictor of survival. The other two studies by Kouzmina et al. [19] and Fields et al. [15] showed statistically nonsignificant results despite HR > 1 which denotes the lower MCC-related death or recurrence in the SLN− as compared to the SLN+ patients. The reasons of statistically nonsignificant results in these studies are most likely the low sample size and statistical power. Our meta-analysis by combining the results of individual studies increased the statistical power and yielded statistically significant results. Anyhow, the direction of effect size (HR) in the included studies is all the same and denotes the survival benefit of SLN− status. Location of the MCC is an important issue which has been brought up in the SLN mapping of MCC. Fritsch et al. [16] reviewed the information of SEER database and reported that SLN+ status was not an independent prognostic factor for predicting DFS. They attributed this finding to different lymphatic pathways and behavior in the head and neck area as compared to the other parts of the body [16, 51]. However, our meta-analysis did not show any difference between head and neck and other parts of the body regarding prognostic value of SLN status in MCC patients. It seems that further studies with long follow-up are needed to further elucidate this issue.
4.2. Does SLN Mapping Actually Improves Survival in cN0 MCC Patients?
Bulk of medical literature is devoted to evaluate the accuracy of SLN mapping in MCC. However, we aimed to know if actually SLN mapping in cN0 MCC patients is associated with improved survival or not. Only four studies had enough survival analyses to answer the abovementioned clinical question and were included in the current systematic review. Three of the included studies compared operative nodal staging by SLN mapping with nodal observation only [14, 17, 18]. As shown in Table 1, all of these studies showed survival benefit of the SLN mapping compared to observation strategy (HR > 1 for DFS). Our systematic review also supported the previous findings with pooled HR of 1.64 for DFS. The reason of this finding is most likely the early diagnosis of regional nodal involvement by SLN mapping which leads to start of the adjuvant treatments in earlier stages of the disease course providing a better DFS.
On the other hand the pooled HR for OS was not statistically significant (HR = 1.08, P = 0.093). This is most likely due to the different design of Tarantola et al. study [3] as they reported the survival benefit of SLN mapping as compared to other nodal treatments (including regional nodal dissection, nodal radiation therapy, and observation) in cN0 MCC patients. Other nodal treatment strategies besides nodal observation can introduce a bias into the study as they can have survival benefit compared to nodal observation alone.
To sum up, it seems that operative nodal staging with SLN mapping provides survival benefit versus nodal observation in cN0 MCC patients. The survival benefit is mostly obvious for DFS. In order to evaluate the effect of SLN mapping on OS, larger studies with long follow-up are still needed.
4.3. Limitations
MCC is a rare tumor and many authors reported their experience in SLN mapping in this tumor with small sample size. In order to overcome this problem, we gathered the prognostic information in the literature performing survival analysis in the final sample of cases (172 patients). Although studies based on SEER database had very large sample size, the other included studies had relatively small sample size and this may limit the statistical power of our meta-analysis.
Comparison of SLN mapping with nodal observation in all but one of the included studies is another limitation of our study. Survival benefit of SLN mapping ought to be compared with other nodal treatment protocols such as prophylactic regional node dissection or irradiation. Thus far only Tarantola et al. study reported survival benefit of SLN mapping as compared to other nodal treatment methods and further multicenter large studies are needed in this regard.
The quality of the included studies can also be considered as a limitation of our study. As shown in Table 1, only two of the included studies reported the details of SLN mapping technique.
Publication bias is a major concern in all systematic reviews. We evaluated this bias by funnel plots and Egger's regression method. Although neither funnel plots nor Egger's test showed possible publication bias, the power of Egger's test is relatively low and we cannot rule out possible important publication bias in our systematic review.
5. Conclusion
SLN mapping can provide strong prognostic information regarding OS and DFS in cN0 MCC patients. SLN+ patients may suffer MCC-related death and recurrence more frequently per unit time than SLN− patients. Further multicenter studies with long follow-up are needed to evaluate the effect of the location of MCC.
More importantly, SLN mapping can improve DFS and possibly OS in cN0 MCC patients as compared to nodal observation. As MCC is a rare tumor, large multicenter prospective studies are still needed to validate the survival benefit of SLN mapping. Further studies ought to compare SLN mapping with other nodal treatment strategies such as prophylactic lymph node dissection and/or irradiation.
Acknowledgments
This study is the result of a residency thesis under the approval number of 920574. The study was financially supported by the Vice Chancellery of research of Mashhad University of Medical Sciences.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Pink R, Ehrmann J, Molitor M, et al. Merkel cell carcinoma. A review. Biomedical Papers. 2012;156(3):213–217. doi: 10.5507/bp.2012.033. [DOI] [PubMed] [Google Scholar]
- 2.Prieto Muñoz I, Pardo Masferrer J, Olivera Vegas J, Medina Montalvo MS, Jover Díaz R, Pérez Casas AM. Merkel cell carcinoma from 2008 to 2012: reaching a new level of understanding. Cancer Treatment Reviews. 2013;39(5):421–429. doi: 10.1016/j.ctrv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. Journal of the American Academy of Dermatology. 2013;68(3):425–432. doi: 10.1016/j.jaad.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Smith VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope. 2012;122(6):1283–1290. doi: 10.1002/lary.23222. [DOI] [PubMed] [Google Scholar]
- 5.Herbert HM, Sun MT, Selva D, et al. Merkel cell carcinoma of the eyelid: management and prognosis. JAMA Ophthalmology. 2014;132(2):197–204. doi: 10.1001/jamaophthalmol.2013.6077. [DOI] [PubMed] [Google Scholar]
- 6.Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK. Merkel cell carcinoma: prognosis and management. Archives of Surgery. 1991;126(12):1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi R, Gholami H, Zakavi SR, Kakhki VRD, Tabasi KT, Horenblas S. Accuracy of sentinel lymph node biopsy for inguinal lymph node staging of penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Journal of Urology. 2012;187(1):25–31. doi: 10.1016/j.juro.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Hassanzade M, Attaran M, Treglia G, Yousefi Z, Sadeghi R. Lymphatic mapping and sentinel node biopsy in squamous cell carcinoma of the vulva: systematic review and meta-analysis of the literature. Gynecologic Oncology. 2013;130(1):237–245. doi: 10.1016/j.ygyno.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Ansari M, Rad MA, Hassanzadeh M, et al. Sentinel node biopsy in endometrial cancer: systematic review and meta-analysis of the literature. European Journal of Gynaecological Oncology. 2013;34(5):387–401. [PubMed] [Google Scholar]
- 10.Gupta SG, Wang LC, Peñas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Archives of Dermatology. 2006;142(6):685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 11.Mehrany K, Otley CC, Weenig RH, Kim Phillips P, Roenigk RK, Nguyen TH. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatologic Surgery. 2002;28(2):113–117. doi: 10.1046/j.1524-4725.2002.02901.x. [DOI] [PubMed] [Google Scholar]
- 12.Shaw JHF, Rumball E. Merkel cell tumour: clinical behaviour and treatment. British Journal of Surgery. 1991;78(2):138–142. doi: 10.1002/bjs.1800780205. [DOI] [PubMed] [Google Scholar]
- 13.Hasan S, Liu L, Triplet J, Li Z, Mansur D. The role of postoperative radiation and chemoradiation in merkel cell carcinoma: a systematic review of the literature. Frontiers in Oncology. 2013;3(article 276) doi: 10.3389/fonc.2013.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kachare SD, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. Sentinel lymph node biopsy is associated with improved survival in merkel cell carcinoma. Annals of Surgical Oncology. 2014;21(5):1624–1630. doi: 10.1245/s10434-013-3434-3. [DOI] [PubMed] [Google Scholar]
- 15.Fields RC, Busam KJ, Chou JF, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: analysis of 153 patients from a single institution. Annals of Surgical Oncology. 2011;18(9):2529–2537. doi: 10.1245/s10434-011-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritsch VA, Camp ER, Lentsch EJ. Sentinel lymph node status in Merkel cell carcinoma of the head and neck: not a predictor of survival. Head and Neck. 2014;36(4):571–579. doi: 10.1002/hed.23334. [DOI] [PubMed] [Google Scholar]
- 17.Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Annals of Surgical Oncology. 2009;16(11):2985–2993. doi: 10.1245/s10434-009-0615-1. [DOI] [PubMed] [Google Scholar]
- 18.Sattler E, Geimer T, Sick I, et al. Sentinel lymph node in Merkel cell carcinoma: to biopsy or not to biopsy? Journal of Dermatology. 2013;40(5):374–379. doi: 10.1111/1346-8138.12072. [DOI] [PubMed] [Google Scholar]
- 19.Kouzmina M, Leikola J, Böhling T, Koljonen V. Positive sentinel lymph node biopsy predicts local metastases during the course of disease in Merkel cell carcinoma. Journal of Plastic Surgery and Hand Surgery. 2013;47(2):139–143. doi: 10.3109/2000656X.2012.736386. [DOI] [PubMed] [Google Scholar]
- 20.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21. http://www.cebm.net/index.aspx?o=5987.
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ames SE, Krag DN, Brady MS. Radiolocalization of the sentinel lymph node in Merkel cell carcinoma: a clinical analysis of seven cases. Journal of Surgical Oncology. 1998;67(4):251–254. doi: 10.1002/(sici)1096-9098(199804)67:4<251::aid-jso8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Wasserberg N, Schachter J, Eyal Fenig MD, Feinmesser M, Gutman H. Applicability of the sentinel node technique to Merkel cell carcinoma. Dermatologic Surgery. 2000;26(2):138–141. doi: 10.1046/j.1524-4725.2000.99213.x. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues LKE, Leong SPL, Kashani-Sabet M, Wong JH. Early experience with sentinel lymph node mapping for Merkel cell carcinoma. Journal of the American Academy of Dermatology. 2001;45(2):303–308. doi: 10.1067/mjd.2001.114749. [DOI] [PubMed] [Google Scholar]
- 27.Pan D, Narayan D, Ariyan S. Merkel cell carcinoma: five case reports using sentinel lymph node biopsy and a review of 110 new cases. Plastic and Reconstructive Surgery. 2002;110(5):1259–1265. doi: 10.1097/01.PRS.0000025287.96915.88. [DOI] [PubMed] [Google Scholar]
- 28.Su LD, Lowe L, Bradford CR, Yahanda AI, Johnson TM, Sondak VK. Immunostaining for cytokeratin 20 improves detection of micrometastatic Merkel cell carcinoma in sentinel lymph nodes. Journal of the American Academy of Dermatology. 2002;46(5):661–666. doi: 10.1067/mjd.2002.119563. [DOI] [PubMed] [Google Scholar]
- 29.Blom A, Kolb F, Lumbroso J, et al. Significance of sentinel lymph node biopsy in Merkel cell carcinoma. Analysis of II cases. Annales de Dermatologie et de Venereologie. 2003;130(4):417–422. [PubMed] [Google Scholar]
- 30.Alex JC. The application of sentinel node radiolocalization to solid tumors of the head and neck: a 10-year experience. Laryngoscope. 2004;114(1):2–19. doi: 10.1097/00005537-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Schmalbach CE, Lowe L, Teknos TN, Johnson TM, Bradford CR. Reliability of sentinel lymph node biopsy for regional staging of head and neck Merkel cell carcinoma. Archives of Otolaryngology—Head and Neck Surgery. 2005;131(7):610–614. doi: 10.1001/archotol.131.7.610. [DOI] [PubMed] [Google Scholar]
- 32.Civantos FJ, Moffat FL, Goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope. 2006;116(3) supplement 109:1–15. doi: 10.1097/01.mlg.0000200750.74249.79. [DOI] [PubMed] [Google Scholar]
- 33.Maza S, Trefzer U, Hofmann M, et al. Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: results of a prospective study and review of the literature. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33(4):433–440. doi: 10.1007/s00259-005-0014-1. [DOI] [PubMed] [Google Scholar]
- 34.Ortin-Perez J, van Rijk MC, Valdes-Olmos RA, et al. Lymphatic mapping and sentinel node biopsy in Merkel’s cell carcinoma. European Journal of Surgical Oncology. 2007;33(1):119–122. doi: 10.1016/j.ejso.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Koljonen V, Suominen S. Sentinel node biopsy in local anaesthesia in patients with head and neck Merkel cell carcinoma. European Journal of Plastic Surgery. 2008;30(5):205–210. [Google Scholar]
- 36.Migliano E, Monarca C, Tedesco M, Rizzo MI, Bucher S. Merkel cell carcinoma and sentinel lymph node dissection: nine cases report. Il Giornale di Chirurgia. 2008;29(1-2):28–32. [PubMed] [Google Scholar]
- 37.Ramón LR, Javier FA, Roberto M, et al. Merkel cell carcinoma of the head and neck: report of seven cases. Medicina Oral, Patologia Oral y Cirugia Bucal. 2008;13(6):E390–E394. [PubMed] [Google Scholar]
- 38.Shnayder Y, Weed DT, Arnold DJ, et al. Management of the neck in Merkel cell carcinoma of the head and neck: university of Miami experience. Head and Neck. 2008;30(12):1559–1565. doi: 10.1002/hed.20899. [DOI] [PubMed] [Google Scholar]
- 39.Maalouf T, George JL, Geoffrois L, Olivier P, Dolivet G. Sentinel lymph node biopsy for lymphophilic conjunctival and eyelid tumours: apropos of 8 cases. Revue de Laryngologie Otologie Rhinologie. 2009;130(4-5):231–234. [PubMed] [Google Scholar]
- 40.Kamo R, Kumei A, Ueoku S, Kusutani N, Sowa J, Ishii M. Importance of sentinel lymph node biopsy in Merkel cell carcinoma. Journal of Dermatology. 2010;37(12):1068–1070. doi: 10.1111/j.1346-8138.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 41.Howle J, Veness M. Sentinel lymph node biopsy in patients with Merkel cell carcinoma: an emerging role and the Westmead hospital experience. Australasian Journal of Dermatology. 2012;53(1):26–31. doi: 10.1111/j.1440-0960.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 42.Matthey-Gie ML, Boubaker A, Letovanec I, Demartines N, Matter M. Sentinel lymph node biopsy in nonmelanoma skin cancer patients. Journal of Skin Cancer. 2013;2013:8 pages. doi: 10.1155/2013/267474.267474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchesi A, Camillo Parodi P, Brioschi M, Sileo G, Marchesi M, Vaienti L. Giant merkel cell carcinoma of the lower limb: case report and review of the literature . Journal of Cutaneous Medicine and Surgery. 2013;17:1–5. doi: 10.2310/7750.2013.12121. [DOI] [PubMed] [Google Scholar]
- 44.Zeitouni NC, Cheney RT, Delacure MD. Lymphoscintigraphy, sentinel lymph node biopsy, and Mohs micrographic surgery in the treatment of merkel cell carcinoma. Dermatologic Surgery. 2000;26(1):12–18. doi: 10.1046/j.1524-4725.2000.99129.x. [DOI] [PubMed] [Google Scholar]
- 45.Düker I, Starz H, Bachter D, Balda B-R. Prognostic and therapeutic implications of sentinel lymphonodectomy and S-staging in merkel cell carcinoma. Dermatology. 2001;202(3):225–229. doi: 10.1159/000051641. [DOI] [PubMed] [Google Scholar]
- 46.Giannotti G, Lazzeri D, Viacava P, et al. Merkel cell carcinoma of the lower extremity: report of four cases and new considerations. Annals of Plastic Surgery. 2009;62(1):83–86. doi: 10.1097/SAP.0b013e31817439a7. [DOI] [PubMed] [Google Scholar]
- 47.Mehrabibahar M, Forghani MN, Memar B, et al. Sentinel lymph node biopsy in melanoma patients: an experience with Tc-99m antimony sulfide colloid. Iranian Journal of Nuclear Medicine. 2010;18(1):1–6. [Google Scholar]
- 48.Aliakbarian M, Memar B, Jangjoo A, et al. Factors influencing the time of sentinel node visualization in breast cancer patients using intradermal injection of the radiotracer. The American Journal of Surgery. 2011;202(2):199–202. doi: 10.1016/j.amjsurg.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 49.McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: controversy despite widespread agreement. Journal of Clinical Oncology. 2001;19(11):2851–2855. doi: 10.1200/JCO.2001.19.11.2851. [DOI] [PubMed] [Google Scholar]
- 50.Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography for the diagnosis of osteomyelitis related to diabetic foot: a systematic review and a meta-analysis. Foot. 2013;23(4):140–148. doi: 10.1016/j.foot.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Miller SJ, Alam M, Andersen J, et al. Merkel cell carcinoma: clinical practice guidelines in oncology. JNCCN Journal of the National Comprehensive Cancer Network. 2006;4(7):704–712. doi: 10.6004/jnccn.2006.0060. [DOI] [PubMed] [Google Scholar]


