Abstract
Despite a large number of molecular epidemiological studies, the association of Mouse Mammary Tumor Virus-Like Virus (MMTV-LV) infection with the risk of human breast cancer remains inconclusive mainly due to the heterogeneity in populations involved. We performed a systematic search of multiple bibliographic databases, up to October 2013, to identify all studies on detection of MMTV-LV DNA in human breast cancer using polymerase chain reaction (PCR) and conducted the first comprehensive meta-analysis of published literature to explore the relevance of MMTV-LV to human breast cancer. As a result, meta-analysis of twelve case-control studies identified from the systematic search revealed a significantly increased risk for breast cancer development after MMTV-LV infection (OR=15.20; 95% CI: 9.98-23.13). However, there was no significant correlation between MMTV-LV infection and the transformation from ductal carcinoma in situ to invasive ductal carcinoma (OR=1.16; 95% CI: 0.27-4.97). In addition, MMTV-LV infection was not associated with the expression of estrogen receptor (ER) (OR=0.89; 95% CI: 0.48-1.65), progesterone receptor (PR) (OR=0.73; 95% CI: 0.22-2.42), HER-2 (OR=0.65; 95% CI: 0.30-1.43) or p53 (OR=1.47; 95% CI: 0.79-2.73). Finally, we found that the prevalence of MMTV-LV in breast carcinoma was significantly higher in patients from Western countries (prevalence=40.4%, 95% CI: 28.9%-51.9%) than in Asian patients (prevalence: 8.5%; 95% CI: -7.1%-24.1%) in a subgroup and meta-regression analysis (p=0.015). In summary, the meta-analysis of published studies revealed a significantly increased risk for breast cancer development after MMTV-LV infection. In addition, the prevalence of MMTV-LV is much higher in breast cancer patients from Western countries than Asian patients.
Keywords: Human breast cancer, mouse mammary tumor virus-like virus, MMTV-LV
Introduction
Although some risk reduction might be achieved with prevention, breast cancer remains the top cancer in women both in the developed and the developing world and the principle cause of death from cancer among women globally. The incidence of breast cancer varies worldwide [1]. It is universally agreed that changes in the environment account for the increased risk for breast cancer [2]. People moving from areas of lower to higher breast cancer incidence show a gradually acquired increasing risk. For example, the Japanese moving to the USA [3], or south Asians to the UK [4] all experience increased incidences of breast cancer over a period of decades. Diet high in fat and xeno-oestrogens have been proposed to increase breast cancer risk [5]. However, the circumpolar Inuit have a high saturated fat diet that is contaminated with high levels of xeno-oestrogens [6], but the breast cancer incidence is low [7]. So some other unrecognized environmental factors with oncogenic potential may explain the geographic differences in human breast cancer incidence.
Viral infection is the most important risk factor for the development of human cancers. Oncovirus that can cause cancers in human contributes to carcinogenesis by carrying viral oncogenes or inserting into the genome to activate proto-oncogenes. Many oncoviruses have been identified already, such as hepatitis B, hepatitis C, human papillomavirus and Epstein-Barr virus.
The majority of mammary tumors in mice are caused by mouse mammary tumor virus (MMTV), a milk-transmitted retrovirus formerly known as Bittner virus [8]. As the infected mice develop mammary tumors in adulthood, MMTV has inspired the search for a human breast cancer virus. MMTV-like sequence has been found in human breast cancer. A complete proviral sequence that was greater than 95% homologous to MMTV, named MMTV-like virus (MMTV-LV) was sequenced out of human breast cancer tissue including a correct integration into the human genome. Since realizing the involvement of MMTV-LV in human breast cancer in the late 1970s [9], a growing number of case-control studies have been performed to evaluate the relevance of MMTV-LV infection to human breast carcinogenesis [10-34].
In an effort to gain a better insight into the correlation of MMTV infection and breast cancer development, we conducted the first systematic review and comprehensive meta-analysis to explore the correlation of MMTV-LV infection and human breast cancer risk and the characteristics of MMTV-LV prevalence in human breast cancers.
Material and methods
This systematic review was conducted following guidance provided by the Cochrane Handbook [35] and is reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [36].
Search strategy
We carried out a comprehensive search strategy in various databases including PubMed, Embase, EBSCO (ASP/BSP), Cochrane Library and China National Knowledge Infrastructure (CNKI) to seek out the articles which were about the association between MMTV-LV infection and human breast cancer risk. We searched “mouse mammary tumor virus” or “MMTV” in title/abstract combined with medical subject headings term “breast neoplasms”. The title and abstract of studies identified in the search were reviewed by two authors independently (FW and JH) to exclude studies that did not answer the research question of interest. The full text of the remaining articles was examined to determine whether it contained relevant information. Additional studies were identified by a manual search from references of original studies or review articles on this topic. All relevant studies published between January 1995, when more specific techniques were available, and October 2013 were included.
Eligibility criteria
Included studies had to meet the following criteria: (i) Studies had to use PCR-based techniques to detect regions of MMTV env gene that have low homology to known HERVs in tissues. Researches with PCR product not homologous to MMTV env gene were excluded [37]. (ii) Only studies on breast cancer in females were included. Data on breast cell lines [17], animal breast tumors [38], male breast cancer [23] or human family breast carcinoma [39] were all excluded. (iii) If data or data subsets were published in more than one article, only the publication with the most explicit description was included [14,23,34]. (iiii) Studies with ambiguous sample prevention method [26,30] or not with a detection target of MMTV env gene [29] were excluded. Inclusion was not otherwise restricted by study size, language or study location. The article selection process used in this study is summarized in Figure 1.
Figure 1.
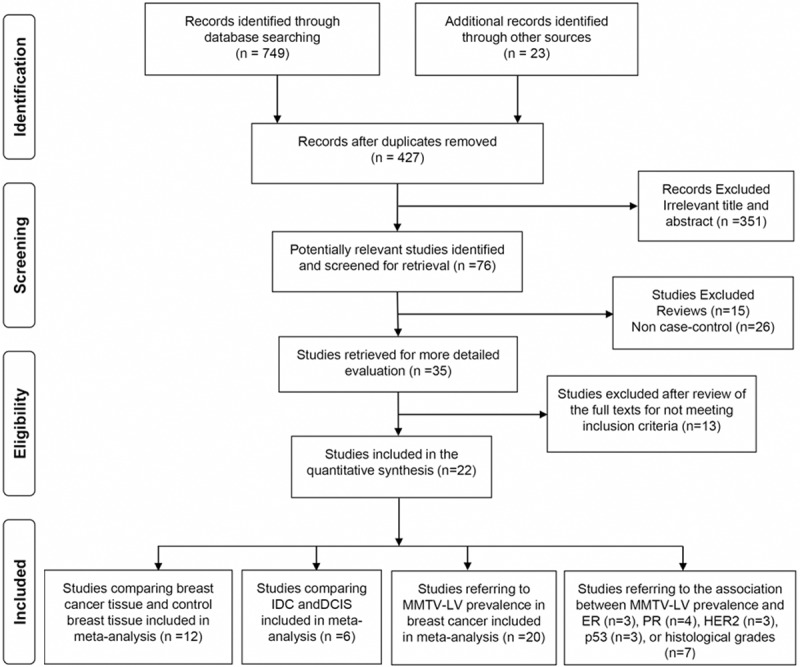
Flow diagram of the literature search strategy and assessment of studies identified for systematic review.
The methodologic quality of case-control studies was assessed by two authors independently (FW and QS) using the Newcastle-Ottawa scale (NOS) [40]. In this scale, studies were scored across three categories: selection (4 items), comparability (2 items) of study groups and exposure (3 items), with all items with a score of one. Any discrepancies were addressed by a joint reevaluation of the original article.
Data abstraction
Two reviewers (FW and YY) independently extracted data and reached consensus on all the terms. We recorded the following information about each eligible trial: first author’s name, study location, language, sample preservation method, cell selecting method, detection method, detection target, breast cancer pathology, matching control type and sample size. The sample preservation methods could be formalin-fixed paraffin-embedded or frozen in -80°C. Cell selecting methods were divided into sections cut from tissue (traditional method) and laser capture microdissection (LCM), which can exactly select cancer cells and exclude stromal and inflammatory cells. Detection methods contained traditional PCR, semi-nested PCR, nested PCR, and fluorescence-nested PCR (FN PCR). The detection targets were all related to MMTV envelope gene, which is not homological to the known HERVs. The pathologic type of breast cancer contained infiltrating lobular carcinoma (ILC), infiltrating ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS), while matching control can be matched adjacent normal breast tissue, breast specimens from reduction mammoplasties or breast fibroadenomas. Detailed information on all included studies was presented in Tables 1 and 6.
Table 1.
Characteristics of included studies assessing the risk of human breast cancer in women with MMTV-LV infection
| Study | Study location | Language | Sample preservation | Cell selecting method | Detection method | Detection target | Breast cancer pathology | MMTV-LV+ / Case (%) | Matching control | MMTV-LV+ / Control (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang [34] 1995 | America | English | Frozen tissue | Sections cut from tissue | PCR | 660 bp env | IBC | 121/314 (38.54) | Con | 4/136 (2.94) | |
| BF | 2/29 (6.90) | ||||||||||
| NBT | 2/107 (1.87) | ||||||||||
| Paraffin-embedded tissue | Sections cut from tissue | PCR | 250 bp env | IBC | 60/151 (39.74) | NBT | 1/27 (3.70) | ||||
| Pogo [33] 1999 | Italy | English | Paraffin-embedded tissue | Sections cut from tissue | PCR | 250 bp env | BC | 26/69 (37.68) | - | ||
| DCIS | 5/17 (29.41) | ||||||||||
| IDC | 14/36 (38.90) | ||||||||||
| Etkind [32] 2000 | America | English | Frozen tissue | Sections cut from tissue | Nested PCR | 660/250 bp env | IBC | 27/73 (36.99) | NBT | 0/35 (0.00) | |
| Melana [31] 2001 | America | English | Paraffin-embedded tissue | Sections cut from tissue | PCR | 250 bp env | IBC | 32/106 (30.19) | NBT | 1/106 (0.94) | |
| Melana [28] 2002 | Argentina | Spanish | Frozen tissue | Sections cut from tissue | PCR | 250 bp env | BC | 23/74 (31.08) | Con | 1/15 (6.67) | |
| NBT | 1/10 (10.00) | ||||||||||
| BF | 0/5 (0.00) | ||||||||||
| Ford [27] 2003 | Vietnam | English | Paraffin-embedded tissue | Sections cut from tissue | PCR | 356 bp env | BC | 1/120 (0.83) | NBT | 0/60 (0.00) | |
| Ford [23] 2004 | Australia | English | Paraffin-embedded tissue | Sections cut from tissue | Semi-nested PCR | 190 bp env | BC | 45/144 (31.25) | Con | 7/136 (5.15) | |
| DCIS | 2/8 (25.00) | NBT | 2/111 (1.80) | ||||||||
| IDC | 43/136 (31.62) | BF | 5/25 (20.00) | ||||||||
| Faedo [24] 2004 | Australia | English | Paraffin-embedded tissue | Sections cut from tissue | Semi-nested PCR | 190 bp env | IBC | 50/128 (39.06) | - | ||
| Levine [21] 2004 | Tunisia | English | Paraffin-embedded tissue | Sections cut from tissue | PCR | 250 bp env | BC | 28/38 (73.68) | - | ||
| Zammarchi [18] 2006 | Italy | English | Frozen tissue | Laser Microdissection | Fluorescent nested-PCR (FN-PCR) | 248 bp env | BC | 15/45 (33.33) | NBT | 0/8 (0.00) | |
| DCIS | 1/1 (100.00) | ||||||||||
| IDC | 13/43 (30.23) | ||||||||||
| Luo [19] 2006 | China | Chinese | Paraffin-embedded tissue | Sections cut from tissue | Nested PCR | 166 bp env | BC | 22/131 (16.79) | - | ||
| DCIS | 2/23 (8.70) | ||||||||||
| IDC | 20/87 (22.99)- | ||||||||||
| Zapata-Benavides [17] 2007 | Mexico | English | Frozen tissue | Sections cut from tissue | PCR | 250/594 bp env | BC | 5/119 (4.20) | - | ||
| Mok [15] 2008 | Australia | English | Paraffin-embedded tissue | Sections cut from tissue | Nested PCR | 255 bp env | IBC | 28/50 (56.00) | - | ||
| Hachana [16] 2008 | Tunisia | English | Frozen tissue | Sections cut from tissue | Nested PCR | 190 bp env | BC | 17/122 (13.93) | NBT | 0/122 (0.00) | |
| Lawson | 2010 [14] | Australia | English | Paraffin-embedded tissue | Sections cut from tissue | Nested PCR | 255 bp env | BC | 33/74 (44.59) | NBT | 0/29 (0.00) |
| 2004 [22] | PCR | 250 bp env | DCIS | 5/22 (22.73) | - | - | |||||
| IDC | 15/20 (75.00) | ||||||||||
| Mazzanti [13] 2011 | Italy | English | Paraffin-embedded tissue | Laser Microdissection | Fluorescent nested-PCR (FN-PCR) | 202 bp env | BC | 47/69 (68.12) | Con | 5/46 (10.87) | |
| DCIS | 40/49 (81.63) | NBT | 0/20 (0.00) | ||||||||
| IDC | 7/20 (35.00) | ANBT | 5/26 (19.23) | ||||||||
| Motamedifar [10] 2012 | Iran | English | Paraffin-embedded tissue | Sections cut from tissue | Nested PCR | 250 bp env | BC | 0/50 (0.00) | - | ||
| Glenn [11] 2012 | Australia | English | Frozen tissue | Sections cut from tissue | PCR | 643 bp env | IBC | 39/50 (78.00) | NBT | 13/40 (32.50) | |
| Morales-Sanchez [47] 2013 | Mexico | English | Frozen tissue | Sections cut from tissue | Nested PCR | 253 bp env | BC | 0/65 (0.00) | ANBT | 0/65 (0.00) | |
| Paraffin-embedded tissue | BC | 0/21 (0.00) | - | ||||||||
| Tabriz [48] 2013 | Iran | English | Paraffin-embedded tissue | Sections cut from tissue | Real time-PCR | 104 bp env | BC | 0/40 (0.00) | - | ||
BC: breast cancer; IBC: infiltrating breast cancer; IDC: infiltrating ductal carcinoma; DCIS: ductal carcinomas in situ; BF: breast fibroadenomas; NBT: normal breast tissue; ANBT: adjacent normal breast tissue.
Table 6.
Included studies assessing the association of clinical parameters and MMTV-LV prevalence in human breast cancer
| Parameters | MMTV-LV+ (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pogo [33] 1999 | Faedo [24] 2004 | Hachana [16] 2008 | Glenn [11] 2012 | Ford [23] 2004 | Zammarchi [18] 2006 | Luo [19] 2006 | Lawson [20] 2006 | ||
| ER | + | - | 10/29 (34.48) | 8/54 (14.81) | 29/38 (76.32) | - | - | - | - |
| - | - | 40/99 (40.40) | 9/68 (13.24) | 8/10 (80.00) | - | - | - | - | |
| PR | + | 13/35 (37.14) | 18/31 (58.06) | 4/58 (6.90) | 24/34 (70.59) | - | - | - | - |
| - | 13/34 (38.24) | 32/97 (32.99) | 13/64 (20.31) | 14/15 (93.33) | - | - | - | - | |
| HER2 | + | 10/24 (41.67) | - | 1/32 (3.13) | 2/3 (66.67) | - | - | - | - |
| - | 16/45 (35.56) | - | 16/90 (17.78) | 27/35 (77.14) | - | - | - | - | |
| P53 | + | - | 15/27 (55.56) | 7/57 (12.28) | 34/44 (77.27) | - | - | - | - |
| - | - | 35/101 (34.65) | 10/75 (13.33) | 5/6 (83.33) | - | - | - | - | |
| IDC Grade | I | - | 12/37 (32.43) | 4/23 (17.39) | 3/5 (60.00) | 9/40 (22.50) | 0/2 (0.00) | 3/16 (18.75) | 5/9 (55.56) |
| II | - | 18/44 (40.91) | 7/43 (16.28) | 11/17 64.71) | 19/56 (33.93) | 2/9 (22.22) | 9/39 (23.08) | 9/18 (50.00) | |
| III | - | 20/47 (42.55) | 4/45 (8.89) | 20/23 (86.96) | 15/40 (37.50) | 11/32 (34.38) | 8/32 (25.00) | 5/16 (31.25) | |
IDC: infiltrating ductal carcinoma.
Statistical analysis
This meta-analysis consisted of four parts. The first part was a statistical pooling of MMTV-LV infection and breast cancer risk estimate. The second part was an estimation of the transformation risk from ductal carcinoma in situ to infiltrating ductal carcinoma affected by MMTV-LV infection. In the third part, we estimated the MMTV-LV prevalence in breast carcinoma tissues and explored possible parameters associated with MMTV-LV detection rate in subgroup analysis. In the last part, we explored the association of clinical parameters, including ER, PR, HER2, P53 and grades of infiltrating ductal carcinoma, and MMTV-LV prevalence in breast cancer tissue.
Fixed-effect and rawere adopted to pool the case-control data based on Mantel-Haenszel [41] and DerSimonian and Laird methods [42], respectively. These two models provide similar results when between-studies heterogeneity is absent. Otherwise, a random-effect model is more appropriate. We assessed heterogeneity between study-specific estimates using 2 methods [43,44]. First, the Cochran’s statistical test for heterogeneity, which tests the null hypothesis that all studies in a meta-analysis have the same underlying magnitude of effect, was measured. Because this test is underpowered to detect moderate degrees of heterogeneity, a p-value of < 0.10 was considered suggestive of significant heterogeneity. Second, to estimate what proportion of total variation across studies was due to heterogeneity rather than chance, I2 statistic was calculated. In this analysis, a value of < 30%, 30-60%, 61-75% and > 75% were suggestive of low, moderate, substantial and considerable heterogeneity, respectively [44]. Once heterogeneity was noted, between-study sources of heterogeneity were investigated using subgroup analyses by grouping original data according to study characteristics (study location, sample prevention method, cell selecting method and detection method). In this analysis, a test of interaction comparing the sub-groups was performed in a meta-regression model. If the p-value for difference between subgroups was < 0.05, it was considered statistically significant (i.e., p < 0.05 suggested that stratifying based on that particular study characteristic partly explained the heterogeneity observed in the analysis). We assessed for publication bias quantitatively using Egger’s regression test(publication bias present if p ≤ 0.10) [45] and visually by inspection of funnel plots of the logarithmic OR versus their standard errors [46]. All p values were two tailed. For all tests (except for total heterogeneity and publication bias), p < 0.05 was considered statistically significant. All calculations and graphs were performed using STATA statistical software, version 12.0.
Results
From a total of 772 unique studies identified using the search strategy, 22 studies fulfilled the inclusion criteria and were included in meta-analysis [10,11,13-24,27,28,31-34,47,48]. Two of the publications were both divided into two parts because of their samples including two types (paraffin-embedded tissue and frozen tissue) [34,47], so there were 24 data sets (Table 1). There were 13 data sets included in the MMTV-LV infection and breast cancer risk estimate analysis [11,13,14,16,18,23,27,28,31,32,34,47], while only 6 data sets were used for the estimation of the transformation risk from ductal carcinoma in situ to infiltrating ductal carcinoma effected by MMTV-LV infection [13,18,19,22,23,33]. There were 22 data sets referring the MMTV-LV prevalence in breast carcinoma tissues, so they were included in the MMTV-LV prevalence meta-analysis [10,11,13-19,21,23,24,27,28,31-34,47,48]. Lastly, there were 3 studies estimating the association of MMTV-LV prevalence with ER and p53 [11,16,24], 4 studies referring PR [11,16,24,33], 3 studies referring HER2 [11,16,33] and 7 studies relating to IDC grades [11,16,18-20,23,24], so all the studies were included in meta-analysis, respectively (Table 6).
Characteristics of included studies
The characteristics of the included studies are shown in Tables 1 and 6. All the studies were population-based. Sixteen studies represented Western populations (6 based in the Americas [17,28,31,32,34,47], 3 based in Europe [13,18,33], 7 based in Oceania [11,14,15,20,22-24]). Two studies were performed in an African population (both based in Tunisia [16,21]). There was an interval of 4 years between two studies conducted in Tunisia and the most obvious distinction between them was that the earlier one was performed on a small series of Tunisian patients with 71% showing objective signs of inflammatory breast cancer [21]. The other four studies were performed in Asian populations (1 based in China [19], 1 based in Vietnam [27] and 2 based in Iran [10,48]). The earliest study was published in 1995 [34], and the latest ended in 2013 [47,48].
All the included publications were case-control studies. The exposure factor in both case and control groups for estimating OR was MMTV-LV infection.
Quality of included studies
The overall methodologic quality of these evidences was moderate to high (Table 2). The results of quality assessment according to NOS for case-control studies are shown in Table 2, respectively. All these studies reported that the diagnoses of all cases and controls were based on pathological and clinical records, and thus all studies got the two scores in the items of “adequate definition of cases” and “definition of controls”. All breast cancer cases in each of the studies were declared to be diagnosed cases during a certain period, in certain medical centers, and thus the representativeness of cases was qualified for another score. MMTV-LV infection was identified by PCR methods, so two more scores were assigned for “ascertainment of exposure” and “same method to ascertain for cases and controls” to all studies. Gender was supposed to be the most important impact factor for detection rate of MMTV-LV, so all studies got one score in the terms of “study controls for symptoms”. However, the same non-response rate between groups was not shown, or non-response rate was not mentioned in all studies, and thus all studies failed to win a score for “non-response rate”.
Table 2.
Newcastle-Ottawa Scores for the Studies Included in the MMTV-LV Infection and Breast Cancer Risk Analysis
| Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Reference | Selection | Comparability | Exposure | TOTAL | ||||||
|
|
||||||||||
| 1 Is the case definition | 2 Representativeness of the cases | 3 Selection of controls | 4 Definition of controls | 5 Study controls for symptoms (yes=1, no=0) | 6 Study controls for systemic therapy (yes=1, no=0)a | 7 Ascertainment of exposure | 8 Same method of ascertainment for cases and controls (yes=1, no=0) | 9 Nonresponse rate | ||
| Wang 1995-1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Wang 1995-2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Pogo 1999 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Etkind 2000 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Melana 2001 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Melana 2002 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Ford 2003 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Ford 2004 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Faedo 2004 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Lawson 2004 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Zammarchi 2006 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Luo 2006 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Lawson 2006 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Hachana 2008 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Lawson 2010 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Mazzanti 2011 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Glenn 2012 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Morales-Sanchez 2013-1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
1 score can be allotted in this category for other controlled factors (proportion of cancer cells in the cut selection, patients location and so on).
MMTV-LV infection and breast cancer risk
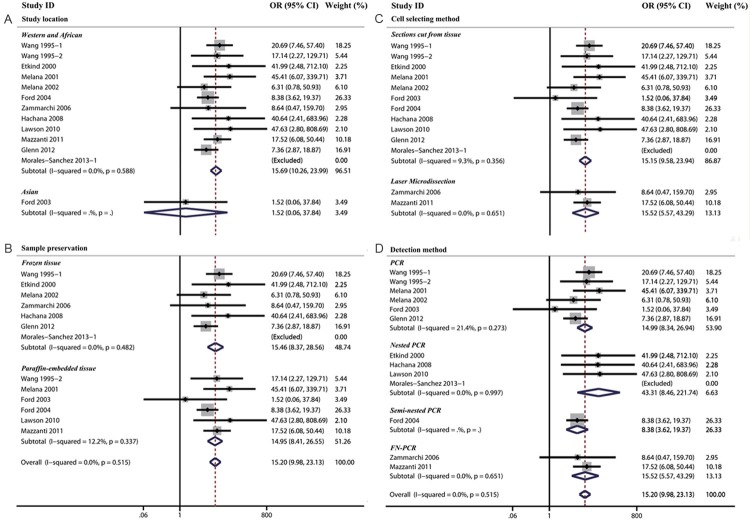
Thirteen data sets were collected from 12 studies that reported on the association between MMTV-LV infection and breast cancer risk. Of all the 13 data sets, 9 demonstrated an apparent hazardous association [11,13,14,16,23,31,32,34], 3 reported no significant relationship [18,27,28], and 1 was automatically excluded by the software for the zero MMTV-LV prevalence in both case and control groups [47]. Overall, meta-analysis of the case-control studies demonstrated that MMTV-LV infection (as compared to non-infection) increased the risk to develop breast cancer by 15.20 fold (OR=15.20, 95% CI: 9.98-23.13).
There was no significant heterogeneity between studies (Cochran’s Q test: p=0.515, I2=0.0%). No significant difference was noted in subgroup analysis based on study location, sample prevention method, cell selecting and detection method (Table 3 and Figure 2).
Table 3.
Subgroup and overall analysis of studies comparing the association of MMTV-LV infection and the risk of human breast cancer
| Subgroup analysis | Human breast cancre risk | p-value for difference between sub-group | |||
|---|---|---|---|---|---|
|
| |||||
| N | OR | 95% CI | |||
| Study location | Asian | 1 | 1.52 | 0.06-37.84 | 0.11 |
| Western and African | 11 | 15.69 | 10.26-23.99 | ||
| Sample preservation | Paraffin-embedded tissue | 6 | 14.95 | 8.41-26.55 | 0.96 |
| Frozen tissue | 6 | 15.46 | 8.37-28.56 | ||
| Cell selecting method | Sections cut from tissue | 10 | 15.15 | 9.58-23.94 | 0.94 |
| Laser Microdissection | 2 | 15.52 | 5.57-43.29 | ||
| Detection method | PCR | 6 | 14.99 | 8.34-26.94 | 0.94 |
| Nested PCR | 3 | 43.31 | 8.46-221.74 | ||
| Semi-nested PCR | 1 | 8.38 | 3.62-19.37 | ||
| FN-PCR | 2 | 15.52 | 5.57-43.29 | ||
| Overall analysis | 12 | 15.20 | 9.98-23.13 | - | |
95% CI: 95% confidence interval, OR: odds ratio.
Figure 2.
The association between MMTV-LV infection and breast cancer risk were analyzed by subgroup analysis based on study location (A), cell selction methods (B), sample preservation (C) and detection methods (D).
MMTV-LV infection and the transformation risk
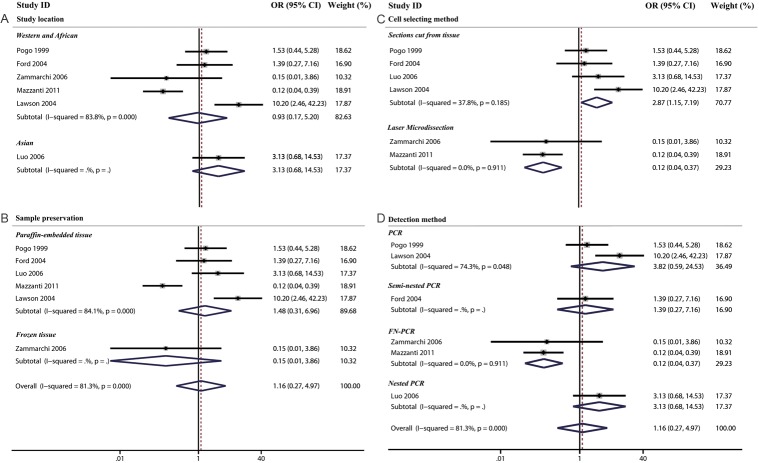
Subsequently, we analyzed whether MMTV-LV infection could increase the risk for developing invasive ductal carcinoma (IDC) from ductal carcinoma in situ (DCIS). Six studies including IDC and DCIS groups were selected for meta-analysis [13,18,19,22,23,33]. Of all the six studies, 1 demonstrated an apparent hazardous association [22], 1 indicated an obvious protective association [13], while the remaining four studies reported no significant relationship [18,19,23,33]. Overall meta-analysis of the case-control studies indicated that MMTV-LV infection (as compared to noninfection) appeared to have a neutral effect on the risk of transformation from DCIS to IDC (OR=1.16, 95% CI: 0.27-4.97).
There was a significant heterogeneity between studies (Cochran’s Q test: p=0.000, I2=81.3%). When we divided all the studies into two subgroups (selections cut from tissue and laser microdissection) by cell selecting method, we found that the association between MMTV-LV infection and transformation risk was significantly protective in the laser microdissection subgroup (OR=0.12, 95% CI: 0.04-0.37), while a harmful association in the subgroup of sections cut from tissue (OR=2.87, 95% CI: 1.15-7.19) (Table 4 and Figure 3).
Table 4.
Subgroup and overall analysis of studies comparing the association of MMTV-LV infection and the risk of transformation from ductal carcinoma in situ to infiltrating ductal carcinoma
| Subgroup analysis | Transformation risk | p-value for difference between sub-group | |||
|---|---|---|---|---|---|
|
| |||||
| N | OR | 95% CI | |||
| Study location | Asian | 1 | 3.13 | 0.68-14.53 | 0.56 |
| Western and African | 5 | 0.93 | 0.17-5.20 | ||
| Sample preservation | Paraffin-embedded tissue | 5 | 1.48 | 0.31-6.96 | 0.33 |
| Frozen tissue | 1 | 0.15 | 0.01-3.86 | ||
| Cell selecting method | Sections cut from tissue | 4 | 2.87 | 1.15-7.19 | 0.02 |
| Laser Microdissection | 2 | 0.12 | 0.04-0.37 | ||
| Detection method | PCR | 2 | 3.82 | 0.59-24.53 | 0.03 |
| Nested PCR | 1 | 3.13 | 0.68-14.53 | ||
| Semi-nested PCR | 1 | 1.39 | 0.27-7.16 | ||
| FN-PCR | 2 | 0.12 | 0.04-0.37 | ||
| Overall analysis | 6 | 1.16 | 0.27-4.97 | - | |
Figure 3.
The association between MMTV-LV infection and the risk of transformation from CDIS to IDC were analyzed by subgroup analysis based on study location (A), cell selction methods (B), sample preservation (C) and detection methods (D).
Characteristics of MMTV-LV prevalence
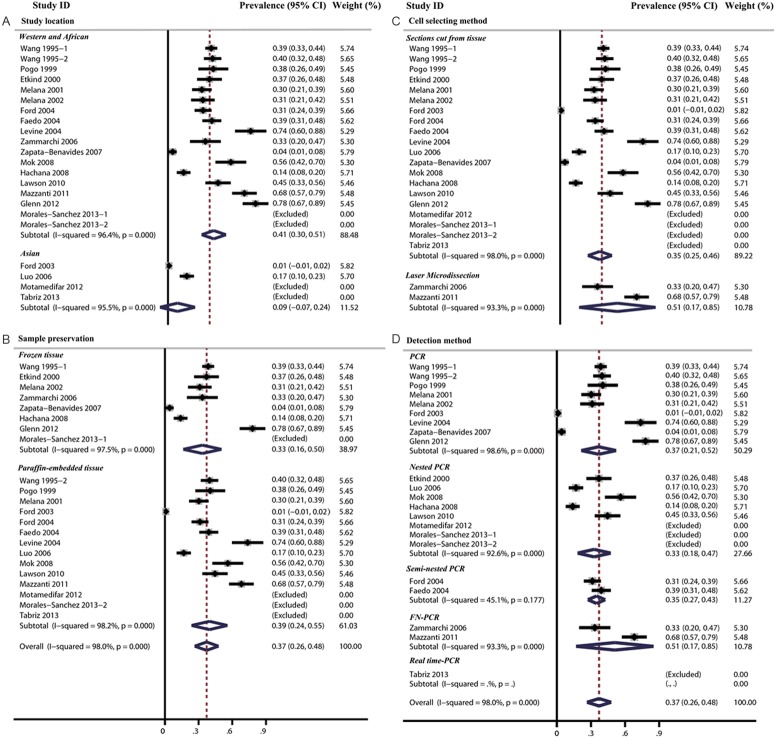
In addition, the prevalence of MMTV-LV in breast cancer tissue ranged from 0% to 78% and the overall MMTV-LV prevalence was 37.0% (95% CI: 26.4-47.6%). The heterogeneity between studies was significant (Cochran’s Q test: p=0.000, I2=98%). In the subgroup analysis, we found that the lowest MMTV-LV prevalence was in Asian subgroup (8.5%, 95% CI: -7.1-24.1%) and the highest prevalence was in Western and African subgroup (40.6%, 95% CI: 29.9-51.4%). The difference between the two subgroups was statistically significant (p=0.02) (Table 5 and Figure 4). Then, we specially compared MMTV-LV prevalence in Asian and Western patients (prevalence=40.4%, 95% CI: 28.9%-51.9%) and also found a significant difference between them (p=0.015).
Table 5.
Subgroup and overall analysis of studies assessing the prevalence of MMTV-LV in human breast cancer
| Subgroup analysis | MMTV-LV prevalence | p-value for difference between sub-group | |||
|---|---|---|---|---|---|
|
| |||||
| N | Prevalence (%) | 95% CI (%) | |||
| Study location | Asian | 2 | 8.5 | -7.1-24.1 | 0.02 |
| Western and African | 16 | 40.6 | 29.9-51.4 | ||
| Sample preservation | Paraffin-embedded tissue | 11 | 39.4 | 23.5-55.4 | 0.74 |
| Frozen tissue | 7 | 33.4 | 16.4-50.4 | ||
| Cell selecting method | Sections cut from tissue | 16 | 35.3 | 24.5-46.0 | 0.31 |
| Laser Microdissection | 2 | 51.0 | 16.9-85.1 | ||
| Detection method | PCR | 9 | 36.5 | 21.2-51.8 | 0.35 |
| Nested PCR | 5 | 32.8 | 18.2-47.4 | ||
| Semi-nested PCR | 2 | 34.9 | 27.3-42.6 | ||
| FN-PCR | 2 | 51.0 | 16.9-85.1 | ||
| Overall analysis | 18 | 37.0 | 26.4-47.6 | - | |
Figure 4.
The MMTV-LV prevalence in women breast cancer tissue were analyzed by subgroup analysis based on study location (A), cell selction methods (B), sample preservation (C) and detection methods (D).
MMTV-LV infection and clinical parameters
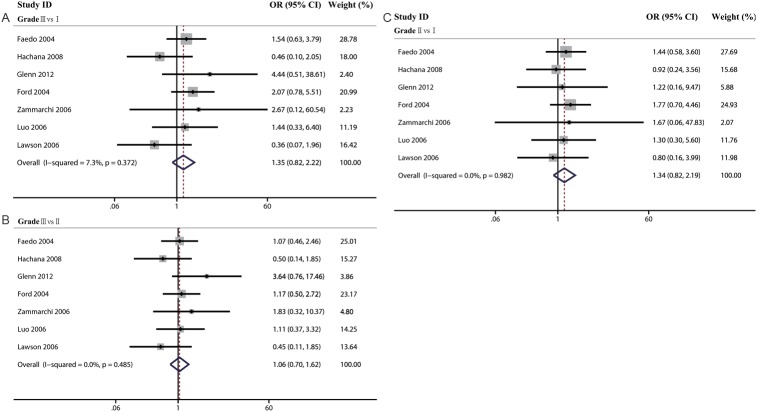
In the last part, we analyzed the association of MMTV-LV prevalence and clinical parameters such as estrogen receptor (ER), progesterone receptor (PR), Her-2, p53 expression and histological grade. In the meta-analysis of pathological parameters, we did not find any positive association between MMTV-LV infection and the expression of ER (OR=0.89, 95% CI=0.48-1.65), PR (OR=0.73, 95% CI: 0.22-2.42), HER2 (OR=0.65, 95% CI: 0.30-1.43) and p53 (OR=1.47, 95% CI: 0.79-2.73). Then, we made three meta-analysis to explore the differences among histological grades (GradeIII vs I, GradeIII vs II, and GradeII vs I). With the same results, we did not find any positive association (GradeIII vs I: OR=1.35, 95% CI: 0.82-2.22; GradeIII vs II: OR: 1.06, 95% CI=0.70-1.62; GradeII vs I: OR: 1.34, 95% CI: 0.82-2.19) (Table 7, Figures 5 and 6).
Table 7.
Meta-analysis of the association of clinical parameters and MMTV-LV prevalence in human breast cancer
| parameters | The association of clinical parameters and MMTV-LV | |||
|---|---|---|---|---|
|
| ||||
| N | OR | 95% CI | ||
| ER | 3 | 0.89 | 0.48-1.65 | |
| PR | 4 | 0.73 | 0.22-2.42 | |
| HER2 | 3 | 0.65 | 0.30-1.43 | |
| P53 | 3 | 1.47 | 0.79-2.73 | |
| Grade | III vs I | 7 | 1.35 | 0.82-2.22 |
| III vs II | 1.06 | 0.70-1.62 | ||
| II vs I | 1.34 | 0.82-2.19 | ||
Figure 5.
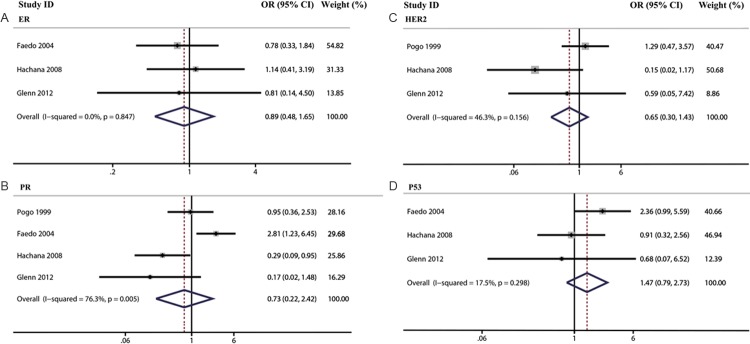
The association of MMTV-LV prevalence with ER (A), HER2 (B), PR (C) and p53 (D) were analyzed.
Figure 6.
The association of MMTV-LV prevalence with different histological grades as indicated were analyzed.
Sensitivity analysis and publication bias
To assess where any one study had a dominant effect on the meta-analytic results, each study was excluded and its effect on the main summary estimate and Cochran’s Q-test p-value for heterogeneity was evaluated. The results were consistent, revealing a relatively low sensitivity to restrictions and credible meta-analysis results. When we excluded the studies with the most weight for each individual analysis (Ford et al for breast cancer risk, Mazzanti et al for transformation risk, Ford et al for MMTV-LV prevalence, Faedo et al for ER, PR and histological grades, Hachana et al for HER2 and p53) [13,16,23,24,27], the conclusions of the main analysis did not change significantly for breast cancer occurring risk (OR=17.63, 95% CI: 10.82-28.73), transformation risk (OR=2.27, 95% CI: 0.81-6.33), prevalence of MMTV-LV in breast cancer tissue (rate=0.39, 95% CI: 0.29-0.49) or the association of MMTV-LV infection with ER (OR=1.04, 95% CI: 0.43-2.49), PR (OR=0.46, 95% I: 0.17-1.25), P53 (OR=1.97, 95% CI: 0.90-4.30), HER2 (OR=1.17, 95% CI: 0.45-3.01) or histological grades (Grade III vs I: OR=1.27, 95% CI: 0.70-2.32; Grade III vs II: OR=1.06, 95% CI: 0.66-1.72, and GradeII vs I: OR=1.30, 95% CI: 0.73-2.33).
There was no evidence revealing publication bias, both quantitatively (p=0.39 for breast cancer occurring risk, p=0.92 for transformation risk, p=0.37 for the prevalence of MMTV-LV, p=0.97 for ER, p=0.20 for PR, p=0.43 for HER2, p=0.56 for p53, p=0.83 for Grade III vs I, p=0.82 for Grade III vs II, p=0.36 for Grade II vs I) and qualitatively, on visual inspection of the funnel plot for studies (figures not shown).
Discussion
Studies on the relevance of MMTV-LV to human breast carcinogenesis started shortly after clarifying the role and mechanism of viruses to cancer development. Early at the 1970s and 1980s, MMTV-like antigens or viral particles have been detected in human milk or breast cancer cells and variable immunological responses to MMTV-LV antigens was observed in women with breast cancer [49-51]. However, hybridization experiments unexpectedly revealed that the DNA detected was homologous to human endogenous retroviruses (HERVs) [52]. Until recently, PCR studies using primers from selected regions of the MMTV env gene which have low homology to known HERVs, detected MMTV-like DNA sequences in human breast carcinoma tissues [34]. Interests in a viral etiology of breast carcinoma were therefore rekindled. Unfortunately, the reported prevalence of MMTV-LV infection in breast carcinoma samples varied geographically, and some researchers even reported their inability to detect MMTV-LV sequence using PCR in human breast cancer tissues. These studies stimulated a new wave of controversy on the relevance of MMTV-LV to human breast cancer.
So we made a comprehensive meta-analysis to summarize the inconsistent results of previous studies. In this meta-analysis of 22 studies analyzing the association between MMTV-LV infection and breast cancer, we found that MMTV-LV infection could significantly increase the risk of breast cancer (15.20 fold), whereas MMTV-LV infection was associated with neutral or possibly slightly increased risk of transformation from DCIS to IDC. However, the meta-analysis of transformation risk was limited by substantial heterogeneity across studies that could be explained by differences in cell selecting method or detection method. When we pooled the MMTV-LV prevalence in breast cancer tissue in this meta-analysis, we found the prevalence was much higher in Western patients than that in Asian, whereas, in addition, we did not find any positive association between MMTV-LV infection and expression of ER, PR, HER2, p53 or histological grades. Based on the data from our study, we speculate that the variable prevalence of MMTV-LV infection in breast cancer tissue may at least partially explain why breast cancer incidence rate was much higher in Western women than that in Asian [53,54].
The tumorigenic effect of MMTV-LV is postulated to be mediated by proviral DNA integration near cellular proto-oncogenes that activates their transcription. Analysis of the proviral sequences demonstrates that the LTRs of MMTV-LV contain all of the enhancer and promoter elements and MMTV-LV genes, 95% homologous to MMTV, have potential for expression, replication and integration into the hosts’ cell genome [55]. Indeed, the mammalian Wnt gene family and several other oncogenes were discovered because of their association with MMTV [56]. Because MMTV integration does not appear to be site-specific [57], the more viruses produced, the more likely it is that proviral DNA will integrate near a proto-oncogene. Thus, latency and incidence of tumor formation are proportional to virus load [58]. In addition, the env protein also participates in MMTV-mediated transformation of mammary epithelial cells, since mutation of the Env protein reduces MMTV-induced mammary tumorigenesis without effecting infection levels [59].
Based on the theory, the incidence rate of breast cancer should roughly parallel the prevalence of MMTV-LV in women breast cancer tissue in different countries. Overall, breast cancer incidence rate varies 4-fold by geographic location between countries with the highest and lowest rates. North American and northern European countries have the highest incidence rates of breast cancer; intermediate levels have been reported in Western Europe and Oceania; while Asia has the lowest levels [53]. In our meta-analysis results, the prevalence of MMTV-LV in Western women breast cancer tissue is 40.4% (95% CI: 28.9%-51.9%), which is much higher than that in Asian (rate=8.5%, 95% CI: -7.1%-24.1%, p=0.015). This result supports the association between MMTV-LV infection and human breast cancer risk.
More intriguing is that the incidence of breast cancer correlates with the geographic distribution of various species of wild mice [60]. Sage et al. treat mice as three separate species, namely Mus domesticus, Mus musculus and Mus castaneus [61]. The ability to transmit MMTV varies in the three species. The common mice, Mus domesticus, as compared with Mus musculus and Mus castaneus are thought to shed a higher viral burden of MMTV [60]. Usually landed from western Iran and North Africa to Western Europe, Mus domesticus mice gradually expanded their range to North and South America, Australia, New Zealand and Hawaii via ships sailing from western European ports. The range of Mus musculus abuts that of Mus domesticus from east Europe to east Asia and Mus castaneus lives from southern China to central Iran [61]. The incidence of breast cancer is much higher in Mus domesticus lands than that in other mice lands [60]. Such a clear geographic pattern of mice distribution is related to the incidence of breast cancer and the prevalence of MMTV-LV in women breast cancer tissue.
Mice may be a zoonotic source of MMTV-LV infection in human breast cancer. The sequence of MMTV-LV is 95% homologous to MMTV [55], and the distribution of mice is associated with breast cancer incidence [60] and MMTV-LV prevalence in women breast cancer tissue. Both two points suggest that MMTV-LV infection should be considered a zoonotic infection, and that MMTV-LV has been transferred to human from mice. As a supportive argument for early human exposure, they cite ancient documents indicating that mouse fecal pellets have been present in stored grains, and thus could have found their way into the human alimentary tract. By another way, cats may become infected by MMTV from mouse and then transmit an adapted virus to humans [62].
In the overall meta-analysis of the association between MMTV-LV infection and the risk of transformation from CDIS to IDC, we found a neutral effect of MMTV-LV infection on the transformation from CDIS to IDC (OR=1.16, 95% CI: 0.27-4.97). But when we divided the studies by cell selecting methods or detection methods, we found that the association between MMTV-LV infection and transformation risk was significantly protective in the laser capture microdissection (LCM) and fluorescence-nested PCR (FN PCR) subgroup (OR=0.12, 95% CI: 0.04-0.37), by contrast, the association in the subgroup of sections cut from tissue was harmful (OR=2.87, 95% CI: 1.15-7.19).
LCM process does not alter or damage the morphology and chemistry of the sample collected, nor the surrounding cells. For this reason, LCM is a useful method of collecting selected cells for DNA, RNA and/or protein analyses. LCM can be performed on a variety of tissue samples including frozen and paraffin embedded archival tissue [63]. There were two studies using this method [13,18] in the meta-analysis. They carefully excluded stromal and inflammatory cells and exactly selected cancer cells. Compared with traditional selecting method, LCM is more exact and reliable. The two studies also adopted FN PCR to detect MMTV ENV gene sequence. This method was proved as sensitive and robust for detecting MMTV-LV in human tissues as other traditional approaches such as combined nested-PCR/agarose gel electrophoresis/southern blot/autoradiography [64,65]. Although the two studies used a more exact and reliable method, their sample sizes were smaller. So the results need to be confirmed by more studies adopting LCM method and FN PCR.
There were several studies referring to the association between MMTV-LV prevalence and clinical parameters of women breast cancer. Their results were not consistent, so we explored the overall effects in this comprehensive meta-analysis. We showed no correlation between the prevalence of MMTV-LV and the histological grades, expression of ER, PR, HER2 or p53.
In addition, we did not find any significant difference between frozen and formalin-fixed paraffin-embedded tissues.
There were two major limitations to our meta-analysis that merit consideration. First, the cancer-modifying association between MMTV-LV infection and breast cancer risk was based on data from case-control studies. There were few perspective studies to include into this meta-analysis. In order to confirm the conclusion that MMTV-LV infection do contribute to the etiology of breast cancer, more perspective studies are needed to perform. Second, there were only two studies using LCM method, while the others all used scalpel blades when scraping sections from paraffin-embedded or frozen tissues. The researchers could not control the proportion of cancer cells when they adopted the latter method. This may be the partial source of heterogeneity between studies. We should pay more attention to the cell selecting method in the future studies.
Based on the results of this meta-analysis, it appears that MMTV-LV infection could increase the risk of breast cancer. We find no positive association between MMTV-LV prevalence and histological grades, expression of ER, PR, HER2 or p53 in breast cancer tissue. However, the prevalence of MMTV-LV is much higher in Western women breast cancer tissue than in tissues of Asian patients. Our results support the zoonotic hypothesis that human acquired MMTV from house mice. These results may provide important guidelines to breast carcinoma diagnosis, prevention and treatment.
Acknowledgements
This work was supported by Ministry of Education (20110101110137), Natural Science Foundation of Zhejiang Province (LR12H16001), and 973 project (No.2012CB526600).
Disclosure of conflict of interest
We declare no conflict of interest. The results included in the submission have not been published and is not under consideration for publication in any forms.
References
- 1.Parkin D. Cancer incidence in five continents. World Health Organization. 2005 [Google Scholar]
- 2.Hunter DJ, Hankinson SE, Laden F, Colditz GA, Manson JE, Willett WC, Speizer FE, Wolff MS. Plasma organochlorine levels and the risk of breast cancer. New Engl J Med. 1997;337:1253–1258. doi: 10.1056/NEJM199710303371801. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer I. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 4.Winter H, Cheng KK, Cummins C, Maric R, Silcocks P, Varghese C. Cancer incidence in the south Asian population of England (1990-92) Brit J Cancer. 1999;79:645–654. doi: 10.1038/sj.bjc.6690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter DJ, Spiegelman D, Adami HO, Beeson L, van den Brandt PA, Folsom AR, Fraser GE, Goldbohm RA, Graham S, Howe GR. Cohort studies of fat intake and the risk of breast cancer-a pooed analysis. New Engl J Med. 1996;334:356–361. doi: 10.1056/NEJM199602083340603. [DOI] [PubMed] [Google Scholar]
- 6.Ayotte P, Dewailly E, Ryan J, Bruneau S, Lebel G. PCBs and dioxin-like compounds in plasma of adult Inuit living in Nunavik (Arctic Quebec) Chemosphere. 1997;34:1459–1468. doi: 10.1016/s0045-6535(97)00442-6. [DOI] [PubMed] [Google Scholar]
- 7.Miller AB, Gaudette LA. Breast cancer in circumpolar Inuit 1969-1988. Acta Oncol. 1996;35:577–580. doi: 10.3109/02841869609096989. [DOI] [PubMed] [Google Scholar]
- 8.Bittner JJ. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science. 1936;84:162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- 9.Poon MC, Tomana , Niedermeier W. Serum antibodies against mouse mammary tumor-virus-associated antigen detected nine months before appearance of a breast carcinoma. Ann Intern Med. 1983;98:937–938. doi: 10.7326/0003-4819-98-6-937. [DOI] [PubMed] [Google Scholar]
- 10.Motamedifar M, Saki M, Ghaderi A. Lack of association of mouse mammary tumor virus-like sequences in Iranian breast cancer patients. Med Princ Pract. 2012;21:244–248. doi: 10.1159/000334572. [DOI] [PubMed] [Google Scholar]
- 11.Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One. 2012;7:e48788. doi: 10.1371/journal.pone.0048788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park DJ, Southey MC, Giles GG, Hopper JL. No evidence of MMTV-like env sequences in specimens from the Australian Breast Cancer Family Study. Breast Cancer Res Treat. 2011;125:229–235. doi: 10.1007/s10549-010-0946-4. [DOI] [PubMed] [Google Scholar]
- 13.Mazzanti CM, Al Hamad M, Fanelli G, Scatena C, Zammarchi F, Zavaglia K, Lessi F, Pistello M, Naccarato AG, Bevilacqua G. A mouse mammary tumor virus env-like exogenous sequence is strictly related to progression of human sporadic breast carcinoma. Am J Pathol. 2011;179:2083–2090. doi: 10.1016/j.ajpath.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson JS, Glenn WK, Salmons B, Ye Y, Heng B, Moody P, Johal H, Rawlinson WD, Delprado W, Lutze-Mann L, Whitaker NJ. Mouse mammary tumor virus-like sequences in human breast cancer. Cancer Res. 2010;70:3576–3585. doi: 10.1158/0008-5472.CAN-09-4160. [DOI] [PubMed] [Google Scholar]
- 15.Mok MT, Lawson JS, Iacopetta BJ, Whitaker NJ. Mouse mammary tumor virus-like env sequences in human breast cancer. Int J Cancer. 2008;122:2864–2870. doi: 10.1002/ijc.23372. [DOI] [PubMed] [Google Scholar]
- 16.Hachana M, Trimeche M, Ziadi S, Amara K, Gaddas N, Mokni M, Korbi S. Prevalence and characteristics of the MMTV-like associated breast carcinomas in Tunisia. Cancer Lett. 2008;271:222–230. doi: 10.1016/j.canlet.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Zapata-Benavides P, Saavedra-Alonso S, Zamora-Avila D, Vargas-Rodarte C, Barrera-Rodriguez R, Salinas-Silva J, Rodriguez-Padilla C, Tamez-Guerra R, Trejo-Avila L. Mouse mammary tumor virus-like gene sequences in breast cancer samples of Mexican women. Intervirology. 2007;50:402–407. doi: 10.1159/000110652. [DOI] [PubMed] [Google Scholar]
- 18.Zammarchi F, Pistello M, Piersigilli A, Murr R, Di Cristofano C, Naccarato AG, Bevilacqua G. MMTV-like sequences in human breast cancer: a fluorescent PCR/laser microdissection approach. J Pathol. 2006;209:436–444. doi: 10.1002/path.1997. [DOI] [PubMed] [Google Scholar]
- 19.Luo T, Wu XT, Zhang MM, Qian K. [Study of mouse mammary tumor virus-like gene sequences expressing in breast tumors of Chinese women] . Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:844–846, 851. [PubMed] [Google Scholar]
- 20.Lawson JS, Tran DD, Carpenter E, Ford CE, Rawlinson WD, Whitaker NJ, Delprado W. Presence of mouse mammary tumour-like virus gene sequences may be associated with morphology of specific human breast cancer. J Clin Pathol. 2006;59:1287–1292. doi: 10.1136/jcp.2005.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine PH, Pogo BGT, Klouj A, Coronel S, Woodson K, Melana SM, Mourali N, Holland JF. Increasing evidence for a human breast carcinoma virus with geographic differences. Cancer. 2004;101:721–726. doi: 10.1002/cncr.20436. [DOI] [PubMed] [Google Scholar]
- 22.Lawson JS, Tran DD, Ford C, Rawlinson WD. Elevated expression of the tumor suppressing protein p53 is associated with the presence of mouse mammary tumor-like env gene sequences (MMTV-like) in human breast cancer. Breast Cancer Res Treat. 2004;87:13–17. doi: 10.1023/B:BREA.0000041573.09142.00. [DOI] [PubMed] [Google Scholar]
- 23.Ford CE, Faedo M, Crouch R, Lawson JS, Rawlinson WD. Progression from normal breast pathology to breast cancer is associated with increasing prevalence of mouse mammary tumor virus-like sequences in men and women. Cancer Res. 2004;64:4755–4759. doi: 10.1158/0008-5472.CAN-03-3804. [DOI] [PubMed] [Google Scholar]
- 24.Faedo M, Ford CE, Mehta R, Blazek K, Rawlinson WD. Mouse mammary tumor-like virus is associated with p53 nuclear accumulation and progesterone receptor positivity but not estrogen positivity in human female breast cancer. Clin Cancer Res. 2004;10:4417–4419. doi: 10.1158/1078-0432.CCR-03-0232. [DOI] [PubMed] [Google Scholar]
- 25.Etkind PR, Stewart AF, Dorai T, Purcell DJ, Wiernik PH. Clonal isolation of different strains of mouse mammary tumor virus-like DNA sequences from both the breast tumors and non-Hodgkin’s lymphomas of individual patients diagnosed with both malignancies. Clin Cancer Res. 2004;10:5656–5664. doi: 10.1158/1078-0432.CCR-03-0364. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Melana SM, Baker B, Bleiweiss I, Fernandez-Cobo M, Mandeli JF, Holland JF, Pogo BG. High prevalence of MMTV-like env gene sequences in gestational breast cancer. Med Oncol. 2003;20:233–236. doi: 10.1385/MO:20:3:233. [DOI] [PubMed] [Google Scholar]
- 27.Ford CE, Tran D, Deng Y, Ta VT, Rawlinson WD, Lawson JS. Mouse mammary tumor virus-like gene sequences in breast tumors of Australian and Vietnamese women. Clin Cancer Res. 2003;9:1118–1120. [PubMed] [Google Scholar]
- 28.Melana SM, Picconi MA, Rossi C, Mural J, Alonio LV, Teyssié A, Holland JF, Pogo BG. [Detection of murine mammary tumor virus (MMTV) env gene-like sequences in breast cancer from Argentine patients] . Medicina (B Aires) 2002;62:323–327. [PubMed] [Google Scholar]
- 29.Wang Y, Pelisson I, Melana SM, Holland JF, Pogo BG. Detection of MMTV-like LTR and LTR-env gene sequences in human breast cancer. Int J Oncol. 2001;18:1041–1044. doi: 10.3892/ijo.18.5.1041. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Pelisson I, Melana SM, Go V, Holland JF, Pogo BG. MMTV-like env gene sequences in human breast cancer. Arch Virol. 2001;146:171–180. doi: 10.1007/s007050170201. [DOI] [PubMed] [Google Scholar]
- 31.Melana SM, Holland JF, Pogo BG. Search for mouse mammary tumor virus-like env sequences in cancer and normal breast from the same individuals. Clin Cancer Res. 2001;7:283–284. [PubMed] [Google Scholar]
- 32.Etkind P, Du J, Khan A, Pillitteri J, Wiernik PH. Mouse mammary tumor virus-like ENV gene sequences in human breast tumors and in a lymphoma of a breast cancer patient. Clin Cancer Res. 2000;6:1273–1278. [PubMed] [Google Scholar]
- 33.Pogo BG, Melana SM, Holland JF, Mandeli JF, Pilotti S, Casalini P, Menard S. Sequences homologous to the mouse mammary tumor virus env gene in human breast carcinoma correlate with overexpression of laminin receptor. Clin Cancer Res. 1999;5:2108–2111. [PubMed] [Google Scholar]
- 34.Wang Y, Holland JF, Bleiweiss IJ, Melana S, Liu X, Pelisson I, Cantarella A, Stellrecht K, Mani S, Pogo BG. Detection of mammary tumor virus env gene-like sequences in human breast cancer. Cancer Res. 1995;55:5173–5179. [PubMed] [Google Scholar]
- 35.JPT CHH, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011] The Cochrane Collaboration. 2011 [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 37.Mant C, Gillett C, D’Arrigo C, Cason J. Human murine mammary tumour virus-like agents are genetically distinct from endogenous retroviruses and are not detectable in breast cancer cell lines or biopsies. Virology. 2004;318:393–404. doi: 10.1016/j.virol.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Glenn WK, Lawson JS, Whitaker NJ. Mouse mammary tumour-like virus gene sequences and specific breast cancer morphology. J Clin Pathol. 2007;60:1071. doi: 10.1136/jcp.2006.044487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etkind PR, Stewart AF, Wiernik PH. Mouse mammary tumor virus (MMTV)-like DNA sequences in the breast tumors of father, mother, and daughter. Infect Agent Cancer. 2008;3:2. doi: 10.1186/1750-9378-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 41.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 42.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanwal F, White D. “Systematic Reviews and Meta-analyses” in Clinical Gastroenterology and Hepatology. Clin Gastroenterol Hepatol. 2012;10:1184–1186. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 47.Morales-Sanchez A, Molina-Munoz T, Martinez-Lopez JL, Hernandez-Sancen P, Mantilla A, Leal YA, Torres J, Fuentes-Panana EM. No association between Epstein-Barr Virus and Mouse Mammary Tumor Virus with Breast Cancer in Mexican Women. Sci Rep. 2013;3:2970. doi: 10.1038/srep02970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabriz HM, Zendehdel K, Shahsiah R, Fereidooni F, Mehdipour B, Hosseini ZM. Lack of Detection of the Mouse Mammary Tumor-like Virus (MMTV) Env Gene in Iranian Women Breast Cancer using Real Time PCR. Asian Pac J Cancer Prev. 2013;14:2945–2948. doi: 10.7314/apjcp.2013.14.5.2945. [DOI] [PubMed] [Google Scholar]
- 49.Ohno T, Mesa-Tejada R, Keydar I, Ramanarayanan M, Bausch J, Spiegelman S. Human breast carcinoma antigen is immunologically related to the polypeptide of the group-specific glycoprotein of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1979;76:2460–2464. doi: 10.1073/pnas.76.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witkin SS, Sarkar NH, Kinne DW, Good RA, Day NK. Antibodies reactive with the mouse mammary tumor virus in sera of breast cancer patients. Int J Cancer. 1980;25:721–725. doi: 10.1002/ijc.2910250606. [DOI] [PubMed] [Google Scholar]
- 51.Moore DH, Charney J, Kramarsky B, Lasfargues EY, Sarkar NH, Brennan MJ, Burrows JH, Sirsat SM, Paymaster JC, Vaidya AB. Search for a human breast cancer virus. Nature. 1971;229:611–614. doi: 10.1038/229611a0. [DOI] [PubMed] [Google Scholar]
- 52.May FE, Westley BR, Rochefort H, Buetti E, Diggelmann H. Mouse mammary tumour virus related sequences are present in human DNA. Nucleic Acids Res. 1983;11:4127–4139. doi: 10.1093/nar/11.12.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973-1997. Int J Epidemiol. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 54.Parkin D, Whelan S, Raymond L, Young J. Cancer incidence in five continents. 1997;Vol VII [Google Scholar]
- 55.Liu B, Wang Y, Melana SM, Pelisson I, Najfeld V, Holland JF, Pogo BG. Identification of a proviral structure in human breast cancer. Cancer Res. 2001;61:1754–1759. [PubMed] [Google Scholar]
- 56.Callahan R, Smith GH. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene. 2000;19:992–1001. doi: 10.1038/sj.onc.1203276. [DOI] [PubMed] [Google Scholar]
- 57.Faschinger A, Rouault F, Sollner J, Lukas A, Salmons B, Günzburg WH, Indik S. Mouse mammary tumor virus integration site selection in human and mouse genomes. J Virol. 2008;82:1360–1367. doi: 10.1128/JVI.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golovkina T, Prescott J, Ross S. Mouse mammary tumor virus-induced tumorigenesis in sag transgenic mice: a laboratory model of natural selection. J Virol. 1993;67:7690–7694. doi: 10.1128/jvi.67.12.7690-7694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross SR, Schmidt JW, Katz E, Cappelli L, Hultine S, Gimotty P, Monroe JG. An immunoreceptor tyrosine activation motif in the mouse mammary tumor virus envelope protein plays a role in virus-induced mammary tumors. J Virol. 2006;80:9000–9008. doi: 10.1128/JVI.00788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart TH, Sage RD, Stewart AF, Cameron DW. Breast cancer incidence highest in the range of one species of house mouse, Mus domesticus. Br J Cancer. 2000;82:446–451. doi: 10.1054/bjoc.1999.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sage RD, Atchley WR, Capanna E. House mice as models in systematic biology. Syst Biol. 1993;42:523–561. [Google Scholar]
- 62.Szabo S, Haislip AM, Garry RF. Of mice, cats, and men: is human breast cancer a zoonosis? Microsc Res Tech. 2005;68:197–208. doi: 10.1002/jemt.20232. [DOI] [PubMed] [Google Scholar]
- 63.Kihara AH, Moriscot AS, Ferreira PJ, Hamassaki DE. Protecting RNA in fixed tissue: an alternative method for LCM users. J Neurosci Methods. 2005;148:103–107. doi: 10.1016/j.jneumeth.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Cammarota G, Da Prato L, Nicoletti E, Matteucci D, Bendinelli M, Pistello M. Quantitation of feline immunodeficiency provinsses in doubly infected cats using competitive PCR and a fluorescence-based RFLP. J Virol Methods. 1996;62:21–31. doi: 10.1016/0166-0934(96)02085-x. [DOI] [PubMed] [Google Scholar]
- 65.Butler JM, McCord BR, Jung JM, Wilson MR, Budowle B, Allen RO. Quantitation of polymerase chain reaction products by capillary electrophoresis using laser fluorescence. J Chromatogr B Biomed Appl. 1994;658:271–280. doi: 10.1016/0378-4347(94)00238-x. [DOI] [PubMed] [Google Scholar]