Abstract
This study aimed to investigate the roles of bone marrow stromal cells (BMSCs) in promoting axonal regeneration after complete transection of spinal cord in adult rats. Transplantation was done 9 days after injury. Only a few BMSCs were detected at the injury site 8 weeks after transplantation, yet there was robust growth of axons. The scarcity of surviving BMSCs may attribute to the adverse conditions in their ambient environment. In this connection, the immediate accumulation of a large number of macrophages/reactive microglia following BMSCs transplantation and subsequent cavitation of tissues may be detrimental to their survival. An unexpected finding following BMSCs transplantation was the marked increase in the nestin, GFAP, NF200, olig 3 and CNP positive cells at the injury site. Immunoelectron microscopy showed CNP cells were oval or fibroblast-like and had multiple perineurial-like compartments with long extending filopodia. The spatial relationship between regenerating axons and CNP-positive cells was also confirmed by double immunofluorescence staining. Our results suggest that transplantation of BMSCs elicits the influx and survival of local cells including CNP positive cells and Schwann cells into injury site, which provide structural support for the axon regeneration and remyelination after spinal cord injury.
Keywords: Bone marrow stromal cells, transplantation, CNP cells, Schwann cells, axonal sprouting
Introduction
The failure of damaged nerve fibers to regenerate is an underlying cause of permanent disability experienced by patients after spinal cord injury (SCI). A number of potential approaches have been employed to enhance the axonal growth and optimize functional recovery. These approaches include the manipulation of neuroinhibitory environment of the spinal cord [1,2], transplantation of cells or tissues that support the axonal elongation [3-6], delivery of neurotrophic factors to promote axonal growth [7], and maximization of the regenerative potential of endogenous progenitor cells [8-10].
To date, different types of cells including Schwann cells [11], olfactory ensheathing cells (OECs) [12-14], neural stem cells (NSCs) [15,16], bone marrow stromal cells (BMSCs) [17,18], and embryonic stem cells (ESCs) [19] have been used to support the axonal growth. This has resulted in progressive functional recovery in animals with SCI, suggesting the great potential of cell transplantation in the therapy of SCI.
BMSCs, referring to as mesenchymal stem cells, are multipotent adult progenitor cells derived from the bone marrow, and have the capability to differentiate into other cell types such as osteoblasts, adipocytes, and chondrocytes [20]. They also have the potential to differentiate into neural cell lineages, such as neurons and astrocytes, both in vivo [21,22] and in vitro [23]. Furthermore, the secretion of growth factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and glial cell line-derived neurotrophic factors (GDNF) by these cells has been demonstrated both in vitro and in vivo [24,25].
Previous studies have indicated that transplanted BMSCs may “form guiding strands” for transected axons regeneration, reduce cavity formation, and promote functional recovery [21,26,27]. The fate of the transplanted BMSCs and their interaction with local cells have not been fully elucidated, although there is evidence showing that some of them may be integrated into the new environment and transdifferentiate into neurons [28] and glial cells [29]. Despite the voluminous studies, the sequential events especially at the cellular level at the lesioned site following the transplantation of BMSCs have remained elusive. By immunofluorescence staining and immunoelectron microscopy, this study aimed to investigate the fate of BMSCs and their role in promoting the axonal sprouting following transplantation into adult rats with complete transection of spinal cord.
Materials and methods
In the handling and care of all animals, the International Guiding Principles for Animals Research, as stipulated by the Council for International Organizations of Medical Sciences (CIOMS) and as adopted by the Laboratory Animal Centre, National University of Singapore, were followed. All efforts were made to minimize the number of rats used and their suffering.
Bone marrow stromal cells culture
BMSCs were separated from adult Wistar rats weighing 200-250 g. Rats were killed by an overdose anesthesia with 7% choral hydrate (500 mg/kg). Both femurs and tibias were collected and the bone marrow cavity was flushed with DMEM. The resultant medium was incubated in the presence of 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. After 24 h, non-adherent cells were removed by refreshing the medium. When the cell confluence reached about 100%, cells were passaged at a ratio of 1:3 following digestion with 0.25% trypsin/1 mM EDTA. After passaging for 4 times, the BMSCs were used for immunocytochemistry and transplantation.
Immunocytochemistry
BMSCs were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 30 min, and then washed three times with PBS (pH 7.4) containing 0.1% Triton X-100 (Sigma-Aldrich, MO, USA). After treatment in 1% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 1 h to block nonspecific antibody binding, BMSCs were incubated overnight at 4°C with corresponding antibodies. The secondary antibodies were Cy3-conjugated goat anti-rabbit, anti-mouse, and rabbit anti-goat IgG (1:200; Chemicon, USA). A mounting medium with DAPI (4’,6 diamidino-2-phenylindole Vector Laboratories, Burlingame, CA, USA) was used to counterstain the cell nuclei. In negative control group, the BMSCs were incubated in a buffer or normal buffered serum instead of the primary antibody.
Surgical procedures
Adult female Wistar rats weighing about 250 g were used as recipients in this study. Rats were intraperitoneally anaesthetized with 7% choral hydrate (350 mg/kg), and then the spinal cord was completely transected at T10 level according to previously reported [30,31]. The skin and muscles of the back were incised to expose the T10 vertebra. A T10 laminectomy was then done by using a microsurgery bone rongeur. With a spinal cord hook, the spinal cord was isolated and transected with a pair of microscissors, causing the two stumps completely separated and creating a 2-3 mm gap. A small piece of gelfoam was then inserted into the lesioned site for hemostasis. The gelfoam may also serve as a substratum for the infiltration of host-derived cells and as a new scaffold to support the survival of transplanted BMSCs at the lesioned site. After surgery, these rats were immediately kept under a heating lamp on the first day. Urine was evacuated thrice daily by direct compression of the lower abdominal wall until an autonomous bladder voidance reflex was present. To prevent the urinary tract infection, procaine penicillin G (20000 units, Sigma) was injected subcutaneously twice daily.
Preparation of cells and transplantation
In order to verify the survival and differentiation of transplanted BMSCs, BMSCs were labeled with 5(6)-carboxyfluorescein diacetate, succinimidyl ester (10 μM CFDA SE; Molecular Probes) because CFDA SE can be inherited by daughter cells after cell division but not be transferred to adjacent cells [32]. Following harvesting, BMSCs were incubated with 10 μM CFDA SE for 15 min, and resuspended in a fresh warm medium for 30 min, resulting in CFDA SE acetate hydrolysis. After trypan blue dye exclusion test, the cell density was adjusted to 50,000 viable cells/μl with culture medium for transplantation.
It has been reported that, when compared with immediate transplantation, delayed transplantation enhances the survival of transplanted cells and exerts a beneficial effect on the functional improvement [33]. In the light of this, all experimental rats were maintained for 9 days before BMSCs transplantation or vehicle treatment. Then, a total of 36 rats were re-anesthetized with 7% choral hydrate 350 mg/kg). The injured site of the spinal cord was re-exposed. After irrigating with normal saline, 10 μl of BMSCs suspension was delivered into the injured site; in addition, two 5 μl cells deposits were injected, respectively, into the spinal cord 1 mm cranial and caudal to the lesioned site using a 50-μl Hamilton syringe (n=20). In control group, animals received an injection of vehicle solution (n=16). The wound was closed, and animals were housed till sacrifice.
Tissue preparation and immunohistochemistry
Rats subjected to transplantation were allowed to survive for 2 (control: n=4; experiment: n=8), 4 (control: n=4; experiment: n=4) or 8 (control: n=8; experiment: n=12) weeks. At each time point, rats were deeply anaesthetized and perfused transcardially with Ringer’s solution, followed by fixation with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The T8 to T12 spinal cord was collected, post-fixed in 4% paraformaldehyde overnight, and then cryoprotected in 20% sucrose for 1 day. The spinal cord was then cryosectioned horizontally at a thickness of 20 mm, mounted onto gelatin-coated glass slides and stored at -20°C until use. The spinal cord sections were processed as described above, and then incubated with a panel of primary antibodies overnight at room temperature. After incubation, these sections were treated with Cy3-conjugated goat anti-rabbit, anti-mouse, or rabbit anti-goat IgG (1:200; Chemicon, USA) for 1 h in dark at room temperature. After washing, the sections were mounted using the mounting medium containing DAPI (4’,6 diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA, USA). In the negative control group, sections were incubated in a buffer or normal buffered serum instead of the primary antibody.
Cell quantification
Sections stained with NF200, GFAP, Olig3, Nestin and CNP were used for cell counting. Three sections from the injured spinal cord were used for cell counting in each rat at 8 weeks after transplantation. A total of three fields were randomly selected from each section. Cell counting was carried out under a microscope at the magnification of ×200. Student’s t-test was used to compare the cell counts between experiment group and control group. A value of P less than 0.05 was considered statistically significant.
Electron microscopy
Four rats receiving BMSCs transplantation were killed at 2 and 8 weeks, respectively; at each time point, two rats were used for routine electron microscopy and the others for immunoelectron microscopy. For routine electron microscopy, rats were killed by perfusion with a mixed aldehyde solution containing 2% paraformaldehyde and 3% glutaraldehyde in 0.1 M PB. For immunoelectron microscopy, rats were killed by perfusion with Ringer’s solution and then with 4% paraformaldehyde containing 0.1% glutaraldehyde in 0.1 M PB. The T8-T12 spinal cord was removed and 100-μm vibratome sections were obtained (transversely and horizontally). For immunoelectron microscopy, sections were then incubated overnight at room temperature with mouse anti-CNP (dilution 1:200; Chemicon, USA). The treatment with secondary antibodies was done with anti-mouse ABC kit (Vector Laboratories, Burlingame, CA). The peroxidase reaction was visualized using the 3, 3’- diaminobenzidine (DAB) containing 0.1% nickel as a peroxidase substrate. Post-fixation was performed in 2% osmium tetroxide. Tissue blocks were embedded in araldite mixture. Ultrathin sections were double stained in uranyl acetate and lead citrate or lead citrate alone and observed under a CM120-biotwin or EM208S electron microscope.
Double immunofluorescence staining
In order to clarify the relationship between cells and regenerating fibres, double immunofluorescence staining was carried out with primary antibodies. Secondary antibodies used were FITC-conjugated goat anti-rabbit and Cy3-conjugated goat anti-mouse antibodies. The procedures for immunofluorescence staining were those described above.
Result
Characterization of MSCs in vitro
BMSCs were subjected to immunocytochemistry after passaging 4 times. Virtually, all of these cells expressed vimentin, fibronectin and laminin, markers of mesenchymal cells (Figure 1A-C). A subpopulation of BMSCs expressed nestin, a marker widely used for proliferating neural progenitor cells (Figure 1D).
Figure 1.
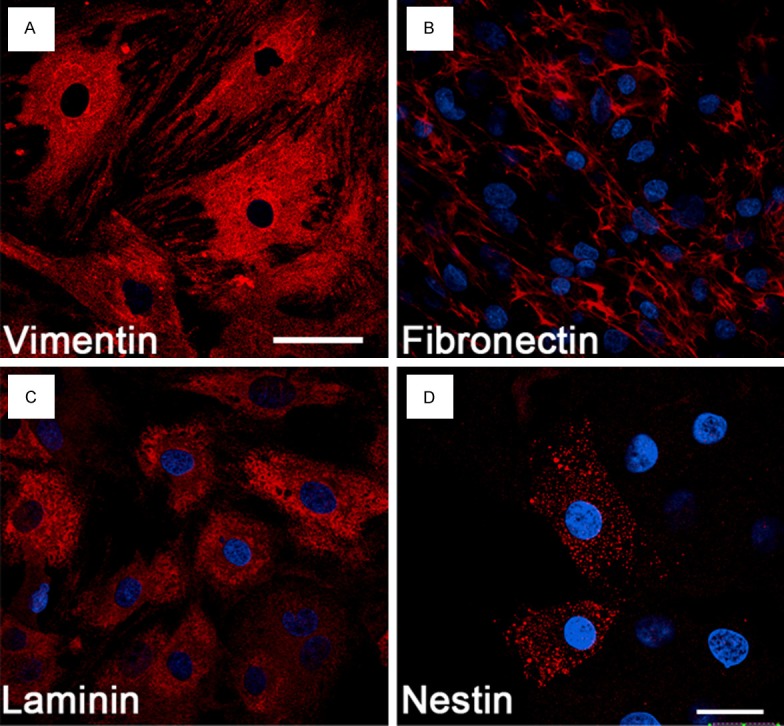
Characterization of BMSCs in vitro. Nearly all cultured BMSCs display vimentin- (A), fibronectin- (B), and laminin-immunoreactivity (C), the markers for mesenchymal cells after passaging 4 times. A subset of BMSCs also express nestin, a marker widely used for proliferating neural progenitor cells (D). Blue: DAPI positive nuclei. Scale bar, 50 μm (A-C) and 20 μm (D).
Survival and differentiation of MSCs in vivo
Although a large number of transplanted BMSCs labeled by CFDA-SE were observed at 2 w after transplantation (Figure 2A), they markedly reduced in rats surviving for a long time, and only a few were detected at the lesioned site at 8 w after transplantation (Figure 2B, 2C). The surviving cells were localized mainly in areas either rostral or caudal to the epicenter and appeared to have integrated into the host spinal cord. At different time points, the surviving BMSCs were often distributed near or surrounded by reactive macrophages as confirmed by OX42 immunofluorescence staining (Figure 2A-C). Indeed, many reactive macrophages were found to infiltrate the lesioned epicenter at 2 w after transplantation in both control and experiment rats. Besides the infiltration of reactive macrophages, another distinct feature at the lesioned epicenter was the formation of cavities beginning at 4 w after transplantation in both control and experiment rats. In experiment rats, however, the cavities noticeably reduced in size as they were traversed by strands of nerve fibers (Figure 2B, 2C). The surviving CFDA-SE labeled BMSCs were oval or spindle-shaped, had long processes and expressed fibronectin (Figure 2D-F) and NeuN (Figure 2G-I). However, none of them showed nestin, vimentin, laminin, GFAP, CNP, and Olig3 immunoreactivities after transplantation.
Figure 2.
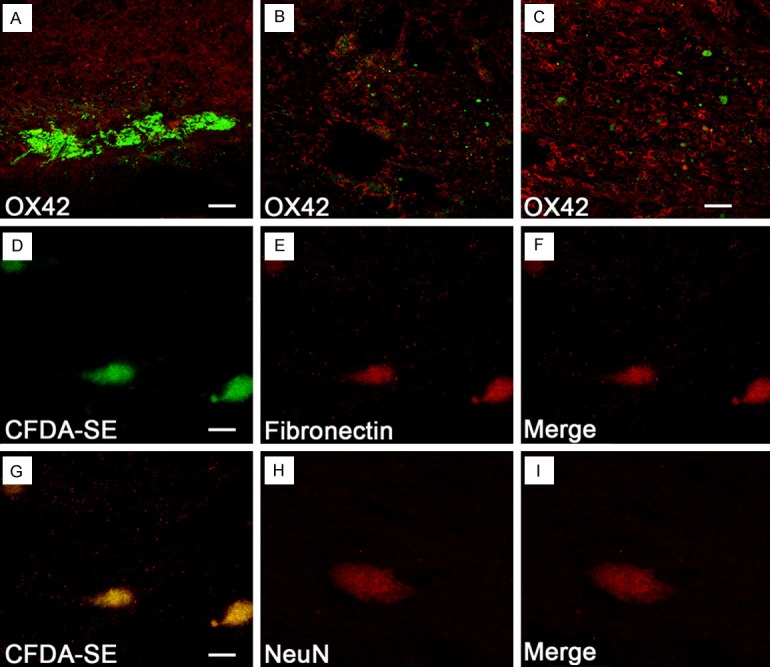
Survival and differentiation of BMSCs in the transected spinal cord at 8 w after transplantation. Although many CFDA-SE labeled BMSCs are surrounded by numerous OX42-positive macrophages at 2 w after transplantation (A), very few cells survive at 8 w at the lesioned epicenter (B). (C) Cells at a higher magnification (B). The BMSCs (D) surviving after transplantation retain fibronectin immunoreactivity (E, F), and a few (G) display faint NeuN-immunoreactivity (H, I). Scale bar, 200 μm (A, B), 100 μm (C), 20 μm (D-F) and 10 μm (G-I).
MSCs transplantation promotes axonal sprouting into the lesioned site
Although very few BMSCs were found at 8 w after transplantation, all rats receiving BMSCs transplantation showed robust growth of NF-200 immnopositive axons into the lesioned site (Figure 3B); similar fibers were barely observed in control rats (Figure 3A). However, a variable number of regenerating axons were found to sprout into the lesioned site where BMSCs were transplanted.
Figure 3.
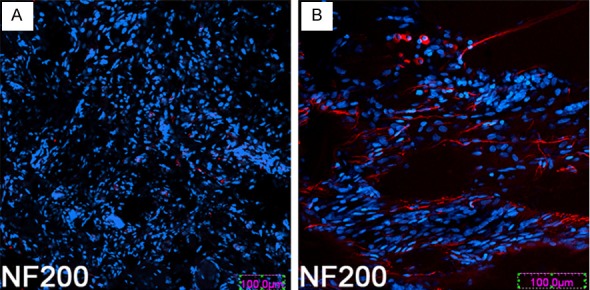
Transplantation of BMSCs enhances the sprouting of axons into the lesioned site. At 8 w after transplantation, few NF200 positive fibers sprouted into and through the lesioned site in control rats (A); however, a large number of NF200 positive fibers are observed in the experiment rats (B). Scale bar, 100 μm (A, B).
MSCs transplantation enhances the infiltration and survival of host-derived cells at the lesioned site
In agreement with Hokari [34], a large number of nestin- and GFAP-positive cells appeared at the lesioned site following BMSCs transplantation when compared with corresponding controls. Our results showed a concomitant increase in host-derived CNP, NF-200 and Olig3 positive cells in the same area of rats killed at 8 w. Similar cells were almost undetectable in the control group. The number of cells positive for nestin, GFAP, NF200, Olig3 and CNP was significantly different (P<0.05) between experiment group and control group (Figure 4).
Figure 4.
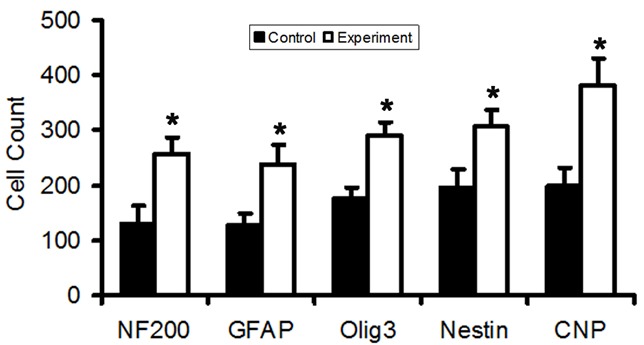
Host-derived cells at the lesioned site. The number of cells positive for nestin, GFAP, NF200, Olig3 and CNP is significantly different between experiment group and control group (P<0.05).
Relationship of CNP positive cells with sprouting axons and other host-derived cells
Many studies have reported the roles of Schwann cells in the remyelination and axonal regeneration after SCI [35-38]. In order to clarify the relationship between host-derived myelinating cells (Schwann cells and oligodendrocytes) and regenerating axons after BMSCs transplantation, immunoelectron microscopy was performed using their common marker CNP. Immunoelectron microscopy confirmed that there were many CNP positive cells at lesioned site at 2 and 8 w after BMSCs transplantation. These cells had long extending filopodia forming a single- (Figure 5) or multi-layered (Figure 6) perineurial-like lamellae delineating bundles of axons and associated Schwann cells into numerous compartments. In a fortuitous longitudinal section of an axon, a CNP positive filopodial process was in direct contact with the axolemma at the Ranvier node as well as in close approximation with the paranodal loop (Figure 7). The spatial relationship between regenerating axons and CNP-positive cells was also confirmed by double immunofluorescence staining (Figure 8).
Figure 5.
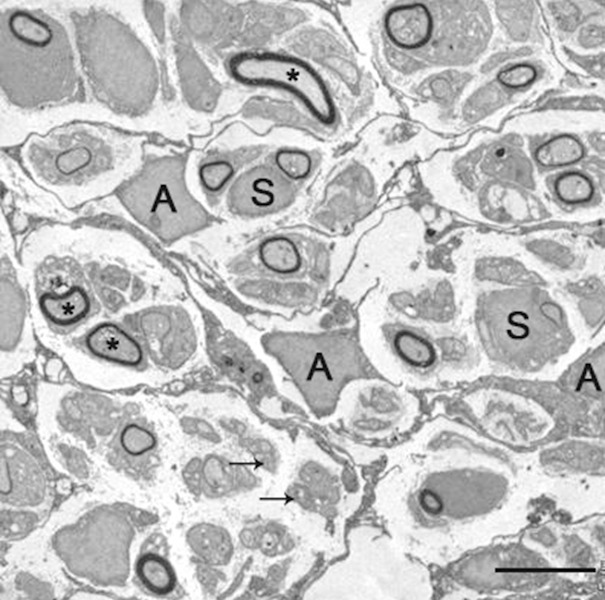
Astrocyte-like CNP positive cells (A) with emanating filopodial processes form multiple perineurial-like compartments containing a varying number of myelinated (asterisks) and unmyelinated (arrows)axons. The axons are associated with Schwann cells (S). Two weeks after BMSCs transplantatioin. Scale bar, 5 μm.
Figure 6.
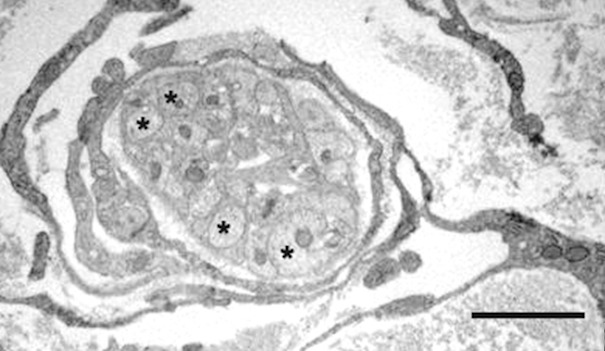
Multilayered perineurial-like sheath encloses a bundle of unmyelinated axons (asterisks). Scale bar, 2 μm.
Figure 7.
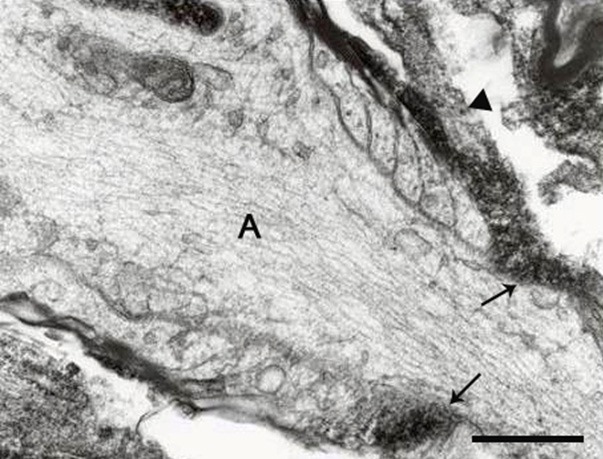
CNP is localized in the paranodal loops as well as in a filopodia (arrowhead) contacting an axon (A). Scale bar, 0.5 μm.
Figure 8.
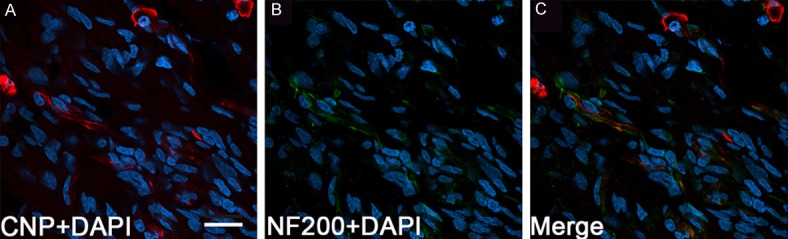
Spatial relationship between regenerating axons and CNP-positive cells. At 8 w after transplantation of BMSCs, many CNP positive cells and their long extending fibers (red, A) show close spatial relationship (C) with NF200 positive fibers (green, B). Blue: DAPI positive nuclei. Scale bar, 20 μm (A-C).
Discussion
Although axonal regeneration is extremely limited in the central nervous system of adult mammalians, spontaneous axonal sprouting and functional improvements may follow a partial lesion of the spinal cord [39,40]. Recent studies using different animal models and stem cells transplantation have shown an enhanced axon sprouting after SCI. There is strong evidence suggesting that stem cell transplantation facilitates the axonal sprouting, yet the relationship between the transplanted cells and regenerating axons, especially at cellular level, remains elusive. For example, it remains to be clarified if cells (such as olfactory ensheathing cells, [OECs]) used for transplantation could transdifferentiate into so-called perineurial-like cells and Schwann cells at the lesioned site [41]. It has been reported that invading Schwann cells rather than implanted OECs are solely responsible for the myelination of axons at the lesioned site [41,42]. Indeed, according to the study of Ramer [42] et al the transplanted OECs were undetectable at the lesioned site.
In the present study, a complete transection of the spinal cord was prepared in which a 2-3 mm gap was created between two stumps of the spinal cord, and the possible axonal regeneration was investigated after BMSCs transplantation. At 8 w after transplantation, very few BMSCs labeled with CFDA SE and expressing fibronectin were detected and appeared to integrate into the host spinal cord at the lesioned site. Meanwhile, a large number of OX42-positive macrophages accumulated at the lesioned site. Reactive microglia/macrophages are known to secrete a plethora of inflammatory cytokines such as TNF-α and IL-β [43] that may be detrimental to the survival of BMSCs at the lesioned epicenter. This would explain the scarcity of BMSCs at later time points. Another possible explanation for the marked reduction of BMSCs is the formation of large cavities that are probably not helpful for the survival and growth of transplanted cells. It has been reported by Li et al [44] that the transplanted OECs decreased in number over time, and that they were undetectable at the lesioned site at 13 w after transplantation, suggesting that the lesioned epicenter was not helpful for the survival of transplanted cells. The occurrence of some surviving BMSCs expressing NeuN suggests their potential of transdifferentiating into neurons. On the other hand, none were labeled with antibodies against GFAP, laminin, vimentin, nestin, Olig3, and CNP [34]. In addition, there was no strong evidence that the BMSCs were spatially related to the regenerating axons. This, along with their rarity in number, argues against the possibility that the cells are directly involved in the myelination and maintenance of sprouting axons.
The present study showed the robust axonal sprouting across the lesioned site despite the fact that it contained only sporadic BMSCs. As similar features were not evident in control rats, it is concluded that transplantation of BMSCs did facilitate the regeneration of fibers but it remains to elucidate the mechanisms. The combined use of BMSC and OEC may provide an improved approach for the treatment of SCI [45]. Hence, the involvement of BMSCs in promoting or facilitating the axonal sprouting, if any, might be indirect. To this end, it is desirable to examine other cellular changes during the axonal sprouting. It is significant to note the appearance and rise of numerous nestin and GFAP positive cells at the lesioned site following the MSCs transplantation, a feature that has also been reported by Hokari et al [34]. This study confirmed this and added fact that there was a concomitant increase in host-derived nestin-, GFAP-, NF200-, Olig3 and CNP positive cells. The coincident occurrence of above-mentioned cells, in particular, the close spatial relation between CNP cells and regenerating fibers, strongly indicates that they functionally link to the regeneration process.
As drastic increase in CNP positive cells at the lesioned site following BMSCs transplantation is a novel and major finding of this study, we further performed immunoelectron microscopy to investigate the role of these cells in nerve regeneration. 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNP) is expressed as two isoforms with an apparent molecular weight of 46 kDa (CNP1) and 48 kDa (CNP2), which are produced by alternative ribosomal initiation at two different AUG codons, thus differing each other only by the 20-amino acid extension at the N terminus [46]. In addition to the myelinating cells such as oligodendrocytes and Schwann cells [47,48], CNP is also expressed in various cell types such as retinal cells [49], liver cells [46], Purkinje cells [50], hippocampal neurons (soma and dendrites) [51], neural stem cells [52], astrocytes [53] and glioblasts [54] indicating more general cellular functions. Despite its lengthy history, relatively little is known about the function of CNP. Recent biochemical evidence indicates that CNP interacts with mitochondria and cytoskeletal proteins in certain non-glial cells, and serves as a membrane anchor for tubulin [55]. In addition, study also revealed that CNP over-expression in cultured cells increased the outgrowth of filopodia [56]. Recently, Cnp1, a gene encoding both CNP1 and CNP2 in the oligodendrocytes, was found to be essential for the axonal survival but not for myelin assembly [57], because CNP1 knockout mice developed axonal swelling and neurodegeneration throughout the brain, but the ultrastructure, periodicity and physical stability of myelins were remained unchanged in these mice. Our results were consistent with those reported by Lappe-Siefke [57].
Immunoelectron microscopy showed the extensive prolongation of filopodia from the CNP positive cells which may be linked to the contents of enzyme known to interact with microtubules [58] as well as housekeeping function in organelle transport [57].
The present results revealed that host-derived CNP positive cells formed perineurial-like compartments containing regenerating axons and associated Schwann cells, a feature also reported after OECs transplantation. Indeed, the CNP and Schwann cells displayed features resembling the so-called A and S cells, respectively, as reported by Li et al [59,60]. Although there is still controversy on whether the OECs can transdifferentiate into so-called perineurial-like cells and Schwann cells at the lesioned site [41], on the basis of the configuration of filopodial processes of CNP cells and their spatial relation with axons presently described, it is likely that they provide structural support in the form of compartments or tunnels for fiber growth. Some CNP positive processes were in close apposition to the axolemma suggesting their direct interaction with some factors for nerve growth. However, this remains to be further elucidated. Despite the above mentioned, our results showed the CNP may function to form perineurial-like compartments and remyelination of axons by Schwann cells.
Although BMSCs may not be directly associated with axonal sprouting, their possible involvement in promoting infiltration and survival of CNP and Schwann cells should be considered. We speculate that some trophic factors [61] secreted by the transplanted BMSCs may provide an optimal environment for the infiltration and survival of CNP and other host-derived cells.
This study shows that very few transplanted BMSCs actually survive and integrate into the local spinal cord following transplantation. The scarcity of surviving BMSCs may be attributed to the adverse environment into which the cells were transplanted. The transplantation site was filled with a large number of macrophages/reactive microglia and the presence of cavities in the spinal cord may not be helpful for their survival. The most dramatic feature following BMSCs transplantation in this study is the presence of a large number of host-derived CNP positive cells. The CNP positive cells form many perineurial compartments delineating a varying number of axons, strongly suggesting their support, perhaps both structurally and developmentally, for the fiber regeneration. It is also evident that Schwann cells are key cells involved in axon remyelination. The mechanistic link between BMSCs and host-derived cells is vital for a better understanding of sequential events promoting axonal growth. Likewise, the source of CNP and Schwann cells as well as their roles and mechanisms in guiding the growth of regenerating axons should be further studied.
Acknowledgements
Peng Ding and Zhi-Yong Yang contributed equally to this work. This study was supported by Fund for High-level talents of Yunnan Province (D201230), Combined Fund Surport of Yunnan Science-Technology Bureau-Kunming Medical University (2013FZ280) and Fund for Research Institute in Yunnan Province (2012ws0023).
References
- 1.Lee TH. Functional effect of mouse embryonic stem cell implantation after spinal cord injury. J Exerc Rehabil. 2013;9:230–233. doi: 10.12965/jer.130004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myckatyn TM, Mackinnon SE, McDonald JW. Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol. 2004;12:343–358. doi: 10.1016/j.trim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Houle JD, Cote MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci. 2013;1279:154–163. doi: 10.1111/nyas.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 2013;22:1309–1323. doi: 10.3727/096368912X657260. [DOI] [PubMed] [Google Scholar]
- 5.Zhilai Z, Hui Z, Anmin J, Shaoxiong M, Bo Y, Yinhai C. A combination of taxol infusion and human umbilical cord mesenchymal stem cells transplantation for the treatment of rat spinal cord injury. Brain Res. 2012;1481:79–89. doi: 10.1016/j.brainres.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 6.Ruff CA, Wilcox JT, Fehlings MG. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012;235:78–90. doi: 10.1016/j.expneurol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 8.Lacroix S, Hamilton LK, Vaugeois A, Beaudoin S, Breault-Dugas C, Pineau I, Levesque SA, Gregoire CA, Fernandes KJ. Central canal ependymal cells proliferate extensively in response to traumatic spinal cord injury but not demyelinating lesions. PLoS One. 2014;9:e85916. doi: 10.1371/journal.pone.0085916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonough A, Martinez-Cerdeno V. Endogenous proliferation after spinal cord injury in animal models. Stem Cells Int. 2012;2012:387513. doi: 10.1155/2012/387513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa-Jeffrey A, Oregel K, Wiggins L, Valera R, Bosnoyan K, Agbo C, Awosika O, Zhao PM, de Vellis J, Woerly S. Strategies for endogenous spinal cord repair: HPMA hydrogel to recruit migrating endogenous stem cells. Adv Exp Med Biol. 2012;760:25–52. doi: 10.1007/978-1-4614-4090-1_3. [DOI] [PubMed] [Google Scholar]
- 11.Wakatsuki S, Araki T, Sehara-Fujisawa A. Neuregulin-1/glial growth factor stimulates Schwann cell migration by inducing alpha5 beta1 integrin-ErbB2-focal adhesion kinase complex formation. Genes Cells. 2014;19:66–77. doi: 10.1111/gtc.12108. [DOI] [PubMed] [Google Scholar]
- 12.Botero L, Gomez RM, Chaparro O. [Pathogenesis of spinal cord injuries and mechanisms of repair induced by olfactory ensheathing cells] . Rev Neurol. 2013;56:521–531. [PubMed] [Google Scholar]
- 13.Roloff F, Ziege S, Baumgartner W, Wewetzer K, Bicker G. Schwann cell-free adult canine olfactory ensheathing cell preparations from olfactory bulb and mucosa display differential migratory and neurite growth-promoting properties in vitro. BMC Neurosci. 2013;14:141. doi: 10.1186/1471-2202-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tharion G, Indirani K, Durai M, Meenakshi M, Devasahayam SR, Prabhav NR, Solomons C, Bhattacharji S. Motor recovery following olfactory ensheathing cell transplantation in rats with spinal cord injury. Neurol India. 2011;59:566–572. doi: 10.4103/0028-3886.84339. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Wen Q, Xia Y, Yang J, Gao P, Zhang N, Li H, Zou S. Osthole augments therapeutic efficiency of neural stem cells-based therapy in experimental autoimmune encephalomyelitis. J Pharmacol Sci. 2014;124:54–65. doi: 10.1254/jphs.13144fp. [DOI] [PubMed] [Google Scholar]
- 16.Wang JM, Zeng YS, Wu JL, Li Y, Teng YD. Cograft of neural stem cells and schwann cells overexpressing TrkC and neurotrophin-3 respectively after rat spinal cord transection. Biomaterials. 2011;32:7454–7468. doi: 10.1016/j.biomaterials.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Song M, Mohamad O, Gu X, Wei L, Yu SP. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 2013;22:2001–2015. doi: 10.3727/096368912X657909. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L, Chen J, Xu W, Lu S, Zhao Q, Peng J. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett. 2012;514:96–101. doi: 10.1016/j.neulet.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 19.Denham M, Parish CL, Leaw B, Wright J, Reid CA, Petrou S, Dottori M, Thompson LH. Neurons derived from human embryonic stem cells extend long-distance axonal projections through growth along host white matter tracts after intra-cerebral transplantation. Front Cell Neurosci. 2012;6:11. doi: 10.3389/fncel.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You W, Gao H, Fan L, Duan D, Wang C, Wang K. Foxc2 regulates osteogenesis and angiogenesis of bone marrow mesenchymal stem cells. BMC Musculoskelet Disord. 2013;14:199. doi: 10.1186/1471-2474-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao T, Yan W, Xu K, Qi Y, Dai X, Shi Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy. 2013;15:792–804. doi: 10.1016/j.jcyt.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Osanai T, Kuroda S, Sugiyama T, Kawabori M, Ito M, Shichinohe H, Kuge Y, Houkin K, Tamaki N, Iwasaki Y. Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats--in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery. 2012;70:435–444. doi: 10.1227/NEU.0b013e318230a795. discussion 444. [DOI] [PubMed] [Google Scholar]
- 23.Ban DX, Ning GZ, Feng SQ, Wang Y, Zhou XH, Liu Y, Chen JT. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6:707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J. Cell based therapies for ischemic stroke: From basic science to bedside. Prog Neurobiol. 2013;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Ma Y. Expression of soluble Nogo-66 receptor and brain-derived neurotrophic factor in transduced rat bone marrow stromal cells. J Clin Neurosci. 2010;17:762–765. doi: 10.1016/j.jocn.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Salomone R, Bento RF, Costa HJ, Azzi-Nogueira D, Ovando PC, Da-Silva CF, Zanatta DB, Strauss BE, Haddad LA. Bone marrow stem cells in facial nerve regeneration from isolated stumps. Muscle Nerve. 2013;48:423–429. doi: 10.1002/mus.23768. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Cui YC, Wang XJ, Wu AL, Hu GF, Luo FL, Sun JK, Sun J, Wu LK. Neural progenitor cells derived from adult bone marrow mesenchymal stem cells promote neuronal regeneration. Life Sci. 2012;91:951–958. doi: 10.1016/j.lfs.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Abbaszadeh HA, Tiraihi T, Delshad AR, Saghedi Zadeh M, Taheri T. Bone marrow stromal cell transdifferentiation into oligodendrocyte-like cells using triiodothyronine as a inducer with expression of platelet-derived growth factor alpha as a maturity marker. Iran Biomed J. 2013;17:62–70. doi: 10.6091/ibj.11162.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin H, Dan QQ, Rong R, Wang TH. [Implication of BDNF expression in transected spinal cord of rats] . Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43:231–235. [PubMed] [Google Scholar]
- 31.Gao L, Li LH, Xing RX, Ou S, Liu GD, Wang YP, Zhang H, Gao GD, Wang TH. Gastrocnemius-derived BDNF promotes motor function recovery in spinal cord transected rats. Growth Factors. 2012;30:167–175. doi: 10.3109/08977194.2012.678842. [DOI] [PubMed] [Google Scholar]
- 32.Simic D, Euler C, Thurby C, Peden M, Tannehill-Gregg S, Bunch T, Sanderson T, Van Vleet T. Assessing cell fusion and cytokinesis failure as mechanisms of clone 9 hepatocyte multinucleation in vitro. Curr Protoc Toxicol. 2012;Chapter 14:Unit 14.9.1–17. doi: 10.1002/0471140856.tx1409s53. [DOI] [PubMed] [Google Scholar]
- 33.Holmes T, Yan F, Ko KH, Nordon R, Song E, O’Brien TA, Dolnikov A. Ex vivo expansion of cord blood progenitors impairs their short-term and long-term repopulating activity associated with transcriptional dysregulation of signalling networks. Cell Prolif. 2012;45:266–278. doi: 10.1111/j.1365-2184.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hokari M, Kuroda S, Shichinohe H, Yano S, Hida K, Iwasaki Y. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J Neurosci Res. 2008;86:1024–1035. doi: 10.1002/jnr.21572. [DOI] [PubMed] [Google Scholar]
- 35.Zaminy A, Shokrgozar MA, Sadeghi Y, Noroozian M, Heidari MH, Piryaei A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran Biomed J. 2013;17:113–122. doi: 10.6091/ibj.1121.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, Maris DO, Horner PJ. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci U S A. 2013;110:4075–4080. doi: 10.1073/pnas.1210293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh M, Tuesta LM, Puentes R, Patel S, Melendez K, El Maarouf A, Rutishauser U, Pearse DD. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia. 2012;60:979–992. doi: 10.1002/glia.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darlot F, Cayetanot F, Gauthier P, Matarazzo V, Kastner A. Extensive respiratory plasticity after cervical spinal cord injury in rats: axonal sprouting and rerouting of ventrolateral bulbospinal pathways. Exp Neurol. 2012;236:88–102. doi: 10.1016/j.expneurol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Heidemann M, Streit J, Tscherter A. Functional regeneration of intraspinal connections in a new in vitro model. Neuroscience. 2014;262C:40–52. doi: 10.1016/j.neuroscience.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 41.Boyd JG, Lee J, Skihar V, Doucette R, Kawaja MD. LacZ-expressing olfactory ensheathing cells do not associate with myelinated axons after implantation into the compressed spinal cord. Proc Natl Acad Sci U S A. 2004;101:2162–2166. doi: 10.1073/pnas.0303842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004;473:1–15. doi: 10.1002/cne.20049. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C, Huang C, Ma TT, Bian EB, He Y, Zhang L, Li J. SOCS1 hypermethylation mediated by DNMT1 is associated with lipopolysaccharide-induced inflammatory cytokines in macrophages. Toxicol Lett. 2014;225:488–97. doi: 10.1016/j.toxlet.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Yu HL, Chen LF, Duan CX, Zhang JY, Li BC. Survival and number of olfactory ensheathing cells transplanted in contused spinal cord of rats. Chin J Traumatol. 2010;13:356–361. [PubMed] [Google Scholar]
- 45.Deng YB, Liu Y, Zhu WB, Bi XB, Wang YZ, Ye MH, Zhou GQ. The co-transplantation of human bone marrow stromal cells and embryo olfactory ensheathing cells as a new approach to treat spinal cord injury in a rat model. Cytotherapy. 2008;10:551–564. doi: 10.1080/14653240802165673. [DOI] [PubMed] [Google Scholar]
- 46.Ma H, Zhao XL, Wang XY, Xie XW, Han JC, Guan WL, Wang Q, Zhu L, Pan XB, Wei L. 2’,3’-cyclic nucleotide 3’-phosphodiesterases inhibit hepatitis B virus replication. PLoS One. 2013;8:e80769. doi: 10.1371/journal.pone.0080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwata K, Cafe-Mendes CC, Schmitt A, Steiner J, Manabe T, Matsuzaki H, Falkai P, Turck CW, Martins-de-Souza D. The human oligodendrocyte proteome. Proteomics. 2013;13:3548–3553. doi: 10.1002/pmic.201300201. [DOI] [PubMed] [Google Scholar]
- 48.Keng VW, Watson AL, Rahrmann EP, Li H, Tschida BR, Moriarity BS, Choi K, Rizvi TA, Collins MH, Wallace MR, Ratner N, Largaespada DA. Conditional Inactivation of Pten with EGFR Overexpression in Schwann Cells Models Sporadic MPNST. Sarcoma. 2012;2012:620834. doi: 10.1155/2012/620834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gotoh H, Ueda T, Uno A, Ohuchi H, Ikenaka K, Ono K. Expression of myelin genes in the developing chick retina. Gene Expr Patterns. 2011;11:471–475. doi: 10.1016/j.gep.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Cho SJ, Jung JS, Jin I, Moon IS. 2’, 3’-cyclic nucleotide 3’-phosphodiesterase is expressed in dissociated rat cerebellar cells and included in the postsynaptic density fraction. Mol Cells. 2003;16:128–135. [PubMed] [Google Scholar]
- 51.Walser M, Sama MT, Wickelgren R, Aberg M, Bohlooly YM, Olsson B, Tornell J, Isgaard J, Aberg ND. Local overexpression of GH and GH/IGF1 effects in the adult mouse hippocampus. J Endocrinol. 2012;215:257–268. doi: 10.1530/JOE-12-0077. [DOI] [PubMed] [Google Scholar]
- 52.Cho SJ, Jung JS, Shin SC, Jin I, Ko BH, Kim Kwon Y, Suh-Kim H, Moon IS. Nonspecific association of 2’,3’-cyclic nucleotide 3’-phosphodiesterase with the rat forebrain postsynaptic density fraction. Exp Mol Med. 2003;35:486–493. doi: 10.1038/emm.2003.63. [DOI] [PubMed] [Google Scholar]
- 53.Naureen I, Waheed KA, Rathore AW, Victor S, Mallucci C, Goodden JR, Chohan SN, Miyan JA. Fingerprint changes in CSF composition associated with different aetiologies in human neonatal hydrocephalus: glial proteins associated with cell damage and loss. Fluids Barriers CNS. 2013;10:34. doi: 10.1186/2045-8118-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braun PE, Sandillon F, Edwards A, Matthieu JM, Privat A. Immunocytochemical localization by electron microscopy of 2’3’-cyclic nucleotide 3’-phosphodiesterase in developing oligodendrocytes of normal and mutant brain. J Neurosci. 1988;8:3057–3066. doi: 10.1523/JNEUROSCI.08-08-03057.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bifulco M, Laezza C, Stingo S, Wolff J. 2’,3’-Cyclic nucleotide 3’-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc Natl Acad Sci U S A. 2002;99:1807–1812. doi: 10.1073/pnas.042678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyermann C, Czaplinski K, Colognato H. Dystroglycan promotes filopodial formation and process branching in differentiating oligodendroglia. J Neurochem. 2012;120:928–947. doi: 10.1111/j.1471-4159.2011.07600.x. [DOI] [PubMed] [Google Scholar]
- 57.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 58.Xia C, Nguyen M, Garrison AK, Zhao Z, Wang Z, Sutherland C, Ma L. CNP/cGMP signaling regulates axon branching and growth by modulating microtubule polymerization. Dev Neurobiol. 2013;73:673–687. doi: 10.1002/dneu.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci. 1998;18:10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 61.Yan K, Zhang R, Sun C, Chen L, Li P, Liu Y, Peng L, Sun H, Qin K, Chen F, Huang W, Chen Y, Lv B, Du M, Zou Y, Cai Y, Qin L, Tang Y, Jiang X. Bone marrow-derived mesenchymal stem cells maintain the resting phenotype of microglia and inhibit microglial activation. PLoS One. 2013;8:e84116. doi: 10.1371/journal.pone.0084116. [DOI] [PMC free article] [PubMed] [Google Scholar]


