Abstract
Working memory training has been the focus of intense research interest. Despite accumulating behavioral work, knowledge about the neural mechanisms underlying training effects is scarce. Here we show that seven days of training on an n back task lead to substantial performance improvements in the trained task; furthermore, the experimental group shows cross modal transfer as compared to an active control group. In addition, there are two neural effects that emerged as a function of training: first, increased perfusion during task performance in selected regions, reflecting a neural response to cope with high task demand; second, increased blood flow at rest in regions where training effects were apparent. We also found that perfusion at rest was correlated with task proficiency, probably reflecting an improved neural readiness to perform. Our findings are discussed within the context of the available neuroimaging literature on n back training.
Keywords: ASL, n-back, cognitive training, longitudinal fMRI, transfer
What happens in the brain when training improves performance on a trained task? We aimed to answer this question by means of an n back intervention that trains working memory (WM), our ability to store and manipulate a limited amount of information for a short period of time (Jonides et al., 2008). WM is the underlying mechanism that drives performance in many complex cognitive tasks, such as fluid intelligence, reading comprehension, and mathematics (e.g. Daneman & Carpenter, 1980; Pickering, 2006).1 Therefore, it is not surprising that training on WM has been repeatedly shown to improve not only WM skills, but also other complex cognitive skills that rely on WM processes (e.g. Chein & Morrison, 2010; Jaeggi, Studer Luethi, et al., 2010; Jaeggi, Buschkuehl, Jonides, & Perrig, 2008; Jaeggi, Buschkuehl, Jonides, & Shah, 2011; Jaeggi, Buschkuehl, Shah, & Jonides, 2013; Jaušovec & Jaušovec, 2012; Rudebeck, Bor, Ormond, O’Reilly, & Lee, 2012; Stephenson & Halpern, 2013). Despite the increasing popularity of WM interventions, there are still very few studies investigating the neural effects of WM training. Most of these have examined functional activation changes (e.g. Dahlin, Neely, Larsson, Bäckman, & Nyberg, 2008; Hempel et al., 2004; Jolles, Grol, Van Buchem, Rombouts, & Crone, 2010; Olesen, Westerberg, & Klingberg, 2004; Schneiders, Opitz, Krick, & Mecklinger, 2011; Westerberg et al., 2007). In addition, there are also studies investigating the effect of training on cerebral perfusion at rest and on functional connectivity (Kundu, Sutterer, Emrich, & Postle, 2013; Mozolic, Hayasaka, & Laurienti, 2010; Takeuchi et al., 2012), on brain structure (Takeuchi et al., 2010, 2011), and on dopaminergic functions (e.g. Bäckman et al., 2011; McNab et al., 2009). Due to the small body of literature and the substantial methodological differences among these studies, it is currently difficult to draw firm conclusions about the underlying neural mechanisms of WM training, and therefore, it is difficult to make predictions about training related activation changes (Buschkuehl, Jaeggi, & Jonides, 2012). In the present study, we focus on the neural effects of an n back intervention that is a relatively common vehicle used in behavioral WM training research and whose effects on untrained tasks have been documented by several independent laboratories and with different populations (Colom et al., 2013; Jaeggi et al., 2008, 2011, 2013; Jaeggi, Studer Luethi, et al., 2010; Jaušovec & Jaušovec, 2012; Owens, Koster, & Derakshan, 2013; Rudebeck et al., 2012; Schweizer, Grahn, Hampshire, Mobbs, & Dalgleish, 2013; Schweizer, Hampshire, & Dalgleish, 2011; Stephenson & Halpern, 2013). To our knowledge, there are only four studies to date that have investigated the neural correlates of n back training by means of fMRI (Hempel et al., 2004; Schneiders et al., 2012, 2011; Schweizer et al., 2013). Here, we aim to build upon this prior work to further elucidate the underlying neural mechanisms that are involved in n back training. Our results reveal that as little as 2.5h of n back training are enough to result in behavioral changes that have a measurable neural correlate.
Task-Relevant Activation Changes
In principle, there are four classes of outcomes that would be of interest when examining brain activations as a function of cognitive training. One is activation of the same brain regions before and after training, but with less overall activation after training. This pattern would reveal a single circuitry underlying the task, a circuit that becomes more efficient in its operation. A second pattern is the opposite: the same circuit is active before and after training, but with greater activation after training. This pattern would reveal a common underlying mechanism that might implicate a larger population of cells consuming greater energy as a function of the training experience. A third potential outcome is a combination of the first two if there are increases of activation in some brain areas, and decreases in others. Finally, a fourth possible result of interest is a qualitatively different pattern of activation that results from the training experience. This would indicate that new and different mechanisms have been called to the table by the training regimen (see Kelly, Foxe, & Garavan, 2006 for a detailed discussion of this topic).
Let us briefly review the available n back training literature in the light of these four potential outcomes. In a study conducted by Hempel et al. (2004) participants were instructed to train on a spatial 0 back, a 1 back, and a 2 back task twice a day over the course of four weeks. Functional brain imaging data were collected while participants performed the trained tasks not only before and after the intervention, but also at an intermediate point after two weeks of training. Hempel et al. reported an activation increase in the first half of training, followed by an activation decrease in the second half of training. This pattern was significant for the right intraparietal sulcus (Brodmann Area (BA) 39, 40) and the superior parietal lobe (BA 40). The authors also report a trend for a similar activation pattern for the right inferior/medial frontal gyrus (BA 9, 45, 46). Schneiders et al. (2011) let their participants train on either an auditory or a visual n back training task for 8 10 sessions over a period of two weeks. Each training session lasted approximately 1h and the training was adaptive in that it adjusted to participants’ performance. Before and after training, participants were scanned while performing a visual 0 back and 2 back task. Irrespective of training condition, participants showed significant activation decreases in the right superior middle frontal gyrus (BA 6) and posterior parietal regions (BA 40). In the group that trained on the visual n back task, Schneiders et al. reported additional activation decreases in the right middle frontal gyrus (BA 9, 46). There was no region in which there were activation increases as a function of training. In another study, Schneiders et al. (2012) trained participants on an adaptive auditory n back task in eight sessions that were distributed across two weeks; training time per day was 50min on average. Before and after training, participants were tested on an auditory and visual 0 back and 2 back task. The authors reported decreased activation in the auditory and the visual n back task in right inferior parietal regions (BA 40) and the right superior frontal gyrus (BA 6). In the auditory n back task, there were additional activation decreases in the right inferior frontal gyrus (BA 46, 47). In a recent study conducted by Schweizer et al. (2013) participants were trained on an affective dual n back task in which participants were presented with emotionally neutral or emotionally negative faces and words. The training lasted for approximately 20 sessions, daily training time was between 20 and 30min, and the training was adaptive. Before and after the intervention, participants were scanned on the trained task at 1 back, 2 back, 3 back, and 5 back levels. Similar to the studies reviewed before, Schweizer et al. reported activation decreases when the data of the 3 back condition were analyzed. These decreases were observed in the left dorsolateral prefrontal cortex, right superior frontal gyrus, left and right supramarginal gyrus, left and right middle temporal gyrus, and left and right middle occipital lobe. However, when the data of the 5 back condition were analyzed, only activation increases were found. These increases were observed in the right orbitofrontal cortex, right inferior frontal gyrus, and right inferior parietal cortex. Schweizer et al. explain their pattern of results by arguing that increased effort is related to increased activation especially in WM relevant brain areas, a result that is in line with previous findings of neural effects of n back training and also in line with studies investigating brain activation as a function of n back load (see Owen, McMillan, Laird, & Bullmore, 2005 for a meta analysis).
To summarize, the available brain imaging literature on n back training seems to agree on several points. All four studies reported task related activation decreases if the scanned n back task was relatively easy, that is, within the WM capacity range of the trainees after training. The finding of Hempel et al. suggests that there could be two distinct neural processes at work that occur in succession: first an increase in activation followed by a decrease in activation. The former could reflect controlled and effortful processes that are replaced by more automatic processes as training goes on and participants get more proficient at the task, manifested as lowered activation levels. This view is largely consistent with the dual process theory of human performance (Chein & Schneider, 2005; Posner & Snyder, 1975) and it is also in line with the findings of Schweizer et al. (2013). Unfortunately, Hempel et al. do not report how long their participants trained per day and therefore it is difficult to compare their results with the two studies from Schneiders et al. and the one by Schweizer et al. However, since Schneiders et al. reported decreased activations after approximately 6.5h and 8h of training, it is conceivable that the overall training time in the Hempel et al. study was within this range as well (they trained twice a day for 4 weeks). Therefore, we hypothesize that training on an n back task for less than 3h results in increased task related activations as long as participants are tested on an n back level that still requires effortful processing. Further, the four studies crudely agree regarding the brain regions in which activation changes were found. Uniformly involved in all four studies is BA 40, which is commonly thought of to be involved in functions of semantic representation and spatial orientation, and which is often activated in spatial WM tasks. Also involved in three of the four studies are BA 6 and BA 9, both areas that are commonly thought to be involved in executive control and WM processing. It is also worth mentioning that only one of the four studies implemented an active control group (Schweizer et al., 2013), so there has been little control for potential unspecific training effects such as expectation effects or effects of motivation, an issue that we address with the present study.
Activation Changes at Rest
Beyond what training might confer on the task challenged brain, there are two interesting patterns of activation that might characterize the resting brain as a result of training. One is greater activation in the regions responsible for the task. This might be an indication of the increased readiness of these areas to participate in the task. A second result might be decreased activation at rest in task engaged regions. This might be an indication of fatigue in these regions caused by the training (e.g., Persson, Larsson, & Reuter Lorenz, 2013).
To our knowledge there are no n back training studies available that investigated activation changes at rest. However, there are two WM training studies that examined activation changes at rest relying on interventions other than n back (Mozolic et al., 2010; Takeuchi et al., 2012). Mozolic et al. trained older adults on an interference focused intervention, and Takeuchi et al. trained younger adults on four different WM tasks. Both studies reported increased baseline activity levels as a result of training. Mozolic et al. in the right inferior frontal cortex2 and Takeuchi et al. in the right lateral prefrontal cortex. These two findings suggest that WM training leads to increased perfusion, i.e., a ‘fitter’ brain as a result of cognitive (or brain) training.
The Current Study
Primarily, our experiment was designed to examine the various possibilities for task related and rest related brain activations as a function of training. Using Arterial Spin Labeling (ASL) as an imaging technique, we tested how cerebral perfusion changes as a function of n back training. ASL allows one to map brain activity quantitatively (Detre, Leigh, Williams, & Koretsky, 1992; Williams, Detre, Leigh, & Koretsky, 1992). Perfusion estimates can be obtained during task activity and at rest by fitting a linear model as in the case of BOLD weighted functional Magnet Resonance Imaging (fMRI) and scaling the parameter estimates appropriately (Hernandez Garcia, Jahanian, & Rowe, 2010). BOLD imaging's sensitivity to scanner drift makes it very challenging to carry out longitudinal designs and designs with very low frequency paradigms. On the other hand, while the noise of an ASL time course within a single run is higher than with BOLD, it has been demonstrated that ASL data are practically insensitive to scanner drifts and, thus, much less variable across sessions than BOLD (Aguirre, Detre, & Alsop, 2002; Wang, Aguirre, Kimberg, & Detre, 2003; Wang, Aguirre, Kimberg, Roc, et al., 2003). Additionally, the self calibrating nature of ASL data reduces the variance of the signals across subjects relative to BOLD, thus requiring fewer subjects to be scanned per experiment (Tjandra et al., 2005). To conclude, ASL is ideally suited for longitudinal studies investigating low frequency signals such as ours because the method quantifies a physiological parameter and is not prone to scanner drifts within and across sessions (cf. Hernandez Garcia & Buschkuehl, 2012 for further discussion about this issue).
In our study, young adults trained for approximately 20min per day over a period of seven consecutive days on an adaptive version of a visuospatial n back task. That is, the task difficulty (i.e. level of n) continuously adjusted to the participants’ performance. Before and after the intervention, functional brain data were collected while participants lay in the scanner and worked on the trained n back task on a 1 back and a 4 back level. The 1 back level served as a control condition because previous research has revealed that participants perform very well at this level (Jaeggi, Studer Luethi, et al., 2010), and thus, we did not expect any group differences or changes as a function of training. The relatively difficult 4 back condition has been chosen because prior work indicated that participants usually struggle with this level at pre test, but are able to perform with adequate accuracy after seven days of training (Jaeggi, Studer Luethi, et al., 2010). Thus, we predicted that participants who trained on the task would outperform the control group in the 4 back task after training; however, they would still be adequately challenged after an accumulated training time of only 2.5h. Based on previous findings, we predicted an increase in perfusion during 4 back, especially in prefrontal and posterior parietal brain areas. Finally, we hypothesized that training on n back would result in increased perfusion at rest, as previous studies have suggested.
As an additional focus, we were interested whether we could replicate an interesting behavioral finding reported by Schneiders et al. (2012). In their paper, these authors presented behavioral transfer effects within modality (from an auditory n back training to a different auditory n back task) but not cross modal (from an auditory n back training to a visual n back task). In our previous work, we demonstrated that training on a visuospatial n back task resulted in transfer on an n back task with random shapes as stimuli; a transfer within the same modality (Jaeggi, Studer Luethi, et al., 2010). Here, we wanted to go a step further and test whether training on a visuospatial n back task leads to transfer to an auditory n back task, a transfer result that would seem rather unlikely given the non existent cross modal effect reported by Schneiders et al. (2012).
In sum, the current study adds to the existing literature by a) using ASL as a desired imaging technique for longitudinal designs, b) using an adequate sample size, c) comparing the training effects to an active control group, d) assessing the training outcome with a measure (4 back) that is adequate in difficulty (i.e., preventing ceiling performance at post test), e) testing for perfusion changes at rest, and finally, f) testing for behavioral cross modal transfer.
Method
A total of 69 participants were recruited for our study. Of these 69 participants, 14 were excluded from data analysis. One participant was excluded because of ceiling performance at pre test in both criterion tasks; one participant was excluded because of claustrophobia; 4 participants were excluded due to equipment problems, and the remaining 8 participants (3 from the experimental group; 5 from the control group) were excluded because they did not adhere to the study schedule. Therefore, for the data analysis we included a total sample of 55 participants (20 women; mean age: 21.8 years, SD=2.7). There were 27 participants in the experimental group (10 women; mean age: 22.3 years, SD=3.1), and 28 in the control group (10 women; mean age: 21.2 years, SD=2.1). There was no statistical difference in age between the groups (t(46)=1.50, p=ns, r=0.22) or in any of the behavioral measures at pre test (all p's=ns). Participants were recruited from the Ann Arbor/University of Michigan community and were compensated $20/hour for the scanning sessions (pre and post); no compensation was provided for training.
Procedure
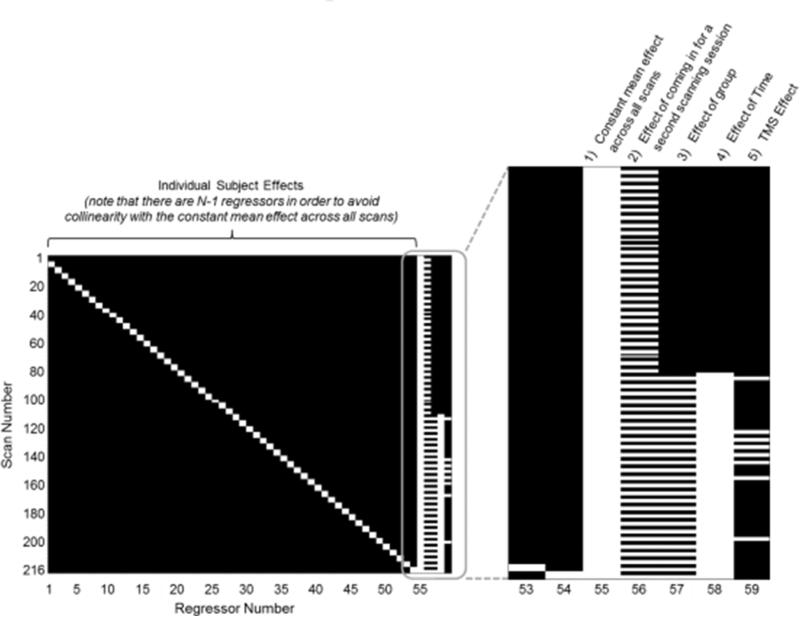
Participants were assigned to either an experimental condition or a control condition3. The two groups differed only in the training task; all other aspects of the study were identical for both groups. In the testing sessions before and after training, both groups were tested on an auditory n back task outside of the scanner and on a visuospatial n back task while we acquired brain imaging data. Participants performed two blocks of the visuospatial n back task in the pre test and two blocks in the post test resulting in a total of 4 scans × 55 participants = 220 total scans (refer to Figure 1). The training lasted seven consecutive days with approximately 20min of training each day. Participants in both groups trained at home and were instructed to send us their training data via email on a daily basis. This procedure allowed us to monitor their training progress and to follow up in the case of training irregularities. The scanning sessions took place two days before and one day after the 7 day intervention. Note that a subset of eight participants from the experimental group completed an additional visuospatial n back testing session after the pre test fMRI session, that is, before the start of training as well as after the post test fMRI and behavioral session. These eight participants were exposed to transcranial magnetic stimulation (TMS) to investigate its impact on the trained task and the corresponding data will be reported elsewhere (Bernard, Jaeggi, Buschkuehl, Hernandez Garcia, & Jonides, in preparation). In order to account for potential functional training effects of this single TMS session in those eight participants, we included an additional regressor in the statistical model (refer to Figure 1 and associated text).
Figure 1.
Design matrix used in the second level analysis. The main effects of interest are represented to the right of the figure: (1) a constant mean effect across all scans, (2) the effect of coming in for a second scanning session (regardless of training), (3) the effect of group (having received WM or control training), (4) the effect of time, and (5) the TMS effect.
Training Task
N-Back Training
Participants were presented with a sequence of single blue squares that could appear at any of eight locations on a computer screen. The task required determining whether the current stimulus appeared at the same location as the stimulus presented n positions back in the sequence. One trial consisted of a blue square that was presented for 500ms followed by a blank screen for 2,500ms. A training session consisted of 15 blocks, and each block entailed 20+n trials of which 6 were targets and 14+n were non targets. Training duration for one session was approximately 20min. Participants responded to targets with their left hand, and to non targets with their right hand. After each block, the participant's individual performance was determined, and if the participant made fewer than 3 errors, the level of n increased by 1; it decreased by 1 if the participant made more than 5 errors; in all other cases, n remained unchanged. Training performance per session was operationalized as the average n back level of the last 12 out of 15 blocks (Jaeggi, Studer Luethi, et al., 2010).
Control Training
. In this computerized task, participants were presented with a series of vocabulary and general knowledge questions one at a time, similar to a control task that we have used previously (Anguera et al., 2012; Jaeggi et al., 2013). Every question was accompanied by 4 answer alternatives presented below the question, one of them being correct. Participants were instructed to select the answer alternative which they thought to be correct by pressing a corresponding key on the keyboard. After making their choice, participants were told whether their answer was correct or not, along with the correct response, followed by the presentation of the next question. Incorrectly answered questions were shown again in the next training session providing a potential learning experience for the participants. Identical to the n back training, each session lasted approximately 20min. We have used this control task in previous work, and typically participants training with this control task are as motivated as the participants who are training on the n back task (Anguera et al., 2012; Jaeggi et al., 2011). This feature and the fact that the control task does not explicitly target WM processes make it an ideal task for the current study in that any improvements can be attributed to improvements in WM skills while excluding the possibility that any improvements are solely due to motivational factors.
Criterion Tasks
Visuospatial N-Back Task
The stimulus material and the timing parameters of this task were the same as the ones of the trained n back task. The task was presented while we acquired functional brain imaging data and consisted of two identical 19min runs. One run consisted of six blocks of 1 back, six blocks of 4 back, and six blocks of rest, presented in the same fixed order to each participant. Block lengths in the 1 back and 4 back condition were counterbalanced and were 48s (16 trials), 72s (24 trials), or 96s (32 trials) in length. Rest periods lasted 30s and participants were not required to do anything but look at a fixation cross in the center of the screen. Each block was preceded by an instruction screen for 5s which informed the participant about the nature of the next block. Task accuracy was measured as the proportion of hits minus false alarms for each block, separately for 1 back blocks and 4 back blocks. The average of the accuracies for the two n back conditions across blocks was used as a dependent measure.
Auditory N-Back Task
This task was very similar to the trained n back task but instead of visuospatial material, spoken letters (C, D, G, K, P, Q, T, V; presented in a female voice) were used. The letters were also presented for 500ms followed by 2,500ms of silence. During stimulus presentation, a fixation cross was shown in the center of the screen (Jaeggi, Buschkuehl, Perrig, & Meier, 2010). Participants were presented with a total of nine blocks: three blocks of 2 back, three blocks of 3 back, and three blocks of 4 back in that order. Each block consisted of 20+n trials. Task accuracy was measured as the proportion of hits minus false alarms for each n back level separately. The average of all accuracies across blocks was then used as a dependent measure.
Brain Imaging Acquisition
Participants were scanned while they performed the visuospatial n back task using a pseudo continuous ASL (PCASL) sequence followed by a spiral image acquisition. We used the following parameters for image acquisition: TR/TE=4,000/3ms; tagging time=2,100ms; post inversion delay=1,500ms; matrix size=64×64 voxels; 12 slices; slice thickness=6mm. The pseudo continuous labeling segment of the sequence consisted of a train of slice selective Hanning window pulses separated by 1.5ms intervals (flip angle=35°). The slice selective gradient was 6mT/m and the fractional moment was 0.9 (Dai, Garcia, de Bazelaire, & Alsop, 2008). In order to maximize label efficiency, a phase correction to the pseudo continuous labeling pulses was employed in order to overcome magnetic susceptibility distortions in the neck region (Jahanian, Noll, & Hernandez Garcia, 2011). The efficiency of the arterial inversion label was measured in a separate scan by collecting a spiral image 50mm above the inversion plane and by calculating the magnetization change in the carotid arteries between control and tagged images (Hernandez Garcia, Lewis, Moffat, & Branch, 2007).
The images were reconstructed, realigned using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002), smoothed with a 4mm Gaussian kernel, and surround subtracted to obtain a perfusion weighted time series of images. Perfusion estimates of the baseline and task levels were obtained using linear regression (Hernandez Garcia et al., 2010). We constructed a general linear model of the experimental paradigm's signal. The observed signal was modeled using the following 16 regressors: (1) 1 back condition (2) 4 back condition (3) instruction screens between the task blocks, (4) rest, and we also used twelve additional confound regressors (5 16). As confounds, we used six rigid body movement parameters generated by the realignment step, as well as six additional parameters generated by a modified version of the CompCor de noising method. CompCor consists of constructing a mask of voxels exhibiting high variance mask outside the gray matter, and conducting principal (or independent) component analysis of that region to identify the main sources of nuisance variance (see Behzadi, Restom, Liau, & Liu, 2007). The model's parameters and their variance were estimated for each participant using least squares. Next, the model's parameter estimates were scaled by a standard kinetic model in order to quantify perfusion. We used the following constants: T1grey matter=1,400ms, T1blood=1,660ms, and transit time=1,200ms. Gray matter M0 was computed from the mean control image by correcting for T1 effects.
This approach yielded the baseline perfusion as well as perfusion in the 1 back and the 4 back conditions for each participant. We used the contrast between 4 back and 1 back as an indicator of WM activation. Having performed this first level analysis on each scanning session for each participant, we analysed the resulting perfusion estimates of the baseline and of the difference between 4 back and 1 back conditions at the group level. We constructed a second level linear model of these effects to estimate the changes in activation in the population as a result of training. This model included the following regressors: (1) a constant mean effect across all scans, (2) the effect of coming in for a second scanning session (regardless of training), (3) the effect of group (having received WM or control training), (4) the effect of time4, and (5) the effect of TMS (whether or not the participant received TMS). Additionally, we included one confound regressor for each participant to capture the variation due to each individual participant's brain activity level. The corresponding design matrix is shown in Figure 1.
Prior to estimation of the model, we identified scans whose signal to noise ratio was unacceptable and excluded them from the analysis. This affected less than 2% of all scans (four scans out of 220 scans). Further, we scaled the data (i.e., the individual first level activation estimates) by the global mean resting cerebral blood flow during each session in order to reduce the variability across participants. Estimation of this model's parameters yielded separate statistical maps for each of the five regressors. In our analysis we will focus mainly on the third regressor because it captures exactly the variance of interest, which is the variance that is introduced by having trained on the n back versus the control task. In order to detect significant activation clusters at an overall significance level of p=.05, we combined an intensity threshold of p<.005 and a cluster size threshold of 19 voxels. These parameters were determined through Monte Carlo simulations using AlphaSim from the AFNI software package (Cox, 1996). Next, we identified overlapping brain regions between altered perfusion in the 4 back condition and altered perfusion during rest, both as a function of training, by calculating a conjunction of the corresponding statistical maps (Nichols, Brett, Andersson, Wager, & Poline, 2005). Finally, we correlated perfusion at rest with performance in the 4 back condition across both test sessions in order to investigate the impact of n back proficiency and perfusion.
Results
Specific Training Effects
Of the 55 participants, 48 trained for 7 sessions as instructed. The remaining 7 participants completed only 6 training sessions but were nonetheless included in the data analyses (3 from the experimental group, 4 from the control group).
Analysis of the n back training data revealed a reliable performance improvement (n back level at the first session: M=4.35, SD=1.07; n back level at the last session: M=5.85, SD=1.35); t(24)=6.54, p<.000, r=0.80).
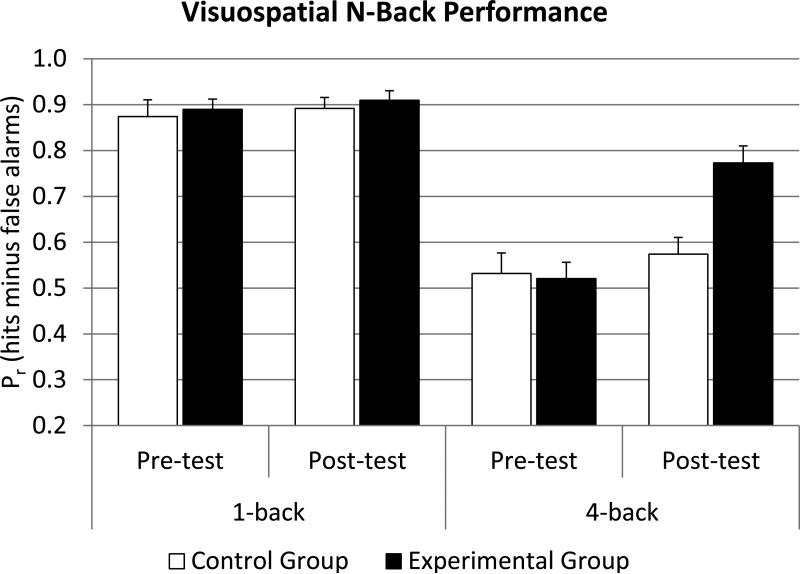
Although it is important to confirm that the experimental group improved on the training task itself, the main goal of the current study was to compare the n back improvement across the n back training group and the control group. The descriptive data are represented in Figure 25. A session (pre vs post) × load (1 back vs 4 back) × group (experimental vs control) analysis of variance (ANOVA) revealed significant main effects for session (F(1,52)=30.44, p<.001, η2partial=.37) and load (F(1,52)=164.99, p<.001, η2partial=.76). Further, there was a significant session by group interaction (F(1,52)=12.41, p<.001, η2partial=.19) and a session by load interaction (F(1,52)=39.68, p<.001, η2partial=.43). Most importantly, there was a significant session × load × group interaction (F(1,52)=26.01, p<.001, η2partial=.33) confirming that the experimental group improved more from pre to post than the control group in the 4 back condition. Furthermore, there was a reliable correlation between the gain in n back training (last session minus first session) and the gain in visuospatial 4 back (r=.55, p<.01) in the experimental group.
Figure 2.
Performance in the visuospatial n back task as a function of test session, n back level, and group. As expected, all participants performed near ceiling level at 1 back in both sessions, but there was a significant improvement in the experimental group in the 4 back condition from pre to post test which was not reliably present in the control group.
Transfer Effects Across Modalities
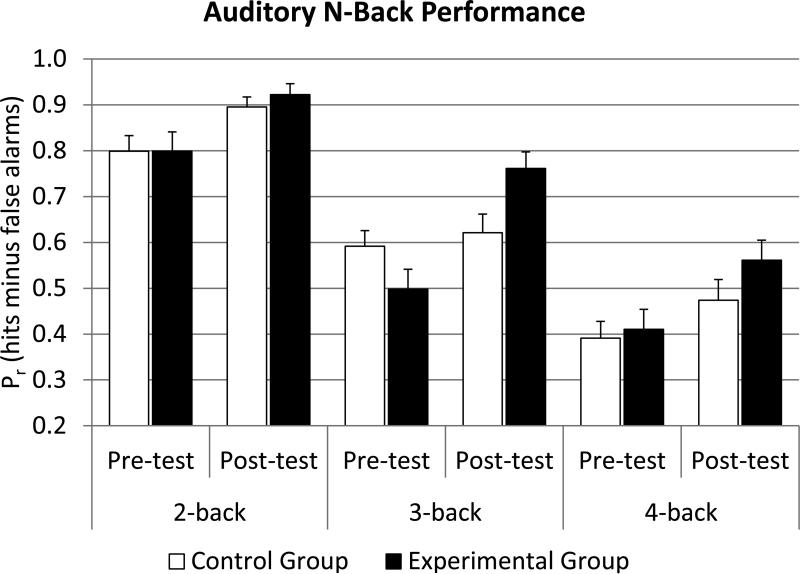
The descriptive data of the auditory n back task are visualized in Figure 36. A session (pre vs post) × load (2 back vs 3 back vs 4 back) × group (experimental vs control) ANOVA revealed significant main effects for session (F(1,94)=48.21, p<.001, η2partial=.51) and load (F(2,94)=162.53, p<.001, η2partial=.76). Further, there was a significant session by group interaction (F(1,94)=9.36, p<.01, η2partial=.17). Again, there was a significant session by load by group interaction (F(2,52)=3.48, p<.05, η2partial=.07). Separate session (pre vs post) × group (experimental vs control) ANOVAs revealed only a significant interaction for the 3 back condition (F(1,47)=13.34 p<.001, η2partial=.31)7 indicating that the significant three way interaction is mainly driven by the 3 back condition. Focusing only on the experimental group, this finding is further strengthened by correlations between the 4 back gain in the visuospatial n back task and the gain in the three different n back levels in the auditory n back task, where the largest correlation was observed in the 3 back condition (r=.61, p<.01), while there were only modest correlations in the 2 back (r=.18, p=ns), and the 4 back condition (r=.14, p=ns).
Figure 3.
Performance in the untrained auditory n back task as a function of test session, n back load, and group. The benefit of visuospatial n back training was especially pronounced in the 3 back condition.
Brain Imaging
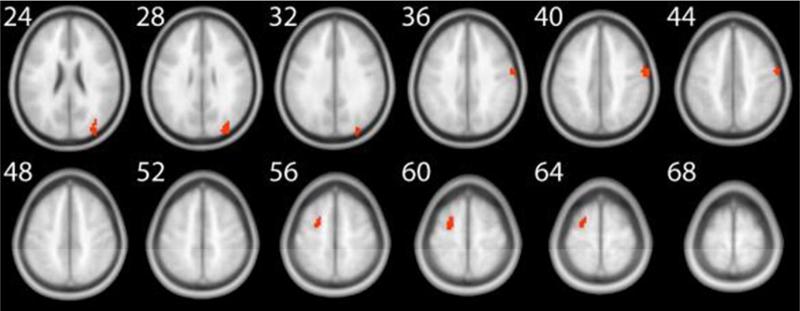
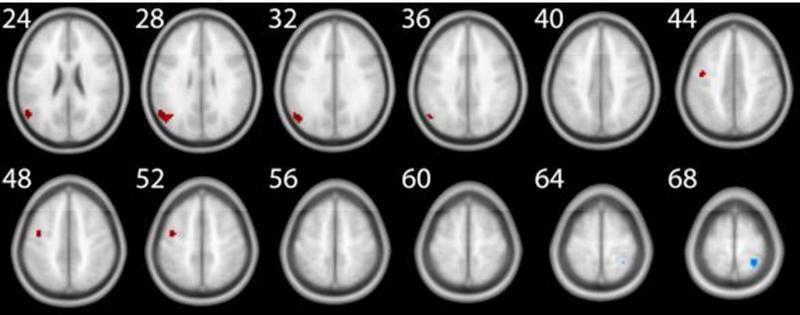
We first investigated the effect of n back training on task related brain areas. For this purpose, we performed a voxel wise whole brain analysis and estimated a regression model with the five regressors as outlined above. Our third regressor captured the variance of the effect of being in the n back training group versus the control group. The explained variance of this regressor is comparable to a group (n back vs control) × time (pre vs post) interaction. The variance of the factors group and time are already accounted for by the other regressors of the model. Applying this model and using perfusion as a surrogate for neural activity, the imaging data revealed an increase in the magnitude of perfusion in several task related brain areas in the 4 back minus 1 back contrast. This activation increase was observed in three different clusters, located in the frontal and occipital cortices (Table 1 and Figure 4). In order to investigate what drives this increase in perfusion, we inspected the beta values of our regression analysis as a function of group (Experimental vs Control) and test occasion (pre test vs post test). As visualized in Figure S1 in the supplementary online material (SOM), the increase in perfusion in right BA 6 and right BA 19 was driven by the experimental group. However, the effect in left BA 6 was only driven by a decrease of perfusion in the control group.
Table 1.
Significant perfusion clusters for the 4-back minus 1-back contrast, contrasting the post- and pre-measurement between the experimental and the control group (z > 2.8; cluster size >= 19).
| Region | Hemisphere | BA | Voxels | Z-Valuepeak | Xpeak | Ypeak | Zpeak | |
|---|---|---|---|---|---|---|---|---|
| Frontal | POST | right | 6 | 45 | 3.53 | 66 | −6 | 39 |
| F1 | left | 6 | 35 | 3.18 | −24 | −3 | 60 | |
| Occipital | O1, O2 | right | 19 | 73 | 3.40 | 33 | −93 | 30 |
Note. BA = Brodmann Area; Xpeak, Ypeak, and Zpeak represent the MNI coordinates with the highest z-values; Z-Valuepeak represents the highest z value in the corresponding cluster. F1 = superior frontal gyrus; O1 = superior occipital gyrus; O2 = middle occipital gyrus; POST = postcentral gyrus.
Figure 4.
Task related perfusion in the 4 back minus 1 back contrast. The numbers next to each slice represent the z coordinates in MNI space. This figure corresponds to the data reported in Table 1.
Our next analyses addressed perfusion changes at rest. We observed mainly perfusion increases after training, again in frontal, but also in parietal brain areas. There was one cluster in the right parietal cortex that showed a decrease in the magnitude of perfusion (Table 2 and Figure 5). A similar inspection of the beta values as in the 4 back minus 1 back contrast revealed that the effects were driven by the experimental group (Figure S2 in the SOM).
Table 2.
Significant perfusion clusters at rest, contrasting the post- and pre-measurement between the experimental and the control group (z > 2.8; cluster size >= 19).
| Region | Hemisphere | BA | Voxels | Z-Valuepeak | Xpeak | Ypeak | Zpeak | |
|---|---|---|---|---|---|---|---|---|
| Frontal | PRE | left | 6 | 20 | 3.23 | −42 | −6 | 48 |
| Parietal | AG | left | 39 | 74 | 3.54 | −54 | −60 | 24 |
| POST | right | 5 | 24 | −3.67 | 27 | −45 | 69 |
Note. BA = Brodmann Area; Xpeak, Ypeak, and Zpeak represent the MNI coordinates with the highest z-values; Z-Valuepeak represents the highest z value in the corresponding cluster. AG = angular gyrus; POST= postcentral gyrus; PRE = precentral gyrus.
Figure 5.
Perfusion at rest. The numbers next to each slice represent the z coordinates in MNI space. This figure corresponds to the data reported in Table 2.
In Figures S3 S6 in the SOM we provide activation maps of the 4 back minus 1 back contrast, before and after the intervention, separately for both groups. Note that these figures are for illustration purposes only since the effects of training are not evident from these maps by eye inspection.
A correlational analysis of 4 back task performance and perfusion at rest revealed positive correlations within lateral frontal brain areas, demonstrating that higher task proficiency corresponds to increased resting perfusion in frontal brain regions (Table 3). In addition, there was one parietal cluster that correlated negatively with 4 back performance.
Table 3.
Brain areas that show significant correlations between 4-back performance and perfusion at rest (z > 2.8; cluster size >= 19). A positive peak Z-Value indicates a positive correlation, a negative value indicates a negative correlation.
| Region | Hemisphere | BA | Voxels | Z-Valuepeak | Xpeak | Ypeak | Zpeak | |
|---|---|---|---|---|---|---|---|---|
| Frontal | F2, PRE, F3OP, F3T | left | 8, 9 | 71 | 3.55 | −48 | 18 | 42 |
| F1, SMA, medFG | right | 6, 8 | 41 | 3.44 | 12 | 24 | 60 | |
| F2 | left | 6 | 21 | 3.06 | −27 | 6 | 60 | |
| Parietal | RO, PRE, POST, SMG | right | 6, 43 | 118 | −3.67 | 54 | −9 | 18 |
| PRE, POST | left | 4, 6 | 33 | 3.78 | −51 | −3 | 51 |
Note. BA = Brodmann Area; Xpeak, Ypeak, and Zpeak represent the MNI coordinates with the highest z-values; Z-Valuepeak represents the highest z value in the corresponding cluster. F1 = superior frontal gyrus; F1M = superior frontal gyrus, medial; F2 = frontal middle gyrus; F3OP = inferior frontal gyrus, percular part; F3T = inferior frontal gyrus, triangular part; POST = postcentral gyrus; PRE = precentral gyrus; RO = rolandic operculum; SMG = supramarginal gyrus.
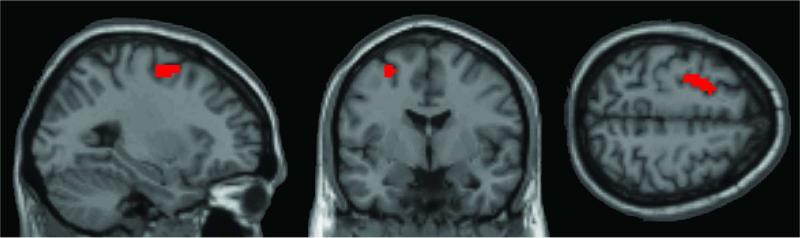
Finally, we ran a conjunction analysis to determine brain regions that showed an increased perfusion during task performance (4 back minus 1 back contrast) after training contrasting the two groups, and the brain regions that showed increased perfusion during rest after training contrasting the two groups. A statistical threshold of z = 2.3 was used for both maps, followed by a logical AND conjunction of the two maps resulting in a conjoint threshold of approximately z = 3.1. Setting a minimal cluster size of 19 contiguous voxels, the resulting map revealed one common brain area, located in the left precentral gyrus/frontal middle gyrus/superior frontal gyrus (BA 6; 42 voxels; approximate center of mass in MNI coordinates: 25, 3, 58; Figure 6).
Figure 6.
Brain region that showed an increased perfusion during task performance after training and an increased perfusion during rest.
Discussion
The behavioral data revealed that the experimental group substantially improved its performance in the trained task. As expected, compared to the control group, the experimental group showed significant performance increases in the 4 back condition which was correlated with improvements during training. That is, all participants initially struggled in the 4 back condition, but the experimental group became quite proficient after seven days of training, although they were not yet at ceiling. In contrast, there were no measurable group differences or improvements in the 1 back condition due to the fact that all participants already excelled at it at pre test, and therefore, there was not enough room for improvement. These results confirm our hypothesized behavioral effects.
The data of the auditory n back task that we used to assess cross modal transfer demonstrated a significant interaction across all n back levels (2 back, 3 back, and 4 back) in favor of the experimental group. The increased improvement from pre to post test was mainly driven by the 3 back condition and to a lesser degree by the 2 back and 4 back conditions. This finding is also reflected by the observation that the highest correlation between the training gain in the visuospatial n back and the three different n back levels of the auditory n back task was observed in the 3 back condition. It seems that the difficulty of the auditory 3 back condition was ideal to document the cross modal transfer. In contrast, the 2 back condition was fairly easy, even for an untrained participant, especially after having done quite a few n back trials in the practice and scanning session. On the other hand, in the auditory 4 back condition, there was more error variance due to the difficulty of the task, and even though the n back training group numerically outperformed the control group at the 4 back level, the interaction was not significant.
The cross modal transfer is in line with our earlier finding that training on an n back task with one set of stimuli transfers to an n back task with different stimulus material (Jaeggi, Studer Luethi, et al., 2010). However, in this earlier study, both sets of stimuli were in the same modality (visuospatial locations and random shapes). Another study by Schneiders et al. (2012) investigated transfer from an auditory n back task to a visual one, but in contrast to the present data, there was no significant transfer across modalities. Schneiders et al. suggested that the specific three tone melodies that they used as stimuli might have prevented cross modal transfer. In particular, Schneiders et al. point out that the three tone stimuli were specifically distinctive and allowed participants to recode them semantically, a strategy that was not easily applicable in their visual task (random black and white patterns). In our tasks, participants might have been able to rely on similar (e.g. verbally oriented) processes in both tasks, and thus, transfer across modalities was successful. This notion is further supported by the fact that these particular n back task versions are well correlated (r=.56, p<.001, data from pre test only), suggesting shared processes which could facilitate transfer. However, it remains an open question whether one would find cross modal transfer if participants trained on the auditory n back task as used in the current study and were then tested on a visuospatial n back task variant. Nonetheless, given the correlations between the two variants, it is likely that transfer would work both ways (Jaeggi, Buschkuehl, et al., 2010; cf. Jaeggi, Studer Luethi, et al., 2010).
We set the stage for our imaging analysis by the significant behavioral group differences in the scanned n back task. Our imaging data revealed two main effects of training. First, following the intervention, participants showed more neural activation in order to perform the n back task at a 4 back level. The most pronounced perfusion increases were observed in right BA 6 and in one cluster in BA 19. In addition we also found an increase in perfusion in left BA 6, however, this effect was driven by a decrease of perfusion in the control group. This is in line with what we have predicted based on previous research: an increase in activation if the training duration is relatively short and if the difficulty level of the scanned task is relatively high and therefore requires executive control. Such a pattern has been previously demonstrated by Schweizer et al. (2013) who reported an increase in activation after participants were tested on a 5 back level following 4 weeks of training. Thus, even though the experimental group significantly improved performance in the 4 back task, they did not perform at ceiling, and they still required considerable mental effort to perform the task rather than relying on automatic processing. Our data together with the data of Schweizer et al. and Schneiders et al. therefore suggest that further training would result in a decrease of activation providing that participants are able to perform the task effortlessly and with a high level of accuracy. Such a pattern would be in line with the dual process theory of human performance (e.g. Chein & Schneider, 2005; Posner & Snyder, 1975) which predicts that training on a task initially requires more effort and controlled resources, and that prolonged training subsequently leads to more automatic and less effortful task processing. This predicted increased neural efficiency might be the result of enhanced interactions between brain regions leading to faster neural processing (Jonides, 2004) and to a shift from relatively effortful to relatively automatic processing as a consequence of the adaptive training regimen (Rypma et al., 2006). This is a hypothesis that is in line with the study of Hempel et al. (2004) that found such an inverted u shaped activation pattern as a function of training time and proficiency.
In general, the activation pattern present in the available literature that investigated neural changes following n back training is remarkably similar to certain aspects of the Compensation-Related Utilization of Neural Circuits Hypothesis (CRUNCH) that originated from work with older adults (Reuter Lorenz & Cappell, 2008). CRUNCH posits an activation increase (or over activation in older adults) in prefrontal brain areas to compensate for neurophysiological challenge due to conditions that compromise neural efficiency (Reuter Lorenz & Cappell, 2008; Rypma et al., 2006). CRUNCH further predicts that effective cognitive training makes relevant brain circuits more efficient and lowers activation so that the activation peak is associated with higher load levels, which is essentially what the available n back training literature confirms in younger adults.
Our analysis of task related activation changes revealed increases in right BA 6 and right BA 19. The effects in BA 6 are in line with previous findings that also reported training related activation changes in this area (Schneiders et al., 2012, 2011; Schweizer et al., 2013) and the observation that BA 6 is commonly activated when increasing load is placed on the WM system (de Fockert, Rees, Frith, & Lavie, 2001; Marvel & Desmond, 2010; Smith & Jonides, 1997). The training induced perfusion changes in BA 6 are well in line with the nature of the training task that requires processes that are usually mediated by these brain areas. Similarly, BA 19 is often activated during visual imagery and visual storage which fits well with the visuospatial nature of our training task in which it is a useful strategy to mentally predict upcoming target locations (Cavanna & Trimble, 2006; Lamm, Windischberger, Leodolter, Moser, & Bauer, 2001; Wager & Smith, 2003).
In addition to task related perfusion changes, we also investigated perfusion changes at rest which are prevalent in frontal brain areas (left BA 6) and also parietal regions (right BA 5 and left BA 39). BA 6 and BA 39 are regions that are often associated with aspects of executive control (Wager & Smith, 2003), and we found increases in perfusion in both of these regions. Contrary to our expectations, we found a reduction in perfusion at rest in right BA 5, which is part of association cortex and often associated with finger and hand movements (Premji, Rai, & Nelson, 2011). Nonetheless, activation changes in this region have also been observed as a function of n back training (Schneiders et al., 2012). Related to this, left BA 5 has been found to be active during a complex version of an n back task (Yoo, Paralkar, & Panych, 2004). Although there is some precedence suggesting that BA 5 plays a role in WM training and n back task performance, it is certainly not one of the prominent brain areas associated with training and proficiency. Therefore, further research is needed to determine its exact role in training on the n back task. It is important to note that perfusion changes at rest were normalized to the global baseline CBF at the group level, and so the changes are relative or regional CBF changes. Therefore, the perfusion changes at rest could reflect either an absolute increase or a redirection of resources.
The second main effect in our imaging results is that blood flow at rest in the trained brain regions increases along with task proficiency, probably reflecting an improved neural readiness to perform. These predominantly positive correlations were present in five brain areas (covering BA 4, BA 6, BA 8, BA 9, and BA 43) and most of these areas are typically considered part of the WM network (Wager & Smith, 2003). A conjunction analysis of brain areas that showed an increase in perfusion at rest with those that showed an increase during task activity revealed left BA 6 as a common brain area. This common region is also very close to an area in which we observed a positive correlation between perfusion at rest and 4 back performance. This overlap lets us speculate that there is coherence between the two neural effects of training, and it is conceivable that improved perfusion at rest could ultimately lead to more efficient task processing. However, as pointed out before, the perfusion increase in the 4 back minus 1 back condition was driven by the control group and not the experimental group (Figure S1, left panel). Therefore, our speculation should be viewed very cautiously.
A potential caveat of our study design is that due to the fact that we used a fixed block order, the effects related to perfusion at rest could potentially reflect task related effects that were carried over from 1 back or 4 back blocks that preceded a rest block. In order to test for this possibility, we assumed that a potential carry over effect is stronger in the first half of the rest period compared to the second half. Therefore, we contrasted the first 15s of all rest blocks with the second 15s of all rest blocks across both test occasions. Our analysis did not reveal a significant difference in any of the brain areas in which we found a training related effect in the current study, suggesting that carry over effects did not influence our findings. Nevertheless, we suggest caution in completely ruling out the occurrence of potential carry over effects.
Previous research has shown that similar interventions as the one used in the present study resulted in improvements in non trained reasoning tasks (Colom et al., 2013; Jaeggi et al., 2008, 2011, 2013; Jaeggi, Studer Luethi, et al., 2010; Jaušovec & Jaušovec, 2012; Rudebeck et al., 2012; Schweizer et al., 2011; Stephenson & Halpern, 2013). One model that might account for this result is that trained and untrained tasks activate overlapping brain regions that implement overlapping psychological processes. The training, then, would exercise and train these processes, and this training would transfer to the implementation of those same processes in the untrained task (Dahlin et al., 2008). This may be the case for the present n back task and for reasoning tasks to which it shows positive transfer. Brain imaging research on matrix reasoning performance has revealed activations in areas that are very similar to the areas uncovered by our conjunction analysis (e.g. Jung & Haier, 2007; Perfetti et al., 2009). Therefore, our results provide an excellent rationale for generalized cognitive improvement in visuospatial reasoning following WM training (Jaeggi et al., 2008, 2011, 2013; Jaeggi, Studer Luethi, et al., 2010).
To conclude, our results reveal that training WM not only improves task relevant skills that extend into non trained modalities, but that there are also accompanying neural effects reflected by perfusion increases during task performance and during rest. These data suggest that the brain changes and becomes more physically fit as a function of training; a mental conditioning effect, which could be one of the prerequisites for transfer.
We note at this point the connection between the research presented in this paper and Ed Smith, whose work this issue commemorates. An anecdote makes this connection perhaps better than anything else. One of us (JJ) had lunch with Ed at a west side restaurant one sunny day in New York in 2007. A napkin served as the blackboard of choice that day, and we laid out on that napkin the finding that WM training had an effect on improving fluid intelligence, as measured by two matrix reasoning tasks. Ed at that time was an editor at PNAS and invited us to submit our work on this phenomenon to the journal through him as editor. He then led the paper through the review process and to eventual publication. This was routine. What was not routine was Ed's spark of recognition of the potential importance of our finding at that lunch. Hardly had the transfer effect been sketched on the napkin when Ed saw the reach and the novelty of this finding. Of this he was a master: recognizing the importance of phenomena and their implications. That quality infused his work and his collaborations with others (JJ included), and he bettered all the work that he touched because of his superb taste. This early work of ours has led to much additional behavioural work and to the current investigation of the neural underpinnings of training. We suspect that Ed would be pleased to see the outcome and the fruit of his editorial adventure when the research program was yet in its infancy.
Supplementary Material
Footnotes
We note at this point, and elaborate below, that Professor Edward Smith devoted a good deal of his career to the study of working memory precisely because he viewed it as a cornerstone for cognitive processing, as do we. Ed saw that the architecture of working memory was central to the architecture of many higher cognitive skills. In that the lion’s share of Ed's scholarly work was devoted to a variety of higher cognitive functions, his investment in understanding the processes that feed into these functions was both considerable and impactful.
Due to significant baseline differences, this finding remains unfortunately inconclusive.
With a few exceptions and due to logistical reasons, participants were first assigned to the experimental group and then to the control group. That is, we first collected data for the experimental group, and later, we collected the data for the control group. However, when recruiting the control group we tried to match participants to the experimental group as closely as possible on age and gender.
This regressor accounted for the fact that most of the control group's data were collected after the experimental group.
Note that we discarded the behavioural data of one participant in the control group because the participant pressed the wrong response buttons starting mid way through the experiment.
Note that six participants of the experimental group did not complete this task.
Note that the significance level remains the same if an ANCOVA is used to control for numerical pre-test differences.
References
- Aguirre GK, Detre JA, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. NeuroImage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. doi:10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Bernard JA, Jaeggi SM, Buschkuehl M, Benson BL, Jennett S,, Seidler RD. The effects of working-memory resource depletion and training on sensorimotor adaptation. Behavioural Brain Research. 2012;228(1):107–115. doi: 10.1016/j.bbr.2011.11.040. doi:10.1016/j.bbr.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Rinne JO. Effects of working memory training on striatal dopamine release. Science. 2011;333(6043):718. doi: 10.1126/science.1204978. doi:10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. doi:10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Jaeggi SM, Buschkuehl M, Hernandez-Garcia L, Jonides J. Working Memory Training Gains Mitigate TMS-Induced Working Memory Performance Disruption. in preparation. [Google Scholar]
- Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Developmental Cognitive Neuroscience. 2012;2(Supplement 1):S167–S179. doi: 10.1016/j.dcn.2011.10.001. doi:10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. doi:10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chein JM, Morrison AB. Expanding the mind's workspace: training and transfer effects with a complex working memory span task. Psychonomic Bulletin & Review. 2010;17(2):193–199. doi: 10.3758/PBR.17.2.193. doi:10.3758/PBR.17.2.193. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Research. Cognitive Brain Research. 2005;25(3):607–623. doi: 10.1016/j.cogbrainres.2005.08.013. doi:10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Colom R, Román FJ, Abad FJ, Shih PC, Privado J, Froufe M, Jaeggi SM. Adaptive n-back training does not improve fluid intelligence at the construct level: Gains on individual tests suggest that training may enhance visuospatial processing. Intelligence. 2013;41(5):712–727. doi:10.1016/j.intell.2013.09.002. [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. doi:10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320(5882):1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2008;60(6):1488–1497. doi: 10.1002/mrm.21790. doi:10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- De Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. doi:10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magnetic Resonance in Medicine. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wüstenberg T, Schröder J. Plasticity of cortical activation related to working memory during training. The American Journal of Psychiatry. 2004;161(4):745–747. doi: 10.1176/appi.ajp.161.4.745. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia L, Buschkuehl M. Advances in Longitudinal MRI Diagnostic Tests. Expert Opinion on Medical Diagnostics. 2012;6(4):1–11. doi: 10.1517/17530059.2012.686995. doi:10.1517/17530059.2012.686995. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia L, Jahanian H, Rowe DB. Quantitative analysis of arterial spin labeling FMRI data using a general linear model. Magnetic Resonance Imaging. 2010;28(7):919–927. doi: 10.1016/j.mri.2010.03.035. doi:10.1016/j.mri.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Garcia L, Lewis DP, Moffat B, Branch CA. Magnetization transfer effects on the efficiency of flow-driven adiabatic fast passage inversion of arterial blood. NMR in Biomedicine. 2007;20(8):733–742. doi: 10.1002/nbm.1137. doi:10.1002/nbm.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6829–6833. doi: 10.1073/pnas.0801268105. doi:10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10081–10086. doi: 10.1073/pnas.1103228108. doi:10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18(4):394–412. doi: 10.1080/09658211003702171. doi:10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Shah P, Jonides J. The role of individual differences in cognitive training and transfer. Memory & Cognition. 2013 doi: 10.3758/s13421-013-0364-z. doi:10.3758/s13421-013-0364-z. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Studer-Luethi B, Buschkuehl M, Su Y-F, Jonides J, Perrig WJ. The relationship between n-back performance and matrix reasoning -- implications for training and transfer. Intelligence. 2010;38(6):625–635. doi:10.1016/j.intell.2010.09.001. [Google Scholar]
- Jahanian H, Noll DC, Hernandez-Garcia L. B0 field inhomogeneity considerations in pseudo-continuous arterial spin labeling (pCASL): effects on tagging efficiency and correction strategy. NMR in Biomedicine. 2011;24(10):1202–1209. doi: 10.1002/nbm.1675. doi:10.1002/nbm.1675. [DOI] [PubMed] [Google Scholar]
- Jaušovec N, Jaušovec K. Working memory training: improving intelligence--changing brain activity. Brain and Cognition. 2012;79(2):96–106. doi: 10.1016/j.bandc.2012.02.007. doi:10.1016/j.bandc.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Grol MJ, Van Buchem MA, Rombouts SARB, Crone EA. Practice effects in the brain: Changes in cerebral activation after working memory practice depend on task demands. NeuroImage. 2010;52(2):658–668. doi: 10.1016/j.neuroimage.2010.04.028. doi:10.1016/j.neuroimage.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Jonides J. How does practice makes perfect? Nature Neuroscience. 2004;7(1):10–11. doi: 10.1038/nn0104-10. doi:10.1038/nn0104-10. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig C, Berman MG, Moore KS. The mind and brain of short-term memory. Annual Review of Psychology. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. doi:10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. The Behavioral and Brain Sciences. 2007;30(2):135–154. doi: 10.1017/S0140525X07001185. discussion 154–187. doi:10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Archives of Physical Medicine and Rehabilitation. 2006;87(12 Suppl 2):S20–29. doi: 10.1016/j.apmr.2006.08.333. doi:10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- Kundu B, Sutterer DW, Emrich SM, Postle BR. Strengthened effective connectivity underlies transfer of working memory training to tests of short-term memory and attention. The Journal of Neuroscience. 2013;33(20):8705–8715. doi: 10.1523/JNEUROSCI.5565-12.2013. doi:10.1523/JNEUROSCI.5565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Windischberger C, Leodolter U, Moser E, Bauer H. Evidence for premotor cortex activity during dynamic visuospatial imagery from single-trial functional magnetic resonance imaging and event-related slow cortical potentials. NeuroImage. 2001;14(2):268–283. doi: 10.1006/nimg.2001.0850. doi:10.1006/nimg.2001.0850. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(7):880–895. doi: 10.1016/j.cortex.2009.08.017. doi:10.1016/j.cortex.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. doi:10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Frontiers in Human Neuroscience. 2010;4:1–10. doi: 10.3389/neuro.09.016.2010. doi:10.3389/neuro.09.016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. doi:10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7(1):75–79. doi: 10.1038/nn1165. doi:10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. doi:10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Koster EHW, Derakshan N. Improving attention control in dysphoria through cognitive training: transfer effects on working memory capacity and filtering efficiency. Psychophysiology. 2013;50(3):297–307. doi: 10.1111/psyp.12010. doi:10.1111/psyp.12010. [DOI] [PubMed] [Google Scholar]
- Perfetti B, Saggino A, Ferretti A, Caulo M, Romani GL, Onofrj M. Differential patterns of cortical activation as a function of fluid reasoning complexity. Human Brain Mapping. 2009;30(2):497–510. doi: 10.1002/hbm.20519. doi:10.1002/hbm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Larsson A, Reuter-Lorenz PA. Imaging fatigue of interference control reveals the neural basis of executive resource depletion. Journal of Cognitive Neuroscience. 2013;25(3):338–351. doi: 10.1162/jocn_a_00321. doi:10.1162/jocn_a_00321. [DOI] [PubMed] [Google Scholar]
- Pickering SJ. Working memory and education. Academic Press; 2006. [Google Scholar]
- Posner MI, Snyder CRR. Attention and cognitive control. In Information processing and cognition: The Loyola symposium. Erlbaum; Hillsdale, NJ: 1975. pp. 55–85. [Google Scholar]
- Premji A, Rai N, Nelson A. Area 5 Influences Excitability within the Primary Motor Cortex in Humans. PLoS ONE. 2011;6(5):e20023. doi: 10.1371/journal.pone.0020023. doi:10.1371/journal.pone.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Current Directions in Psychological Science. 2008;17(3):177–182. doi:10.1111/j.1467-8721.2008.00570.x. [Google Scholar]
- Rudebeck SR, Bor D, Ormond A, O'Reilly JX, Lee ACH. A potential spatial working memory training task to improve both episodic memory and fluid intelligence. PloS One. 2012;7(11):e50431. doi: 10.1371/journal.pone.0050431. doi:10.1371/journal.pone.0050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D'Esposito M. Neural correlates of cognitive efficiency. NeuroImage. 2006;33(3):969–979. doi: 10.1016/j.neuroimage.2006.05.065. doi:10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Schneiders JA, Opitz B, Krick CM, Mecklinger A. Separating Intra-Modal and Across-Modal Training Effects in Visual Working Memory: An fMRI Investigation. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr037. doi:10.1093/cercor/bhr037. [DOI] [PubMed] [Google Scholar]
- Schneiders JA, Opitz B, Tang H, Deng Y, Xie C, Li H, Mecklinger A. The impact of auditory working memory training on the fronto-parietal working memory network. Frontiers in Human Neuroscience. 2012;6:173. doi: 10.3389/fnhum.2012.00173. doi:10.3389/fnhum.2012.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. Training the emotional brain: improving affective control through emotional working memory training. The Journal of Neuroscience. 2013;33(12):5301–5311. doi: 10.1523/JNEUROSCI.2593-12.2013. doi:10.1523/JNEUROSCI.2593 12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Hampshire A, Dalgleish T. Extending brain-training to the affective domain: increasing cognitive and affective executive control through emotional working memory training. PloS One. 2011;6(9):e24372. doi: 10.1371/journal.pone.0024372. doi:10.1371/journal.pone.0024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cognitive Psychology. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. doi:10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Stephenson CL, Halpern DF. Improved Matrix Reasoning is Limited to Improving Working Memory Capacity Using Intensive N-back Tasks with a Visuospatial Component. Intelligence. 2013;41:341–357. doi:10.1016/j.intell.2013.05.006. [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Kawashima R. Training of Working Memory Impacts Structural Connectivity. The Journal of Neuroscience. 2010;30(9):3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. doi:10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Hashizume H, Sekiguchi A, Kotozaki Y, Kawashima R. Effects of working memory training on functional connectivity and cerebral blood flow during rest. Cortex. 2012 doi: 10.1016/j.cortex.2012.09.007. doi:10.1016/j.cortex.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Working Memory Training Using Mental Calculation Impacts Regional Gray Matter of the Frontal and Parietal Regions. PLoS ONE. 2011;6(8):e23175. doi: 10.1371/journal.pone.0023175. doi:10.1371/journal.pone.0023175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandra T, Brooks JCW, Figueiredo P, Wise R, Matthews PM, Tracey I. Quantitative assessment of the reproducibility of functional activation measured with BOLD and MR perfusion imaging: implications for clinical trial design. NeuroImage. 2005;27(2):393–401. doi: 10.1016/j.neuroimage.2005.04.021. doi:10.1016/j.neuroimage.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Detre JA. Empirical analyses of null-hypothesis perfusion FMRI data at 1.5 and 4 T. NeuroImage. 2003;19(4):1449–1462. doi: 10.1016/s1053-8119(03)00255-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine. 2003;49(5):796–802. doi: 10.1002/mrm.10437. doi:10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Östensson M-L, Bartfai A, Klingberg T. Computerized working memory training after stroke -- A pilot study. Brain Injury. 2007;21(1):21–29. doi: 10.1080/02699050601148726. doi:10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proceedings of the National Academy of Sciences. 1992;89(1):212. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-S, Paralkar G, Panych LP. Neural substrates associated with the concurrent performance of dual working memory tasks. The International Journal of Neuroscience. 2004;114(6):613–631. doi: 10.1080/00207450490430561. doi:10.1080/00207450490430561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.