Abstract
The cystatins are inhibitors of papain- and legumain-like cysteine proteinases, classified in MEROPS subfamilies I25A-I25C. This study shows that 84 % (42/50) of tick cystatins are putatively extracellular in subfamily I25B and the rest are putatively intracellular in subfamily I25A. On the neighbor joining phylogeny guide tree, subfamily I25A members cluster together, while subfamily I25B cystatins segregate among prostriata or metastriata ticks. Two Ixodes scapularis cystatins, AAY66864 and ISCW011771 that show 50–71 % amino acid identity to metastriata tick cystatins may be linked to pathways that are common to all ticks, while ISCW000447 100 % conserved in I. ricinus is important among prostriata ticks. Likewise metastriata tick cystatins, Dermacentor variabilis-ACF35512, Rhipicephalus microplus-ACX53850, A. americanum-AEO36092, R. sanguineus-ACX53922, D. variabilis-ACF35514, R. sanguineus-ACX54033 and A. macula-tum-AEO35155 that show 73–86 % amino acid identity may be essential to metastriata tick physiology. RT-PCR expression analyses revealed that I. scapularis cystatins were constitutively expressed in the salivary glands, midguts and other tissues of unfed ticks and ticks that were fed for 24–120 h, except for ISCW017861 that are restricted to the 24 h feeding time point. On the basis of mRNA expression patterns, I. scapularis cystatins, ISCW017861, ISCW011771, ISCW002215 and ISCW0024528 that are highly expressed at 24 h are likely involved in regulating early stage tick feeding events such as tick attachment onto host skin and creation of the feeding lesion. Similarly, ISCW018602, ISCW018603 and ISCW000447 that show 2–3 fold transcript increase by 120 h of feeding are likely associated with blood meal up take, while those that maintain steady state expression levels (ISCW018600, ISCW018601 and ISCW018604) during feeding may not be associated with tick feeding regulation. We discuss our findings in the context of advancing our knowledge of tick molecular biology.
Keywords: Ixodes scapularis, Cystatins and tick physiology, Tick cystatin molecular relationship
Introduction
In public health, an increasing number of human tick borne diseases have been discovered (Bratton and Corey 2005; Fish and Childs 2009) since the discovery of Borrelia burgdorferi as the causative agent of Lyme disease in the 1980s (Burgdorfer et al. 1982; Burgdorfer 1986). The USA Centers for Disease Control (CDC) April 6th 2012 (http://www.cdc.gov/ticks/diseases) update listed 12 human tick borne diseases (TBD) in the USA. Causative agents of 4 of the 12 human TBDs, borrelisosis, anaplasmosis, babesiosis and Powassan virus infections are vectored by Ixodes spp (Bratton and Corey 2005; Fish and Childs 2009). The importance of Ixodes tick species in public health was the justification for sequencing the I. scapularis genome (Pagel et al. 2007; Nene 2009). The availability of the I. scapularis genome sequence data coupled with multiple tick EST resources in GeneBank have opened up opportunities to understand molecular pathways that are at play in tick physiology. Using sequence resources from the I. scapularis genome data, we are interested in understanding the roles of proteases and protease inhibitors in regulating tick feeding physiology, acquisition, maintenance and transmission of disease agents by ticks as a means to find vaccine development targets. In previous studies, we have characterized protease (Mulenga and Erikson 2011) and, temporal and spatial profiling of serine protease inhibitors (serpins) family (Mulenga et al. 2009) in the I. scapularis genome. In this study the goal was to characterize cystatin superfamily in the I. scapularis genome and other ticks.
The cystatin superfamily is composed of a large group of cystatin domain-containing proteins that function as tight-binding and reversible inhibitors of the papain-like and legumain cysteine proteases (Barrett 1985, 1986; Rawlings and Barrett 1990). On the basis of structure, cystatins have been classified into three families, 1, 2 and 3 or stefins, cystatins and kininonongen respectively (Ochieng and Chaudhuri 2010). On the MEROPS database cystatins have been placed into family I25, which contains three subfamilies, I25A, B and C (Rawlings et al. 2012). In other parasitic organisms (Klotz et al. 2011) majority cystatins were putatively annotated in subfamily I25B. Originally cystatins were characterized as inhibitors of lysosomal cathepsin cysteine proteases (Kopitar-Jerala 2006), which from the standpoint of tick vaccine development will be unattractive. Recent data have however, revealed alternative biological functions of cystatins in the extracellular environment Turk and Bode 1991; Abrahamson 1994) that make them appealing targets for tick vaccine development. These functions include cytokine induction role in tumorigenesis, tissue remodeling, renal function, immune-regulation (Ochieng and Chaudhuri 2010; Kopitar-Jerala 2006).
Cystatins have been identified in multiple tick species (Sonenshine et al. 2011; Zhou et al. 2009, 2010; Yamaji et al. 2009, 2010; Francischetti et al. 2008a, b; 2009; Grunclová et al. 2006a, b; Lima et al. 2006). Several lines of research point to the importance of cystatins in tick physiology (Schwarz et al. 2012; Horka et al. 2012). RNAi silencing of cystatins in I. scapularis (Kotsyfakis et al. 2007) and A. americanum (Karim et al. 2005) or feeding I. scapularis ticks or Guinea pigs (Kotsyfakis et al. 2008) or Ornithodoros moubata (Salát et al. 2010) that were immunized with a recombinant tick salivary gland cystatin caused significant reductions in tick feeding efficiency. In a recent study an I. scapularis tick salivary gland cystatin that retained the consensus cystatin secondary structure fold was shown involved in B. burgdorferi transmission (Kotsyfakis et al. 2010a, b). Studies based on recombinant tick cystatins have provided insight that native tick-encoded cystatins are functional inhibitors of cathepsin-like cysteine proteases (Kotsyfakis et al. 2006; Lima et al. 2006; Zhou et al. 2006, 2009, 2010; Grunclová et al. 2006a, b; Yamaji et al. 2009). In other studies, recombinant cystatins affected the function of immune cell functions (Salát et al. 2010; Sá-Nunes et al. 2009). In this study we have used bioinformatics analyses to identify cystatins that are conserved in most ticks and RT-PCR expression analyses to describe the relationship of I. scapularis cystatins to the tick feeding cycle. We discuss our findings in the context of advancing our knowledge of the molecular physiology of ticks and discovery of target antigens for tick vaccine development.
Materials and methods
Bioinformatics analyses of tick cystatins
Bioinformatics analyses of tick cystatins were done using Geneious Pro version 5.63 (http://www.geneious.com/web/geneious/geneious-pro) and the MacVector (Accelrys, Inc, San Diego, CA) sequence analyses software. Tick cystatin sequences used in this study were downloaded from Genebank were downloaded using the Geneious software to create a local database. Downloaded cystatin sequences are from I. scapularis, Rhipicephalus sanguineus, Amblyomma variegatum, A. maculatum, Dermacentor variabilis, Ornithodoros moubata, O. coriaceus, Haemaphysalis longicornis and R. microplus (Table 1). To gauge the phylogeny relationship among tick cystatins, 50 tick cystatin amino acid sequences (Table 1) were subjected to multiple sequence alignment analyses using the Geneious aligner (http://www.geneious.com). The Geneious aligner used in this study is based on the Needleman-Wunsch and Smith-Waterman pairwise alignment algorithm (ref) and the implementation of a progressive pairwise algorithm for multiple alignments in the Geneous software package. Aligned sequences were used to construct a bootstrap value supported phylogeny tree using the Neighbor joining method. Sequences that clustered together on the phylogeny tree were subjected to pairwise alignment analysis using MacVector sequence analysis software to determine sequence relationships within each cluster. Sequences were considered highly cross-tick conserved if >50–100 % amino acid identity levels were observed among cystatin sequences in different tick species. To gain insight on features that characterize tick cystatins, amino acid motif scanning and signal peptide prediction were done using the Genieous Pro software package.
Table 1. Annotated tick cystatins used in this study.
| Tick species | Accession #s |
|---|---|
| Dermacentor silvarum | ADZ23478 |
| Dermacentor variabilis | ACF35514 |
| Amblyomma variegatum | DAA34288 |
| Amblyomma maculatum | AEO36320 |
| AEO36722 | |
| AEO36321 | |
| AEO36319 | |
| AEO36092 | |
| AEO35899 | |
| AEO35689 | |
| AEO35688 | |
| AEO35650 | |
| AEO35364 | |
| AEO35156 | |
| AEO35155 | |
| AEO34426 | |
| AEO34425 | |
| AEO33631 | |
| AEO32440 | |
| AEO33735 | |
| AEO32139 | |
| Haemaphysalis longicornis | ABC94582 |
| ABZ89553 | |
| ABZ89554 | |
| ABV71390 | |
| BAI59105 | |
| ACF35512 | |
| Ornithodoros moubata | AAS01021 |
| AAS55948 | |
| Ornithodoros coriaceus | ACB70345 |
| ACB70343 | |
| Ornithodoros parkeri | ABR23498 |
| ACX53922 | |
| ACX53862 | |
| ACX53850 | |
| Rhipicephalus microplus | ABG36931 |
| Ixodes ricinus | CAD68002 |
| Ixodes scapularis | ICSW010785 |
| ISCW018604 | |
| ISCW018603 | |
| ISCW018602 | |
| ISCW017861 | |
| ISCW002216 | |
| ISCW018600 | |
| ISCW002036 | |
| ISCW000447 | |
| ISCW018601 | |
| ISCW002037 | |
| ISCW011771 | |
| ISCW024528 | |
| AAY66846 |
Tick feeding, dissections and total RNA extraction
Adult I. scapularis ticks that were used in this experiment were purchased from the University of Oklahoma tick-rearing laboratory. A New Zealand White rabbit without prior exposure to tick infestation was used to feed ticks under humane conditions according to an approved animal use protocol. During feeding, ticks were placed into a two-inch orthopedic cotton stockinet cell that was attached on top of the rabbit ear using Kamar adhesive (Kamar, Steamboat Springs, CO). For dissections, unfed ticks and those that were fed for 24, 72 and 120 h were dissected according to previously published methods (Mulenga et al. 2003). Ticks were placed on a glass slide that was treated with molecular grade water DEPC water. The dorsal shield was removed utilizing a sterile razor blade and a soft tissue forceps. Tick organs, salivary gland (SG,) and midgut (MG), and remnant tissues (OT) were teased out using forceps and 18 gauge needles. Dissected SG, MG and OT tissues were pooled from 18 unfed ticks, 15, 10 and 6 24 h, 72 h and 120 h ticks, respectively. Tissues were dissected directly into the RNA extraction reagent Trizol (Invitrogen) and stored at −80 °C until used for total RNA extraction. To extract RNA, tissue samples were thawed at room temperature and homogenized using a Sonic dismembrator model 100 (Thermo Fisher Scientific). Total RNA was extracted using the Trizol reagent following manufacturer's protocol (Life Technologies, Carlsbad, CA). RNA concentration and purity was determined using Beckman DU640B spectrophotometer (Beckman Coulter, Brea, CA).
Temporal and spatial expression analyses
To determine temporal and spatial gene expression patterns of I. scapularis cystatins during the first 5 days of tick feeding, specific primers in Table 2 were subjected to two-step titration semi-quantitative RT-PCR as previously described (Mulenga et al. 2008). We restricted our analyses to the first 5 days of tick feeding because of our lab interest to investigate early stages of the tick feeding process. Total RNA (0.5 μg) was used for first-strand cDNA synthesis with the qScript™ (Quanta Biosciences), using oligo dT priming, following the manufacturer's protocol (Quanta Bioscience, Gaithersburg, MD) in 20 μl reaction. The cDNA template was then diluted 10 fold with molecular grade water to ∼200 ng/μl. Prior to conducting the semi-quantitative PCR analysis, we determined PCR product saturation points for our target genes and the internal control tick actin gene using primers in Table 2. Subjecting samples to 25, 30 and 35 PCR cycles did this. This analysis revealed that, PCR product saturation points were reached after >28 PCR cycles. Thus, ubsequent PCR amplification was conducted for 28 cycles below the PCR product saturation points for all targets. Routinely, ∼200 ng of the cDNA template was used in a PCR reaction containing MyTaq PCR Master Mix (Promega, Madison, WI, USA), cystatin primers (Table 2) at 0.1 μM final concentration in a 30 μl reaction. The cycling conditions were an initial denaturation of 94 °C for 2 min, followed by 28 amplification cycles of 94 °C for 30′, 55 °C for 30′, and 72 °C for 1 min, and a final extension of 72 °C for 5 min. For template normalization control, actin primers (Table 2) at 0.1 μM final concentrations in a 30 μl reaction were used. Equal volume, of 15 μl of each PCR product were electrophoresed on a 2 % agarose gel with added ethidium bromide. To determine cystatin transcript abundance, densitograms of PCR bands were normalized against tick actin PCR band densities. Densitograms were determined using the Image J software (http://rsb.info.nih.gov/ij). Transcript abundance in each sample was normalized according to the formula: Y = V + V(H-X)/X, where Y is the normalized mRNA density, V is the observed cystatins PCR band density in individual samples, H is the highest tick actin PCR band among tested samples and X is the tick actin PCR band for the test sample (Mulenga et al. 2008).
Table 2. Ixodes scapularis cystatin primers used in this study.
| Gene ID | Forward | Reverse |
|---|---|---|
| ISCW000447 | 5′ATGCAGGTGATTGCGGGTGTCGA3′ | 5′TTAAGAGTTGTTGACAGGTTCGCC3′ |
| ISCW002215 | 5′ATGAGCCTCCCCAAGGTAGCCC3′ | 5′TCAAGACTCGACGTTGAAGAG3′ |
| ISCW010785 | 5′GCCCTCAGGATGTTACCAGTTTG3′ | 5′GTAGACACGATCGCTGCAGTTG3′ |
| ISCW011771 | 5′CGGGGGACAGCGAGTTCTATGAC3′ | 5′TCAAACGCACTCGTAACTTGTGAC3′ |
| ISCW017861 | 5′GCAGGAGTGACTTCAGAACGG3′ | 5′CACGATGTGTTCGCAGGGCATTTC3′ |
| ISCW018600 | 5′GGAACCAACTATAGACTGACGC3′ | 5′TTACGGTACGCAGTCGTAGGAGCT3′ |
| ISCW018601 | 5′CTCCCTCGCTTTGGTCCTTCTGCT3′ | 5′GCGTCATTCTGTAGTTGGTTCC3′ |
| ISCW018602 | 5′ATGACTTCCTCCCTCGCTTTGG3′ | 5′TTATGCGGCCGCACACTCGAAGG3′ |
| ISCW018603 | 5′ATGACTTCTTCCTTCGCTTTGG3′ | 5′CTATGCGGCTTCACACTCGAAGGA3′ |
| ISCW018604 | 5′CGCTCACGCTGGTCATTTTCCTGA3′ | 5′CGCAGACAGACTCCGCCACC3′ |
| ISCW024528 | 5′AAGACGCAGGACCTAACCAACCCC3′ | 5′GGAGTATTCCACGCCGGCTTCG3′ |
Results
Bioinformatics analysis of tick cystatins
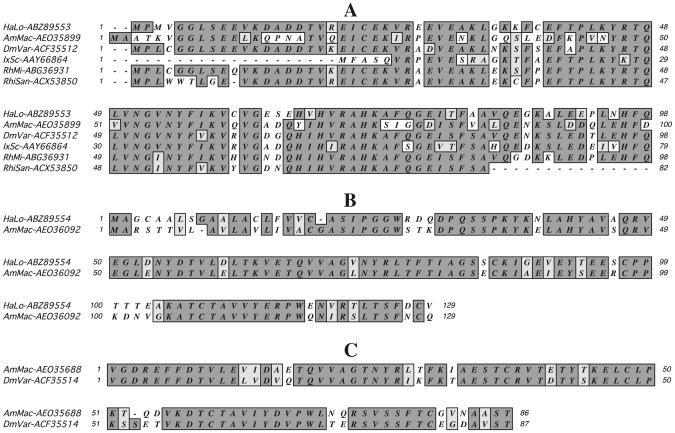
At the time of this analysis, 50 unique tick cystatin sequences (Table 1) were deposited in GeneBank. The 50 amino acid sequences were aligned using the Geneious aligner and then the guide tree constructed using the neighbor-joining method. On the guide tree that was out-routed from the human cystatin sequence (AAA36115), 50 tick cystatin sequences segregated into two clades, “A” containing eight of the 50 sequences and clade “B” containing the rest (Fig. 1). In clade “A” the lone I. scapularis cystatin (AY66864) sequence segregated together with metastriata tick sequences from A. maculatum (AEO35899), H. longicornis (ABZ89553), D. variabilis (ACF35512), R. sanguineus (ACX53850) and R. microplus (ABG36931). The rest of the I. scapularis cystatins segregated with the majority of other tick cystatins in clade B. In clade B, sequences further segregate into specific sub-clusters (SC) 1-11. Except for ISCW011771 in SC1 that segregate with O. moubata (AAS010201 and AAS55948) and A. maculatum (AEO35689) as well as ISCW017681 in SC4 that segregate with A. maculatum (AEO32440), the rest of the I. scapularis cystatins cluster alone in SC9 or with I. ricinus (CAD68002) in SC11. Amino acid motif scanning analysis and signal peptide prediction revealed that sequences in clade “A” have characteristic subfamily I25A cystatin sequence features (Rawlings et al. 2012; Ochieng and Chaudhuri 2010) that consensus cystatin “QXVXG” amino acid motif and lack of a signal peptide and putative disulfide bonds (Fig. 2A). Cystatin sequences in clade “B” putatively belong to subfamily I25B (Rawlings et al. 2012; Ochieng and Chaudhuri 2010) in that, except for partial sequences, all have signal peptides with the “QXVXG” and “PW” amino acid motifs and the four consensus cysteine residues in the amino terminus region conserved (Fig. 2B, C). We would like to note here the “PW” amino acid motif has been replaced in other tick cystatin sequences with “IR”, “HR”, “PL”, PT, PA, “LQ”, “VW”, “SK”, “SV”, TG, in other sequences (not shown). It is important to note that two A. maculatum cystatins (AEO32139 and AEO33735) in clade A do have the “PW” motif, which is typical for subfamily I25B members (Rawlings et al. 2012).
Fig. 1.
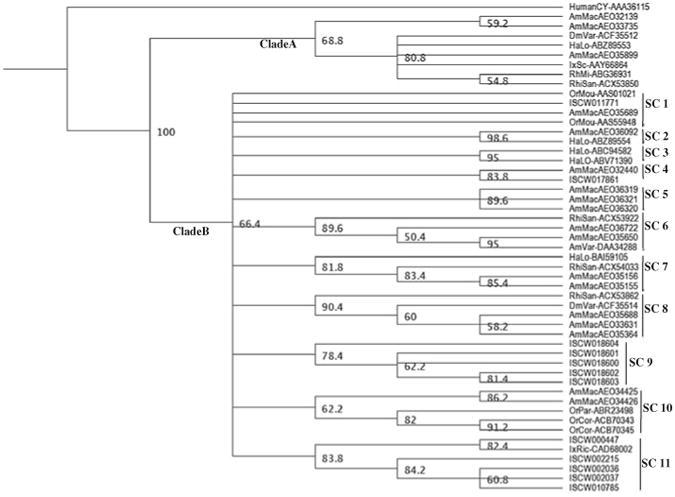
Phylogeny relationship of Ixodes scapularis cystatins to other tick cystatins. I. scapularis tick cystatins and other tick cystatins (Table 1) were downloaded from GeneBank. The downloaded sequences were subjected to guide phylogeny analysis using the neighbor-joining method in the Geneious sequence analysis software. ISCW Ixodes scapularis, AmMac Amblyomma americanum, AmVar A. variegatum, DmVar Demacentor variabilis, OrMou Ornithodoros moubata, OrPar O. parkeri, OrCor O. cor, HaLo Haemaphysalis longicornis, RhiSan Rhipicephalus sanguineus, RhiMi Rhipicephalus microplus. SC subclade
Fig. 2.
Amino acid sequence alignment of orthologous tick cystatins showing >50 % amino acid identity: A I. scapularis cystatin AAY66864 is aligned with metastriata tick cystatin sequences, A. maculatum (AamMacAEO35899), D. variabilis (DmVarACF35512), H. longicornis (HaLoABZ89553), R. sanguineus (RhiSanACX53850) and R. microplus (RHiMiABG369312), B A. americanum (AEO36092) aligned with H. longicornis (ABZ89554), C D. variabilis (ACF35514) aligned with A. americanum (AEO35688)
With exception of sequences shown in Fig. 2, overall amino acid identity levels between I. scapularis and other tick cystatins ranged between 10 and 30 % (not shown). Figure 2 summarizes cross-tick species conserved cystatins with amino acid levels above 50 %. In Fig. 2A, I. scapularis cystatin AAY66864 is cross-conserved in metastrata ticks as it showed 54 % amino acid identity to A. maculatum (AEO35899) and ∼ 66-71 % to D. variabilis (ACF35512), H. longicornis (ABZ89553), R. sanguineus (ACX53850) and R. microplus (ABG369312). Similarly, I. scapularis cystatin ISCW011771 in SC1 shows 50 % amino acid identity to O. moubata (AAS55948) cystatin (not shown). It is also notable that I. scapularis cystatin, ISCW000447 in SC10 is 100 % identical I. ricinus (CAD68002) (not shown). A similar analysis among metastriata tick cysatins identified four highly conserved clusters including A. americanum (AEO36092) and H. longicornis (ABZ89554) that show 73 % amino acid identity (Fig. 2B) and D. variabilis (ACF35514) and A. americanum (AEO35688) that show ∼78 % amino acid identity (Fig. 2C). Another interesting notable observation from our sequence analysis is that we observed that five I. scapularis cystatins ISCW018600, ISCW018601, ISCW018602, ISCW018603 and ISCW018604 occur in tandem with I. scapularis serpin (ISCW018607) (not shown) in the same region of the genome. It is also noteworthy that we identified clusters of highly identical I. scapularis cystatins including SC 9 sequences, ISCW018601, ISCW018602 and ISCW018603 that show 70–75 % amino acid identity and SC11 sequences, ISCW010785, ISCW002036, ISCW002037 and ISCW002215 that showed 89–92 % amino acid identity (not shown).
Temporal and spatial expression patterns of Ixodes scapularis cystatins
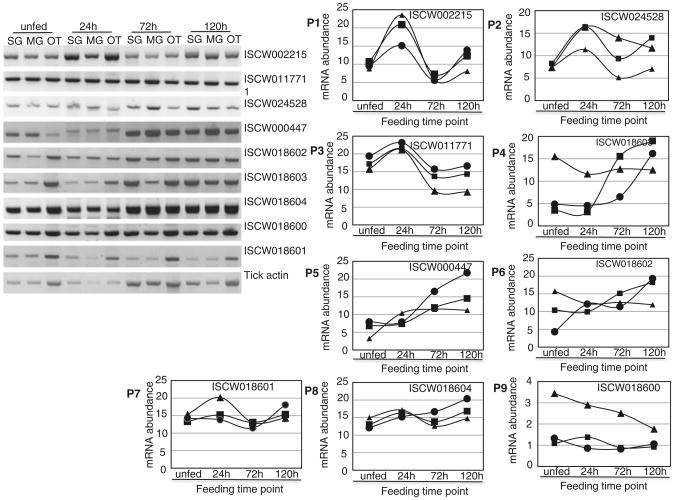
To gauge insight into the biological relationship of I. scapularis cystatins to the tick feeding cycle, temporal and spatial mRNA expression were determined using semi-quantitative RT-PCR as summarized in Fig. 3. We would like to note here that 12 instead of 14 I. scapularis cystatins were involved in this analysis. The reason is that during primer design we noticed that ISCW002215 and ISCW002216 as well as ISCW002036 and ISCW002037 were highly identical. Thus we eliminated ISCW002215 and ISCW002036 from this analysis. Except for ISCW017861 and ISCW010785 mRNA that appeared to be induced exclusively during the 24 h tick feeding time point (not shown) the rest of I. scapularis tick cystatins assayed in this study were constitutively and ubiquitously expressed in that they were amplified in salivary glands (SG), midguts (MG) and other tissue (OT) of unfed ticks and ticks that were fed for 24, 72 and 120 h feeding time points (Fig. 3A). Normalized mRNA abundance summarized in Fig. 3B reveal three I. scapularis cystatin gene expression patterns during the first 5 days of tick feeding. In the first pattern, ISCW011771, ISCW002215 and ISCW0024528 (Fig. 3B, panels P1–P3), transcript abundance increases by ∼1.5-twofold at the 24 h feeding time point before dropping to near steady state by the 72 h time point. Transcript abundance starts to go back up from the 120 h time point in the case of ISCW002215 and ISCW0024528 (Fig. 3B, panels P1 and P2). In the second pattern (P4–P6), transcript abundance of ISCW000447, ISCW018602 and ISCW018603 is apparently up regulated as ticks continue to feed as indicated by an ∼2–4 fold increase in transcript abundance by the 120 h feeding time point. In the third group mRNA of ISCW018600, ISCW018601 and ISCW018604 (P7–P9) are apparently constitutively expressed and not responsive to tick feeding activity as their mRNA abundance did not significantly vary during tick feeding. It is interesting to note that while ISCW018601 show steady state mRNA expression in the SG and MG, its mRNA abundance appear to be down regulated in response to feeding in other tissues. ISCW017861 and ISCW010785 mRNA are weakly expressed during the first 120 h of I. scapularis feeding in that even after 40 PCR cycles, PCR products were barely detectable on agarose gels and thus we did not quantify.
Fig. 3.
RT-PCR expression analysis of Ixodes scapularis cystatins. A Spatial and temporal mRNA expression analysis of I. scapularis cystatins: Total RNA of salivary glands (SG), midguts (MG) and tick remnants after removal of SG and MG (OT) dissected from unfed and adult ticks that were partially fed on rabbits for 24, 72 and 120 h were subjected to two step RT-PCR analysis as described on materials and methods. B Normalized I.scapularis cystatin mRNA abundance: PCR band intensity as proxy for cystatin mRNA in assayed samples was normalized against tick actin abundance using the formula, Y = V + V(H− X)/X. In this formula Y is the normalized mRNA density, V is the observed cystatins PCR band density in individual samples, H is the highest tick actin PCR band density among tested samples and X is the tick actin PCR band density for the test sample. filled circle = MG, filled square = SG, filled triangle = OT
Discussion
A recent study by Schwarz et al. (2012) up dated the status of knowledge on the role of cystatins in tick physiology. Findings in this study confirm previous reports (Schwarz et al. 2012; Karim et al. 2010) that consistent with other parasites (Klotz et al. 2011), the majority of tick cystatins are in subfamily I25B followed by few members in subfamily I25A. From the perspective of tick vaccine development research, subfamily I25B cystatins are attractive in that they are extracellular (Turk and Bode 1991; Abrahamson 1994) and are likely to be bio-accessible by host immune response factors. Our finding of tick cystatins that show high amino acid conservation in multiple tick species is significant in that they are likely to regulate biological functions that are important to all ticks. High amino acid conservation is a characteristic for proteins that are under purifying or negative selection where retention of function through speciation is critical to the survival of stability of the organism (Pacheco et al. 2012). If this is consistent, conserved cystatins being reported in this study represent important target antigens for tick vaccine or drug development. It is interesting to note that, in certain parts of the world animals are infested by multiple different tick species at most times. Strategically it is appealing to target highly conserved targets such as cystatins described in this study for development of novel control strategies to protect animals against any tick species. We would like to note here that I. scapularis cystatins AAY66864 which is conserved in both prostriata and metastriata ticks is putatively intracellular and thus making it not less attractive as a target antigen for tick vaccine development. However, its conservation between prostriata and metastriata ticks warrants further study into its biological functions. Further understanding of its functions could reveal important pathways that are essential to all ticks.
The functional redundancy phenomenon where a biochemical process is regulated by multiple highly identical protein factors has been observed in many organisms (Bedner et al. 2011; Swarnakar et al. 2012). Although empirical functional analysis data is needed for validation, the high amino acid conservation between some of the I. scapularis cystatins observed in this study could signal functional redundancy. Its been observed in cases of functional redundancy that losing function of one enzyme had minimal or no adverse effect on an organism (Wagner 2000). Willadsen (2006) recognized the potential limitation of targeting redundant molecular systems such as the highly conserved I. scapularis cystatins in this study for tick vaccine development in that targeting one or a few candidates may not necessarily affect tick physiology. Thus in targeting potential functionally redundant groups of proteins for tick vaccine development, the strategy will be to target the all group in multivalent vaccine development approach. From the perspective of increasing of our knowledge of tick molecular biology, another interesting observation in this study is the observed occurrence of five I. scapularis cystatins in tandem with a serpin. Some serpins are cross-class inhibitors of both serine and cysteine proteases (Wladyka et al. 2011; Higgins et al. 2010; Herrera-Mendez et al., 2009; Hwang and Hook 2007). It will be interesting to investigate whether or not the I. scapularis serpin observed here in tandem with cystatins is a functional cysteine protease inhibitor.
Temporal and spatial mRNA expression profiling during tick feeding has routinely been used to gauge insight into the roles of candidate genes in regulation of the tick feeding process (Sonenshine et al. 2011; Zhang et al. 2011; Tian et al. 2011; Anisuzzaman et al. 2011). Consistent with other tick cystatins that showed ubiquitous expression patterns (Schwarz et al. 2012) and the observation of I. scapularis being expressed in all tested organs is indicative of their potential to regulate important biological functions in tick physiology. On the basis of expression profiles observed in this study, I scapularis cystatins were likely associated with early stage tick feeding events such as attachment and/or creation of the tick feeding site when highest transcript abundance was observed at 24 h post-attachment. Those genes with transcript abundance increasing in response to feeding are potentially involved in regulating blood meal up take. There is also a possibility that some of the cystatins are not associated with tick feeding regulation in that their transcript abundance did not vary in response to tick feeding activity. It is important to note here that two I. scapularis cystatins, ISCW018602 and ISCW018603 were also previously shown to be expressed in SG and MG of partially fed I. scapularis ticks (Schwarz et al. 2012). Data in this study indicates these cystatins are also expressed in unfed ticks and that their transcript abundance increases with tick feeding. We would like to note here that biased by our long-term interest in secreted tick saliva proteins, we did not determine the expression profile of AAY66864, which is apparently an intracellular cystatin. However, in a previous study, AAY66864 was shown to be expressed in tick SG (Lima et al. 2006). In assuming that cystatins are involved in tick feeding regulation, the assumption is that they are injected into the host during tick feeding. Thus, the next phase of this project is to validate secretion of candidate cystatins into the host during tick feeding.
Acknowledgments
The authors acknowledge research support to AM from NIH/NIAID (NIH-R21AI081093, NIHRO1AI093858). NIH/NIAID diversity in life Science Research supplement to AI081093 supported CGL, an undergraduate researcher. ME was an undergraduate researcher who also participated in this research for her undergraduate research internship. AMGI was an international visiting student supported by a CNPq scholarship (201690/2010-1) from the Brazilian government.
Contributor Information
Adriana Mércia Guaratini Ibelli, Department of Entomology, Texas A & M University AgriLife Research, 2475 TAMU, College Station, TX 77843, USA; Graduate Program in Genetics and Evolution, Federal University of São Carlos, São Carlos, Brazil.
Meghan M. Hermance, Department of Entomology, Texas A & M University AgriLife Research, 2475 TAMU, College Station, TX 77843, USA
Tae Kwon Kim, Department of Entomology, Texas A & M University AgriLife Research, 2475 TAMU, College Station, TX 77843, USA.
Cassandra Lee Gonzalez, Department of Entomology, Texas A & M University AgriLife Research, 2475 TAMU, College Station, TX 77843, USA.
Albert Mulenga, Email: a-mulenga@tamu.edu, Department of Entomology, Texas A & M University AgriLife Research, 2475 TAMU, College Station, TX 77843, USA.
References
- Abrahamson M. Cystatins. Methods Enzymol. 1994;244:685–700. doi: 10.1016/0076-6879(94)44051-4. [DOI] [PubMed] [Google Scholar]
- Anisuzzaman Islam MK, Alim MA, Miyoshi T, Hatta T, Yamaji K, Matsumoto Y, Fujisaki K, Tsuji N. Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks. PLoS Pathog. 2011;7(3):e1001312. doi: 10.1371/journal.ppat.1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ. The cystatins: small protein inhibitors of cysteine proteinases. Prog Clin Biol Res. 1985;180:105–116. [PubMed] [Google Scholar]
- Barrett AJ. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta. 1986;45:1363–1374. [PubMed] [Google Scholar]
- Bedner P, Steinhäuser C, Theis M. Functional redundancy and compensation among members of gap junction protein families? Biochim Biophys Acta. 2011;1818:1971–1984. doi: 10.1016/j.bbamem.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323–2330. [PubMed] [Google Scholar]
- Burgdorfer W. Discovery of the Lyme disease spirochete: a historical review. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263:7–10. doi: 10.1016/s0176-6724(86)80094-3. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Fish D, Childs JE. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricide to white-tailed deer: background and rationale. Vector Borne Zoonotic Dis. 2009;9:357–364. doi: 10.1089/vbz.2009.0022. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Mans BJ, Meng Z, Gudderra N, Veenstra TD, Pham VM, Ribeiro JM. An insight into the sialome of the soft tick Ornithodorus parkeri. Insect Biochem Mol Biol. 2008a;38:1–21. doi: 10.1016/j.ibmb.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Meng Z, Mans BJ, Gudderra N, Hall M, Veenstra TD, Pham VM, Kotsyfakis M, Ribeiro JM. An insight into the salivary transcriptome and proteome of the soft tick and vector of epizootic bovine abortion Ornithodoros coriaceus. J Proteomics. 2008b;71:493–512. doi: 10.1016/j.jprot.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunclová L, Horn M, Vancová M, Sojka D, Franta Z, Mares M, Kopácek P. Two secreted cystatins of the soft tick Ornithodoros moubata: differential expression pattern and inhibitory specificity. Biol Chem. 2006a;387:1635–1644. doi: 10.1515/BC.2006.204. [DOI] [PubMed] [Google Scholar]
- Grunclová L, Horn M, Vancová M, Sojka D, Franta Z, Mares M, Kopácek P. Two secreted cystatins of the soft tick Ornithodoros moubata: differential expression pattern and inhibitory specificity. Biol Chem. 2006b;387:1635–1644. doi: 10.1515/BC.2006.204. [DOI] [PubMed] [Google Scholar]
- Herrera-Mendez CH, Becila S, Blanchet X, Pelissier P, Delourme D, Coulis G, Sentandreu MA, Boudjellal A, Bremaud L, Ouali A. Inhibition of human initiator caspase 8 and effector caspase 3 by cross-class inhibitory bovSERPINA3-1 and A3–3. FEBS Lett. 2009;583:2743–2748. doi: 10.1016/j.febslet.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Higgins WJ, Fox DM, Kowalski PS, Nielsen JE, Worrall DM. Heparin enhances serpin inhibition of the cysteine protease cathepsin L. J Biol Chem. 2010;285:3722–3729. doi: 10.1074/jbc.M109.037358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horka H, Staudt V, Klein M, Taube C, Reuter S, Dehzad N, Andersen JF, Kopecky J, Schild H, Kotsyfakis M, Hoffmann M, Gerlitzki B, Stassen M, Bopp T, Schmitt E. The tick salivary protein sialostatin L inhibits the Th9-derived production of the asthma-promoting cytokine IL-9 and is effective in the prevention of experimental asthma. J Immunol. 2012;188:2669–2676. doi: 10.4049/jimmunol.1100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SR, Hook VY. Multiple domains of endopin 2A for serpin cross-class inhibition of papain. Arch Biochem Biophys. 2007;461:219–224. doi: 10.1016/j.abb.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Miller NJ, Valenzuela J, Sauer JR, Mather TN. RNAi-mediated gene silencing to assess the role of synaptobrevin and cystatin in tick blood feeding. Biochem Biophys Res Commun. 2005;334:1336–1342. doi: 10.1016/j.bbrc.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Karim S, Troiano E, Mather TN. Functional genomics tool: gene silencing in Ixodes scapularis eggs and nymphs by electroporated dsRNA. BMC Biotechnol. 2010;10:1. doi: 10.1186/1472-6750-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz C, Ziegler T, Daniłowicz-Luebert E, Hartmann S. Cystatins of parasitic organisms. Adv Exp Med Biol. 2011;712:208–221. doi: 10.1007/978-1-4419-8414-2_13. [DOI] [PubMed] [Google Scholar]
- Kopitar-Jerala N. The role of cystatins in cells of the immune system. FEBS Lett. 2006;580(27):6295–6301. doi: 10.1016/j.febslet.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Sá-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281:26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Karim S, Andersen JF, Mather TN, Ribeiro JM. Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J Biol Chem. 2007;282:29256–29263. doi: 10.1074/jbc.M703143200. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IM, Mather TN, Valenzuela JG, Ribeiro JM. Cutting edge: immunity against a “silent” salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. J Immunol. 2008;181:5209–5212. doi: 10.4049/jimmunol.181.8.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsyfakis M, Horka H, Salat J, Andersen JF. The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol Microbiol. 2010a;77:456–470. doi: 10.1111/j.1365-2958.2010.07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsyfakis M, Horka H, Salat J, Andersen JF. The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol Microbiol. 2010b;77:456–470. doi: 10.1111/j.1365-2958.2010.07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CA, Sasaki SD, Tanaka AS. Bmcystatin, a cysteine proteinase inhibitor characterized from the tick Boophilus microplus. Biochem Biophys Res Commun. 2006;347:44–50. doi: 10.1016/j.bbrc.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Erikson K. A snapshot of the Ixodes scapularis degradome. Gene. 2011;482:78–93. doi: 10.1016/j.gene.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Macaluso KR, Simser JA, Azad AF. The American dog tick, Dermacentor variabilis, encodes a functional histamine release factor homolog. Insect Biochem Mol Biol. 2003;33:911–919. doi: 10.1016/s0965-1748(03)00097-3. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Chalaire KC, Strey O, Teel P. Molecular and biological characterization of the Amblyomma americanum organic anion transporter polypeptide. J Exp Biol. 2008;211:3401–3408. doi: 10.1242/jeb.022376. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Chalaire KC. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics. 2009;10:217. doi: 10.1186/1471-2164-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V. Tick genomics: coming of age. Front Biosci. 2009;14:2666–2673. doi: 10.2741/3404. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Chaudhuri G. Cystatin superfamily. J Health Care Poor Underserved. 2010;21:51–70. doi: 10.1353/hpu.0.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Elango AP, Rahman AA, Fisher D, Collins WE, Barnwell JW, Escalante AA. Evidence of purifying selection on merozoite surface protein 8 (MSP8) and 10 (MSP10) in Plasmodium spp. Infect Genet Evol. 2012;12:978–986. doi: 10.1016/j.meegid.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel VZJ, Geraci NS, Guerrero FD, Wikel SK, Stuart JJ, Nene VM, Hill CA. Tick genomics: the Ixodes genome project and beyond. Int J Parasitol. 2007;37:1297–1305. doi: 10.1016/j.ijpara.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. Evolution of proteins of the cystatin superfamily. J Mol Evol. 1990;30:60–71. doi: 10.1007/BF02102453. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012;40(Database issue):D343–D350. doi: 10.1093/nar/gkr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salát J, Paesen GC, Rezácová P, Kotsyfakis M, Kovárová Z, Sanda M, Majtán J, Grunclová L, Horká H, Andersen JF, Brynda J, Horn M, Nunn MA, Kopácek P, Kopecký J, Mares M. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem J. 2010;429:103–112. doi: 10.1042/BJ20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Nunes A, Bafica A, Antonelli LR, Choi EY, Francischetti IM, Andersen JF, Shi GP, Chavakis T, Ribeiro JM, Kotsyfakis M. The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J Immunol. 2009;182:7422–7429. doi: 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Valdés JJ, Kotsyfakis M. The role of cystatins in tick physiology and blood feeding. Ticks Tick Borne Dis. 2012;3:117–127. doi: 10.1016/j.ttbdis.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE, Bissinger BW, Egekwu N, Donohue KV, Khalil SM, Roe RM. First transcriptome of the testis-vas deferens-male accessory gland and proteome of the spermatophore from Dermacentor variabilis (Acari: Ixodidae) PloS one. 2011;6(9):e24711. doi: 10.1371/journal.pone.0024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarnakar S, Mishra A, Chaudhuri SR. The gelatinases and their inhibitors: the structure-activity relationships. EXS. 2012;103:57–82. [PubMed] [Google Scholar]
- Tian Z, Liu G, Zhang L, Yin H, Wang H, Xie J, Zhang P, Luo J. Identification of the heat shock protein 70 (HLHsp70) in Haemaphysalis longicornis. Vet Parasitol. 2011;181:282–290. doi: 10.1016/j.vetpar.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Turk V, Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991;285:213–219. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- Wagner A. The role of population size pleiotropy and fitness effects of mutations in the evolution of overlapping gene functions. Genetics. 2000;154:1389–1401. doi: 10.1093/genetics/154.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadsen P. Vaccination against ectoparasites. Parasitology. 2006;133:S9–S25. doi: 10.1017/S0031182006001788. [DOI] [PubMed] [Google Scholar]
- Wladyka B, Kozik AJ, Bukowski M, Rojowska A, Kantyka T, Dubin G, Dubin A. α1-Antichymotrypsin inactivates staphylococcal cysteine protease in cross-class inhibition. Biochimie. 2011;93:948–953. doi: 10.1016/j.biochi.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Yamaji K, Tsuji N, Miyoshi T, Islam MK, Hatta T, Alim MA, Anisuzzaman M, Kushibiki S, Fujisaki K. A salivary cystatin, HlSC-1, from the ixodid tick haemaphysalis longicornis play roles in the blood-feeding processes. Parasitol Res. 2009;106:61–68. doi: 10.1007/s00436-009-1626-3. [DOI] [PubMed] [Google Scholar]
- Yamaji K, Tsuji N, Miyoshi T, Hatta T, Alim MA, Anisuzzaman M, Kushibiki S, Fujisaki K. Hlcyst-1 and Hlcyst-2 are potential inhibitors of HlCPL-A in the midgut of the ixodid tick Haemaphysalis longicornis. J Vet Med Sci. 2010;72:599–604. doi: 10.1292/jvms.09-0561. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tian Z, Liu G, Xie J, Luo J, Zhang L, Shen H. Characterization of acid phosphatase from the tick Haemaphysalis longicornis. Vet Parasitol. 2011;182:287–296. doi: 10.1016/j.vetpar.2011.05.053. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ueda M, Umemiya R, Battsetseg B, Boldbaatar D, Xuan X, Fujisaki K. Asecreted cystatin from the tick Haemaphysalis longicornis and its distinct expression patterns in relation to innate immunity. Insect Biochem Mol Biol. 2006;36:527–535. doi: 10.1016/j.ibmb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liao M, Ueda M, Gong H, Xuan X, Fujisaki K. Characterization of an intracellular cystatin homolog from the tick Haemaphysalis longicornis. Vet Parasitol. 2009;160:180–183. doi: 10.1016/j.vetpar.2008.10.086. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liao M, Gong H, Xuan X, Fujisaki K. Characterization of Hlcyst-3 as a member of cystatins from the tick Haemaphysalis longicornis. Exp Appl Acarol. 2010;51:327–333. doi: 10.1007/s10493-010-9336-1. [DOI] [PubMed] [Google Scholar]