Abstract
Approximately 5–10% of subjects with prediabetes become diabetic every year. Inflammation is involved in the development of obesity-related type 2 diabetes (T2D). However, to date, the relationship between inflammation and prediabetes, defined by hemoglobin A1c (HbA1c) ≥ 5.7 and < 6.5%, remains largely unexplored, especially in African Americans. Therefore, in this study we examined a comprehensive panel of 13 cytokines involved in the inflammatory response in overweight/obese subjects with prediabetes. A total of 21 otherwise healthy, overweight/obese, young adult African American females with prediabetes, together with 20 matched overweight/obese controls, were selected for this study. Plasma cytokines were assessed by multiplex cytokine profiling. Plasma concentrations of interleukin (IL)-5, IL-6, IL-7, tumor necrosis factor-α (TNF-α), and granulocyte-monocyte colony-stimulating factor (GM-CSF) were significantly higher in the prediabetic group, as compared to the control group (all p < 0.05). Plasma concentrations of all the other cytokines, interferon-γ (IFN-γ), IL-1β, IL-2, IL-4, IL-8, IL-10, IL-12p70 and IL-13, seemed to be elevated in the prediabetic group, but failed to reach statistical significances. Upon merging both groups, HbA1c was found to be positively correlated with IFN- γ, IL-1β, IL-2, IL-5, IL-7, IL-8, TNF-α and GM-CSF. This study demonstrates elevated levels of various pro-inflammatory cytokines in overweight/obese young subjects with prediabetes, which place them at higher risk of developing T2D and cardiovascular diseases. Our data also call for further investigations in animal models and population cohorts to establish the roles of a variety of pro-inflammatory cytokines in the early development of obesity-related T2D.
Keywords: Cytokines, prediabetes, HbA1c, African American, obesity, inflammation, type 2 diabetes, cardiovascular diseases
Introduction
Type 2 diabetes (T2D) associated with obesity is a rapidly growing public health problem and a major cause of morbidity and mortality. Prediabetes is characterized by an elevation of plasma glucose level above the normal range, but below that of diabetes, which can be identified as either impaired fasting glucose [IFG: 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L)] or impaired glucose tolerance [IGT: 2-hour values in the oral glucose tolerance test of 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L)] [1]. The American Diabetes Association has recently defined prediabetes as hemoglobin A1c (HbA1c) between 5.7% and 6.4%, as HbA1c has higher repeatability versus fasting glucose, and does not require fasting [2].
Incidence of T2D rises steeply as HbA1c increases from the 5.0% to the 6.5% range [3]. Currently, approximately 79 million adults aged 20 years or older in the United States have prediabetes [4]. Epidemiological evidence clearly indicates the presence of diabetes-related complications in the prediabetic state [5–9]. The age-adjusted rates of diagnosed T2D and cardiovascular diseases (CVD) among adults 20 years or older are significantly higher in African Americans than in Caucasians, although the rates of prediabetes are similar (35%) in both ethnic groups [4]. These data indicate that a higher proportion of African Americans with prediabetes progresses to T2D than Caucasians. Moreover, incidence and mortality related to CVD is high among African-American females [10]. Although a recent study indicates significantly poorer preventive and riskier lifestyle behaviors in African Americans than in Caucasians [11], it is clear that, apart from altered glucose metabolism, other factors might also contribute to the observed differences in predisposition towards the development of T2D between these ethnic groups. As such, a better understanding of the pathogenic mechanisms underlying the progression from prediabetes to T2D, especially in African Americans, is of the utmost clinical importance.
Chronic inflammation plays an important role in the pathophysiology of T2D [1, 12, 13]. Bertoni et al. reported that higher baseline levels of interleukin-6 (IL-6), C-reactive protein (CRP), and fibrinogen were associated with increased incidence of T2D in a multiethnic American cohort [14]. To date, only few studies have examined pro-inflammatory parameters in prediabetic individuals. Gupta et al. observed increased levels of interferon (IFN)- γ, IL-6, tumor necrosis factor-α (TNF-α), and IL-1β in healthy Irish prediabetics compared to non-diabetics [15]. Increasing levels of CRP have been shown to be associated with progressively higher risk of IFG and IGT in Chinese subjects [16]. Plasma IL-6 was found to be elevated in Italian Caucasians with IGT and T2D [17]. Another study reported increased concentrations of TNF-α in Turkish females with IGT versus normal glucose tolerance (NGT) [18]. In contrast, Choi et al. [19] observed no significant differences in TNF-α and IL-6 concentrations in Korean women with IGT and NGT. Of interest, one study found markedly elevated plasma IL-8 concentration after glucose load in obese subjects with IGT as compared to NGT [20]. Moreover, hyperglycemia is known to increase the plasma concentrations of IL-6 and TNF-α within few hours and this effect is more distinct in individuals with IGT [21]. However, whether prediabetes, defined by HbA1c, is characterized by an increased presence of various pro-inflammatory cytokines has not been clearly demonstrated to date, particularly in African Americans, and therefore represented the main aim of this study. We profiled 13 cytokines, involved in the inflammatory response, in overweight/obese subjects, as compared with matched controls. In addition, we explored the relationships between HbA1c and pro-inflammatory cytokines in the entire cohort.
Material and Methods
Study population
Twenty-one apparently healthy young (18–45 years of age) adult African American females with prediabetes were recruited from the local communities of Augusta, Georgia and neighborhood areas. Overweight or obesity was defined by body mass index (BMI) ≥ 25.0 kg/m2. Prediabetes was defined by HbA1c ≥ 5.7 and < 6.5%. Twenty young adult African American females with HbA1c < 5.7%, matched for BMI and age with these prediabetic cases, were selected as control subjects. Subjects were excluded if they were taking any prescription or over-the-counter medication including oral contraceptives, hormone replacement therapy, vitamins, herbals, or mineral supplements or had any acute or chronic medical illnesses. In addition, pregnant and breast-feeding females were also excluded. All subjects provided written informed consent and the study was approved by the Human Assurance Committee of the Georgia Regents University.
Anthropometrics, vitals, and blood collection
Height (cm) and weight (kg) measurements were obtained to calculate BMI. Waist circumference was measured by measuring tape at the level of umbilicus. Three blood pressure (BP) and heart rate (HR) readings, each one minute apart, were obtained by automated Dinamap monitor (Critikon, Tampa, FL) after 5–10 minutes of rest in the seating position with the use of an appropriate sized cuff wrapped on the non-dominant arm. The last two readings of BP and HR were averaged and recorded. Non-fasting blood samples were collected, frozen at −80°C and were later used for the assessment of cytokine measurements.
HbA1c measurement
HbA1c was either measured from capillary blood by finger prick using Bayer’s Professionals A1CNow+ kit or from venous blood sample by Ion-Exchange Chromatography at the clinical laboratory of the Georgia Regents University. Both methods were certified by the National Glycohemoglobin Standardization Program (NGSP).
Plasma cytokine measurement
A panel of 13 pro-inflammatory cytokines [IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, TNF-α, and granulocyte-monocyte colony-stimulating factor (GM-CSF)] was assessed in triplicates in 50 μL plasma from the study subjects, using a highly sensitive cytokine bead assay (MILLIPLEX MAP High Sensitivity Human Cytokine Panel – Premixed 13 Plex, EMD Millipore) [22, 23]. This assay has a high sensitivity, typically with a detection limit in the range from 0.01 to 0.48 ng/L. Data obtained using this cytokine bead assay technology have been shown to be highly reproducible and to be correlated well with values obtained using classical Enzyme-linked immunosorbent assay [24, 25].
Statistical analyses
Descriptive statistics for continuous variables are presented as mean ± standard error of the mean. Continuous data were checked for normality using the Shapiro-Wilk test and were log transformed when needed. Group differences for age, anthropometrics, BP, and cytokines were determined by independent-t test if data were normally distributed or by Mann-Whitney U test otherwise. Associations of HbA1c with cytokines were assessed by Pearson’s bivariate correlation coefficients in the total population. All analyses were performed using SPSS software (version 19.0; SPSS Inc., Chicago, IL) and statistical significance was set at p < 0.05.
Results
Clinical characteristics of study subjects in control and prediabetic groups
As demonstrated in Table 1, there were no statistical differences in age, weight, BMI, systolic BP, HR, or waist circumference between the two groups (all p > 0.05). However, the mean diastolic BP was found to be significantly higher in the prediabetic versus control group (p = 0.05).
Table 1.
Clinical characteristics of African-American female participants
| Variables | Control Group (N=20) |
Prediabetic Group (N=21) |
p valuea |
|---|---|---|---|
| Age (years) | 29.30 ± 1.93 | 30.14 ± 1.70 | 0.74 |
| Weight (kg) | 86.68 ± 4.60 | 99.37 ± 6.35 | 0.11 |
| BMI (kg/m2) | 33.98 ± 1.54 | 35.13 ± 1.91 | 0.64 |
| Systolic BP (mm Hg) | 122 ± 5 | 123 ± 4 | 0.87 |
| Diastolic BP (mm Hg) | 74 ± 3 | 80 ± 2 | 0.05 |
| Heart rate (bpm) | 69 ± 2 | 70 ± 3 | 0.82 |
| Waist circumference (m) | 1.03 ± 0.04 | 1.02 ± 0.06 | 0.95 |
| HbA1c (%) | 5.4 ± 0.07 | 6.1 ± 0.05 | <0.01 |
Values are displayed as mean ± SEM; BMI: body mass index; BP: blood pressure;
Tests of significance between groups were based on independent-samples t test.
Comparison of pro-inflammatory cytokines between both groups
We compared plasma levels of a panel of cytokines associated with inflammation, including IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, TNF-α and GM-CSF, between apparently healthy overweight/obese African American adult females with prediabetes and their matched controls. As demonstrated in Table 2 and Figure 1, plasma concentrations of IL-5, IL-6, IL-7, TNF-α, and GM-CSF were significantly higher in the prediabetic group than in the control group. Plasma concentrations of other cytokines, including IFN-γ, IL-1β, IL-2, IL-4, IL-8, IL-10, IL-12p70, and IL-13 seemed to be elevated in the prediabetic versus control group (Table 2), but failed to reach statistical significances.
Table 2.
Comparison of cytokines between control and prediabetic groups
| Cytokines (ng/L) | Control Group (N=20) |
Prediabetic Group (N=21) |
p value |
|---|---|---|---|
| IFN-γa | 2.24 ± 0.45 | 3.57 ± 0.59 | 0.21 |
| IL-1βa | 0.09 ± 0.02 | 0.15 ± 0.04 | 0.30 |
| IL-2b | 1.14 ± 0.29 | 1.98 ± 0.41 | 0.11 |
| IL-4a | 9.37 ± 2.98 | 12.42 ± 2.86 | 0.32 |
| IL-5a | 0.40 ± 0.11 | 1.19 ± 0.26 | 0.01 |
| IL-6a | 1.39 ± 0.31 | 2.61 ± 0.46 | 0.03 |
| IL-7a | 1.43 ± 0.38 | 2.85 ± 0.53 | 0.01 |
| IL-8b | 1.83 ± 0.19 | 2.47 ± 0.30 | 0.08 |
| IL-10a | 5.42 ± 2.01 | 8.58 ± 2.25 | 0.19 |
| IL-12p70a | 1.14 ± 0.53 | 3.61 ± 126 | 0.28 |
| IL-13a | 1.13 ± 0.51 | 2.13 ± 0.71 | 0.22 |
| TNF-αb | 1.77 ± 0.24 | 2.87 ± 0.48 | 0.05 |
| GM-CSFa | 0.94 ± 0.24 | 1.92 ± 0.45 | 0.02 |
Values are displayed as mean ± SEM; IFN-γ: interferon-gamma; IL: interleukin; TNF: tumor necrosis factor; GM-CSF granulocyte-monocyte colony-stimulating factor
Tests of significance between groups were based on the Mann-Whitney U test
Tests of significance between groups were based on independent-samples t test.
Figure 1.
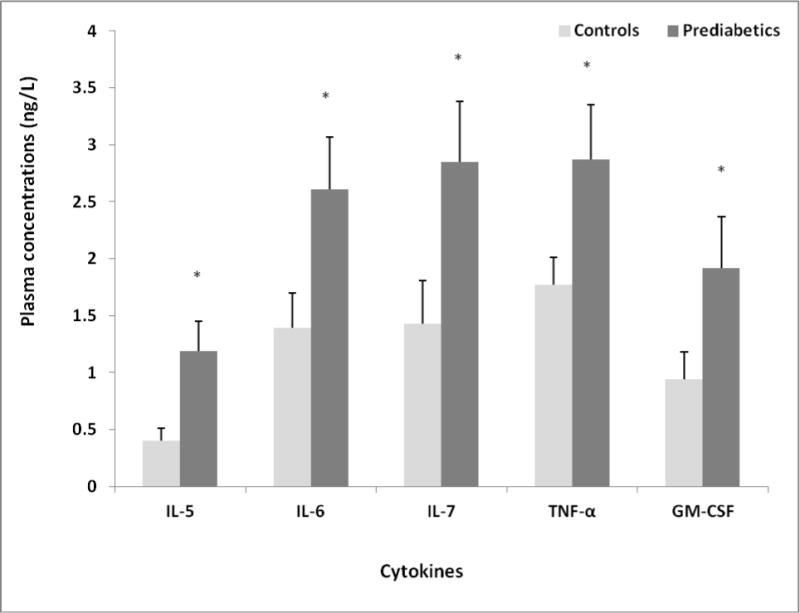
Comparison of plasma cytokines between controls and prediabetics
Associations of HbA1c with cytokines in the overall population
After combining the two groups, HbA1c was found to be positively correlated with IFN-γ, IL-1β, IL-2, IL-5, IL-7, IL-8, TNF-α, and GM-CSF (Table 3).
Table 3.
Correlations of HbA1c with cytokines in the entire cohort
| Cytokines | r | p value |
|---|---|---|
| IFN-γa | 0.32 | 0.04 |
| IL-1βa | 0.44 | 0.01 |
| IL-2 | 0.33 | 0.03 |
| IL-4a | 0.03 | 0.85 |
| IL-5a | 0.38 | 0.02 |
| IL-6a | 0.23 | 0.16 |
| IL-7a | 0.42 | 0.01 |
| IL-8 | 0.35 | 0.02 |
| IL-10a | 0.20 | 0.39 |
| IL-12p70a | 0.25 | 0.34 |
| IL-13a | −0.09 | 0.67 |
| TNF-α | 0.30 | 0.05 |
| GM-CSFa | 0.34 | 0.03 |
r = Pearson’s bivariate correlation coefficient
= log transformed values were used
Correlations among cytokines in the overall population
All cytokines were correlated with one another (p ≤ 0.05), except IL-12 and IL-8. IL-12 was only significantly correlated with TNF-α, yet not any other cytokines. IL-8 was significantly correlated with IL-1β, IL-2, IL-4, IL-5, TNF-α and GM-CSF, yet not the remaining ones.
Discussion
To the best of our knowledge, this is the first study to comprehensively evaluate various pro-inflammatory cytokines in African American subjects with prediabetes. We compared plasma levels of 13 pro-inflammatory cytokines between 21 prediabetic overweight/obese young African American females and 20 matched overweight/obese controls. The prediabetic group displayed a pro-inflammatory state, with significantly increased plasma levels of TNF-α, GM-CSF, IL-5, IL-6 and IL-7. These findings suggest that chronic subclinical inflammation is already in progress in the early stage of obesity-related T2D, implying that inflammation is a component of prediabetes. This observation highlights the importance of early intervention in the prediabetic state to prevent progression to T2D and reduce excessive CVD risk.
Obesity-related prediabetic conditions can give rise to the development of T2D and CVD, even in the absence of severe hyperglycemia and its chronic complications. Indeed, as compared to subjects with normal glucose levels, subjects with prediabetes have a 5 to 15 times higher tendency to develop T2D [12]. Thus, prediabetes, which commonly associates with metabolic syndrome, represents an early therapeutic window to reverse glucose and insulin abnormalities, in order to prevent T2D development. Recent studies have demonstrated that inflammation is involved in the development of T2D [26, 27]. T2D is now recognized as an immune-mediated disease leading to impaired insulin signaling and selective destruction of insulin producing β-cells, in which cytokines play an important role [13, 28, 29]. TNF-α and IL-6 were previously reported to be involved in the development of T2D [28–30]. Our results support the previous reports of higher levels of TNF-α and IL-6 in individuals with IGT [15, 17, 18], but contradict the findings of Choi et al. [19] who observed no differences in TNF-α and IL-6 concentrations between Korean women with IGT and NGT. The discrepancy in the findings could be attributed to the variations in sample size, BMI status, race, geographical conditions, and the definition of prediabetes, as we used HbA1c, rather than IGT criteria, to define prediabetes. TNF-α and IL-6 were also reported to be significantly increased in obese Caucasian females, who are at risk of increased CVD [31]. GM-CSF, which was elevated in our prediabetic female African American subjects, as compared to the control group, is a pro-inflammatory cytokine with growth factorlike properties for monocytes and dendritic cells (DCs). Activated DCs were suggested to play a crucial role in the development of CVD [32]. A recent study reported increased plasma levels of GM-CSF in T2D patients, which correlated with an activated state of myeloid and plasmocytoid DCs [33]. Moreover, in conditions of insulin-resistance and obesity an up-regulation of myeloid DCs was reported, which might contribute to pathological vascular remodeling [34]. As such, increased levels of GM-CSF in prediabetic subjects, together with the augmented TNF-α and IL-6 concentrations can place them at a higher risk for developing CVD.
The detection of increased IL-5 levels in prediabetic subjects was rather interesting. IL-5 is a TH2 homodimeric cytokine involved in the differentiation, maturation, migration, development, survival, trafficking and effector function of blood and local tissue eosinophils, in addition to basophils and mast cells [35]. A recent study reported a potential link between IL-5 actions and diabetic nephropathy, since eosinophil count was positively correlated with albumin excretion rate in men with T2D [36]. Moreover, an 8-year prospective cohort analysis of 1327 Chinese patients with T2D reported that polymorphisms in the IL-5 receptor α subunit correlated with ischemic stroke [37]. Another rather interesting observation in our study was the increased presence of IL-7 in plasma of prediabetic African American females, as compared to matched control subjects. Most T cell subsets depend on IL-7 for survival [38]. Polymorphisms in the IL-7 receptors have been shown to be a risk factor for autoimmune diseases, including type 1 diabetes and graft-versus host disease [39]. Moreover, monoclonal antibody blockade of IL-7 receptor-α was recently shown to reverse autoimmune diabetes, by means of promoting inhibition of effector/memory T cells [40, 41]. This indicates that IL-7 can contribute to the pathogenesis of autoimmune diabetes by enabling effector/memory T cells to remain in a functionally competent state. In a recent study, an aberrant recruitment and activation of effector T cells in T2D-associated nephropathy was demonstrated, and found to be correlated with the amount of proteinuria [42]. As such, the increased IL-5 and IL-7 levels detected in the prediabetic African American females in our study might indicate that they are at increased risk to develop diabetic nephropathy.
There is a scarcity of literature on the relationship between HbA1c and pro-inflammatory cytokines, especially in African American population. One study found positive correlations of HbA1c with CRP, fibrinogen, albumin, erythrocyte sedimentation rate, and white blood cell counts in older patients with coronary artery disease [43]. However, Martins et al. did not correlation of HbA1c with CRP in older adults [44]. Although the mechanisms underlying the relationship between inflammation, obesity, and impaired glucose homeostasis or elevated HbA1c are still incompletely understood, certain pro-inflammatory cytokines have been recognized as central players [45]. TNF-α in the adipose tissue is associated with an increased recruitment of activated macrophages [46]. While the IκB kinase-β (IKKβ) pathway is identified as a target for TNF-α-induced insulin resistance [47], TNF-α is found to down-regulate the tyrosine kinase activity of the insulin receptor [48]. IL-6 is involved in the regulation of the acute phase response and insulin resistance [45]. Subcutaneous and visceral fat has been demonstrated as an important site for IL-6 secretion in humans. Previous studies demonstrated that macrophage-activation-deficient GM-CSF-knockout mice prevent adipose tissue inflammation induced by a high-fat diet (HFD) [49]. A rise in GM-CSF appeared to be mediated by activation of protein kinase C, probably acting on extracellular signal-regulated kinases 1 and 2 and being calcium dependent [50]. Signal transducers and activators of transcription 5 mediated energy homeostasis in response to endogenous GM-CSF [51]. The remaining two cytokines that significantly differed between our prediabetic individuals and matched overweight/obese controls, IL-5 and IL-7, are not yet extensively investigated in this regard. As newly identified adipokines, however, they both have been associated with adiposity and glucose intolerance, when mice were placed on a HFD [52, 53]. In our study, after both study groups were merged, IL-5, IL-7, TNF-α, and GM-CSF positively correlated with HbA1c, as did IFN-γ, IL-1β, IL-2, and IL-8. IL-1β, a key mediator of inflammation, is known to exert harmful effects on human and rodent pancreatic islets under in vitro conditions [54]. Evidence from an experimental model indicates β-cell destruction in autoimmune diabetes is mediated in part by IL-2 [55]. IL-8 is one of the key players of obesity-related insulin resistance [56]. Evidence also suggests that IFN-γ might contribute to the development of T2D [57]. In addition, the degrees of correlations among the cytokines could be due to numerous pathophysiological factors such as their roles in the inflammation pathway, their interactions in response to inflammatory stimuli, various related transcriptional factors, and the balance between Th1 and Th2 phenotypes. How their correlations are varied and involved in obesity and glucose homeostasis deserves further investigation in cells, animal models, and human populations.
Strengths and weaknesses in the present study need to be taken into account. First, there are only a few previous studies analyzing TNF-α, IL-6 and IL-8 in prediabetes, defined by either IFG or IGT [15, 17, 18, 20], whereas the present study included a panel of cytokines and used HbA1c to define prediabetes. While confirming the involvement of TNF and IL-6 in prediabetes, our data for the first time suggest the involvement of less commonly studied cytokines including IL-5, IL-7 and GM-CSF. Plasma levels of other cytokines, such as IL-4, IL-10, IL-12p70, and IL-13 appeared to be higher in the prediabetic group than the control group, but the differences were not statistically significant. Their roles in the pathophysiology of early glucose impairment certainly warrant further investigations in animal models and large population cohort studies. A strength of this work is the stringent study design. We recruited overweight/obese prediabetic cases free of medications and carefully matched them with controls, albeit a small number of study subjects per group. All the subjects were either overweight or obese, which makes the study population relatively homogenous. Due to cross-sectional nature of this study, we cannot determine the causal relationship between HbA1c and pro-inflammatory markers. Moreover, interpretation of observed differences in plasma cytokine concentrations between prediabetic and control group might be limited by non-fasting status of blood samples, although non-fasting inflammatory factors have been previously used [58, 59]. In addition, because we studied only African American females, our results cannot be generalizable to males, and female subjects of other ethnicities.
Conclusion
In conclusion, obesity has dramatically increased in the last decade. Impaired glucose homeostasis in case of obesity is commonly accompanied by low-grade systemic inflammation and adipose tissue inflammation. Our study for the first time demonstrates that African American females with prediabetes as defined by elevated HbA1c display a pro-inflammatory state, which is characterized by increased levels of TNF-α, IL-6, IL-5 and IL-7and GM-CSF. Moreover, upon merging both groups, HbA1c is found to be positively correlated with IFN-γ, IL-1β, IL-2, IL-5, IL-7, IL-8, TNF-α and GM-CSF. As such, these cytokines might help identify prediabetic individuals at risk of developing overt obesity-related T2D and complications. However, more research and larger sample groups will be required in order to strengthen this hypothesis.
Highlights.
A total of 13 cytokines were profiled in prediabetcs and matched controls
IL-5, IL-6, IL-7, TNF-α, and GM-CSF were significantly higher in prediabetic group
HbA1c correlated with IFN-γ, IL-1β, IL-2, IL-5, IL-7, IL-8, TNF-α, and GM-CSF
Acknowledgments
The authors would like to thank the study staff and the subjects who made this study possible. YBD, RL, SJP and HZ contributed to the study concept and design. RL, SS, and DG measured cytokines. YBD, SJP, and RL analyzed and interpreted the data. YBD, SJP, JB, and HZ recruited the subjects and acquired the data. RL, SJP, and YBD drafted the manuscript. SJP, RL, SS, DG, JB, YTD, RC, AM, WC, HZ, and YBD critically revised the manuscript for intellectual content. YBD had primary responsibility for final content. All authors read and approved the final manuscript.
Source of support
The study was supported by the National Heart, Lung, and Blood Institute Grant HL077230 and the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK094765-01.
Abbreviations
- T2D
Type 2 diabetes
- CVD
Cardiovascular diseases
- IL
Interleukin
- IFN
Interferon
- TNF
Tumor necrosis factor
- GM-CSF
Granulocyte-monocyte colony-stimulating factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors reported no conflict of interest.
References
- 1.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 2.Standards of Medical Care in Diabetes-2012. Diabetes Care. 2012;35 doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33:1665–73. doi: 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 5.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23:1113–8. doi: 10.2337/diacare.23.8.1113. [DOI] [PubMed] [Google Scholar]
- 6.Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14:933–46. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM, Stern MP, Gruber MK, Hazuda HP, Mitchell BD, Patterson JK. Microalbuminuria. Potential marker for increased cardiovascular risk factors in nondiabetic subjects? Arteriosclerosis. 1990;10:727–31. doi: 10.1161/01.atv.10.5.727. [DOI] [PubMed] [Google Scholar]
- 8.Plantinga LC, Crews DC, Coresh J, Miller ER, 3rd, Saran R, Yee J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673–82. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Player MS, Diaz VA, Mainous AG, 3rd, Gregorie SH, Knoll ME, Everett CJ. Ethnic differences in the relationship of prediabetes with the presence of target-organ disease. Diabetes Metab. 2011;37:403–9. doi: 10.1016/j.diabet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 11.Zhou QP, Remsburg R, Caufield K, Itote EW. Lifestyle behaviors, chronic diseases, and ratings of health between black and white adults with pre-diabetes. The Diabetes educator. 2012;38:219–28. doi: 10.1177/0145721712440334. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 13.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33:804–10. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Maratha A, Gajanayake T, Siednienko J, Natarajan A, Hoashi S, et al. Cytokine profiling of pre-diabetic patients. Presented at Society for Endocrinology BES 2011; Birmingham, UK. 2011. Endocrine Abstracts. [Google Scholar]
- 16.Lin J, Zhang M, Song F, Qin J, Wang R, Yao P, et al. Association between C-reactive protein and pre-diabetic status in a Chinese Han clinical population. Diabetes Metab Res Rev. 2009;25:219–23. doi: 10.1002/dmrr.923. [DOI] [PubMed] [Google Scholar]
- 17.Cardellini M, Andreozzi F, Laratta E, Marini MA, Lauro R, Hribal ML, et al. Plasma interleukin-6 levels are increased in subjects with impaired glucose tolerance but not in those with impaired fasting glucose in a cohort of Italian Caucasians. Diabetes Metab Res Rev. 2007;23:141–5. doi: 10.1002/dmrr.679. [DOI] [PubMed] [Google Scholar]
- 18.Konukoglu D, Hatemi H, Bayer H, Bagriacik N. Relationship between serum concentrations of interleukin-6 and tumor necrosis factor alpha in female Turkish subjects with normal and impaired glucose tolerance. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2006;38:34–7. doi: 10.1055/s-2006-924974. [DOI] [PubMed] [Google Scholar]
- 19.Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, et al. Comparison of serum concentrations of C-reactive protein, TNF-alpha, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Res Clin Pract. 2004;64:99–106. doi: 10.1016/j.diabres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Szelachowska M, Kinalska I. Plasma interleukin 8 concentrations in obese subjects with impaired glucose tolerance. Cardiovasc Diabetol. 2003;2:5. doi: 10.1186/1475-2840-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 22.Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci U S A. 2012;109:2084–9. doi: 10.1073/pnas.1121075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17:3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- 24.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richens JL, Urbanowicz RA, Metcalf R, Corne J, O’Shea P, Fairclough L. Quantitative validation and comparison of multiplex cytokine kits. Journal of biomolecular screening. 2010;15:562–8. doi: 10.1177/1087057110362099. [DOI] [PubMed] [Google Scholar]
- 26.Cai D. One step from prediabetes to diabetes: hypothalamic inflammation? Endocrinology. 2012;153:1010–3. doi: 10.1210/en.2011-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta AK, Johnson WD. Prediabetes and prehypertension in disease free obese adults correlate with an exacerbated systemic proinflammatory milieu. J Inflamm (Lond) 2010;7:36. doi: 10.1186/1476-9255-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The rolelof cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 29.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 30.Arnalich F, Hernanz A, Lopez-Maderuelo D, Pena JM, Camacho J, Madero R, et al. Enhanced acute-phase response and oxidative stress in older adults with type II diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2000;32:407–12. doi: 10.1055/s-2007-978662. [DOI] [PubMed] [Google Scholar]
- 31.Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, et al. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur J Med Res. 2010;15(Suppl 2):120–2. doi: 10.1186/2047-783X-15-S2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishizawa T, Bornfeldt KE. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann Med. 2011 doi: 10.3109/07853890.2011.585346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surendar J, Mohan V, Pavankumar N, Babu S, Aravindhan V. Increased levels of serum granulocyte-macrophage colony-stimulating factor is associated with activated peripheral dendritic cells in type 2 diabetes subjects (CURES-99) Diabetes Technol Ther. 2012;14:344–9. doi: 10.1089/dia.2011.0182. [DOI] [PubMed] [Google Scholar]
- 34.Musilli C, Paccosi S, Pala L, Gerlini G, Ledda F, Mugelli A, et al. Characterization of circulating and monocyte-derived dendritic cells in obese and diabetic patients. Mol Immunol. 2011;49:234–8. doi: 10.1016/j.molimm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42:712–37. doi: 10.1111/j.1365-2222.2011.03854.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukui M, Tanaka M, Hamaguchi M, Senmaru T, Sakabe K, Shiraishi E, et al. Eosinophil count is positively correlated with albumin excretion rate in men with type 2 diabetes. Clin J Am Soc Nephrol. 2009;4:1761–5. doi: 10.2215/CJN.03330509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luk AO, Wang Y, Ma RC, Tam CH, Ng MC, Lam V, et al. Predictive role of polymorphisms in interleukin-5 receptor alpha-subunit, lipoprotein lipase, integrin A2 and nitric oxide synthase genes on ischemic stroke in type 2 diabetes–an 8-year prospective cohort analysis of 1327 Chinese patients. Atherosclerosis. 2011;215:130–5. doi: 10.1016/j.atherosclerosis.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Mazzucchelli RI, Riva A, Durum SK. The human IL-7 receptor gene: deletions, polymorphisms and mutations. Semin Immunol. 2012;24:225–30. doi: 10.1016/j.smim.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoe E, McKay FC, Schibeci SD, Gandhi K, Heard RN, Stewart GJ, et al. Functionally significant differences in expression of disease-associated IL-7 receptor alpha haplotypes in CD4 T cells and dendritic cells. J Immunol. 2010;184:2512–7. doi: 10.4049/jimmunol.0902900. [DOI] [PubMed] [Google Scholar]
- 40.Lee LF, Logronio K, Tu GH, Zhai W, Ni I, Mei L, et al. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1203795109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penaranda C, Kuswanto W, Hofmann J, Kenefeck R, Narendran P, Walker LS, et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1203692109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon JY, Jeong KH, Lee TW, Ihm CG, Lim SJ, Lee SH. Aberrant recruitment and activation of T cells in diabetic nephropathy. Am J Nephrol. 2012;35:164–74. doi: 10.1159/000334928. [DOI] [PubMed] [Google Scholar]
- 43.Gustavsson CG, Agardh CD. Markers of inflammation in patients with coronary artery disease are also associated with glycosylated haemoglobin A1c within the normal range. European heart journal. 2004;25:2120–4. doi: 10.1016/j.ehj.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Martins RA, Jones JG, Cumming SP, Coelho e Silva MJ, Teixeira AM, Verissimo MT. Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol. 2012;11:13. doi: 10.1186/1475-2840-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Archivum immunologiae et therapiae experimentalis. 2013;61:119–25. doi: 10.1007/s00005-012-0210-1. [DOI] [PubMed] [Google Scholar]
- 46.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 49.Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, et al. The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab. 2008;295:E1038–46. doi: 10.1152/ajpendo.00061.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahramian N, Ostergren-Lunden G, Bondjers G, Olsson U. Fatty acids induce increased granulocyte macrophage-colony stimulating factor secretion through prote ase C-activation in THP-1 macrophages. Lipids. 2004;39:243–9. doi: 10.1007/s11745-004-1226-2. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PloS one. 2008;3:e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lucas S, Taront S, Magnan C, Fauconnier L, Delacre M, Macia L, et al. Interleukin-7 regulates adipose tissue mass and insulin sensitivity in high-fat diet-fed mice through lymphocyte-dependent and independent mechanisms. PloS one. 2012;7:e40351. doi: 10.1371/journal.pone.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Major CD, Wolf BA. Interleukin-1beta stimulation of c-Jun NH(2)-terminal kinase activity in insulin-secreting cells: evidence for cytoplasmic restriction. Diabetes. 2001;50:2721–8. doi: 10.2337/diabetes.50.12.2721. [DOI] [PubMed] [Google Scholar]
- 55.Zhou W, Zhang F, Aune TM. Either IL-2 or IL-12 is sufficient to direct Th1 differentiation by nonobese diabetic T cells. J Immunol. 2003;170:735–40. doi: 10.4049/jimmunol.170.2.735. [DOI] [PubMed] [Google Scholar]
- 56.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. European journal of endocrinology / European Federation of Endocrine Societies. 2003;148:535–42. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 57.Tsiavou A, Hatziagelaki E, Chaidaroglou A, Koniavitou K, Degiannis D, Raptis SA. Correlation between intracellular interferon-gamma (IFN-gamma) production by CD4+ and CD8+ lymphocytes and IFN-gamma gene polymorphism in patients with type 2 diabetes mellitus and latent autoimmune diabetes of adults (LADA) Cytokine. 2005;31:135–41. doi: 10.1016/j.cyto.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Herder C, Baumert J, Zierer A, Roden M, Meisinger C, Karakas M, et al. Immunological and cardiometabolic risk factors in the prediction of type 2 diabetes and coronary events: MONICA/KORA Augsburg case-cohort study. PloS one. 2011;6:e19852. doi: 10.1371/journal.pone.0019852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicholson W, Wang NY, Baptiste-Roberts K, Chang YT, Powe NR. Association between adiponectin and tumor necrosis factor-alpha levels at eight to fourteen weeks gestation and maternal glucose tolerance: the Parity, Inflammation, and Diabetes Study. Journal of women’s health. 2013;22:259–66. doi: 10.1089/jwh.2012.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]


