Abstract
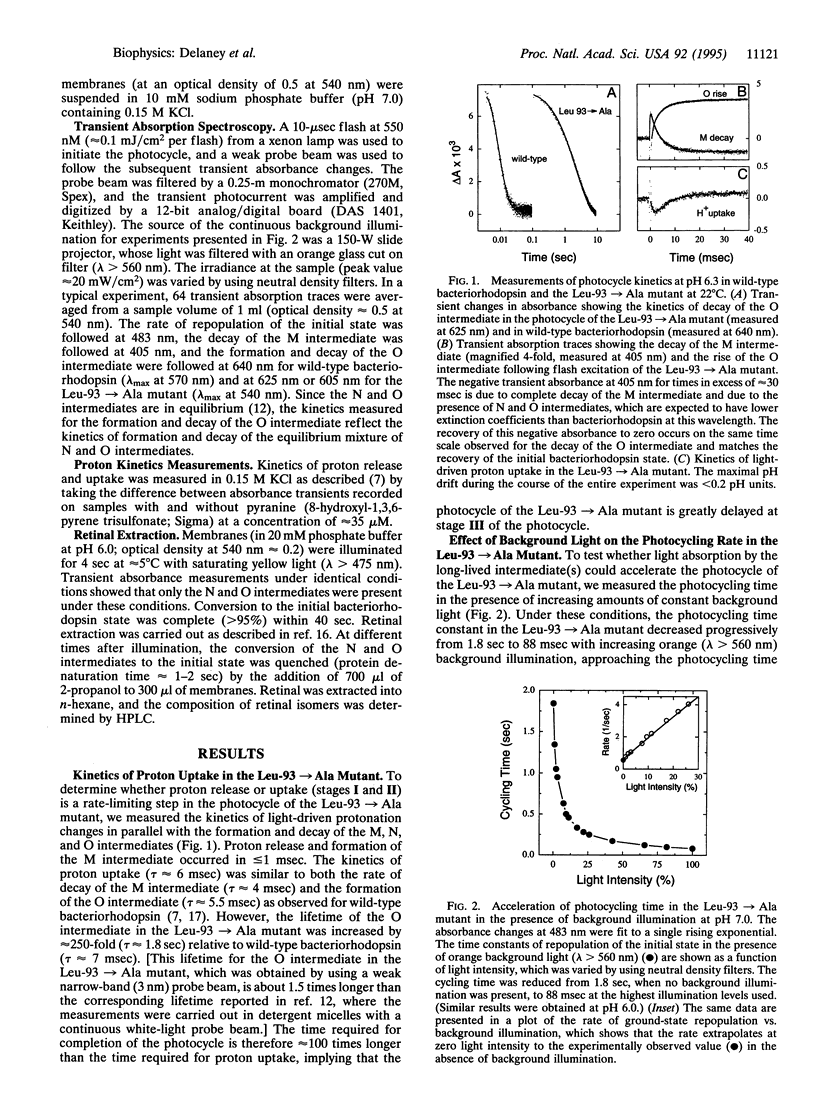
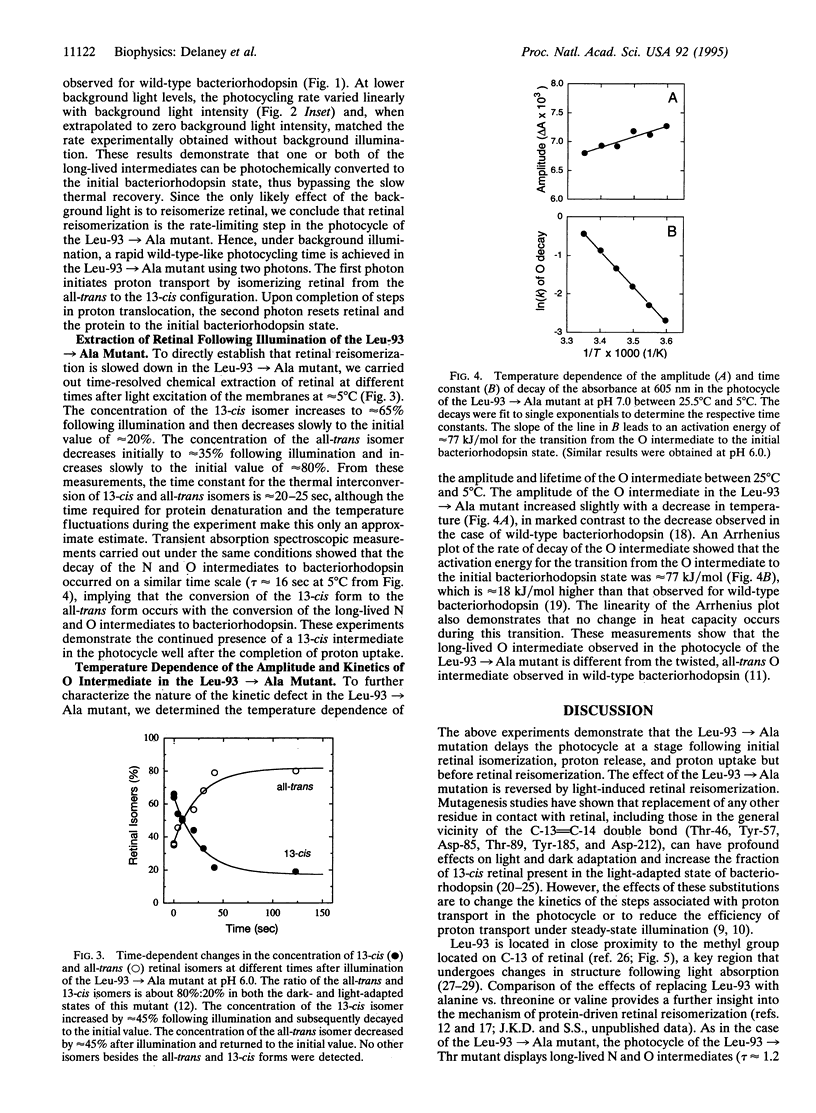
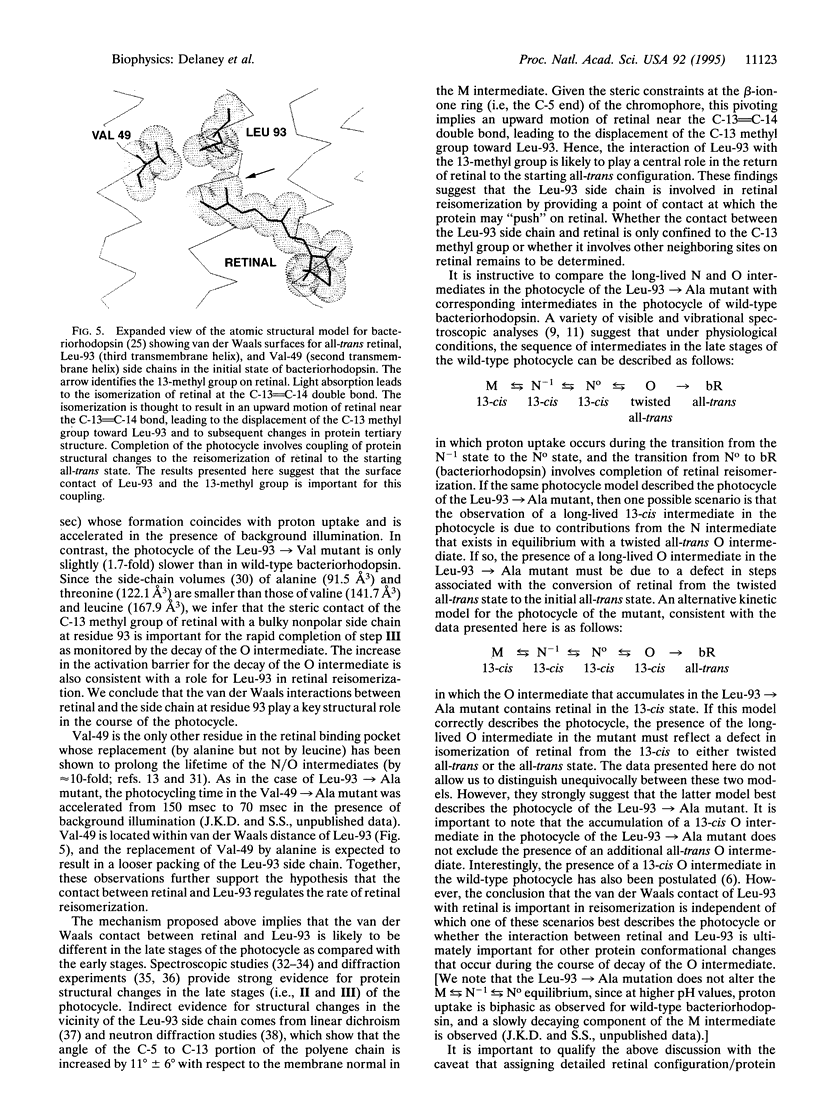
Bacteriorhodopsin is a membrane protein that functions as a light-driven proton pump. Each cycle of proton transport is initiated by the light-induced isomerization of retinal from the all-trans to 13-cis configuration and is completed by the protein-driven reisomerization of retinal to the all-trans configuration. Previous studies have shown that replacement of Leu-93, a residue in close proximity to the 13-methyl group of retinal, by alanine, resulted in a 250-fold increase in the time required to complete each photocycle. Here, we show that the kinetic defect in the photocycle of the Leu-93-->Ala mutant occurs at a stage after the completion of proton transport and can be overcome in the presence of strong background illumination. Time-resolved retinal-extraction experiments demonstrate the continued presence of a 13-cis intermediate in the photocycle of the Leu-93-->Ala mutant well after the completion of proton release and uptake. These results indicate that retinal reisomerization is kinetically the rate-limiting step in the photocycle of this mutant and that the slow thermal reisomerization can be bypassed by the absorption of a second photon. The effects observed for the Leu-93-->Ala mutant are not observed upon replacement of any other residue in van der Waals contact with retinal or upon replacement of Leu-93 by valine. We conclude that the contact between Leu-93 and the 13-methyl group of retinal plays a key role in controlling the rate of protein conformational changes associated with retinal reisomerization and return of the protein to the initial state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balashov S. P., Govindjee R., Kono M., Imasheva E., Lukashev E., Ebrey T. G., Crouch R. K., Menick D. R., Feng Y. Effect of the arginine-82 to alanine mutation in bacteriorhodopsin on dark adaptation, proton release, and the photochemical cycle. Biochemistry. 1993 Oct 5;32(39):10331–10343. doi: 10.1021/bi00090a008. [DOI] [PubMed] [Google Scholar]
- Brown L. S., Gat Y., Sheves M., Yamazaki Y., Maeda A., Needleman R., Lanyi J. K. The retinal Schiff base-counterion complex of bacteriorhodopsin: changed geometry during the photocycle is a cause of proton transfer to aspartate 85. Biochemistry. 1994 Oct 11;33(40):12001–12011. doi: 10.1021/bi00206a001. [DOI] [PubMed] [Google Scholar]
- Cao Y., Brown L. S., Needleman R., Lanyi J. K. Relationship of proton uptake on the cytoplasmic surface and reisomerization of the retinal in the bacteriorhodopsin photocycle: an attempt to understand the complex kinetics of the pH changes and the N and O intermediates. Biochemistry. 1993 Sep 28;32(38):10239–10248. doi: 10.1021/bi00089a046. [DOI] [PubMed] [Google Scholar]
- Ferrando E., Schweiger U., Oesterhelt D. Homologous bacterio-opsin-encoding gene expression via site-specific vector integration. Gene. 1993 Mar 15;125(1):41–47. doi: 10.1016/0378-1119(93)90743-m. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Ganter U. M., Schmid E. D., Perez-Sala D., Rando R. R., Siebert F. Removal of the 9-methyl group of retinal inhibits signal transduction in the visual process. A Fourier transform infrared and biochemical investigation. Biochemistry. 1989 Jul 11;28(14):5954–5962. doi: 10.1021/bi00440a036. [DOI] [PubMed] [Google Scholar]
- Govindjec R., Kono M., Balashov S. P., Imasheva E., Sheves M., Ebrey T. G. Effects of substitution of tyrosine 57 with asparagine and phenylalanine on the properties of bacteriorhodopsin. Biochemistry. 1995 Apr 11;34(14):4828–4838. doi: 10.1021/bi00014a040. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Farrens D. L., Subramaniam S., Khorana H. G. Hydrophobic amino acids in the retinal-binding pocket of bacteriorhodopsin. J Biol Chem. 1993 Sep 25;268(27):20305–20311. [PubMed] [Google Scholar]
- Hauss T., Büldt G., Heyn M. P., Dencher N. A. Light-induced isomerization causes an increase in the chromophore tilt in the M intermediate of bacteriorhodopsin: a neutron diffraction study. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11854–11858. doi: 10.1073/pnas.91.25.11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M. P., Khorana H. G. Mechanism of light-dependent proton translocation by bacteriorhodopsin. J Bacteriol. 1993 Mar;175(6):1555–1560. doi: 10.1128/jb.175.6.1555-1560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti T., Otto H., Mogi T., Rösselet S. J., Heyn M. P., Khorana H. G. Bacteriorhodopsin mutants containing single substitutions of serine or threonine residues are all active in proton translocation. J Biol Chem. 1991 Apr 15;266(11):6919–6927. [PubMed] [Google Scholar]
- Needleman R., Chang M., Ni B., Váró G., Fornés J., White S. H., Lanyi J. K. Properties of Asp212----Asn bacteriorhodopsin suggest that Asp212 and Asp85 both participate in a counterion and proton acceptor complex near the Schiff base. J Biol Chem. 1991 Jun 25;266(18):11478–11484. [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., He Y. W., Sonar S., Marti T., Khorana H. G. Vibrational spectroscopy of bacteriorhodopsin mutants. Evidence that Thr-46 and Thr-89 form part of a transient network of hydrogen bonds. J Biol Chem. 1992 Jan 25;267(3):1615–1622. [PubMed] [Google Scholar]
- Rothschild K. J., Marti T., Sonar S., He Y. W., Rath P., Fischer W., Khorana H. G. Asp96 deprotonation and transmembrane alpha-helical structural changes in bacteriorhodopsin. J Biol Chem. 1993 Dec 25;268(36):27046–27052. [PubMed] [Google Scholar]
- Sasaki J., Shichida Y., Lanyi J. K., Maeda A. Protein changes associated with reprotonation of the Schiff base in the photocycle of Asp96-->Asn bacteriorhodopsin. The MN intermediate with unprotonated Schiff base but N-like protein structure. J Biol Chem. 1992 Oct 15;267(29):20782–20786. [PubMed] [Google Scholar]
- Scherrer P., Mathew M. K., Sperling W., Stoeckenius W. Retinal isomer ratio in dark-adapted purple membrane and bacteriorhodopsin monomers. Biochemistry. 1989 Jan 24;28(2):829–834. doi: 10.1021/bi00428a063. [DOI] [PubMed] [Google Scholar]
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Sonar S., Krebs M. P., Khorana H. G., Rothschild K. J. Static and time-resolved absorption spectroscopy of the bacteriorhodopsin mutant Tyr-185-->Phe: evidence for an equilibrium between bR570 and an O-like species. Biochemistry. 1993 Mar 9;32(9):2263–2271. doi: 10.1021/bi00060a019. [DOI] [PubMed] [Google Scholar]
- Souvignier G., Gerwert K. Proton uptake mechanism of bacteriorhodopsin as determined by time-resolved stroboscopic-FTIR-spectroscopy. Biophys J. 1992 Nov;63(5):1393–1405. doi: 10.1016/S0006-3495(92)81722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff H. J., Mollaaghababa R., Altenbach C., Hideg K., Krebs M., Khorana H. G., Hubbell W. L. Time-resolved detection of structural changes during the photocycle of spin-labeled bacteriorhodopsin. Science. 1994 Oct 7;266(5182):105–107. doi: 10.1126/science.7939627. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Greenhalgh D. A., Rath P., Rothschild K. J., Khorana H. G. Replacement of leucine-93 by alanine or threonine slows down the decay of the N and O intermediates in the photocycle of bacteriorhodopsin: implications for proton uptake and 13-cis-retinal----all-trans-retinal reisomerization. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6873–6877. doi: 10.1073/pnas.88.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Glaccum M., Nelson B., Ebrey T. G. Light isomerizes the chromophore of bacteriorhodopsin. Nature. 1980 Sep 25;287(5780):351–353. doi: 10.1038/287351a0. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Effects of the crystalline structure of purple membrane on the kinetics and energetics of the bacteriorhodopsin photocycle. Biochemistry. 1991 Jul 23;30(29):7165–7171. doi: 10.1021/bi00243a018. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Yan B., Nakanishi K., Spudich J. L. Mechanism of activation of sensory rhodopsin I: evidence for a steric trigger. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9412–9416. doi: 10.1073/pnas.88.21.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]



