Abstract
Extracorporeal pulsed electromagnetic field (PEMF) has been shown the ability to improve regeneration in various ischemic episodes. Here, we examined whether PEMF therapy facilitate cardiac recovery in rat myocardial infarction (MI), and the cellular/molecular mechanisms underlying PEMF-related therapy was further investigated. The MI rats were exposed to active PEMF for 4 cycles per day (8 minutes/cycle, 30 ± 3 Hz, 5 mT) after MI induction. The data demonstrated that PEMF treatment significantly inhibited cardiac apoptosis and improved cardiac systolic function. Moreover, PEMF treatment increased capillary density, the levels of vascular endothelial growth factor (VEGF) and hypoxic inducible factor-1α in infarct border zone. Furthermore, the number and function of circulating endothelial progenitor cells were advanced in PEMF treating rats. In vitro, PEMF induced the degree of human umbilical venous endothelial cells tubulization and increased soluble pro-angiogenic factor secretion (VEGF and nitric oxide). In conclusion, PEMF therapy preserves cardiac systolic function, inhibits apoptosis and trigger postnatal neovascularization in ischemic myocardium.
Keywords: Pulsed electromagnetic field, cardiac function, angiogenesis, apoptosis, ischemic myocardium
Introduction
Coronary artery disease is a leading cause of morbidity and mortality in modern society. Massive loss of cardiac muscle after several ischemic episodes lead to compromised cardiac function, remodeling and low quality life of patients. A growing body of evidence in experimental models of cardiac injury suggests that early re-establishment of blood perfusion to the injured myocardium would restrict infarct expansion, prevent cardiac remodeling and maintain cardiac function [1-3]. Although several strategies for therapeutic angiogenesis including the delivery of growth factors, gene therapy and stem cell implantation have been investigated, unsolvable theoretical limitations are still remaining [4-8]. For instance, the limited survival of implanted stem cell, uncontrolled angiogenesis and others [9-11]. Therefore, a safe, effective and non-invasive treatment for myocardial ischemia may be an ideal approach.
The therapeutic efficacy of various forms of electromagnetic stimulations, including capacitative coupling, direct current, combined magnetic fields, and pulsed electromagnetic field (PEMF), have been intensely investigated [12]. Among them, extracorporeal PEMF is the most widely tested techniques in the topic of osteanagenesis [13], skin rapture healing [14] and neuronal regeneration [15,16]. Recently, several study also indicated that PEMF exhibited the capability to stimulate angiogenesis and endothelial proliferation [17-19], however the detailed mechanism remains modest understood.
In the present study, we investigated whether extracorporeal PEMF therapy was able to rescue ischemic myocardium through inhibiting cardiac apoptosis as well as promoting postnatal neovascularization in a rat model of myocardial infarction (MI).
Material and methods
Animals
Male Sprague-Dawley (SD) rats weighing 250-300 g were provided by Sino-British SIPPR/BK Laboratory Animal (Shanghai, China). Animals were housed with controlled temperature (22-25°C) and lighting (08:00-20:00 light, 20:00-08:00 dark), and free access to tap water and standard rat chow. All the animals in this work received humane care in compliance with institutional guidelines for health and care of experimental animals of Shanghai Jiao Tong University.
MI model
All rats (n=36) were subjected to permanent left anterior descending artery ligation to establish MI model. Briefly, left thoracotomy and pericardiectomy were performed, and the hearts were gently exteriorized. Left anterior descending artery was ligated 4 mm below the left atrium with a 5-0 silk suture. The chest wall was then closed and the animals were returned to home cages. MI rats were then randomly divided into PEMF treated and untreated groups.
PEMF treatment
PEMF were generated by a commercially available healing device purchased from Biomobie Regenerative Medicine Technology (Shanghai, China). Fields were asymmetric and consisted of 4.5 ms pulses at 30 ± 3 Hz, with a magnetic flux density increasing from 0 to 5 mT in 400 μs. The MI rats were housed in custom-designed cages and exposed to active PEMF for 4 cycles per day (8 minutes for 1 cycle), while the control rats were housed in identical cages with inactive PEMF generator. For in vitro study, culture dishes were directly exposed to PEMF for 1-4 cycles as indicated (8 minutes for 1 cycle, 30 ± 3 Hz, 5 mT).
Echocardiography
Trans-thoracic echocardiographic analysis was performed using an animal specific instrument (VisualSonics, Vevo770; VisualSonicsInc, Toronto, Canada), at postoperative day 7, 14 and 28. Rats were anesthetized with 10% chloral hydrate solution. After shaving the chest, pre-warmed ultrasound transmission gel was applied to the chest and two dimensional-directed M-mode and Doppler echocardiographic studies were carried out. The ejection fraction (EF) and fractional shortening (FS) were used to assess left ventricular systolic function. All measurements were averaged for consecutive cardiac cycles and triplicated.
Capillary density
Capillary density in peri-infarcted zone (PIZ) was determined by anti-CD31 staining (R&D Systems, San Diego, CA, USA). Briefly, 14 days after MI, rats were euthanized and hearts were perfused with a 0.9% NaCl solution followed by 4% solution of paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4), and then dissected and fixed in this solution for 24 h. Next, samples were washed, dehydrated in a graded ethanol series and embedded in paraffin. 5 μm-sections were cut transversely at 200 μm intervals from into 5 slices from the ligation site to the apex. Endothelial capillaries were identified by goat anti-rat antibody of CD31 (5 μg/ml, Becton-Dickinson Biosciences, Franklin Lakes, NJ, USA), and followed by a secondary antibody (Invitrogen, Carlsbad, CA, USA). Capillary density was determined by counting of 10 randomly selected fields and is expressed as numbers of capillary/field (×400 magnification) [20,21].
Enzyme-linked immunosorbent assay (ELISA)
The concentration of vascular endothelial growth factor (VEGF) and nitric oxide (NO) contained in conditional media of cultured HUVECs was measured using ELISA kit purchased from R&D Systems (San Diego, CA, USA). The concentrations of VEGF contained in PIZ was determined by ELISA kits purchased from Raybiotech (Norcross, GA, USA) [22].
Western blotting
PIZ tissue and HUVECs were homogenized with ice-cold homogenizing buffer (20 μl/gram tissue, 50 mmol/l Tris-HCl, 150 mmol/l NaCl, 1 mmol/l EDTA, and 0.5 mmol/l Triton X-100, pH 7.4) and protease inhibitor cocktail (5 mM, Roche, Berlin, Germany). Proteins were measured with Pierce BCA Protein Assay Kit (Thermo, Asheville, North Carolina, USA). Hippocampal protein lysates (50 mg/well) were separated using SDS-PAGE under reducing conditions. Following electrophoresis, the separated proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, Massachusetts, USA). Subsequently, nonspecific proteins were blocked using blocking buffer (5% nonfat dried milk in T-TBS containing 0.05% Tween 20), followed by incubation with primary rabbit anti-rat antibodies specific for phospho-Akt (p-Akt), total Akt, hypoxic-inducible factor (HIF)-1α (Santa Cruz, California, USA), phospho-endothelial nitric oxide synthase (p-eNOS), total eNOS and β-actin (Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C. Blots were washed four times with 0.1% Tween 20 in PBS and incubated with HRP-conjugated secondary antibody (1/5000; Biochain, Newark, California, USA) for 1 h at room temperature. The bands were visualized using enhanced chemiluminescence method (Bioimaging System; Syngene, Cambridge, UK). Intensity of the tested protein bands was quantified by densitometry.
Detection of apoptosis
Heart samples were fixed in 10% formalin and then paraffin embedded at day 14. Then, the hearts were cut into 5-μm sections. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was carried out using a commercially available kit according to the manufacturer’s instructions (Promega, Madison, Wisconsin, USA). Nuclei were stained by DAPI (Roche) [23]. Three mid-ventricular sections of each heart (from the apex to the base) were analyzed. Ten fields in the PIZ were randomly selected from each section for the calculation of the percentage of apoptotic nuclei (apoptotic nuclei/total nuclei) and the obtained ratios were averaged for statistical analysis.
Isolation of circulating endothelial progenitor cells (EPCs)
Circulating EPCs were obtained by cardiac puncture after animals were anesthetized. Peripheral blood-derived mononuclear cells (PB-MNCs) were then purified by Histopaque-1083 (Sigma-Aldrich, St. Louis, MO, USA) density gradient centrifugation at 400 g for 30 min. The mononuclear layer was then collected and re-suspended in endothelial growth medium-2 (EGM-2, Clonetics, San Diego, CA, USA). Antibodies to the stem cell antigen-1 (Sca-1) and Flk-1 were used to mark EPC as described before), and the isotype specific conjugated anti-IgG was used as a negative control. Sca-1+ and Flk-1+ cells were gated in the mononuclear cell fraction.
EPC migration assay
Migratory activity of PB-EPCs from PEMF-treated and untreated rats was evaluated by a 24-well modified Boyden chamber assay (Transwell, Corning, NY, USA) [24]. After cultured with EGM-2 for 4 days, PB-EPCs were trypsinized and 5×105 cells in 100 μl of EBM-2 with 0.1% BSA in placed in the upper compartments. 50 ng/mL recombinant vascular endothelial growth factor (VEGF, Clonetics) in 600 μL of chemotaxis buffer (serum-free EBM-2, 0.1% BSA) was added to the lower compartment. The chamber was incubated at 37°C for 6 hrs. The cells were then fixed and stained with hematoxylin and eosin (H&E). Non-migrated cells on the filter’s upper surface were removed using a cotton swab. The numbers of migrated cells were counted in 4 random high-power fields (HPF, ×400 magnification) and averaged for each sample.
Tube formation assay
Matrigel-Matrix (BD Biosciences, Franklin Lakes, New Jersey, USA) was inserted in the well of a 48-well cell culture plate and a number of 5×104 EPCs or HUVECs were seeded [25].
After incubation in EGM-2, images of tube morphology was taken and tube number was counted at random under four low power fields (magnifications ×40) per sample. Capillary tube branch points were counted in six randomly selected fields per well, and used as an index for tube formation.
Cell culture
Human umbilical vein endothelial cells (HUVECs, passage 3) were purchased from Clonetics (San Diego, CA, USA) and EGM-2 in a humidified atmosphere of 5% CO2 and 95% air. HUVECs were reseeded into plates coated with Matrix gel and stimulated for 1-4 cycles of PEMF stimulation (5.5 mT, 8 minutes per cycle). Supernatant and cell lysates were collected at 24 hrs after reseeding. Additionally, HUVECs-formed vasculature was quantified by calculating its length under microscopic photography 24 hrs after reseeding [26].
Statistical analysis
Data are expressed as means ± standard deviation (SD). Student’s t-test was used for statistical analyses. SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) was used. A value of p<0.05 was considered significant.
Results
PEMF promotes cardiac function after MI
To determine whether PEMF could increase myocardial function in MI rats, echocardiographic studies were carried out at postoperative day 7, 14 and 28. We observed that PEMF had no effects on body weight and heart rates when compared with control group (Table 1). Meanwhile, higher EF and FS values were detected in PEMF-treated rats than control (Figure 1), indicating that PEMF preserves left ventricular contractility after MI damage.
Table 1.
Effect of PEMF on cardiac functions of MI rats
| Parameter | Day 7 | Day 14 | Day 28 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | PEMF | Control | PEMF | Control | PEMF | |
| BW (g) | 314.36 ± 45 | 329.39 ± 38 | 298.64 ± 24 | 308.74 ± 29 | 289.72 ± 25 | 294.93 ± 28 |
| HR (bpm) | 419.81 ± 39 | 430.58 ± 29 | 436.50 ± 31 | 429.17 ± 27 | 417.64 ± 35 | 408.73 ± 26 |
| LVAWs (mm) | 1.02 ± 0.4 | 0.97 ± 0.7 | 0.79 ± 1.2 | 2.14 ± 0.5 | 0.75 ± 0.5 | 1.31 ± 0.9 |
| LVAWd (mm) | 1.07 ± 1.0 | 1.19 ± 1.4 | 0.63 ± 1.3 | 1.57 ± 1.8 | 0.67 ± 0.7 | 1.24 ± 0.8 |
| IVSTs (mm) | 2.04 ± 1.6 | 2.54 ± 1.8 | 2.62 ± 2.1 | 2.14 ± 1.6 | 3.06 ± 2.1 | 3.28 ± 1.6 |
| IVSTd (mm) | 1.33 ± 1.0 | 1.72 ± 2.0 | 1.67 ± 1.7 | 1.93 ± 1.4 | 1.86 ± 1.6 | 2.12 ± 1.9 |
| LVPWs (mm) | 6.79 ± 2.4 | 5.31 ± 2.7 | 5.09 ± 2.5 | 4.21 ± 1.8 | 4.06 ± 1.6 | 3.96 ± 1.7 |
| LVPWd (mm) | 6.73 ± 1.0 | 5.28 ± 1.7 | 5.92 ± 0.8 | 5.78 ± 2.1 | 6.77 ± 2.5 | 6.01 ± 2.7 |
| LV Vol s (μl) | 297.41 ± 29 | 246.79 ± 24 | 348.24 ± 37 | 285.81 ± 42 | 351.54 ± 43 | 317.56 ± 47 |
| LV Vol d (μl) | 326.54 ± 31 | 345.11 ± 34 | 544.36 ± 39 | 397.42 ± 36 | 486.66 ± 48 | 493.08 ± 40 |
| EF (%) | 29.45 ± 2.2 | 36.73 ± 3.1† | 28.23 ± 1.1 | 32.83 ± 1.7§ | 28.80 ± 0.7 | 35.00 ± 1.4# |
| FS (%) | 14.34 ± 1.8 | 15.24 ± 0.9 | 15.63 ± 2.8 | 18.94 ± 1.6§ | 18.54 ± 2.6 | 22.54 ± 1.2# |
Data were presented as mean ± SEM. PEMF, Pulsed Electromagnetic Field; MI, myocardial infarction; BW, body weight; HR, heart rate; LVAW, left ventricular anterior wall; IVST, interventricular septal thickness; LVPW, left ventricular posterior wall; s, systolic; d, diastolic; Vol, volume; EF, ejection fraction; FS, fractional shortening.
p<0.05 versus control (day 7);
p<0.05 versus control (day 14);
p<0.01 versus control (day 28).
Figure 1.
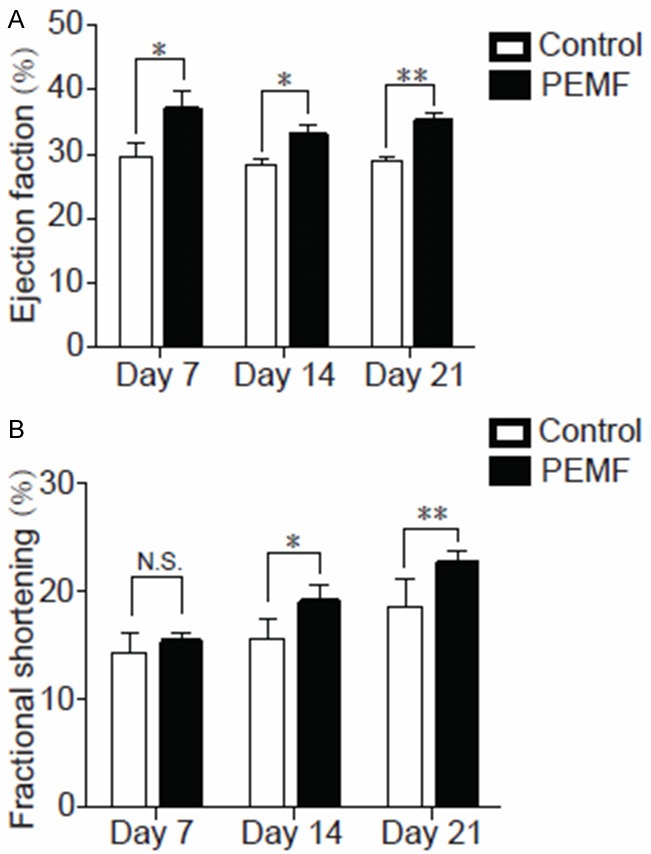
Echocardiography after PEMF therapy. All rats were subjected to MI and randomly separated to control and PEMF group. The data of (A) ejection fractions and (B) fractional shorting in both groups collected in day 7, 14 and 28. Values are mean ± SEM; n=6, N.S. means no significant difference, *means p<0.05, **means p<0.01.
PEMF enhances angiogenesis in PIZ
To examine whether the changes in the cardiac function are associated with changes in capillary EC formation, we measured capillary densities of PEMF and control rats in PIZ through anti-CD31 immunofluorescence staining at postoperative day 14. Representative photomicrographs are shown in Figure 2A. Quantitative analyses by counting the CD31+ capillary ECs revealed that PEMF treatment significantly increases capillary densities in PIZ than control rats (Figure 2B). PEMF treatment also increased the protein levels of VEGF and HIF-1α in damaged hearts (Figure 2C and 2D), as well as enhancing the phosphorylation of Akt signal pathway in ischemic myocardium at postoperative day 14 (Figure 2E).
Figure 2.
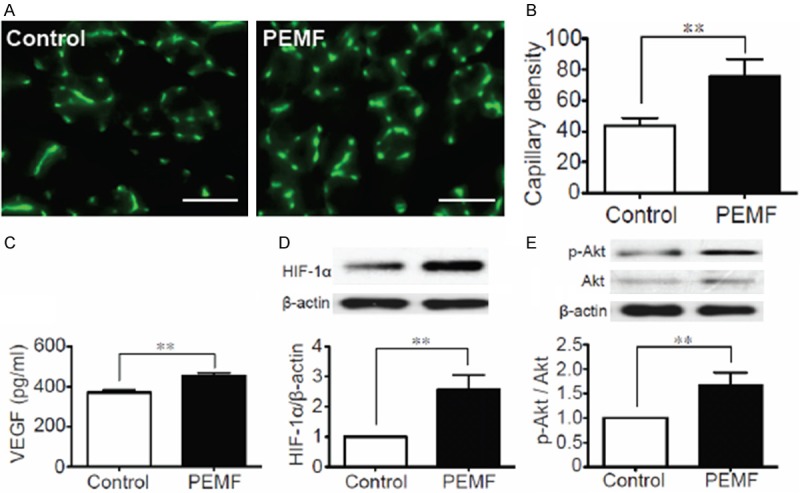
Pro-angiogenic effect of PEMF in ischemic myocardium. A: Immunofluorescence staining of CD31-positive cells in the infarct border zone at postoperative day 14 in PEMF-treated and control rats. B: Quantitative analyses of capillary density between 2 groups in border area. Capillary density was identified with capillary per field (×400 magnification). C: Quantitative analysis of VEGF concentrations in the experimental groups in the ischemic myocardium in day 14. D, E: Quantitative analysis of protein content of HIF-1α and phosphorylated Akt (p-Akt) 14 days after MI in 2 groups. Data of Western blotting were represented as fold of control. Values are mean ± SEM; n=6. **means p<0.01. Scale bar=50 μm.
Protective effect of PEMF to MI-induced cardiac apoptosis
We evaluated the effect of PEMF on the survival of myocardium in response to hypoxia in vivo at postoperative day 7. The number of TUNEL positive nucleus in PIZ significantly increased in PEMF-treated rats compared with the non-treated ones (Figure 3), indicating that PEMF treatment decreases the susceptibility of cardiomyocytes to hypoxic damage.
Figure 3.
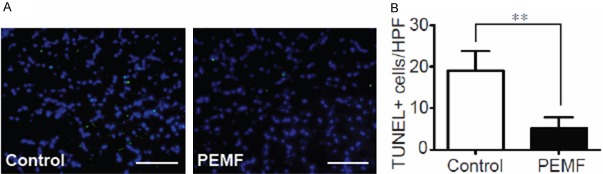
Anti-apoptotic benefit of PEMF in damaged myocardium. A: TUNEL staining for cardiac cell apoptosis (green) and DAPI (blue) for nuclear staining in the border zone 14 days after AMI (×400 magnification). B: Quantitative analysis of the TUNEL-positive cells versus high-power field (HPF) between 2 groups. Values are mean ± SEM; n=6, **means p<0.01 vs. control group. Scale bar=50 mm.
PEMF augments EPC-mediated neovascularization
EPC-mediated neovascularization after myocardial infarction supported their therapeutic potential [27]. Thus, the strategy to amplify EPC abundance and function is an active focus of research. The number of circulating EPCs was identified by stem cell antigen-1 (Sca-1)/fetal liver kinase-1 (flk-1) dual positive cells as described. We found that PEMF treatment increased the number of Sca-1+/flk-1+ cells in peripheral blood at postoperative day 7 and 14 (Figure 4A). Additionally, EPCs isolated from PEMF-treated rats exhibited enhanced tube formative capacity and migratory ability when compared with control ones in vitro (Figure 4B and 4C), which suggesting that PEMF increases the abundance and regenerative capacity of EPCs.
Figure 4.
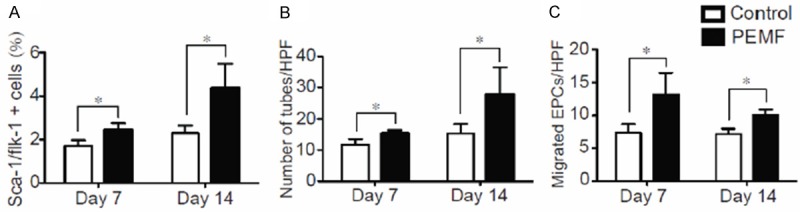
PEMF enhanced circulating endothelial progenitor cells (EPCs) function in MI Rats. 7 and 14 days after AMI induction, peripheral blood was collected from rats in both groups. A: Quantitative analysis of Sca-1/flk-1 dual positive PB-EPCs isolated from the rats with or without PEMF treatment. B: Quantitative capacity of the number of circulating EPCs tube formative capacity. C: Quantitative analysis of the migratory capability of EPCs in PEMF and control groups. Values are mean ± SEM; n=6, *means p<0.05 vs. control group.
Pro-angiogenic beneficial of PEMF in vitro
Cultured HUVECs were treated with PEMF stimulation for 1 to 4 cycles and the supernatant and cell lysate were collected. PEMF promoted VEGF and NO releasing from cultured HUVECs in a dose-dependent manner (Figure 5A and 5B). Additionally, the phosphorylation of eNOS in HUVECs was also enhanced in response to PEMF following a dose dependent manner (Figure 5C). Finally, the HUVEC-formed tubes were lengthened by PEMF in a dose dependent manner (Figure 6).
Figure 5.
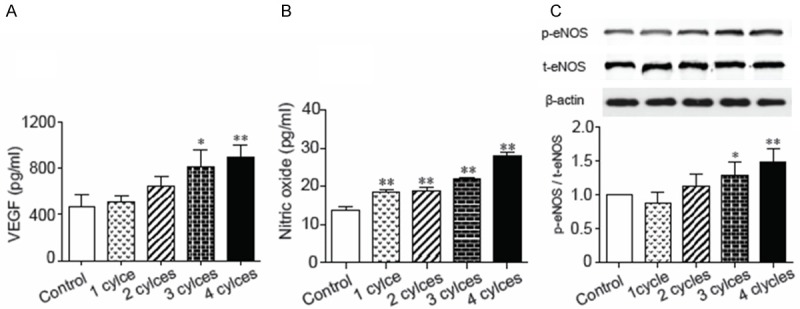
Enhancement of the expression of VEGF and nitric oxide in PEMF-treated HUVECs. PEMF stimulated vascular endothelial growth factors secretion concentration dependently. Bar graph of the concentrations of (A) VEGF and (B) nitric oxide released from HUVECs in response to PEMF stimulation. (C) Quantitative results of protein expression of endothelial nitric oxide synthase (eNOS). Data of Western blotting were represented as fold of control. Values are mean ± SEM; n=5, *means p<0.05 and **means p<0.01 vs. control group.
Figure 6.
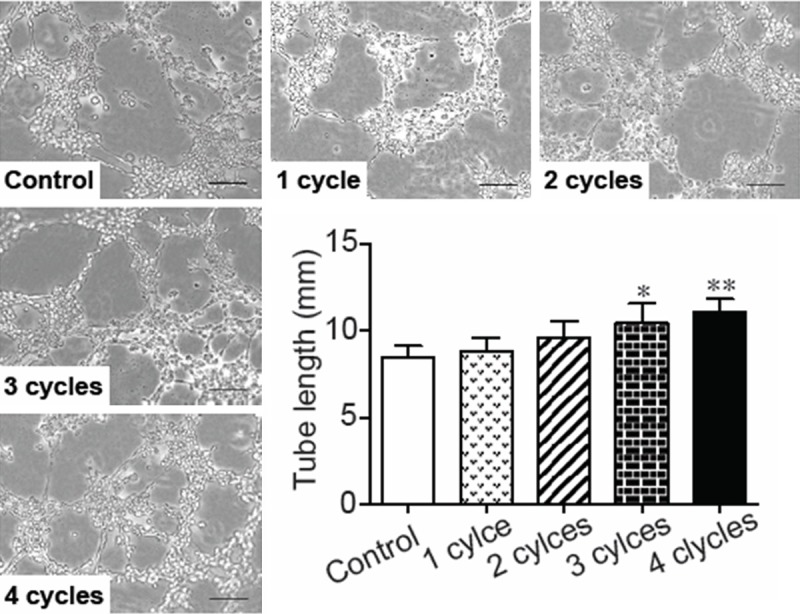
Effects of PEMF on tube formation of cultured HUVECs. Representative images of tube formation in HUVECs by stimulated PEMF for 1-4 cycles and quantitative analysis of tube length formed by PEMF-treated HUVECs. Values are mean ± SEM; n=4. *means p<0.05 and **means p<0.01 vs. control group. Scale bars=500 mm.
Discussion
Major findings of our study are: (1) PEMF prevents cardiomyocytes against hypoxia-induced apoptosis and preserves cardiac systolic function in a rat MI model; (2) PEMF induces angiogenesis and vasculogenesis through activating VEGF-eNOS system and promoting EPCs mobilized to the ischemic myocardium.
We demonstrated that PEMF treatment preserved the cardiac systolic function after MI and prevented cardiac apoptosis. Previous report demonstrated that PEMF treatment activated voltage-gated calcium channels (VGCC) [28], which is crucial for maintaining cardiac contractility and cell survival [29,30]. Increased intracellular Ca2+ produced by PEMF-mediated VGCC activation may lead to increase of NO through the action of eNOS, which is dominant modulator to prevent cardiomyocytes from apoptosis and enhance revascularization in PIZ after MI [31]. Consistent with the previous work, we demonstrated that the HIF-1α/Akt axis was activated in PIZ in PEMF rats. In addition, PEMF induced eNOS phosphorylation in vitro, which is a key molecular served in the survival pathway in both myocardium and endothelial cell lineage [32].
Another possible mechanism in cardiac protecting effect of PEMF is to stimulate neovascularization. Increasing evidence suggests that neovascularization limits infarct expansion and extension, improves cardiac remodeling [1,2]. Recent data demonstrated that PEMF stimulation induced angiogenesis and amplified endothelial cells function [17,20]. Some researchers believe that PEMF induces cellular proliferation, as evidenced by cAMP activation and uptake of tritiated thymidine [33]. In present study, we demonstrated that the capillary density in PIZ was increased after PEMF treatment. Moreover, PEMF therapy triggered the Akt/HIF-1α/VEGF cascade was activated in ischemic myocardium. In in vitro study, we confirmed PEMF-treated HUVECs released more VEGF and NO, which are the key factors response to endothelial proliferation and survival, suggesting that PEMF activates both autocrine and paracrine function of mature endothelial cells. Furthermore, Tepper and colleagues also reported that PEMF stimulated fibroblastic growth factor-2 (FGF-2) releasing and augment angiogenesis [14].
Recent evidence indicates that adult blood vessels may result from not only expansion of existing endothelial cells (angiogenesis), but also the recruitment of endothelial progenitor cells or EPCs (vasculogenesis) [24]. We hypothesized that besides mature endothelial cells, PEMF might also act as a stimulator of progenitor (EPC). To confirm the hypothesis, we examined the effect of PEMF on ex vivo angiogenesis. Our data demonstrated the number of Sca-1/flk-1 dual positive EPCs in peripheral blood increased in response to PEMF. Using the well-established Matrigel assay, we demonstrated that PEMF was able to dramatically enhance the tube formative capacity of either EPCs or mature endothelial cells in vitro. PEMF also accelerated the migratory ability of EPCs. Moreover, Goto et al reported that PEMF stimulation up-regulated the expression of angiopoietin-2 and FGF-2 in bone marrow, suggesting PEMF could promote the regenerative capacity of myeloid-derived cells (such as EPCs) in damaged tissue when recruited. From all these findings, we conclude that PEMF sufficiently re-establishes blood supply to the ischemic and hypoxic cardiomyocytes via enhancing both angiogenesis and vasculogenesis.
In conclusion, our findings indicate that extracorporeal PEMF treatment increases cardiac systolic function through inhibiting cardiac apoptosis and stimulating neovascularization in PIZ. These findings suggest that PEMF deserves further consideration of investigation in its regulation on the signaling pathway and new clinical strategies for ischemic vascular diseases.
Acknowledgements
This work was supported by the Shanghai Science and Technology Committee (11 nm 0503600), the China National Natural Science Foundation (11374213) and Foundation of National Lab for Infrared Physics (200901).
Disclosure of conflict of interest
The authors have nothing to disclose.
References
- 1.Hynes B, Kumar AH, O’Sullivan J, Buneker CK, Leblond AL, Weiss S, Schmeckpeper J, Martin K, Caplice NM. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34:782–789. doi: 10.1093/eurheartj/ehr435. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Zhao L, Shen L, Xu D, Huang B, Wang Q, Lin J, Zou Y, Ge J. Comparison of Various Niches for Endothelial Progenitor Cell Therapy on Ischemic Myocardial Repair Coexistence of Host Collateralization and Akt-Mediated Angiogenesis Produces a Superior Microenvironment. Arterioscler Thromb Vasc Biol. 2012;32:910–923. doi: 10.1161/ATVBAHA.111.244970. [DOI] [PubMed] [Google Scholar]
- 3.Hao CN, Shi YQ, Huang JJ, Li HY, Huang ZH, Cheng XW, Lu W, Duan JL. The power combination of blood-pressure parameters to predict the incidence of plaque formation in carotid arteries in elderly. Int J Clin Exp Med. 2013;6:461–469. [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 5.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene Transfer of Stromal Cell-Derived Factor-1α Enhances Ischemic Vasculogenesis and Angiogenesis via Vascular Endothelial Growth Factor/Endothelial Nitric Oxide Synthase-Related Pathway Next-Generation Chemokine Therapy for Therapeutic Neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 6.Duan JL, Hao CN, Lu W, Han L, Pan ZH, Gu Y, Liu PJ, Tao R, Shi YQ, Du YY. A new method for assessing variability of 24 h blood pressure and its first application in 1526 elderly men. Clin Exp Pharmacol Physiol. 2009;36:1093–1098. doi: 10.1111/j.1440-1681.2009.05196.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama K, Takaji K, Kataoka K, Kurihara Y, Yoshimura M, Kato A, Ogawa H, Kurihara H. Id1 gene transfer confers angiogenic property on fully differentiated endothelial cells and contributes to therapeutic angiogenesis. Circulation. 2005;112:2840–2850. doi: 10.1161/CIRCULATIONAHA.104.516898. [DOI] [PubMed] [Google Scholar]
- 8.Hao CN, Huang ZH, Shi YQ, Lu W, Duan JL. A new index to predict the incidence of cerebral infarction. CNS Neurosci Ther. 2011;17:783–784. doi: 10.1111/j.1755-5949.2011.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Xu DF, Li K, Wang HT, Shen PC, Lin M, Cao XH, Wang R. Neonatal exposure to fluoxetine and fluvoxamine alteres spine density in mouse hippocampal CA1 pyramidal neurons. Int J Clin Exp Pathol. 2011;4:162–168. [PMC free article] [PubMed] [Google Scholar]
- 10.Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH, Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 1991;68:856–869. doi: 10.1161/01.res.68.3.856. [DOI] [PubMed] [Google Scholar]
- 11.Liu AJ, Zang P, Guo JM, Wang W, Dong WZ, Guo W, Xiong ZG, Wang WZ, Su DF. Involvement of Acetylcholine-α7nAChR in the Protective Effects of Arterial Baroreflex against Ischemic Stroke. CNS Neurosci Ther. 2012;18:918–926. doi: 10.1111/cns.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalidis B, Sachinis N, Assiotis A, Maccauro G. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications. Int J Immunopathol Pharmacol. 2011;24:17–20. doi: 10.1177/03946320110241S204. [DOI] [PubMed] [Google Scholar]
- 13.Cheing GL, Li X, Huang L, Kwan RL, Cheung KK. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics. 2014;35:161–169. doi: 10.1002/bem.21832. [DOI] [PubMed] [Google Scholar]
- 14.Kim SS, Shin HJ, Eom DW, Huh JR, Woo Y, Kim H, Ryu SH, Suh PG, Kim MJ, Kim JY, Koo TW, Cho YH, Chung SM. Enhanced expression of neuronal nitric oxide synthase and phospholipase C-gamma1 in regenerating murine neuronal cells by pulsed electromagnetic field. Exp Mol Med. 2002;34:53–59. doi: 10.1038/emm.2002.8. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub MI, Herrmann DN, Smith AG, Backonja MM, Cole SP. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:1102–1109. doi: 10.1016/j.apmr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S, Gan J, Simon B, Hopper RA, Levine JP, Gurtner GC. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004;18:1231–1233. doi: 10.1096/fj.03-0847fje. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Y, Wei L, Li F, Guo W, Li W, Luan R, Lv A, Wang H. Pulsed magnetic field induces angiogenesis and improves cardiac function of surgically induced infarcted myocardium in Sprague-Dawley rats. Cardiology. 2010;117:57–63. doi: 10.1159/000321459. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Dong Y, Hou W, Ji Z, Zhi K, Yin Z, Wen H, Chen Y. Effects of PEMF on microcirculation and angiogenesis in a model of acute hindlimb ischemia in diabetic rats. Bioelectromagnetics. 2013;34:180–188. doi: 10.1002/bem.21755. [DOI] [PubMed] [Google Scholar]
- 19.Delle Monache S, Alessandro R, Iorio R, Gualtieri G, Colonna R. Extremely low frequency electromagnetic fields (ELF-EMFs) induce in vitro angiogenesis process in human endothelial cells. Bioelectromagnetics. 2008;29:640–648. doi: 10.1002/bem.20430. [DOI] [PubMed] [Google Scholar]
- 20.Duan J, Murohara T, Ikeda H, Sasaki K, Shintani S, Akita T, Shimada T, Imaizumi T. Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2000;20:2579–2585. doi: 10.1161/01.atv.20.12.2579. [DOI] [PubMed] [Google Scholar]
- 21.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:III370–376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther. 2013;19:813–819. doi: 10.1111/cns.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Zhao L, Yi-Ming W, Yu YS, Xia CY, Duan JL, Su DF. Sirt1 hyperexpression in SHR heart related to left ventricular hypertrophy. Can J Physiol Pharmacol. 2009;87:56–62. doi: 10.1139/Y08-099. [DOI] [PubMed] [Google Scholar]
- 24.Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, Nakamura K, Di Q, Sasaki T, Tsuzuki M, Shi GP, Okumura K, Murohara T. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation. 2010;122:707–716. doi: 10.1161/CIRCULATIONAHA.109.909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 26.Huang ZH, Guo W, Zhang LL, Song SW, Hao CN, Duan JL. Donepezil protects endothelial cells against hydrogen peroxide-induced cell injury. CNS Neurosci Ther. 2012;18:185–187. doi: 10.1111/j.1755-5949.2011.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanelli C, Coppola S, Barone R, Colussi C, Gualandi G, Volpe P, Ghibelli L. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J. 1999;13:95–102. doi: 10.1096/fasebj.13.1.95. [DOI] [PubMed] [Google Scholar]
- 29.Bers DM, Perez-Reyes E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc Res. 1999;42:339–360. doi: 10.1016/s0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Zhang Y, Li C, Xie J, Liu Y, Zhu W, Zhang X, Jiang S, Liu L, Ding Z. HSPA12B attenuates cardiac dysfunction and remodelling after myocardial infarction through an eNOS-dependent mechanism. Cardiovasc Res. 2013;99:674–684. doi: 10.1093/cvr/cvt139. [DOI] [PubMed] [Google Scholar]
- 31.Hopper RA, VerHalen JP, Tepper O, Mehrara BJ, Detch R, Chang EI, Baharestani S, Simon BJ, Gurtner GC. Osteoblasts stimulated with pulsed electromagnetic fields increase HUVEC proliferation via a VEGF-A independent mechanism. Bioelectromagnetics. 2009;30:189–197. doi: 10.1002/bem.20459. [DOI] [PubMed] [Google Scholar]
- 32.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto T, Fujioka M, Ishida M, Kuribayashi M, Ueshima K, Kubo T. Noninvasive up-regulation of angiopoietin-2 and fibroblast growth factor-2 in bone marrow by pulsed electromagnetic field therapy. J Orthop Sci. 2010;15:661–665. doi: 10.1007/s00776-010-1510-0. [DOI] [PubMed] [Google Scholar]


