INTRODUCTION
Westernized life style is associated with a higher prevalence of allergies and asthma and this is thought to be related at least in part to decreased microbial exposure.1,2 This westernized life style is also associated with widespread exposure to pesticides. The US Environmental Protection Agency reported finding 800 pesticide active ingredients in about 21,000 different consumer products.3 Pesticide exposure has been reported to be associated with asthma and rhinitis.4–7
One specific group of organochlorine pesticides, dichlorophenols, are compounds with potent bactericidal effects.8 They are typically used as herbicides, fungicides, and disinfectants9–11 and can be found in contaminated water, air, and in pesticide-treated crops.9–12 In addition, chlorine-based cleaning agents can react with wood and paper leading to generation of dichlorophenols in packing materials, containers, and storage boxes,13 resulting in contamination of milk, cocoa powder, fruit juices, and dried fruits.9,13–15
Recent studies have indicated that organochlorine compounds may play a role in the increased incidence of atopic diseases because of their immunomodulatory effects.16 Previous research showed that exposure to dichlorodiphenylethylene was linked to a higher total immunoglobulin E level and to an increased risk of asthma in children.16,17 Higher dichlorophenol levels in urine were associated with increased allergic sensitization to foods.18 In addition, previous reports suggested that certain organochlorines preferentially induce the production in vitro of Th2- cytokines associated with increased allergic reactivity such as interleukin-4, and suppress the production of TH-1 cytokines that tend to block allergic reactivity, such as interferon-γ, interleukin2-, and interleukin-10.19,20
In addition to these immunomodulatory effects, dichlorophenols are also upper and lower airway irritants.21 Dichlorophenols are metabolites of 2,4-dichlorophenoxyacetic acid and 1,4-dichlorobenzene. Both of these latter compounds are used in production of insecticides, herbicides and exposure to them may occur through contaminated air. Breathing vapors of 1,4-dichlorobenzene was associated with reduced lung function in adults and higher odds for asthma in children.22,23 However, there was no particular association between atopy and 1,4-dichlorobenzenes among asthmatic children.23 In addition, repeated exposure to chlorine-containing irritant gases increased the risk of adult-onset asthma and wheeze regardless of the history of hay fever (as a marker of atopy).24
Although these associations between organochlorines, lung function, asthma and atopy have been shown, less is known about the effect of these compounds on different forms of asthma and wheeze. Since asthma and wheeze have distinct atopic and non-atopic forms,25 in this study we proposed to investigate whether dichlorophenols were associated with wheezing or asthma and if so, whether atopy played a role in these associations.
METHODS
Study population and data collection
We analyzed the association between urine levels of two dichlorophenols (2,5-dichlorophenol and 2,4-dichlorophenol), self-reported wheezing, self-reported asthma as diagnosed by a doctor, asthma morbidity, and total serum IgE level, in the sample examined in 2005–2006 by the US National Health and Nutrition Examination Survey (NHANES). The NHANES is a database funded by the United States Centers for Disease Control and Prevention that is intended to assess the health and nutritional status of adults and children in the United States. All NHANES data are publically available and can be found at http://www.cdc.gov/nchs/nhanes.htm. NHANES has a complex, multistage, clustered, and stratified probability sample design. The sample for the survey is selected to represent the US civilian non-institutionalized population of all ages. Persons 60 years of age and older, as well as African Americans and Hispanics are oversampled to produce reliable statistics.
Dichlorophenols were measured in urine of a randomly chosen sub-sample of NHANES 2005–2006 participants ≥6 years of age. Details of the laboratory protocol and measurements can be found on the NHANES web site.26 Dichlorophenol levels were measured using solid phase extraction coupled to high performance liquid chromatography and tandem mass spectrometry.26 The detection level for 2,4- and 2,5-dichlorophenols was 0.14 μg/L. We did not include trichlorophenols or O-phenylphenol (these chemicals were a part of the pesticide group in NHANES 2005–06) in our analyses since the majority of the observations were below the lower limit of detection for these chemicals.27
We divided the population on whom urinary dichlorophenols were measured into three groups:
No self-reported wheezing in the past year (non-wheezers);
Self-reported wheezing in the past year, atopic (atopic wheezers);
Self-reported wheezing in the past year, non-atopic (non-atopic wheezers).
Wheezing in the past year was defined by the answer to the following question: In the past 12 months (have you/ has study participant (SP) had wheezing or whistling in (your/his/her) chest? Participants who reported wheezing or whistling in the chest in the last 12 months were asked to answer questions about wheezing-related problems during the preceding 12 months. They were questioned about medication intake for wheezing, missed work or school due to wheezing, and exercise-induced wheezing.28,29 A dichotomous variable was created which characterized subjects as having either no days missed from work or school due to wheezing, or at least one day missed from work or school due to wheezing.
The exact questions that were used in the NHANES questionnaires and that we included into our study are listed in supplemental Table 1S. There were additional questions on asthma and wheezing in the database (e.g., regarding asthma attacks in the last year, emergency room visits in the last year, and about still having asthma), however, there were only a limited number (less than 50%) of responses to these questions in our subsample and therefore a meaningful analysis of those questions was not possible.
The atopic status of participants was also assessed. Atopic subjects were defined as having at least one positive allergen-specific IgE (≥0.35 kU/L) to the environmental and/ or food allergens measured in NHANES 2005–06 (allergen-specific IgE was measured using the Pharmacia Diagnostics ImmunoCAP 1000 System (Kalamazoo, MI)): Dermatophagoides farinae, Dermatophagoides pteronyssinus, cat, dog, German cockroach, Alternaria alternata, short ragweed, ryegrass, Bermuda grass, white oak, birch, Aspergillus fumigatus, Russian thistle, mouse, rat, shrimp, egg, milk, or peanut. Non-atopic subjects had no sensitizations to any of these allergens.
In an exploratory analysis, the above-described investigation of asthma-related morbidity was performed on participants with self-reported physician-diagnosed asthma defined by the answer to the question: has a doctor or other health professional ever told you that (you have/s/he/SP has) asthma?
Only participants with non-missing covariables were studied. Missing covariable participants constituted 19.4%.
Statistical analysis
Associations between the dichlorophenols and doctor-diagnosed asthma, asthma morbidities, such as prescriptions for medications for wheezing, wheezing during exercise, and days missed from work or school due to wheezing, were analyzed in logistic regression models. The association between urinary dichlorophenols and total IgE was analyzed in adjusted linear regression models (after log-transformation of both continuous variables). All analyses were performed using sample weights to account for the complex survey design (including oversampling), survey non-response, and post-stratification, thus providing nationally representative estimates. We adjusted all models for urine creatinine and potential confounders: age, gender, race (non-Hispanic White, non-Hispanic Black, Hispanic, and other), atopic status of participants, and poverty-income ratio (PIR, a ratio of <1 defined low socio-economic status). Dichlorophenol levels were analyzed as tertiles. We tested for interactions between dichlorophenol levels and age, sex, and atopy by including a multiplicative term in the model. We also analyzed the correlation between urinary concentration of both dichlorophenols and between dichlorophenols and triclosan, another chlorinated phenol measured in NHANES, since the latter compound has been reported to be associated with allergic sensitization and because 2,4-dichlorophenol is a byproduct of triclosan.30,31 All statistical analyses were performed with STATA 11.2 software (StataCorp, College Station, TX). P-values of <0.05 were considered significant for all analyses (including interaction effects). The study was classified as exempt by the Albert Einstein College of Medicine Institutional Review Board.
RESULTS
Demographic characteristics of the study population
2,125 NHANES participants ≥6 years of age had data on dichlorophenol levels and all of the relevant covariables we studied. Among the 2,125 participants, 1,852 (87.2%) participants reported no wheezing in the past year. Two hundred seventy three (273) participants (12.8%) reported wheezing in the preceding twelve months and were offered to fill out questionnaires on wheezing-related problems. Two hundred fifty (250) participants completed questionnaires, of which 120 (48%) had been given diagnosis of asthma by a doctor. 2,5-dichlorophenol was detected in the urine of 2,098 (98.7%) participants and 2,4-dichlorophenol was detected in the urine of 1,941 (91.3%) participants. Participants’ characteristics are presented in Table 1. Atopic wheezers were significantly younger than non-wheezers and non-atopic wheezers, p<0.001 and there were fewer Mexican-Americans among the wheezers, p<0.01. Mean dichlorophenol levels were lower in the group of non-atopic wheezers, (p<0.001 for the 2,5-dichlorophenol level and p<0.01 for 2,4-dichlorophenols).
Table 1.
Characteristics of study participantsa, by the presence of wheezing
| Characteristic | Non-wheezers (n=1,852) | Atopicb wheezers (n=156) | Non-atopicb wheezers (n=94) | p-value |
|---|---|---|---|---|
| Age (y), mean (SEc) | 38.9 (1.1) | 37.1 (2.4) | 39.9 (1.3) | <0.001 |
| Male sex (% [SE]) | 44.9 (0.04) | 55.7 (0.07) | 48.9 (0.04) | 0.3 |
| Race/ethnicity (% [SE]) | 0.01 | |||
| Non-Hispanic white | 70.3 (0.03) | 72.1 (0.05) | 80.4 (0.04) | |
| Non-Hispanic black | 11.9 (0.2) | 13.8 (0.03) | 4.9 (0.02) | |
| Mexican American | 9.7 (0.01) | 5.1 (0.01) | 3.3 (0.01) | |
| Other | 8.2 (0.01) | 9.0 (0.03) | 11.4 (0.02) | |
| Low SESd (% [SE]) | 12.6 (0.01) | 15.6 (0.04) | 10.9 (0.03) | 0.6 |
| Asthmae (% [SE]) | 7.5 (0.01) | 48.7 (0.08) | 36.2 (0.05) | <0.001 |
| Atopyb (% [SE]) | 43.2 (0.01) | 100 (0) | - | <0.001 |
| 2,5-Dichlorophenol concentration in urine (geometric mean, μg/L [95% CIf]) | 33.1 (27.4–39.9) | 30.0 (19.2–46.8) | 25.6 (16.0–41.1) | <0.001 |
| Lower tertile | 1.9 (1.6–2.3) | 2.4 (1.8–3.2) | 1.3 (0.7–2.4) | 0.1 |
| Mid-tertile | 12.9 (12.2–13.7) | 14.0 (11.6–16.9) | 11.5 (9.2–14.5) | <0.001 |
| Upper tertile | 142.6 (112.1–181.4) | 134.0 (74.6–240.4) | 115.8 (76.6–175.0) | <0.001 |
| 2,4-Dichlorophenol concentration in urine (geometric mean, μg/L [95% CI]) | 2.4 (2.1–2.8) | 2.4 (1.7–3.4) | 2.2 (1.5–3.1) | <0.01 |
| Lower tertile | 0.30 (0.28–0.34) | 0.35 (0.28–0.45) | 0.27 (0.22–0.34) | <0.001 |
| Mid-tertile | 1.18 (1.1–1.2) | 1.22 (1.1–1.4) | 1.20 (1.1–1.3) | <0.01 |
| Upper tertile | 6.0 (4.8–7.6) | 5.1 (3.3–8.1) | 6.1 (5.3–7.0) | <0.001 |
| Medications for wheezing g (% [SE]) | - | 52.4 (0.07) | 56.0 (0.05) | 0.7 |
| Wheezing during exercise h (% [SE]) | - | 48.5 (0.05) | 52.4 (0.06) | 0.7 |
| Missing work or school due to wheezing i (% [SE]) | - | 26.8 (0.04) | 16.8 (0.05) | 0.2 |
All estimates are weighted to the sampling weights
Atopy was defined as the presence of allergen specific IgE ≥0.35 kU/L to at least one environmental and/ or food allergen
SE – standard error
Percentage of participants with a poverty/income ratio of <1.0
Asthma was defined by the answer to the question: “Has a doctor or other health professional ever told that (you have/s/he/survey participant (SP) has) asthma?”
95% CI – 95% confidence interval
In the past 12 months, (have you/ has study participant) taken medication, prescribed by a doctor, for wheezing or whistling?
In the past 12 months has (your/ study participant’s) chest sounded wheezy during or after exercise or physical activity?
During the past 12 months, did (you/ study participant) miss any days of work or school due to wheezing or whistling?
Among the 2,125 participants, 280 participants (13.2%) reported having asthma diagnosed by a doctor. One hundred thirty (130) of them reported wheezing in the preceding twelve months and 120 filled out questionnaires on wheezing-related problems.
Association between urine dichlorophenol levels and asthma morbidity in wheezers
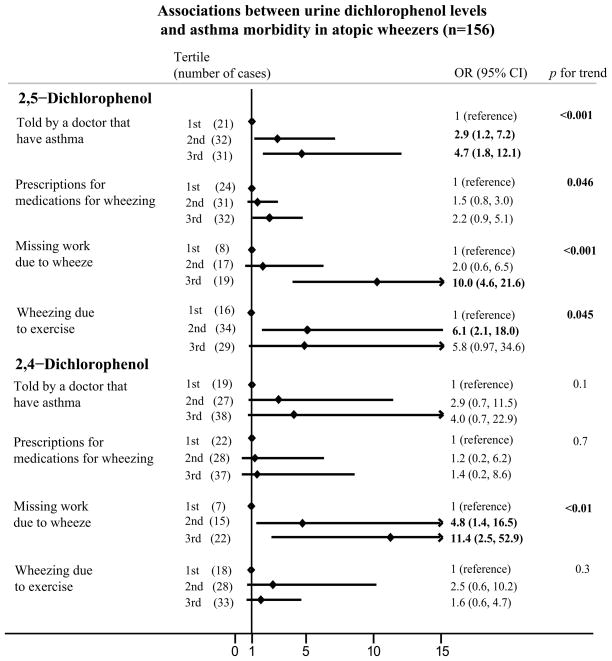
On multivariable analysis, higher urinary 2,5-dichlorophenol levels in the atopic wheezers were strongly associated with more asthma morbidity. These individuals were more likely to have physician-diagnosed asthma (OR for the highest tertile vs. lowest tertile of 2,5-dichlorophenol = 4.7, p for trend <0.001); more likely to require prescriptions for medications for wheezing (OR for the highest tertile vs. lowest tertile = 2.2, p for trend 0.046); more likely to miss work or school due to wheezing (OR for the highest tertile vs. lowest tertile = 10.0, p for trend <0.001); and to wheeze due to exercise (OR for the highest tertile vs. lowest tertile = 5.8, p for trend 0.045) (Table 2, Figure 1A). In the atopic group, 2,4-dichlorophenol was also associated with missing work or school due to wheezing (OR for the highest tertile vs. lowest tertile = 11.4, p for trend <0.01) (Table 3, Figure 1A).
Table 2.
Odds ratioa for 2,5-dichlorophenol levels and asthma morbidity in wheezers, stratified by atopicb status
| Outcome | Atopicb wheezers (n=156) | Non-atopicb wheezers (n=94) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) c | p for trend | Odds ratio (95% CI) | p for trend | |
| Asthma, diagnosed by a doctor d | <0.001 | 0.9 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 2.9 (1.2, 7.2) | 1.0 (0.3, 4.1) | ||
|
| ||||
| Upper tertile | 4.7 (1.8, 12.1) | 1.0 (0.1, 9.8) | ||
|
| ||||
| Medications for wheezingf | 0.046 | 0.3 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 1.5 (0.8, 3.0) | 0.7 (0.2, 2.8) | ||
|
| ||||
| Upper tertile | 2.2 (0.9, 5.1) | 2.9 (0.7, 12.4) | ||
|
| ||||
| Missing work or school due to wheezing e | <0.001 | 0.9 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 2.0 (0.6, 6.5) | 0.7 (0.2, 2.6) | ||
|
| ||||
| Upper tertile | 10.0 (4.6, 21.6) | 1.4 (0.3, 5.8) | ||
|
| ||||
| Wheezing during exercise g | 0.045 | 0.9 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 6.1 (2.1, 18.0) | 1.1 (0.3, 4.0) | ||
|
| ||||
| Upper tertile | 5.8 (0.97, 34.6) | 0.8 (0.2, 3.4) | ||
All estimates are weighted to the sampling weights
Atopy was defined as the presence of allergen specific IgE ≥0.35 kU/L to at least one environmental and/ or food allergen
95% CI – 95% confidence interval
Asthma was defined by the answer to the question: “Has a doctor or other health professional ever told that (you have/s/he/survey participant (SP) has) asthma?”
During the past 12 months, did (you/ study participant) miss any days of work or school due to wheezing or whistling?
In the past 12 months, (have you/ has study participant) taken medication, prescribed by a doctor, for wheezing or whistling?
In the past 12 months has (your/ study participant’s) chest sounded wheezy during or after exercise or physical activity?
Results were adjusted for sample weights, urine creatinine, age, gender, race, and poverty-income ratio.
Values in bold are statistically significant.
Figure 1.
Relationship between asthma morbidity and urine 2,5- and 2,4-dichlorophenol levels in atopic (A) and non-atopic (B) wheezers. Adjusted for sample weights, urine creatinine, age, gender, race, and poverty-income ratio.
Table 3.
Odds ratioa for 2,4-dichlorophenol levels and asthma morbidity in wheezers, stratified by atopicb status
| Outcome | Atopicb wheezers (n=156) | Non-atopicb wheezers (n=94) | ||
|---|---|---|---|---|
| Odds ratio (95% CI c) | p for trend | Odds ratio (95% CI) | p for trend | |
| Asthma, diagnosed by a doctor d | 0.1 | 0.7 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 2.9 (0.7, 11.4) | 0.8 (0.2, 3.1) | ||
|
| ||||
| Upper tertile | 4.0 (0.7, 22.9) | 1.7 (0.2, 14.4) | ||
|
| ||||
| Medications for wheezingf | 0.7 | 0.1 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 1.2 (0.2, 6.2) | 1.9 (0.5, 7.1) | ||
|
| ||||
| Upper tertile | 1.4 (0.2, 8.6) | 6.1 (0.6, 57.4) | ||
|
| ||||
| Missing work or school due to wheezinge | <0.01 | 0.9 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 4.8 (1.4, 16.5) | 1.2 (0.4, 3.5) | ||
|
| ||||
| Upper tertile | 11.4 (2.5, 52.9) | 1.0 (0.2, 4.8) | ||
|
| ||||
| Wheezing during exercise g | 0.3 | 0.4 | ||
|
| ||||
| Lower tertile | 1.0 | 1.0 | ||
|
| ||||
| Mid-tertile | 2.5 (0.6, 10.2) | 2.7 (0.8, 9.6) | ||
|
| ||||
| Upper tertile | 1.7 (0.6, 4.6) | 1.3 (0.2, 7.1) | ||
All estimates are weighted to the sampling weights
Atopy was defined as the presence of allergen specific IgE ≥0.35 kU/L to at least one environmental and/ or food allergen
95% CI – 95% confidence interval
Asthma was defined by the answer to the question: “Has a doctor or other health professional ever told that (you have/s/he/survey participant (SP) has) asthma?”
During the past 12 months, did (you/ study participant) miss any days of work or school due to wheezing or whistling?
In the past 12 months, (have you/ has study participant) taken medication, prescribed by a doctor, for wheezing or whistling?
In the past 12 months has (your/ study participant’s) chest sounded wheezy during or after exercise or physical activity?
Results were adjusted for sample weights, urine creatinine, age, gender, race, and poverty-income ratio.
Values in bold are statistically significant.
There was a significant interaction between atopy and the presence of 2,5-dichlorophenols in urine (p<0.02 for interaction term), with atopic wheezers being more likely to take days off work or school due to wheezing.
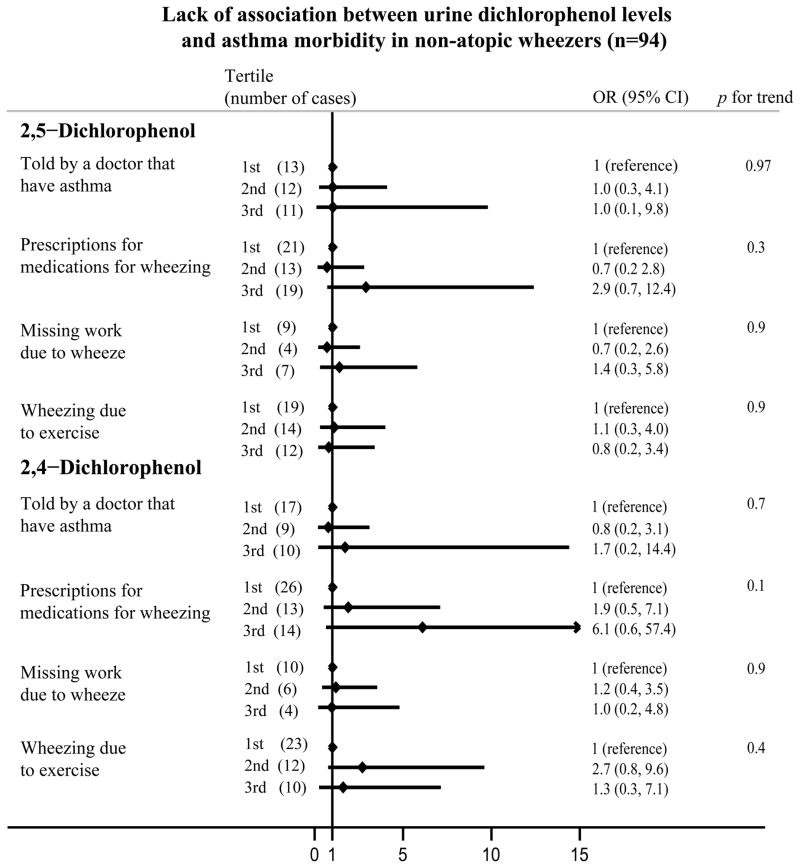
In contrast, in non-atopic wheezers, there were no significant associations with any of the asthma morbidity outcomes (all p for trend were >0.05) (Tables 2 and 3, Figure 1B).
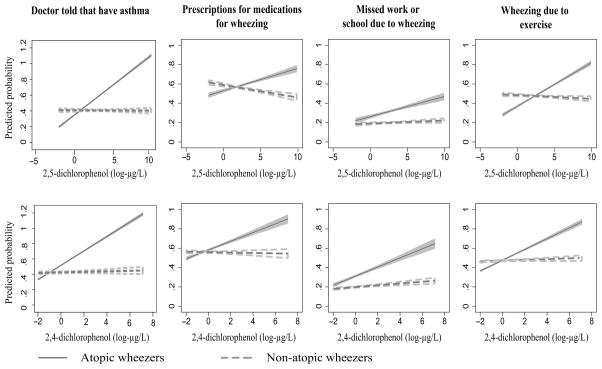
The predicted probability of asthma and wheezing-related problems for atopic and non-atopic participants based on 2,5-dichlorophenol or 2,4-dichlorophenol levels is shown in Figure 2.
Figure 2.
Predicted probability of asthma morbidity by urinary dichlorophenol levels (log-transformed) with 95% CI.
Exploratory analysis of association between urine dichlorophenol levels and asthma morbidity in asthmatics
On multivariable analysis, atopic asthmatics were more likely to miss work or school due to wheezing (OR for the highest tertile vs. lowest tertile = 92.0, 95%CI 13.6–619.0, p for trend <0.01) while there was no significant association with missing work or school in non-atopic asthmatics, p=0.8, data not shown. There were no significant associations with medication use or wheezing during exercise in either atopic or non-atopic asthmatics (data not shown). However, due to the small number of asthmatics who responded to the questions on medication use and wheezing during exercise, a meaningful analysis of associations with these outcomes was not possible as the models became “unstable” and several covariables were omitted from the models.
Association between dichlorophenol levels and total serum IgE
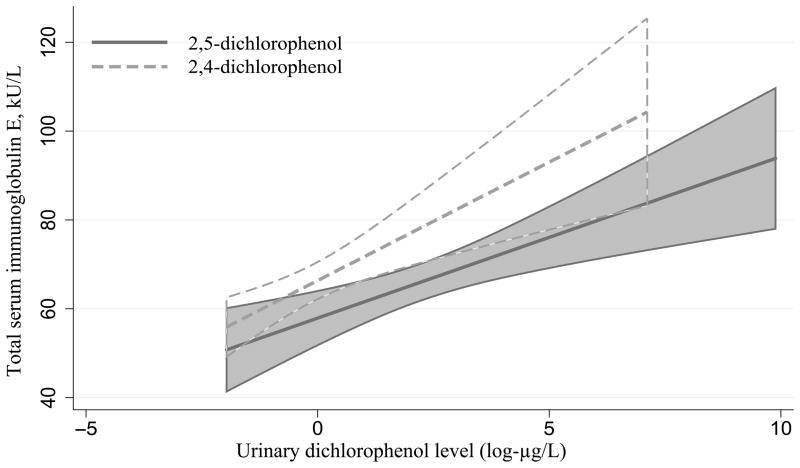
Since asthma morbidity was positively associated with the presence of dichlorophenols only in atopic wheezers and the presence of asthma was previously linked to the total serum IgE levels,32 we examined the association between dichlorophenol levels and total serum IgE. In multivariable linear regression analyses conducted on the entire sample (n=2,125), we found a positive association between total serum IgE with both 2,5- and 2,4-dichlorophenols (beta-coefficients for association were 0.047 and 0.055, respectively, p-value 0.03 for either association) (Figure 3).
Figure 3.
Positive association between urine dichlorophenol levels and total serum IgE in the study population (n=2,125) with 95% CI, p-value 0.03 for either association. Adjusted for sample weights, urine creatinine, age, gender, race, and poverty-income ratio.
Correlation between 2,5- and 2,4-dichlorophenols and triclosan
Concentrations between urine levels of 2,5- and 2,4-dichlorophenols among the entire study population (n=2,125) were highly correlated (Pearson correlation coefficient (R) = 0.87, p<0.001) (data not shown). Since 2,4-dichlorophenol is a byproduct of triclosan (another chlorophenol-based chemical with anti-bacterial properties) correlation between dichlorophenol levels and triclosan was analyzed. The correlation between 2,5-, 2,4-dichlrophenols and triclosan was poor: (R = 0.06, p<0.01 and 0.28, p<0.001, respectively) (data not shown).
DISCUSSION
Our results indicate that atopic wheezers with urinary dichlorophenol levels in the upper tertile are more likely to have doctor-diagnosed asthma, to miss days from work or school due to wheezing, to require medications for wheezing, or to have wheezing with exercise. There were no significant associations with these outcomes in non-atopic wheezers. In addition, we found a positive direct association between 2,4- and 2,5-dichlorophenol and total serum IgE levels. Atopic asthmatics with urinary dichlorophenol levels in the upper tertile were also more likely to miss work or school due to wheezing.
These findings are consistent with several previous reports on atopic disorders and pesticides. Exposure to several insecticides and herbicides was reported to be associated with the presence of allergic rhinitis and atopic asthma4,5,7 and dichlorophenols were associated with increased allergic sensitization to foods.18
The mechanisms by which dichlorophenols may be increasing atopy and asthma are not well understood. Previous reports suggested that organochlorine compounds had direct immunomodulatory effects and were associated with decreased γ-interferon production, increased levels of interleukin-4, and higher serum IgE levels, possibly leading to the increased incidence of atopic diseases in children and in adults.16,17,19,20,33 Our finding of a positive association between urine dichlorophenol levels and total serum IgE levels supports this hypothesis. In addition, it is also possible that dichlorophenols could be associated with atopy indirectly, due to their anti-microbial properties8 and possibly through a change in microbiome. By analogy, a chlorine-containing bactericidal agent suppressed diversity of the intestinal flora in animals.34
Previous reports indicated that exposure to triclosan, a chlorophenol-based chemical with antibacterial properties used in toothpaste and hand sanitizers, was associated with allergic sensitization and hay fever but not with atopic asthma or atopic wheeze.30,31 Interestingly, 2,4-dichlorophenol is a byproduct of triclosan in chlorinated water and both compounds seem to coexist in urine.35,36 We found that there was a high correlation between both dichlorophenol metabolites, suggesting that they are possibly sharing a common source of exposure and/or the same metabolic path. However, there was a low correlation between triclosan and dichlorophenols implying that triclosan is probably not the main source of exposure to dichlorophenols in the study population. This assumption is also supported by a recent study on dichlorophenols in the US population.37 In addition, dichlorophenols seem to be different from triclosan with respect to its effect on IgE levels. While triclosan was reported not to be significantly associated with total serum IgE levels,30 we found that dichlorophenols were positively associated with total serum IgE levels in the entire study sample. Atopy is independently associated with asthma and wheezing, and the prevalence of asthma increases with total serum IgE levels among atopic subjects.38 Therefore, dichlorophenols might be associated with asthma and asthma morbidity in atopic wheezers through their association with total serum IgE levels. The reasons for the difference in relationships between triclosan, dichlorophenols, and total serum IgE are not known, but this difference is interesting and certainly merits further exploration.
Dichlorophenols have a relatively short half-life time in human body and exposure to them varies overtime.39 Thus estimation of a long-term exposure to dichlorophenols based on one-time urine sample is limited. However, most of our study outcomes were symptoms reported within past 12 months and they are likely less affected by the daily fluctuations in exposure to dichlorophenols. In addition, exposure to 2,5-dichlorohenol was reported to have less temporal variability and to be more consistent over time than that of other phenols.40 It is reasonable to question whether serum IgE level is affected over time. Little is known about how environmental exposures can affect its variability. A de novo formation of food-specific IgE in adults has been reported after only three months of exposure to anti-acid treatments, such as H2-receptor blockers or proton-pump inhibitors. In addition, total serum IgE levels nearly tripled after this treatment.41 These findings persisted at least several months after the treatment with anti-acid agents was stopped. Thus it is plausible that other environmental exposures could affect serum IgE in a similar fashion.
Dichlorophenols have other associations with human health. For example, 2,4- and 2.5-dichlorophenol levels in urine were higher in obese adults than in non-obese adults.42 Our study has several limitations: its cross-sectional design, exclusion of children <6 years on whom dichlorophenols were not measured in the 2005–2006 NHANES dataset, and the relatively small sample size in this analysis (resulting in wide confidence intervals). Since asthma in school-aged children and adolescents commonly starts during the preschool years, not having dichlorophenols measured in children <6 years is a major limitation to the present analysis. All data on wheezing-related problems were self-reported, thus are possibly affected by the recall bias. Due to the small number of asthmatics who responded to the questionnaires in this sample, only a limited exploratory analysis of the associations with asthma morbidity was possible. Such analysis could be a potential future research topic. Since our study is based on a cross-sectional analysis, there is also a possible reverse causality in the relationship between atopy and dichlorophenols. Dichlorophenols are found in toilet deodorizers, moth balls, insecticides, and herbicides, and are used in daily life.10,11 It is possible that atopic individuals may be more likely to be exposed to dichlorophenols due to their increased use of chlorine-containing pesticides and cleaning products in an attempt to maintain a less allergenic environment.
Despite these limitations, the findings from this study highlight the unique association between urine dichlorophenol levels and wheezing in a nationally representative sample of participants.
In summary, our findings suggest that higher levels of dichlorophenols in urine may contribute to greater asthma morbidity among atopic wheezers and atopic asthmatics. Prospective studies are necessary to determine the cause-effect relationship underlying these associations in wheezers and in asthmatics.
Supplementary Material
Acknowledgments
Grant information
This study was supported in part by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), through Clinical and Translational Science Award (grant numbers UL1 RR025750, KL2 RR025749 and TL1 RR025748).
Authors would like to thank Simon Spivack and Robert Y. Lin for their critical comments and review of the manuscript.
Sources of Support: None
List of abbreviations
- IgE
serum immunoglobulin E
- NHANES
National Health and Nutrition Examination Survey
- PIR
poverty-income ratio
- OR
odds ratio
- CI
confidence interval
- SE
standard error
Footnotes
- Elina Jerschow – conception and design of the study, data collection, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Purvi Parikh – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Aileen McGinn – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Gabriele de Vos – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Sunit Jariwala – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- Golda Hudes – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
- David Rosenstreich – conception and design of the study, analysis and interpretation of the data, preparation and critical revision of the manuscript.
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloomfield SF, Stanwell-Smith R, Crevel RW, Pickup J. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2006;36:402–25. doi: 10.1111/j.1365-2222.2006.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. New England Journal of Medicine. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Laboratory Component. 2011 at http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/lab_comp_g.pdf.)
- 4.Chatzi L, Alegakis A, Tzanakis N, Siafakas N, Kogevinas M, Lionis C. Association of allergic rhinitis with pesticide use among grape farmers in Crete, Greece. Occupational and environmental medicine. 2007;64:417–21. doi: 10.1136/oem.2006.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoppin JA, Umbach DM, London SJ, et al. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;34:1296–303. doi: 10.1183/09031936.00005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slager RE, Poole JA, LeVan TD, Sandler DP, Alavanja MC, Hoppin JA. Rhinitis associated with pesticide exposure among commercial pesticide applicators in the Agricultural Health Study. Occupational and environmental medicine. 2009;66:718–24. doi: 10.1136/oem.2008.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoppin JA, Umbach DM, London SJ, et al. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. American journal of respiratory and critical care medicine. 2008;177:11–8. doi: 10.1164/rccm.200706-821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechold H, Ehrlich P. Beziehungen zwischen chemischer Konstitution und Desinfektionswirkung. Z Physiol Chem. 1906;47:173–99. [Google Scholar]
- 9. [Accessed 12/22/2013];2,4-Dichlorophenol. 2012 at http://toxmap.nlm.nih.gov/toxmap/main/index.jsp.
- 10. [Accessed 10/06/2013];Chemical Information. 2,4-Dichlorophenol. 2013 at http://www.cdc.gov/biomonitoring/24D_BiomonitoringSummary.html.)
- 11. [Accessed 12/22/2013];Chemical Information. 2,5-Dichlorophenol. 2013 at http://www.cdc.gov/biomonitoring/25D_BiomonitoringSummary.html.)
- 12.Caliz J, Vila X, Marti E, et al. Impact of chlorophenols on microbiota of an unpolluted acidic soil: microbial resistance and biodegradation. FEMS microbiology ecology. 2011;78:150–64. doi: 10.1111/j.1574-6941.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 13.Organohalogen Taints in Foods. Australian Food and Grocery Council; 2007. [Accessed 12/22/2013]. at http://www.afgc.org.au/doc-library/.) [Google Scholar]
- 14.Whitfield FB, Nguyen TH, Last JH. Effect of relative humidity and chlorophenol content on the fungal conversion of chlrophenols to chloroanisoles in fibreboard cartons containing dried fruits. J Sci Food Agric. 1991;54:595–604. [Google Scholar]
- 15.Whitfield FB, Tindale CR, Shaw KJ, Stanley G. Contamination of cocoa powder by chlorophenols and cloroanisoles absorbed from packaging materials. Chem Ind. 1984:772–4. [Google Scholar]
- 16.Karmaus W, Brooks KR, Nebe T, Witten J, Obi-Osius N, Kruse H. Immune function biomarkers in children exposed to lead and organochlorine compounds: a cross-sectional study. Environmental health : a global access science source. 2005;4:5. doi: 10.1186/1476-069X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmaus W, Kuehr J, Kruse H. Infections and atopic disorders in childhood and organochlorine exposure. Archives of environmental health. 2001;56:485–92. doi: 10.1080/00039890109602896. [DOI] [PubMed] [Google Scholar]
- 18.Jerschow E, McGinn A, de Vos G, et al. Dichlorophenol-containing pesticides and allergies: results from the US National Health and Nutrition Examination Survey 2005–2006. Ann Allergy Asthma Immunol. 2012;109:420–5. doi: 10.1016/j.anai.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel V, Huber W, Bauer K, et al. Association of elevated blood levels of pentachlorophenol (PCP) with cellular and humoral immunodeficiencies. Archives of environmental health. 2001;56:77–83. doi: 10.1080/00039890109604057. [DOI] [PubMed] [Google Scholar]
- 20.Daniel V, Huber W, Bauer K, Suesal C, Conradt C, Opelz G. Associations of dichlorodiphenyltrichloroethane (DDT) 4.4 and dichlorodiphenyldichloroethylene (DDE) 4. 4 blood levels with plasma IL-4. Archives of environmental health. 2002;57:541–7. doi: 10.1080/00039890209602086. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed 12/22/2013];2,5-Dichlorophenol. 2009 at http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+4287.)
- 22.Elliott L, Longnecker MP, Kissling GE, London SJ. Volatile organic compounds and pulmonary function in the Third National Health and Nutrition Examination Survey, 1988–1994. Environmental health perspectives. 2006;114:1210–4. doi: 10.1289/ehp.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59:746–51. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson E, Olin AC, Hagberg S, Nilsson R, Nilsson T, Toren K. Adult-onset asthma and wheeze among irritant-exposed bleachery workers. American journal of industrial medicine. 2003;43:532–8. doi: 10.1002/ajim.10203. [DOI] [PubMed] [Google Scholar]
- 25.Court CS, Cook DG, Strachan DP. Comparative epidemiology of atopic and non-atopic wheeze and diagnosed asthma in a national sample of English adults. Thorax. 2002;57:951–7. doi: 10.1136/thorax.57.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed 12/22/2013];Environmental Pesticides (2,4-dichlorophenol, 2,5-dichlorophenol, ortho-phenylphenol, 2,4,5-trichlorophenol, and 2,4,6-trichlorophenol (PP_D) 2009 at http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/PP_D.htm.)
- 27.Biomonitoring summary. [Accessed 12/22/2013];Trichlorophenols. 2013 at http://www.cdc.gov/biomonitoring/Trichlorophenols_BiomonitoringSummary.html.)
- 28. [Accessed 12/22/2013];2005–2006 Data Documentation, Codebook, and Frequencies. Respiratory Disease. 2007 at http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/RDQ_D.htm.)
- 29. [Accessed 09/19/2013];2005–2006 Data Documentation, Codebook, and Frequencies. Medical Conditions. 2007 at http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/MCQ_D.htm.)
- 30.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. The Journal of allergy and clinical immunology. 2012;130:453–60. e7. doi: 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environmental health perspectives. 2011;119:390–6. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. The New England journal of medicine. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 33.Daniel V, Huber W, Bauer K, Suesal C, Conradt C, Opelz G. Associations of blood levels of PCB, HCHS, and HCB with numbers of lymphocyte subpopulations, in vitro lymphocyte response, plasma cytokine levels, and immunoglobulin autoantibodies. Environmental health perspectives. 2001;109:173–8. doi: 10.1289/ehp.01109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaway TR, Anderson RC, Genovese KJ, et al. Sodium chlorate supplementation reduces E. coli O157:H7 populations in cattle. Journal of animal science. 2002;80:1683–9. doi: 10.2527/2002.8061683x. [DOI] [PubMed] [Google Scholar]
- 35.Canosa P, Morales S, Rodriguez I, Rubi E, Cela R, Gomez M. Aquatic degradation of triclosan and formation of toxic chlorophenols in presence of low concentrations of free chlorine. Analytical and bioanalytical chemistry. 2005;383:1119–26. doi: 10.1007/s00216-005-0116-4. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed 12/22/2013];National Health and Nutrition Examination Survey 2005–2006, Laboratory Data. 2013 at http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2005.)
- 37.Ye X, Wong LY, Zhou X, Calafat AM. Urinary Concentrations of 2,4-Dichlorophenol and 2,5-Dichlorophenol in the U.S. Population (National Health and Nutrition Examination Survey, 2003–2010): Trends and Predictors. Environmental health perspectives. 2014 doi: 10.1289/ehp.1306816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gergen PJ, Arbes SJ, Jr, Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005–2006. The Journal of allergy and clinical immunology. 2009;124:447–53. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassen TH, Frederiksen H, Jensen TK, et al. Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environmental research. 2013 doi: 10.1016/j.envres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology. 2013;47:3439–47. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Untersmayr E, Bakos N, Scholl I, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:656–8. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 42.Wei Y, Zhu J, Nguyen A. Urinary concentrations of dichlorophenol pesticides and obesity among adult participants in the U.S. National Health and Nutrition Examination Survey (NHANES) 2005–2008. International journal of hygiene and environmental health. 2014;217:294–9. doi: 10.1016/j.ijheh.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.