Abstract
Baroreflex dysfunction has been considered an important mortality predictor after myocardial infarction (MI). However, the impact of baroreflex deficiency prior to MI on tonic autonomic control and cardiac function, and on the profile of proteins associated with intracellular calcium handling has not yet been studied. The aim of the present study was to analyze how the impairment of baroreflex induced by sinoaortic denervation (SAD) prior to MI in rats affects the tonic autonomic control, ventricular function and cardiomyocyte calcium handling proteins. After 15 days of following or SAD surgery, rats underwent MI. Echocardiographic, hemodynamic, autonomic and molecular evaluations were performed 90 days after MI. Baroreflex impairment led to additional damage on: left ventricular remodeling, diastolic function, vagal tonus and intrinsic heart rate after MI. The loss of vagal component of the arterial baroreflex and vagal tonus were correlated with changes in the cardiac proteins involved in intracellular calcium homeostasis. Furthermore, additional increase in sodium calcium exchanger expression levels was associated with impaired diastolic function in experimental animals. Our findings strongly suggest that previous arterial baroreflex deficiency may induce additional impairment of vagal tonus, which was associated with calcium handling proteins abnormalities, probably triggering ventricular diastolic dysfunction after MI in rats.
Keywords: Arterial baroreflex, myocardial infarction, autonomic tone, cardiac function, cardiac remodeling, calcium handling proteins
Introduction
Coronary artery disease together with myocardial infarction (MI) is the most prevalent cardiovascular disease, and commonly progresses to heart failure (HF) in affected individuals [1]. Both during and after MI, neurohumoral changes occur in order to minimize the consequences of reduced ventricular function. On the other hand, chronically, autonomic imbalance is usually followed by abnormalities in the cardiorespiratory reflex control, i.e., impairment of baroreflex sensitivity and function, and increased activation of ergoreflex and chemoreflex [2-4]. Thus, autonomic imbalance seems to be a key element in the pathophysiology of heart failure (HF) [5].
Arterial baroreflex control of circulation, governed by arterial pressoreceptors, has been recognized as an important predictor of cardiovascular risk following a cardiac event. The ATRAMI study (Autonomic Tone and Reflexes after Myocardial Infarction) has provided clinical evidence of the prognostic value of baroreflex sensitivity and heart rate variability in the mortality rate after MI, regardless of ejection fraction and ventricular arrhythmias [2]. However, the impact of the baroreflex impairment prior to an ischemic event has been poorly investigated. In this sense, in a study with Japanese type 2 diabetic patients without any structural heart disease, Okada et al. [6] have demonstrated that depressed baroreflex sensitivity at baseline has a long-term predictive value for cardiovascular event.
In order to evaluate the role of baroreflex in cardiovascular homeostasis, sinoaortic denervation (SAD) in animals has been widely applied. Our group has shown that baroreceptor deletion by SAD promotes cardiac remodeling, pulmonary hypertension, collagen content increase, diastolic dysfunction, regardless of potential risk factors [7-10]. In order to evidence the importance of the integrity of the baroreflex prior to an ischemic event, Yu et al. [11] have demonstrated that depressed baroreflex sensitivity by SAD could attenuate the angiogenesis and increase mortality rate after MI in rats. Accordingly, we have previously observed that when SAD is carried out before the induction of MI, it promotes additional changes on the heart rate variability, which was correlated with cardiac remodeling impairment in rats [8]. However, the impact of baroreflex integrity prior to MI on tonic autonomic control and cardiac function, and on the profile of proteins associated with intracellular calcium handling has not yet been studied and requires elucidation. Therefore, the aim of the present study was to analyze how the impairment of baroreflex induced by SAD prior to MI in rats affects the tonic autonomic control, ventricular function and cardiomyocyte calcium handling proteins in these animals.
Materials and methods
Animals
Experiments were performed in adult male Wistar rats (240-260 g) from the Animal House of the Medical School of University of Sao Paulo, São Paulo, Brazil. Rats were fed standard laboratory chow and water ad libitum. The animals were housed in collective polycarbonate cages in a temperature-controlled room (22-23°C) and under 54-55% humidity with a 12-h dark-light cycle (light 07:00-19:00 h). The experimental protocol was approved by the institutional animal care and use committee of the Medical School of the University of Sao Paulo, and this investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals: National Research Council (Eighth Edition. Washington, DC: The National Academies Press, 2011).
The rats were randomly assigned to three groups: control (C, n=12), myocardial infarction (MI, n=39), sinoaortic denervation+myocardial infarction (SAD+MI, n=35).
Sinoaortic denervation surgery
Sinoaortic baroreceptor denervation (SAD) was performed with rats under pentobarbital anaesthesia (50 mg/kg, i.p.). Briefly, a 3 cm midline incision was made and the sternocleidomastoid muscles were reflected laterally, exposing the neurovascular sheath. The common carotid arteries and the vagal trunk were isolated and the aortic depressor fibres, either travelling with the sympathetic nerve or as an isolated aortic nerve, were cut.
The communicating branch of the aortic fibres was also resected. The third contingent of aortic baroreceptor fibres travelling with the inferior laryngeal nerve was interrupted by resection of the superior laryngeal nerve after the carotid bifurcation had been exposed for carotid striping. To complete SAD, the sinus nerve, as well as all the carotid branches and the carotid body, were resected [7-10]. The C and MI animals underwent the same surgery procedures except that SAD was not induced (Sham surgery).
Myocardial infarction
After 15 days of following or SAD surgery, anaesthetized rats (80 mg/kg ketamine and 12 mg/kg xylazine, i.p.) underwent surgical occlusion of the left coronary artery, which resulted in MI as described previously [12-15]. Briefly, after intubation, animals were positive-pressure ventilated with room air at 2.5 mL, 65 strokes/min with a pressure-cycled rodent ventilator (Harvard Apparatus, Model 683, Holliston, MA, USA). For induction of MI, a 2-cm left lateral thoracotomy was performed in the third intercostal space, and the left anterior descending coronary artery was occluded with a nylon (6.0) suture at approximately 1 mm from its origin below the tip of the left atrium. The chest was closed with a silk suture. During the protocol, mortality rate was 0% to C, 25% to MI, and 28% to SAD+MI groups. Thus, all evaluations in the present study were performed with: C (n=12), MI (n=10), and SAD+MI (n=10).
Echocardiographic evaluation
Echocardiography was performed by a blinded observer, under the guidelines of the American Society of Echocardiography 90 days after MI. Rats were anesthetized with Ketamine (Parke-Davis, 50 mg/kg) and Xylazine (Bayer, 10 mg/kg) and images were obtained with a 10-14 mHz linear transducer in a SEQUOIA 512 (ACUSON Corporation, Mountain View, CA) for measurements of morphometric parameter: left ventricular mass (LV mass) and relative wall thickness (RWT); systolic function: ejection fraction (EF) and velocity of circunferencial fiber shortening (VCF); diastolic function: normalized isovolumetric relaxation time (IVRT), normalized Peak E desacceleration time (EDT), and E/A wave ratio (E/A ratio); global function: myocardial performance index (MPI). Echocardiographic parameters were measured as described in detail elsewhere [12-15].
The MI area was delimited leading to analysis of the movement of the LV walls. Regions with systolic shortening classified as absent were considered infarcted, as described in detail elsewhere [8,12-15].
Hemodynamic measurements and autonomic evaluation
One day after the echocardiographic evaluation, 2 catheters filled with 0.06 mL of saline were implanted into the femoral artery and femoral vein of the anesthetized rats (Ketamine, Parke-Davis, 70 mg/kg and Xylazine, Bayer, 10 mg/kg). After 24 hours of catheter implants, the arterial cannula was connected to a strain-gauge transducer (Blood Pressure XDCR, Kent Scientific, USA), and arterial pressure (AP) signals were recorded in conscious rats over a 30-minute period by a microcomputer equipped with an analog-to-digital converter board (WinDaq, 2-kHz, DATAQ, Springfield, OH). The recorded data were analyzed on a beat-to-beat basis to quantify changes in mean AP (MAP) and heart rate (HR) [8,12-15].
After basal period, sequential bolus injections (0.1 mL) of increasing doses of phenylephrine (0.25, 0.5, 1, 2, 4 and 8 μg/Kg) and sodium nitroprusside (0.05, 0.1, 0.2, 0.4, 0.8 and 1.6μg/Kg) were given to induce increases or decreases in MAP in conscious rats, ranging from 5 to 40 mmHg of variation. Baroreflex sensitivity (BRS) was expressed as bradycardic (BR) and tachycardic (TR) responses in beats per minute per millimeter of mercury, as described elsewhere [8,12-15].
After BRS assessment, AP and HR were continuously recorded at basal state and after methylatropine (3 mg/kg, IV) injection (0.2 mL). Because the HR response to the drug reaches its peak within 3 to 5 min, this time interval was allowed to elapse before HR measurement. Atenolol (8 mg/kg, IV) was injected (0.2 mL) 10 min after methylatropine, and again the response was evaluated after simultaneous blockade with atenolol and methylatropine. On the subsequent day, the sequence of injections was inverted (first atenolol and then methylatropine). The intrinsic heart rate (IHR) was evaluated after simultaneous blockade with atenolol and methylatropine. Sympathetic tonus was determined as the difference between maximum HR after methylatropine injection and IHR. Vagal tonus was obtained by the difference between the lowest HR after atenolol injection and IHR [15].
Western blot analysis
In order to evaluate the expression of sarcoplasmic reticulum calcium ATPase pump (SERCA2, 110 kDa), phospholamban (PLN, 25 kDa), phosphorylated-PLN at serine 16 (PLNser16, 25 kDa), phosphorylated-PLN at threonine 17 (PLNthr17, 25 kDa), phosphatase protein 1 (PP1, 38 kDa), protein phosphatase type 2A (PP2A) and sodium calcium exchanger (NCX, 120 kDa), left ventricular homogenates were analyzed by Western blotting as described elsewhere [9,12]. Mouse monoclonal antibodies to SERCA2 (1:2500), PLN (1:500), and NCX (1:2000) were obtained from Affinity BioReagents (Golden, CO); rabbit polyclonal antibody to protein phosphatase type 1 (PP1, 1:1000) and protein phosphatase type 2A (PP2A, 1:1000) were obtained from Upstate (Lake Placid, NY); PLNser16 (1:5000) and PLNthr17 (1:5000) were obtained from Badrilla (Leeds, UK). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:2000) (38 kDa) was obtained from Advanced Immunochemical (Long Beach, CA). Targeted bands were normalized to cardiac GAPDH.
Statistical analysis
Data are reported as means ± SEM. One-way ANOVA was used to compare the groups followed by the Student-Newman-Keuls post-test. Survival curve was estimated by the Kaplan-Meier method and compared by the log-rank test. Spearman’s nonparametric test and Pearson’s correlation were used to study the association between variables. Significance level was established at p<0.05.
Results
Left ventricular function
Echocardiographic parameters are shown in Table 1. Although MI area was similar between MI and SAD+MI groups, LV mass and RWT values were higher in SAD+MI group when compared to C and MI groups. EF and VCF were reduced in MI and SAD+MI rats when compared to C rats. IVRT and EDT parameters were increased in SAD+MI group when compared to C. Furthermore, IVRT was additionally increased in SAD+MI animals when compared to MI animals. No differences were observed in E/A ratio between studied groups. MPI, an index that represents global myocardial stress, was increased in SAD+MI group when compared to C group.
Table 1.
Echocardiographic evaluations in control (C), myocardial infarction (MI) and sinoaortic sinoaortic denervation+MI (SAD+MI) groups
| Parameters/group | C | MI | SAD+MI |
|---|---|---|---|
| MI area (%) | ---- | 37.3 ± 3 | 39.4 ± 4 |
| LV mass (g) | 0.84 ± 0.03 | 0.90 ± 0.02* | 0.98 ± 0.04*,† |
| RWT | 0.34 ± 0.01 | 0.36 ± 0.02 | 0.43 ± 0.03*,† |
| EF (%) | 76 ± 0.9 | 42 ± 3.0* | 41 ± 2.9* |
| VCF (circ/s 10-4) | 50 ± 3 | 35 ± 2* | 39 ± 3* |
| IVRT (ms) | 1.85 ± 0.09 | 1.88 ± 0.05 | 2.15 ± 0.05*,† |
| EDT (ms) | 1.81 ± 0.09 | 2.09 ± 0.12 | 2.37 ± 0.11*,† |
| E/A ratio | 1.61 ± 0.14 | 1.54 ± 0.39 | 1.46 ± 0.01 |
| MPI | 0.38 ± 0.09 | 0.45 ± 0.09 | 0.50 ± 0.07* |
Values are expressed as mean ± SEM. Results of MI area, left ventricular mass (LV mass), relative wall thickness (RWT), ejection fraction (EF), velocity of circunferencial fiber shortening (VCF), normalized isovolumetric relaxation time (IVRT), normalized Peak E desacceleration time (EDT), and E/A wave ratio (E/A ratio), myocardial performance index (MPI).
p<0.05 vs. C;
p<0.05 vs. MI.
Hemodynamic and autonomic evaluations
The results of hemodynamic and autonomic evaluations are presented in Table 2. Systolic arterial pressure values were reduced in MI rats when compared to C rats. No differences in diastolic and mean arterial pressures, and heart rate were observed between groups. Baroreflex sensitivity, evaluated by TR and BR, was worsened in all experimental groups when compared to C. Furthermore, BR and TR values were further reduced in SAD+MI when compared to MI animals.
Table 2.
Hemodynamic and autonomic variables in control (C), myocardial infarction (MI) and sinoaortic denervation+MI (SAD+MI) groups
| Parameters/group | C | MI | SAD+MI |
|---|---|---|---|
| Hemodinamics | |||
| SAP (mmHg) | 121 ± 2 | 110 ± 4* | 117 ± 2 |
| DAP (mmHg) | 80 ± 4 | 78 ± 4 | 84 ± 2 |
| MAP (mmHg) | 93 ± 4 | 92 ± 5 | 98 ± 3 |
| HR (bpm) | 363 ± 9 | 361 ± 9 | 352 ± 10 |
| Baroreflex sensitivity | |||
| BR (bpm/mmHg) | -2.3 ± 0.2 | -1.5 ± 0.1* | -0.5 ± 0.1*,† |
| TR (bpm/mmHg) | 3.8 ± 0.5 | 1.7 ± 0.2* | 0.7 ± 0.2*,† |
| Autonomic blockade | |||
| IHR (bpm) | 372 ± 8 | 352 ± 7 | 340 ± 4* |
| VT (bpm) | 65 ± 14 | 32 ± 5* | 11 ± 7*,† |
| ST (bpm) | 31 ± 5 | 52 ± 4* | 40 ± 5* |
Values are expressed as mean ± SEM. Results of systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), heart rate (HR), bradycardic response (BR), tachycardic response (TR), intrinsic heart rate (IHR), vagal tonus (VT), sympathetic tonus (ST).
p<0.05 vs. C;
p<0.05 vs. MI.
Intrinsic heart rate was reduced in SAD+MI rats when compared to C rats. The sympathetic tonus increased and vagal tonus reduced in MI and SAD+MI groups when compared to C group. However, SAD+MI animals displayed additional vagal tonus reduction when compared to MI animals (Table 2).
Regulatory proteins involved in intracellular Ca2+ homeostasis
GAPDH protein levels were not different among the three studied groups (Figure 1A). SERCA2 expression was reduced in MI and SAD+MI when compared to C group (Figure 1B). PLN, PLNser16 and PLNthr17 were similar among experimental groups (Figure 1C-E). Adittionaly, when SERCA2/PLN ratio was calculated, MI and SAD+MI groups presented reduced values when compared to C group (Figure 1F).
Figure 1.

Expression levels of regulatory proteins related to intracellular calcium homeostasis from Control (C), myocardial infarction (MI) and sinoaortic denervation+MI (SAD+MI). Targeted bands were normalized to cardiac GAPDH. A: Representative blot of GAPDH; B: Sarcoplasmic reticulum calcium ATPase pump (SERCA2); C: Phospholamban (PLN); D: PLN phosphorylated at serine 16 (PLNser16 ); E: PLN phosphorylated at threonine 17 (PLNthr17); F: SERCA2/PLN ratio. *p<0.05 vs. C.
NCX expression levels were increased only in SAD+MI group when compared to C group (Figure 2A). MI and SAD+MI animals displayed decreased SERCA2/NCX (Figure 2B) ratio when compared to C animals. It is important to highlight that SAD+MI rats presented additional reduction of SERCA2/NCX ratio when compared to MI rats. Moreover, PP1 (Figure 2C) and PP2A (Figure 2D) values were not different between experimental groups.
Figure 2.
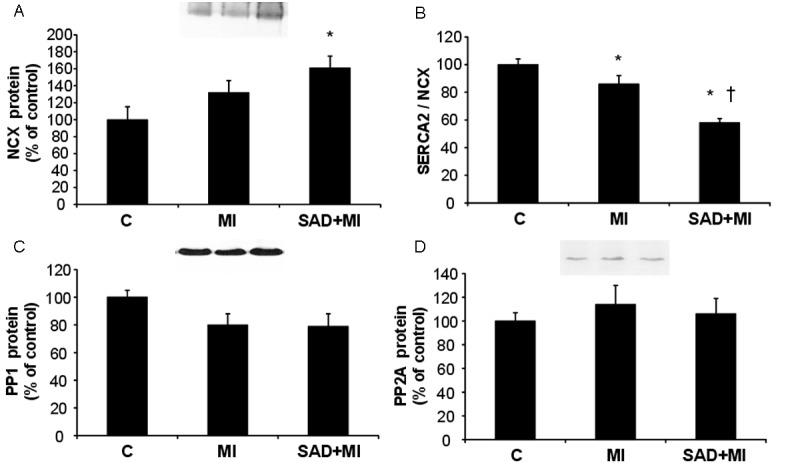
Expression levels of intracellular calcium efflux mediators from Control (C), myocardial infarction (MI) and sinoaortic denervation+MI (SAD+MI). Targeted bands were normalized to cardiac GAPDH (blot represented in Figure 1A). A: Sodium calcium exchanger (NCX); B: SERCA2/NCX ratio; C: Phosphatase protein 1 (PP1); D: Phosphatase protein 2 (PP2A). *p<0.05 vs. C; †p<0.05 vs. MI.
Correlation analysis
Positive correlations were observed between vagal tonus with: SERCA2/PLN (r=0.54; P=0.002), and with SERCA2/NCX (r=0.67; P=0.007). Positive correlations were also observed between BR of baroreflex sensitivity with: SERCA/PLN (r=0.51; P=0.004), and with SERCA/NCX (r=0.75; P=0.001). Furthermore, NCX expression levels were positively correlated with IVRT and EDT echocardiographic diastolic indexes (r=0.71; P=0.001 and r=0.74; P<0.001).
Discussion
The main finding of the present study lies in the fact that prior arterial baroreflex impairment led to additional damage on: left ventricular remodeling, diastolic function, vagal tonus and intrinsic heart rate after MI. The loss of vagal component of the arterial baroreflex and vagal tonus were correlated with changes in the cardiac proteins involved in intracellular calcium homeostasis. Furthermore, additional increase in NCX expression levels was associated with impaired diastolic function in experimental animals.
Several studies have demonstrated that MI in humans or animals is followed by autonomic dysfunction with decreased vagal modulation and baroreflex sensitivity, and increased sympathetic modulation [2-4,12-17]. Corroborating these data, in the present study, MI led to impaired baroreflex sensitivity, lower systolic arterial pressure, decreased vagal tonus, and increased sympathetic tonus in rats. However, it should be stressed that when SAD was undertaken prior to MI, additional vagal tonus impairment and intrinsic heart rate were observed. Possible explanations for the additional loss of tonic vagal activity may lie in the fact that the parasympathetic component is quantitatively the major (78%) component of baroreflex sensitivity; also, the vagal arm of baroreflex is more vulnerable, and has been shown to be damaged in many cardiovascular diseases [18].
After MI, cardiac remodeling is usually characterized by increased interstitial collagen deposition and fibrosis, myocyte hypertrophy, and ventricular dilatation [19-21]. Furthermore, it has been recently demonstrated that the additional reduction of vesicular acetylcholine transporter expression in SAD rats was associated with impaired capillary density, blood flow, and vascular endothelial growth factor expression after MI [11]. In our study, LV mass was increased in infarcted animals when compared to C group; however, previous baroreflex dysfunction induced additional increase in LV mass and relative wall thickness in SAD+MI group. This additional impairment of LV geometry may be closely associated with the abolishment of baroreflex by SAD in MI rats. In fact, Miao and Su [22] have previously demonstrated that the increase in sympathetic outflow and blood pressure variability may lead to aortic and LV hypertrophy.
Echocardiographic assessment indicated that, besides changes in cardiac morphometry, MI led to systolic and diastolic dysfunction in experimental groups, as previously demonstrated [8,12-15,17]. It should be noted that previous SAD induction was responsible for an additional impairment of diastolic and global function in SAD+MI rats, as demonstrated by IVRT, EDT and MPI echocardiographic indexes, regardless of MI area extension. Our group has previously demonstrated the relationship between SAD and ventricular diastolic function in rats [9]; however, to our knowledge, this is the first study to demonstrate that previous impairment of baroreflex sensitivity may induce additional worsening on diastolic function after MI. This finding may have great clinical impact, since many risk factors for cardiovascular disease are associated with that loss of baroreflex sensitivity, such as hypertension [23], diabetes [24], obesity and metabolic syndrome [25].
As cardiac dysfunction observed in heart failure is strongly associated with changes in intracellular calcium net balance [26], we tested whether the additional impairment on diastolic function observed in SAD+MI group would be related to changes in expression levels of proteins associated with intracellular calcium handling. Our results demonstrated that MI and SAD+MI groups displayed reduced SERCA2, SERCA2/PLN and SERCA2/NCX ratios when compared to C group. However, SAD+MI animals presented increased NCX when compared to C, and an additional reduction of SERCA2/NCX ratio when compared to MI animals. These findings indicate that there is an imbalance between the uptake of calcium by the sarcoplasmic reticulum and its extrusion from the cell, thus favoring a reduction in the concentration of intracellular calcium. Elucidating the mechanisms underlying impaired calcium handling in the failing heart is quite challenging, since there are many proteins involved. In the present study, positive correlations were observed between vagal tonus with SERCA2/PLN, as well as with SERCA2/NCX. Additionally, positive correlations were also observed between bradycardic response of baroreflex sensitivity with SERCA/PLN, and with SERCA/NCX. These data may suggest, at least in part, that additional changes in calcium cycling proteins may be related to cardiac vagal impairment after MI in SAD animals.
On the other hand, diastolic function is associated with the passive elastic properties of infarcted myocardium, the calcium uptake by mitochondria and with the beat-to-beat regulation of diastolic calcium by sarcoplasmic reticulum reuptake and transsarcolemmal NCX [27-30]. The present finding of higher NCX protein levels in SAD+MI group supports the hypothesis that an increase in protein levels of NCX represents an important mechanism for regulation of diastolic calcium elimination and diastolic function in the failing myocardium, as previously observed by Hasenfuss et al. [30] in humans. To some extent, NCX overexpression can compensate for compromised SERCA2 function and allow calcium homeostasis to be maintained [31]. However, we suggest that this compensatory mechanism failed in our experiment because the increased expression of NCX was closely associated with the loss of diastolic function in experimental groups.
In conclusion, our findings strongly suggest that previous arterial baroreflex deficiency may induce additional impairment of ventricular remodeling and diastolic function, vagal tonus and intrinsic heart rate, along with sodium calcium exchanger expression levels after MI in rats. Although these cause-effect relationships can only be inferred, rather than confirmed, in our study, we suggest that the previous loss of the baroreflex would promote a reduction in vagal tone, which in turn would lead to an imbalance in the expression of some proteins associated with intracellular calcium homeostasis, triggering a decrease in diastolic function after MI in rats. However, other systemic and local mechanisms cannot be ruled out. Thus, these findings encourage enhancing baroreflex sensitivity and function as a therapeutic strategy for the treatment of MI.
Acknowledgements
This work was funded by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAP-ESP-2007/58942-0; 2012/20141-5), Conselho Nacional de Pesquisa e Desenvolvimento (CNPq-482520/2009-4; 306011/2010-7; 479766/2011-8). B.R., P.C.B., K.D.A., and M.C.I. received financial support from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq-BPQ). All authors approved final version to be published.
Disclosure of conflict of interest
None.
Abbreviations
- CM
Data acquisition, analysis, interpretation, draft the article
- BR
Data acquisition, analysis, interpretation and draft the article
- AM
Data acquisition, analysis and interpretation
- EDM
Data acquisition and analysis
- ICMS
Data acquisition and analysis
- PCB
interpretation of data and contributed with reagents/materials/analysis tools
- MCI
conception and design of the work, help to draft the article
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [Google Scholar]
- 6.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J. 2010;74:1379–83. doi: 10.1253/circj.cj-09-0960. [DOI] [PubMed] [Google Scholar]
- 7.Moraes-Silva IC, De La Fuente RN, Mostarda C, Rosa K, Flues K, Damaceno-Rodrigues NR, Caldini EG, De Angelis K, Krieger EM, Irigoyen MC. Baroreflex deficit blunts exercise training-induced cardiovascular and autonomic adaptations in hypertensive rats. Clin Exp Pharmacol Physiol. 2010;37:e114–20. doi: 10.1111/j.1440-1681.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 8.Mostarda C, Rodrigues B, Vane M, Moreira ED, Rosa KT, Moraes-Silva IC, Lacchini S, Casarini DE, De Angelis K, Irigoyen MC. Autonomic impairment after myocardial infarction: role in cardiac remodeling and mortality. Clin Exp Pharmacol Physiol. 2010;37:447–52. doi: 10.1111/j.1440-1681.2009.05327.x. [DOI] [PubMed] [Google Scholar]
- 9.Mostarda C, Moraes-Silva IC, Moreira ED, Medeiros A, Piratello AC, Consolim-Colombo FM, Caldini EG, Brum PC, Krieger EM, Irigoyen MC. Baroreflex sensitivity impairment is associated with cardiac diastolic dysfunction in rats. J Card Fail. 2011;17:519–25. doi: 10.1016/j.cardfail.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Mostarda C, Moraes-Silva IC, Moreira ED, Medeiros A, Piratello AC, Consolim-Colombo FM, Caldini EG, Brum PC, Krieger EM, Irigoyen MC. Cardiac and pulmonary arterial remodeling after sinoaortic denervation in normotensive rats. Auton Neurosci. 2012;166:47–53. doi: 10.1016/j.autneu.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Yu JG, Song SW, Shu H, Fan SJ, Liu AJ, Liu C, Guo W, Guo JM, Miao CY, Su DF. Baroreflex deficiency hampers angiogenesis after myocardial infarction via acetylcholine-α7-nicotinic ACh receptor in rats. Eur Heart J. 2013;34:2412–2420. doi: 10.1093/eurheartj/ehr299. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues B, Jorge L, Mostarda CT, Rosa KT, Medeiros A, Malfitano C, de Souza AL Jr, Viegas KA, Lacchini S, Curi R, Brum PC, De Angelis K, Irigoyen MC. Aerobic exercise training delays cardiac dysfunction and improves autonomic control of circulation in diabetic rats undergoing myocardial infarction. J Card Fail. 2012;18:734–44. doi: 10.1016/j.cardfail.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues B, Mostarda CT, Jorge L, Barboza CA, Grans CF, De Angelis K, Irigoyen MC. Impact of myocardial infarction on cardiac autonomic function in diabetic rats. J Diabetes Complications. 2013;27:16–22. doi: 10.1016/j.jdiacomp.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Barboza CA, Rocha LY, Mostarda CT, Figueroa D, Caperuto EC, De Angelis K, Irigoyen MC, Rodrigues B. Ventricular and autonomic benefits of exercise training persist after detraining in infarcted rats. Eur J Appl Physiol. 2013;113:1137–1146. doi: 10.1007/s00421-012-2533-3. [DOI] [PubMed] [Google Scholar]
- 15.de La Fuente RN, Rodrigues B, Moraes-Silva IC, Souza LE, Sirvente R, Mostarda C, De Angelis K, Soares PP, Lacchini S, Consolim-Colombo F, Irigoyen MC. Cholinergic stimulation with pyridostigmine improves autonomic function in infarcted rats. Clin Exp Pharmacol Physiol. 2013;40:610–616. doi: 10.1111/1440-1681.12121. [DOI] [PubMed] [Google Scholar]
- 16.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues B, Rosa KT, Medeiros A, Schaan BD, Brum PC, De Angelis K, Irigoyen MC. Hyperglycemia can delay left ventricular dysfunction but not autonomic damage after myocardial infarction in rodents. Cardiovasc Diabetol. 2011;10:26. doi: 10.1186/1475-2840-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo M, Su DF, Julien C, Cerutti C, Vincent M, Sassard J. Influence of hypertension and age on the sympathetic and parasympathetic components of cardiac baroreflex in the conscious rat. Arch Mal Coeur Vaiss. 1988;81:113–117. [PubMed] [Google Scholar]
- 19.Bäcklund T, Palojoki E, Saraste A, Eriksson A, Finckenberg P, Kytö V, Lakkisto P, Mervaala E, Voipio-Pulkki LM, Laine M, Tikkanen I. Sustained cardiomyocyte apoptosis and left ventricular remodeling after myocardial infarction in experimental diabetes. Diabetologia. 2004;47:325–330. doi: 10.1007/s00125-003-1311-5. [DOI] [PubMed] [Google Scholar]
- 20.de Macedo Braga LM, Lacchini S, Schaan BD, Rodrigues B, Rosa K, De Angelis K, Borges LF, Irigoyen MC, Nardi NB. In situ delivery of bone marrow cells and mesenchymal stem cells improves cardiovascular function in hypertensive rats submitted to myocardial infarction. J Biomed Sci. 2008;15:365–374. doi: 10.1007/s11373-008-9237-z. [DOI] [PubMed] [Google Scholar]
- 21.Jorge L, Rodrigues B, Rosa KT, Malfitano C, Loureiro TC, Medeiros A, Curi R, Brum PC, Lacchini S, Montano N, De Angelis K, Irigoyen MC. Cardiac and peripheral adjustments induced by early exercise training intervention were associated with autonomic improvement in infarcted rats: role in functional capacity and mortality. Eur Heart J. 2011;32:904–912. doi: 10.1093/eurheartj/ehq244. [DOI] [PubMed] [Google Scholar]
- 22.Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens. 2002;20:1865–1872. doi: 10.1097/00004872-200209000-00033. [DOI] [PubMed] [Google Scholar]
- 23.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 24.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;28:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 25.Skrapari I, Tentolouris N, Katsilambros N. Baroreflex function: determinants in healthy subjects and disturbances in diabetes, obesity and metabolic syndrome. Curr Diabetes Rev. 2006;2:329–338. doi: 10.2174/157339906777950589. [DOI] [PubMed] [Google Scholar]
- 26.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 27.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 28.Sys SU, Brutsaert DL. Diagnostic significance of impaired LV systolic relaxation in heart failure. Circulation. 1995;92:3377–3380. doi: 10.1161/01.cir.92.12.3377. [DOI] [PubMed] [Google Scholar]
- 29.Meyer M, Keweloh B, Güth K, Holmes JW, Pieske B, Lehnart SE, Just H, Hasenfuss G. Frequency-dependence of myocardial energetics in failing human myocardium as quantified by a new method for the measurement of oxygen consumption in muscle strip preparations. J Mol Cell Cardiol. 1998;30:1459–1470. doi: 10.1006/jmcc.1998.0706. [DOI] [PubMed] [Google Scholar]
- 30.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 31.Terracciano CM, Philipson KD, MacLeod KT. Overexpression of the Na(+)/Ca(2+) exchanger and inhibition of the sarcoplasmic reticulum Ca(2+)-ATPase in ventricular myocytes from transgenic mice. Cardiovasc Res. 2001;49:38–47. doi: 10.1016/s0008-6363(00)00205-4. [DOI] [PubMed] [Google Scholar]


