Abstract
Lacrimal glands provide the important function of lubricating and protecting the ocular surface. Failure of proper lacrimal gland function results in a number of debilitating dry eye diseases. Lacrimal glands secrete lipids, mucins, proteins, salts and water and these secretions are at least partially regulated by neurotransmitter-mediated cell signaling. The predominant signaling mechanism for lacrimal secretion involves activation of phospholipase C, generation of the Ca2+-mobilizing messenger, IP3, and release of Ca2+ stored in the endoplasmic reticulum. The loss of Ca2+ from the endoplasmic reticulum then triggers a process known as store-operated Ca2+ entry, involving a Ca2+ sensor in the endoplasmic reticulum, STIM1, which activates plasma membrane store-operated channels comprised of Orai subunits. Recent studies with deletions of the channel subunit, Orai1, confirm the important role of SOCE in both fluid and protein secretion in lacrimal glands, both in vivo and in vitro.
Introduction
The major function of lacrimal glands is to provide water, electrolytes, proteins and mucins to lubricate and protect the environmentally exposed surfaces of the eye (cornea and conjunctiva) [1]. Mammals have a major gland associated with each eye, and a number of minor glands (i.e., goblet cells, meibomian gland), which contribute to constitutive and neurogenic tears and all of which may be involved in pathological conditions when functionally impaired. An understanding of the basic mechanisms underlying lacrimal gland secretion may provide insights to the treatment of debilitating age-related dry eye diseases, as well as the more general exocrine dysfunction in Sjögren’s syndrome [2]. Here we review the basic cell biology underlying the signaling pathways leading to secretion of proteins and fluid from the major lacrimal glands.
The flow of tears has long been known to be under both parasympathetic and sympathetic control [1;3;4]. Early studies demonstrated that stimulation of muscarinic-cholinergic receptors increased the discharge of granule stored protein, largely peroxidase, from rat exoribital lacrimal gland [5-7]. While not fully defined, the cholinergic-induced calcium signal likely exerts multiple effects during protein secretion to coordinate the mobilization of secretory vesicles to the lacrimal apical membrane where they fuse and release their contents. Synaptotagmin is a likely target to detect the cholinergic-induced calcium signal which works in concert with SNARE proteins (including VAMP8) to complete and open the vesicle fusion pore [8].
Ca2+-dependent activation of monovalent channels (K− and Cl−) plays a critical role in fluid secretion, generating electrochemical and osmotic gradients to drive the movement of water and accumulation of electrolytes into the lumen of acinar clusters and the ductal system [9]. In in vitro preparations, muscarinic receptors increased the efflux of K+ [7], a response thought to reflect ionic movements related to fluid secretion. Both protein secretion and K+ efflux responses depended at least partially upon extracellular Ca2+, and were associated with increased uptake of radioactive Ca2+ into the glands [6;7].
Subsequently, it was demonstrated that an α-adrenoceptor mechanism similarly activated both protein discharge and increased K+ permeability [10-12], although curiously the α-adrenergic protein secretion was somewhat less sensitive to removal of extracellular Ca2+. Unlike parotid salivary glands, lacrimal glands do not apparently contain β-adrenoceptors, but do contain adenylyl cyclase activating vasoactive intestinal peptide receptors [13] and melanotropin receptors [14]. Other Ca2+-linked receptor types shown to significantly modulate lacrimal secretion include multiple types of purinergic P2X and P2Y receptors [15-18], substance P, serotonin, histamine [19] and protease-activated receptors [20].
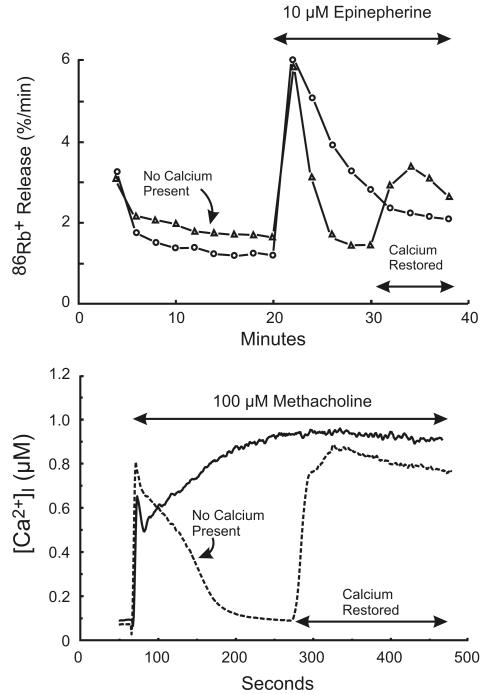
In early studies of Ca2+ signaling, direct measurement of intracellular Ca2+ with chemical or genetically encoded indicators was not available. Changes in intracellular Ca2+ were often inferred from the time course and magnitude of Ca2+-mediated responses, and for lacrimal glands and certain other epithelial cells, Ca2+-activated K+ channels provided this link [21]. The rate of K+ efflux from lacrimal cells was assessed by an isotope washout technique whereby slices of lacrimal gland were equilibrated with 86Rb+, a surrogate for K+ [22]. By stepwise transfer of the slices through a series of non-radioactive incubations, released radioactivity could be measured and time-based changes in the first order rate constant for 86Rb+ efflux calculated. By use of an experimental sequence first described for studies in parotid gland [23;24], protocols omitting and restoring extracellular Ca2+ revealed a biphasic response: the initial transient increase in 86Rb+ efflux was independent of extracellular Ca2+, while the sustained efflux response depended on extracellular Ca2+ being present [25] (Figure 1). The Ca2+ independent component of the response was thought to result from an intracellular release of Ca2+, because only one such response could be obtained in the absence of external Ca2+, and then an incubation in Ca2+-containing medium was necessary to restore the response. This latter finding will be discussed in more detail in relation to its relevance to the mechanism of Ca2+ influx.
Figure 1. Two phases of Ca2+ signaling in in vitro lacrimal gland preparations.
Top: Changes in intracellular Ca2+ in slices of rat lacrimal gland are inferred from the efflux rate of 86Rb+. Redrawn from data originally presented in [11]. Bottom: Changes in intracellular Ca2+ in mouse lacrimal acinar cells are measured with the Ca2+ indicator, Fura-2. In both cases, in the absence of extracellular Ca2+, the response is transient, and subsequently restored by addition of Ca2+.
Release of intracellular Ca2+
Ca2+ signaling in lacrimal acinar cells was initially seen to result from a biphasic mobilization of Ca2+ to the cytoplasm, an initial release of intracellular Ca2+ which was followed by or accompanied by an increase in Ca2+ entry across the plasma membrane [7]. The intracellular release mechanism was the first to be solved. From as early as the 1950’s, it was known that certain receptors, including muscarinic cholinergic receptors, stimulated a turnover of inositol lipids [26]. In 1975, Bob Michell [27] published his classic review on inositol lipids in which he proposed that this turnover in some manner served to link receptor activation to Ca2+ signaling. In 1983, Mike Berridge demonstrated that following receptor activation, the head group of phosphatidylinositol 4,5-bisphosphate, inositol 1,4,5-trisphosphate (IP3), rapidly appeared in fly salivary glands, and suggested that this molecule served as a second messenger for Ca2+ release [28]. Soon thereafter, in a collaboration between Berridge, Irene Schulz and Robin Irvine, IP3 was shown to release Ca2+ from non-mitochondrial stores in a preparation of permeabilized pancreatic acinar cells [29]. Consistent with this idea, in lacrimal glands Ca2+-mobilizing agonists stimulated turnover of inositol lipids and this involved degradation of phosphatidylinositol 4,5-bisphosphate and formation of soluble inositol phosphates [30]. IP3 was later shown to release intracellular Ca2+ in lacrimal acinar cells, by a technique involving introduction of the molecule in intact acinar cells via a patch pipet [31]. IP3 has also been shown to release intracellular Ca2+ in permeabilized lacrimal acinar cells [32], and following microinjection into lacrimal acinar cells [33]. This release of Ca2+ appears to come from a relatively homogenous pool of Ca2+ within the endoplasmic reticulum. Thus, in permeabilized cell experiments in other exocrine glands, inhibition of mitochondrial uptake of Ca2+ does not impair loading of the pool sensitive to IP3 [29]. Interestingly, spatial measurement of acetylcholine-induced Ca2+ signals in clusters of rat lacrimal cells demonstrate a distinct gradient of [Ca2+]i that appears to be maximal at the luminal pole of the cell [34]. Thus, while the agonist-sensitive Ca2+ signal appears to be released from a homogeneous ER Ca2+ pools, the spatial characteristics of the Ca2+ signal may be determined by InsP3 receptors localized to specific regions of the cell. This pattern of calcium release may result in differential physiological effects at luminal versus basolateral membranes, for example in control of lacrimal secretion.
As will be discussed below, a useful tool for studying Ca2+ pools is the plant toxin, thapsigargin, that inhibits the endoplasmic reticulum Ca2+ pump (SERCA) and specifically releases endoplasmic reticulum Ca2+ [35]. In permeabilized lacrimal acinar cells, prior discharge of thapsigargin-sensitive Ca2+ stores precluded any further release by IP3, confirming that the source in lacrimal cells is the endoplasmic reticulum. The homogeneity of this pool was demonstrated in a study utilizing fura-2-loaded attached primary mouse lacrimal acinar cells [36]. Intracellular stores were discharged, in a Ca2+ depleted medium, by one of three agents: methacholine, presumed to release the IP3-sensitive pool; thapsigargin, which would release the total endoplasmic reticulum pool; and the calcium ionophore, ionomycin, which would discharge essentially all intracellular Ca2+ pools. Each of these three strategies essentially prevented further release by either of the other two. For example, after Ca2+ release by methacholine, not further release was seen with either thapsigargin or ionomycin. However, when Ca2+ was elevated for a prolonged period, with a high concentration of methacholine, a pool of Ca2+ appeared in excess of that which could be released by thapsigargin. Loading of this pool was prevented by injection of the mitochondrial Ca2+ uptake inhibitor, ruthenium red. Thus, consistent with other studies, the mitochondria contain little Ca2+ at rest, but actively accumulate it when it is released by IP3 [37].
IP3 releases Ca2+ from the endoplasmic reticulum by activating a receptor-ion channel, the IP3 receptor. The receptor was first described by Spät et al. [38] in permeabilized hepatocytes and was cloned by Mikoshiba [39], who subsequently described three gene products termed types 1, 2 and 3 IP3 receptor [40]. Knockout in mice of the type 1 receptor produces a severe ataxia, but double knockout of the types 2 and 3 results in an exocrine secretion deficit and pups become malnourished [41]. In that same animal model, double knockout of types 2 and 3 IP3 receptor also reduces salivary gland amylase secretion. With evidence of all three IP3 receptor types expressed in mouse lacrimal tissue [42], it will be interesting to study the consequences of their knockdown on lacrimal gland function.
Calcium oscillations in lacrimal acinar cells and feedback regulation of signaling
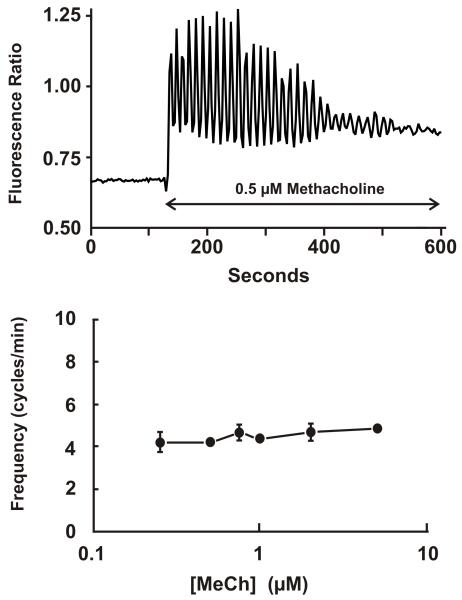
In many cell types utilizing phospholipase C-mediated Ca2+ signaling, low physiological concentrations of agonists do not produce sustained Ca2+ signals, as shown in Figure 1, but rather bring about a complex pattern of cytoplasmic Ca2+ transients termed Ca2+ oscillations [43;44]. The shapes and properties of these oscillations can vary depending upon cell type and the nature of the agonist. In some cases, agonists acting on different phospholipase C-linked receptors, but in the same cell type, can produce oscillations with markedly different properties [45]. The two most likely contributors to these oscillatory behaviors are feedback regulation of the Ca2+ release mechanism, producing oscillations in Ca2+ release at a constant level of IP3, and feedback regulation of steps upstream of phospholipase C resulting in oscillating production of IP3 [44]. When oscillations involve regenerative activation mechanisms, they are generally of constant magnitude (all-or-none) and vary in frequency as a function of the stimulus strength (agonist concentration). However, in lacrimal acinar cells, precisely the opposite is seen; in this cell type, oscillations in response to muscarinic receptor activation occur on an elevated basal level of Ca2+ and are relatively constant in frequency at different agonist concentrations (Figure 2) [46]. With increasing agonist concentration, the average cytoplasmic concentration rises until at near saturating concentrations the oscillations disappear and the cells respond with a sustained elevation as in Figure 1.
Figure 2. Muscarinic receptor-induced sinusoidal Ca2+ oscillations in mouse lacrimal cells.
Top: A single, fura-2-loaded mouse lacrimal acinar cell was exposed to 0.5 μM methacholine (MeCh) inducing sinusoidal Ca2+ oscillations on an elevated basal level of Ca2+. MeCh-induced oscillations occur over a narrow concentration range, and with frequencies that are relatively constant at different agonist concentrations (Bottom figure). These findings were originally published in [46].
Oscillations of this nature, of relatively constant frequency, would likely involve only a simple negative feedback mechanism the time constant of which is slower than the processes regulating cytoplasmic Ca2+. In lacrimal acinar cells, this feedback mechanism appears to be protein kinase C [46]. The oscillations are completely lost when protein kinase C is either strongly activated or inhibited [46]. There is considerable evidence that protein kinase C can serve as a regulator of G-protein coupled receptors [47;48], and in lacrimal acinar cells, activation of protein kinase C by phorbol esters strongly inhibits Ca2+ signaling, IP3 production, but does not inhibit Ca2+ signaling due to direct injection of IP3 into acinar cells [46].
The physiological significance of frequency modulated, baseline Ca2+ spikes is widely appreciated, the general consensus being that such a pattern can produce a digital signal with high signal-to-noise [49;50]. However, for the muscarinic receptor-mediated Ca2+ signals in lacrimal cells, the constant frequency oscillations are only seen when experiments are carried out at room temperature, likely due to a strong temperature dependence of protein kinase C. They thus have little functional importance as oscillations per se, yet understanding their mechanism reveals an important feedback regulator of Ca2+ signaling in this cell type. Since muscarinic receptor activation in exocrine gland undoubtedly occurs through localized acetylcholine release from nearby parasympathetic nerves, it is possible that digitized signaling results from the magnitude and timing of neurosecretion at parasympathetic-acinar cell connections.
Ca2+ entry
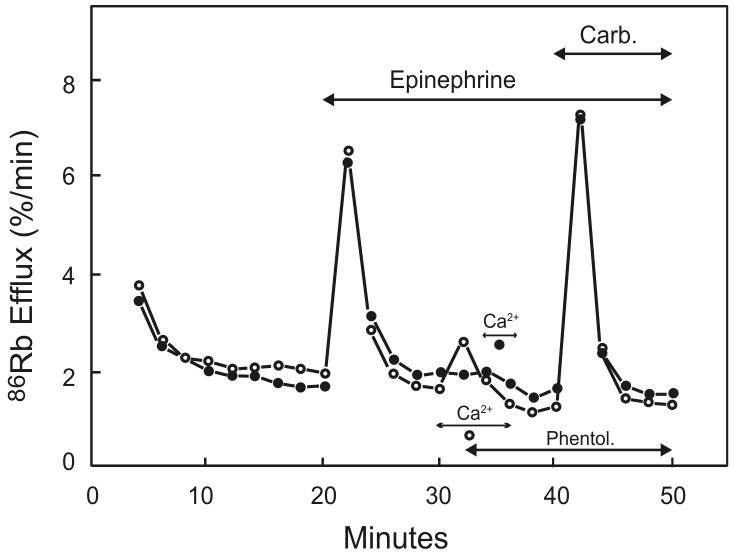
The Ca2+ entry phase of Ca2+ signaling in lacrimal glands is believed to involve the process of capacitative calcium entry or store-operated calcium entry (SOCE) [51;52]. Some of the origins of this concept lie in early experiments carried out with lacrimal gland cells (or slices). A key experiment was a variation on the one already mentioned that established that the first phase of 86Rb+ release was due to intracellular Ca2+ release. In order to refill the intracellular Ca2+ store following its discharge in a Ca2+ depleted medium, it was necessary to briefly incubate slices in a medium containing Ca2+. This experiment was first carried out with parotid gland, but in this case, owing perhaps to a prejudice as to how the Ca2+ would enter the cells, the “loading” in extracellular Ca2+ was tested while agonist receptors were occupied [24]. The logic was that the Ca2+ would need the receptor-activated channels to be open in order to enter the cell and refill the store. However, in a subsequent study with lacrimal gland, a control was added to the experiment: Ca2+ was also briefly added to the preparation after blocking the receptor [25]. Surprisingly, the intracellular stores loaded just as completely whether the receptor was occupied or not (Figure 3). This provided the first experimental evidence that it was not a direct receptor-dependent signal that kept the channels open, but rather they remained open as long as the intracellular stores were empty.
Figure 3. Evidence for the independence of Ca2+ influx from receptor activation.
In both experiments, Ca2+ stores were discharged by addition of epinephrine in the absence of extracellular Ca2+, the α-adrenergic receptors were then blocked by phentolamine (Phentol.), and the status of Ca2+ stores assessed by addition of carbachol (Carb.). In the experiment with open circles, Ca2+ was added before phentolamine, so Ca2+ could flow into the cell through presumed receptor activated channels. In the experiment with closed circles, phentolamine was added before Ca2+ such that receptor-activated channels would presumably be closed. Nonetheless, the stores were refilled with similar efficiency in both cases, indicating that it is not receptor activation per se that is responsible for Ca2+ entry and refilling of intracellular stores. Redrawn from data originally presented in [25].
Eventually, this and other pieces of circumstantial evidence would lead to the formalization of the capacitative model [51]. When first described, there was some uncertainty as to whether the loading of intracellular Ca2+ stores occurred by some discrete route bypassing the cytoplasm [53], or through a series of membrane permeations followed by uptake into the endoplasmic reticulum. A number of experimental observations accumulated to support the latter view. Perhaps the clearest, and most important for many other reasons was the action of the SERCA inhibitor, thapsigargin [54]. Thapsigargin was shown to quantitatively recapitulate agonist-induced Ca2+ entry, first in parotid gland [54], and subsequently in lacrimal gland [32]. According to the direct reloading model, influx into the cytoplasm would only occur following continuous release from the endoplasmic reticulum. In the case of exocrine glands, this would be through the activated IP3 receptor. However, thapsigargin emptied intracellular endoplasmic reticulum stores, but did not increase cellular IP3 levels [32;54]. Thus, the rate of permeation of Ca2+ into the cytoplasm was unrelated to the permeability of the endoplasmic reticulum to Ca2+, and Ca2+ must come to the cytoplasm directly via store-operated plasma membrane Ca2+ channels. Consistent with this conclusion, soon thereafter a transmembrane electrophysiological Ca2+ current underlying SOCE was described in mast cells, termed Icrac for calcium-release-activated calcium current [55].
With the exception of hematopoietic cells, Icrac is often too small to detect when Ca2+ is the charge carrier. It can be detected, however, by exploiting a property such that the deletion of all external divalent cations removes its divalent selectivity permitting Na+ to permeate, and thereby giving substantially larger and readily detectable currents [56]. An inwardly rectifying Na+ current, under conditions of Ca2+ store depletion and a divalent cation free external solution was recently described for mouse lacrimal acinar cells [57].
Inositol tetrakisphosphate and Ca2+ entry in lacrimal acinar cells
Inositol 1,4,5-trisphosphate is formed when agonists, through either a G-protein dependent or tyrosine kinase dependent mechanism, activate a phospholipase C to cleave the head group from phosphatidylinositol 4,5-bisphosphate. IP3 is then rapidly metabolized by two enzymes. A 5-phosphatase cleaves the phosphate from position 5 of the inositol ring, giving inositol 1,4-bisphosphate which is incapable of releasing Ca2+. A 3-kinase phosphorylates IP3 at the 3 position, resulting in the formation of inositol 1,3,4,5-tetrakisphosphate (IP4) [58]. The rapidity of formation of IP4 led to the suggestion that it might have some signaling function distinct from that of IP3, specifically, the activation of the second, Ca2+ entry phase of signaling [59]. This idea was examined by experiments in which IP3, IP4 or a combination of the two was dialyzed into lacrimal acinar cells by patch pipet perfusion, while Ca2+ changes were assessed from outward K+ currents known to be Ca2+-activated [31]. Perfusion of the cells with IP3 produced variable results ranging from no response to rapidly inactivating transient responses. Perfusion with IP4 caused no effect when used alone. However, inclusion of both IP3 and IP4 in the pipet resulted in robust and sustained increases in K+ conductance ([Ca2+]i). The authors interpreted this result as indicating a role for IP4 in the sustained, Ca2+ entry component of lacrimal acinar cell signaling [31]. However, in a subsequent publication, the same group showed that in fact IP4 could also augment the ability of IP3 to release Ca2+ [60]. IP4 is a substrate for the same 5-phosphatase that metabolizes IP3, and has a lower Km but a slow turnover rate [61]. Thus, IP4 would efficiently block the metabolism of IP3, and this could explain the effects of IP4 in the patch perfusion experiments; i.e., the sustained response requires sustained depletion of stores by IP3, and IP4 allows this by protecting IP3 from the 5-phosphatase [62]. In support of this interpretation, perfusion by patch pipet, or injection into intact lacrimal cells of a non-metabolizable but fully efficacious isomer of IP3, (2,4,5)IP3, fully activated sustained Ca2+ entry, whether measured as Ca2+-activated K+ conductance, or by use of the Ca2+ indicator, Fura-2 [63]. The interpretation was that the effect of IP4 in the previous studies was indeed likely due to protection of IP3 from metabolism. A subsequent publication demonstrated this by more direct measurements of the interactions of IP3 and IP4 [62].
Lacrimal secretion in an Orai1 knockout mouse
Throughout the 1990’s and 2000’s, considerable research focused on searching for candidates for the signal that activates store-operated channels, and for the channel itself (see numerous examples in [64]). A number of reports suggested the presence of a diffusible messenger, termed “CIF” for calcium influx factor [65;66]. With regard to the channel, much attention was focused on TRPC channels, which are clearly activated by phospholipase C-dependent mechanisms, and can pass considerable Ca2+ [67]. Although still somewhat controversial, it appears that at least a component of the mechanism for activating TRPC channels, under some conditions, can involve depletion of endoplasmic reticulum stores [68;69]. However, TRPC channels clearly do not share the biophysical properties of Icrac. Nonetheless, knockdown or knockout of specific TRPC channels has been shown to impair exocrine secretion in salivary glands [70] and pancreas [71].
The major molecular components of Icrac, STIM1 and Orai1, were discovered by a series of targeted and whole-genome RNAi screens [72]. STIM1 (or STIM2 under some circumstances), serves as the Ca2+ sensor in the endoplasmic reticulum. STIM1 is a single pass membrane spanning protein which contains a Ca2+-binding (and Ca2+ sensing) EF-hand in the lumenally-directed N-terminus. Loss of Ca2+ from the endoplasmic reticulum results in dissociation of Ca2+ from STIM1, aggregation of STIM1, and accumulation of STIM1 in junctions between endoplasmic reticulum and the plasma membrane [73;74]. There, STIM1 can bind to and activate store-operated channels comprised of Orai1 subunits [75]. Mammals also express two other Orai proteins, Orai2 and Orai3 [76], whose functions are less well understood (but see [77]).
Mice lacking Orai1 tended to die perinatally, presumably due to compromised skeletal muscle development [78;79], but some pups survive with special housing conditions [78], or when the mice are crossed into an outbred strain [79]. The lacrimal glands of Orai1 knockout mice appeared to develop normally, but the secretion in vivo of cholinergically-induced overflow tears was substantially curtailed [57]. In vitro, agonist-activated protein (peroxidase) secretion was reduced to the level seen in the absence of external Ca2+. Sustained Ca2+ entry, whether due to a cholinergic agonist or thapsigargin, was essentially absent. Quantitative PCR demonstrated that of the known SOCE mediators, only Orai1 message was decreased (essentially gone), while message for Orai3 and for STIM1 and STIM2 were not statistically changed. Interestingly, message for Orai2 was substantially increased, yet this failed to compensate for the loss of Orai1, as SOCE was not detectable. The store-operated current, Icrac, measured as a Na+ current under divalent-free conditions (see above), was also lost in the knockout mice.
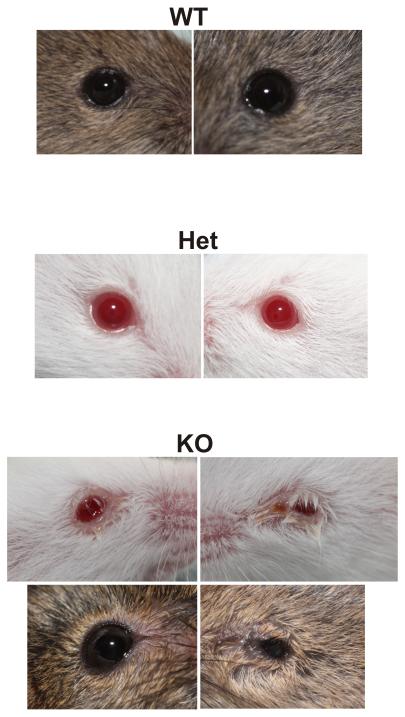
In an earlier report, T-cell specific knockout of both STIM1 and STIM2 resulted in a Sjögren’s syndrome-like condition such that salivary glands degenerated due to an increased autoimmunity and extensive lymphocytic invasion [80]. Orai1 knockout mice would be expected to have compromised T-cell function as well, but these mice showed no evidence of glandular degeneration or lymphocytic invasion [57]. Significantly, the component of protein secretion that did not depend on external Ca2+ was quantitatively similar in glands from knockout mice, indicating that basic upstream signaling, as well as downstream exocytotic machinery remained intact, and the only detectable defect was in the Ca2+ influx mechanism. Gwack et al. reported that Orai1 knockout mice showed signs of eyelid irritation [79], and in the study by Xing et al. many, but not all mice, showed signs of inflammation in the eyes [57] (Figure 4). Since the mice are immune compromised, it is not possible to determine if this is a primary result of impaired lacrimal secretion, lack of immune function, or a combination of both. However, since many mice showed no such symptoms, yet all mice tested exhibited loss of SOCE, it is clear that the SOCE phenotype is not secondary to this inflammation. It is interesting that defects in SOCE can affect exocrine function in two important ways, by triggering a pathological autoimmunity [80], or by failure of signaling for protein and fluid secretion [57].
Figure 4. Appearance of eyes from wild type (WT), heterozygous (Het) and homozygous Orai1 knockout (KO) mice.
The eyes from wild type and heterozygous mice appeared normal, while in many (but not all) cases the eyes from knockout animals showed signs of inflammation.
Summary
Studies on Ca2+ signaling in lacrimal glands have provided important clues for our understanding of basic signaling mechanisms, especially with regard to store-operated Ca2+ entry mechanisms. In addition, these mechanistic studies provide possible insights to the causes and possible treatments of debilitating dry eye diseases.
Acknowledgments
Work from the authors’ laboratory discussed in this review was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. Drs. Jerrel Yakel and Stephen Shears read the manuscript and provided helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf. 2004;2:76–91. doi: 10.1016/s1542-0124(12)70146-5. [DOI] [PubMed] [Google Scholar]
- 3.Bothelo SY. Tears and the lacrimal gland. Scientific American. 1964;211:78–86. doi: 10.1038/scientificamerican1064-78. [DOI] [PubMed] [Google Scholar]
- 4.Bothelo SY, Hisada M, Fuenmayor N. Functional innervation of the lacrimal gland in the cat. Origin of secretomotor fibers in the lacrimal nerve. Arch Opthalmol. 1966;76:581–588. doi: 10.1001/archopht.1966.03850010583019. [DOI] [PubMed] [Google Scholar]
- 5.Herzog V, Sies H, Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. The Journal of Cell Biology. 1976;70:692–706. doi: 10.1083/jcb.70.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keryer G, Rossignol B. Effect of carbachol on 45Ca uptake and protein secretion in rat lacrimal gland. Am J Physiol. 1976;230:99–104. doi: 10.1152/ajplegacy.1976.230.1.99. [DOI] [PubMed] [Google Scholar]
- 7.Putney JW, Parod RJ, Marier SH. Control by calcium of protein discharge and membrane permeability to potassium in the rat lacrimal gland. Life Sciences. 1977;20:1905–1911. doi: 10.1016/0024-3205(77)90227-2. [DOI] [PubMed] [Google Scholar]
- 8.Wu K, Jerdeva GV, da C, Sr., Sou E, Schechter JE, Hamm-Alvarez SF. Molecular mechanisms of lacrimal acinar secretory vesicle exocytosis. Exp Eye Res. 2006;83:84–96. doi: 10.1016/j.exer.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Ambudkar IS. Polarization of calcium signaling and fluid secretion in salivary gland cells. Curr Med Chem. 2012;19:5774–5781. doi: 10.2174/092986712804143321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putney JW, VanDeWalle CM, Leslie BA. Stimulus-secretion coupling in the rat lacrimal gland. Am J Physiol. 1978;235:C188–C198. doi: 10.1152/ajpcell.1978.235.5.C188. [DOI] [PubMed] [Google Scholar]
- 11.Parod RJ, Putney JW. An alpha-adrenergic receptor mechanism controlling potassium permeability in the rat lacrimal gland acinar cell. J Physiol (Lond) 1978;281:359–369. doi: 10.1113/jphysiol.1978.sp012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parod RJ, Leslie BA, Putney JW. Muscarinic and alpha-adrenergic stimulation of Na and Ca uptake by dispersed lacrimal cells. Am J Physiol. 1980;239:G99–105. doi: 10.1152/ajpgi.1980.239.2.G99. [DOI] [PubMed] [Google Scholar]
- 13.Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Invest Ophthalmol Vis Sci. 1997;38:610–619. [PubMed] [Google Scholar]
- 14.Entwistle ML, Hann LE, Sullivan DA, Tatro JB. Characterization of functional melanotropin receptors in lacrimal glands of the rat. Peptides. 1990;11:477–483. doi: 10.1016/0196-9781(90)90046-8. [DOI] [PubMed] [Google Scholar]
- 15.Ohtomo K, Shatos MA, Vrouvlianis J, Li D, Hodges RR, Dartt DA. Increase of intracellular Ca2+ by purinergic receptors in cultured rat lacrimal gland myoepithelial cells. Invest Ophthalmol Vis Sci. 2011;52:9503–9515. doi: 10.1167/iovs.11-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dartt DA, Hodges RR. Interaction of alpha1D-adrenergic and P2X(7) receptors in the rat lacrimal gland and the effect on intracellular [Ca2+] and protein secretion. Invest Ophthalmol Vis Sci. 2011;52:5720–5729. doi: 10.1167/iovs.11-7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dartt DA, Hodges RR. Cholinergic agonists activate P2X7 receptors to stimulate protein secretion by the rat lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:3381–3390. doi: 10.1167/iovs.11-7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges RR, Vrouvlianis J, Scott R, Dartt DA. Identification of P2X(3) and P2X(7) purinergic receptors activated by ATP in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:3254–3263. doi: 10.1167/iovs.10-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draper CE, Singh J, Adeghate E. Effects of age on morphology, protein synthesis and secretagogue-evoked secretory responses in the rat lacrimal gland. Mol Cell Biochem. 2003;248:7–16. doi: 10.1023/a:1024159529257. [DOI] [PubMed] [Google Scholar]
- 20.Oikawa M, Saino T, Kimura K, Kamada Y, Tamagawa Y, Kurosaka D, Satoh Y. Effects of protease-activated receptors (PARs) on intracellular calcium dynamics of acinar cells in rat lacrimal glands. Histochem Cell Biol. 2013;140:463–476. doi: 10.1007/s00418-013-1082-0. [DOI] [PubMed] [Google Scholar]
- 21.Putney JW. Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978;30:209–245. [PubMed] [Google Scholar]
- 22.Kikuchi M, Rabinovitch A, Blackard WG, Renold AE. Perifusion of pancreas fragments. A system for the study of dynamic aspects of insulin secretion. Diabetes. 1974;23:550–559. doi: 10.2337/diab.23.6.550. [DOI] [PubMed] [Google Scholar]
- 23.Putney JW. Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther. 1976;198:375–384. [PubMed] [Google Scholar]
- 24.Putney JW. Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol (Lond) 1977;268:139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parod RJ, Putney JW. The role of calcium in the receptor mediated control of potassium permeability in the rat lacrimal gland. J Physiol (Lond) 1978;281:371–381. doi: 10.1113/jphysiol.1978.sp012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hokin MR, Hokin LE. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953;203:967–977. [PubMed] [Google Scholar]
- 27.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975;415:81–147. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- 28.Berridge MJ. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial store in pancreatic cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–68. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 30.Godfrey PP, Putney JW. Receptor-mediated metabolism of the phosphoinositides and phosphatidic acid in rat lacrimal acinar cells. Biochem J. 1984;218:187–195. doi: 10.1042/bj2180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris AP, Gallacher DV, Irvine RF, Petersen OH. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987;330:653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- 32.Kwan CY, Takemura H, Obie JF, Thastrup O, Putney JW. Effects of methacholine, thapsigargin and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990;258:C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- 33.Bird G.St.J., Putney JW. Effect of inositol 1,3,4,5-tetrakisphosphate on inositol trisphosphate-activated Ca2+ signalling in mouse lacrimal acinar cells. J Biol Chem. 1996;271:6766–6770. doi: 10.1074/jbc.271.12.6766. [DOI] [PubMed] [Google Scholar]
- 34.Elliott AC, Cairns SP, Allen DG. Subcellular gradients of intracellular free calcium concentration in isolated lacrimal acinar cells. Pflüg Arch. 1992;422:245–252. doi: 10.1007/BF00376209. [DOI] [PubMed] [Google Scholar]
- 35.Thastrup O. Role of Ca2+-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2+-ATPase inhibitor, thapsigargin. Agents and Actions. 1990;29:8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- 36.Bird G.St.J., Obie JF, Putney JW. Functional homogeneity of the non-mitochondrial Ca2+-pool in intact mouse lacrimal acinar cells. J Biol Chem. 1992;267:18382–18386. [PubMed] [Google Scholar]
- 37.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 38.Spät A, Bradford PG, McKinney JS, Rubin RP, Putney JW. A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986;319:514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- 39.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa T, Okano H, Furuichi T, Aruga J, Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally specific manner. Proc Nat Acad Sci USA. 1991;88:6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 42.Medina-Ortiz WE, Gregg EV, Brun-Zinkernagel AM, Koulen P. Identification and functional distribution of intracellular ca channels in mouse lacrimal gland acinar cells. Open Ophthalmol J. 2007;1:8–16. doi: 10.2174/1874364100701010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berridge MJ. Calcium oscillations. J Biol Chem. 1990;265:9583–9586. [PubMed] [Google Scholar]
- 44.Thomas AP, Bird G.St.J., Hajnóczky G, Robb-Gaspers LD, Putney JW. Spatial and temporal aspects of cellular calcium signalling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 45.Petersen CCH, Toescu EC, Petersen OH. Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cells: Dependence on receptor type, agonist concentration and intracellular Ca2+ buffering. EMBO J. 1991;10:527–533. doi: 10.1002/j.1460-2075.1991.tb07979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bird GS, Rossier MF, Obie JF, Putney JW. Sinusoidal oscillations in intracellular calcium requiring negative feedback by protein kinase C. J Biol Chem. 1993;268:8425–8428. [PubMed] [Google Scholar]
- 47.Orellana S, Solski PA, Brown JH. Guanosine 5′-O-(thiophosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987;262:1638–1643. [PubMed] [Google Scholar]
- 48.Llano I, Marty A. Protein kinase C activators inhibit the inositol trisphosphate-mediated muscarinic current responses in rat lacrimal cells. J Physiol (Lond) 1987;394:239–248. doi: 10.1113/jphysiol.1987.sp016868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52:585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 50.Dupont G, Combettes L, Bird GS, Putney JW. Calcium oscillations. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Putney JW, Huang Y, Bird G.St.J. Calcium signalling in lacrimal acinar cells. Adv Exp Med Biol. 1998;438:123–128. doi: 10.1007/978-1-4615-5359-5_16. [DOI] [PubMed] [Google Scholar]
- 53.Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells of rabbit ear artery. J Physiol (Lond) 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takemura H, Hughes AR, Thastrup O, Putney JW. Activation of calcium entry by the tumor promoter, thapsigargin, in parotid acinar cells. Evidence that an intracellular calcium pool, and not an inositol phosphate, regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 55.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–355. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 56.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol (Lond) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing J, Petranka JG, Davis FM, Desai PN, Putney JW, Bird GS. Role of orai1 and store-operated calcium entry in mouse lacrimal gland signaling and function. J Physiol (Lond) 2013 doi: 10.1113/jphysiol.2013.267740. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batty IR, Nahorski SR, Irvine RF. Rapid formation of inositol 1,3,4,5-tetrakisphosphate folowing muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985;232:211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irvine RF, Moor RM. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+ Biochem J. 1986;240:917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Changya L, Gallacher DV, Irvine RF, Potter BVL, Petersen OH. Inositol 1,3,4,5-trisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused lacrimal cells. J Membrane Biol. 1989;109:85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- 61.Connolly TM, Bansal VS, Bross TE, Irvine RF, Majerus PW. The metabolism of tris- and tetraphosphates of inositol by 5- phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987;262:2146–2149. [PubMed] [Google Scholar]
- 62.Hermosura MC, Takeuchi H, Fleig A, Riley AM, Potter BVL, Hirata M, Penner R. InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature. 2000;408:735–740. doi: 10.1038/35047115. [DOI] [PubMed] [Google Scholar]
- 63.Bird G.St.J., Rossier MF, Hughes AR, Shears SB, Armstrong DL, Putney JW. Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991;352:162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- 64.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 65.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 66.Bolotina VM, Csutora P. CIF and other mysteries of the store-operated Ca2+-entry pathway. Trends Biochem Sci. 2005;30:378–387. doi: 10.1016/j.tibs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc Nat Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca(2)+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca(2)+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ong EC, Nesin V, Long CL, Bai CX, Guz JL, Ivanov IP, Abramowitz J, Birnbaumer L, Humphrey MB, Tsiokas L. A TRPC1 protein-dependent pathway regulates osteoclast formation and function. J Biol Chem. 2013;288:22219–22232. doi: 10.1074/jbc.M113.459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proceedings of the National Academy of Sciences. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterol. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnstone LS, Graham SJ, Dziadek MA. STIM proteins: integrators of signalling pathways in development, differentiation and disease. J Cell Mol Med. 2010;14:1890–1903. doi: 10.1111/j.1582-4934.2010.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 77.Shuttleworth TJ. Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium. 2009;45:602–610. doi: 10.1016/j.ceca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng KT, Alevizos I, Liu X, Swaim WD, Yin H, Feske S, Oh-hora M, Ambudkar IS. STIM1 and STIM2 protein deficiency in T lymphocytes underlies development of the exocrine gland autoimmune disease, Sj+|gren’s syndrome. Proceedings of the National Academy of Sciences. 2012;109:14544–14549. doi: 10.1073/pnas.1207354109. [DOI] [PMC free article] [PubMed] [Google Scholar]