Abstract
Background
White matter hyperintensity (WMH), a common radiographic finding associated with stroke risk and outcome, has been linked to matrix metalloproteinase (MMP) activity and increased levels of oxidative stress in non-stroke populations. We sought to determine whether WMH severity is associated with plasma levels of MMPs and oxidative stress (F2-isoprostane) in subjects with acute ischemic stroke (AIS).
Methods
We measured plasma biomarker levels at baseline and 48 hours in consecutive AIS subjects. WMH volume (WMHv) was quantified on admission MRI using a validated semi-automated protocol, and Spearman correlation coefficients were derived for all measured biomarkers.
Results
We enrolled 405 AIS subjects (mean age 70±15 years; 58% male; median WMHv 3.4cm3, IQR 1.4-9.5). WMHv and age were strongly correlated (ρ=0.57, p<0.0001). WMHv and MMP-2 levels were correlated at baseline (ρ= 0.23, p<0.0001) and at 48-hours post-stroke (ρ=0.19, p=0.002). In multivariate analysis, 48-hour MMP-2 levels were independently associated with WMHv (β=0.12, p=0.04). MMP-9 and F2-isioprostane levels did not correlate with WMHv.
Conclusion
In AIS patients, MMP-2 levels are associated with the pre-existing burden of WMH. If validated, these findings may further elucidate the role of MMP-2 in pathophysiology of chronic cerebrovascular injury such as WMH and in brain susceptibility to acute ischemia.
Search terms: leukoaraiosis, white matter hyperintensity, stroke, F2-isoprostane(s), matrix metalloproteinase 2, oxidative stress, matrix metalloproteinase 9
INTRODUCTION
The extent of cerebral injury resulting from acute ischemic stroke (AIS) and post-stroke outcomes are independently associated with pre-existing burden of cerebrovascular disease seen on T2-weighted brain MRI as white matter hyperintensity (WMH).1-3 Pathophysiology of WMH is complex and poorly understood.4-6 One proposed cause is primary blood brain barrier (BBB) dysfunction in the setting of brain matrix metalloproteinases (MMPs) upregulation.4, 7-9 Because oxidative stress has also been shown to upregulate MMPs in the brain, 10 the link between WMH severity and biomarkers of oxidative stress in humans11 have been used to support this hypothesis, including the association with decreased levels of the antioxidants lycopene and α-tocopherol,12 as well as F2-isoprostane (F2-isoP), a product of free radicals and arachidonic acid that had demonstrated particular utility as a biomarker of oxidative stress in humans.13. MMP-2 and MMP-9, also known as gelatinase A and B, play an important role in central nervous system (CNS) injury and repair.14 Inhibiting MMP-2 activity or deleting MMP-2 in a knockout mutation decreases white matter damage, glial activation, and BBB destruction in rodent models of cerebral hypoperfusion.15, 16 In patients with vascular cognitive impairment and WMH,17 there is evidence of a reduced cerebrospinal fluid (CSF)-to-plasma ratio of MMP-2.
In this paper, we examine the associations between MMPs, oxidative stress, and WMH severity in AIS patients. We hypothesized that in AIS patients, the volume of WMH is a marker of chronic oxidative stress and BBB disruption. If true, these factors may amplify the acute response to injury in ischemic stroke and may serve as future targets for intervention. To test this hypothesis, we measured plasma levels of MMP-2, MMP-9, and F2-isoP at the time of AIS and assessed their correlations with WMH volume (WMHv) quantified on brain MRI using validated, semi-automatic method.
METHODS
Subject Selection, Clinical Characteristics, and MRI analysis
We analyzed the relationship between WMHv and biomarkers of oxidative stress in AIS as part of a larger, prospective, observational NIH Specialized Program Of Translational Research In Acute Stroke (SPOTRIAS) study.18 Consecutive patients presenting to the two participating academic medical centers within 9 hours of AIS symptom onset, who had plasma biomarkers collected as well as brain MRI obtained and available for WMHv assessment were included in this analysis.
For each patient, we recorded baseline demographics [age, sex, race, ethnicity] and vascular risk factors [hypertension (HTN), body mass index (BMI), alcohol use status, hyperlipidemia (HL), atrial fibrillation (AF), smoking status, diabetes mellitus (DM), prior stroke or transient ischemic attack (TIA), coronary artery disease (CAD)]. Stroke severity measured as NIH stroke scale (NIHSS) score on admission and at 48 hours, as well as acute treatment with intravenous tissue plasminogen activator (IV tPA) were also recorded.
All MRIs were performed within 96 hours from symptom onset on 1.5 Tesla scanners (GE Medical Systems, Milwaukee, WI). Axial T2 FLAIR, sagittal T1, and DWI sequences were used to quantify WMHv using a previously described and validated semi-automated, multistep protocol developed specifically for analysis of clinical brain MRI scans.19, 20 This volumetric algorithm derives WMH maps from an overlap between automated signal intensity thresholding and supratentorial region-of-interest (ROI) outlines, which is followed by detailed manual editing. We exclude the cerebral structures that are prone to T2-hyperintensity artifact, such as basal ganglia and thalamus (calcifications), as well as the mesial temporal areas, cortico-medullary junction line, and ventricular (ependymal) lining from this analysis. Furthermore, hyperintense signal from prior cerebral infarcts are not considered WMH and the corresponding brain regions are masked during the process, as are those with motion artifact. To avoid confounding by hyperintensity signal resulting from acute cerebral ischemia in this WMH protocol, the total WMHv is derived by doubling the WMHv obtained from the hemisphere contralateral to AIS.20 Similarly, this protocol was adopted to measure acute infarct volume on diffusion-weighted (DWI) MRI, using the apparent diffusion coefficient (ADC) sequences for quality assurance.
All participating subjects or their healthcare proxys provided informed consent to be enrolled as part of the ongoing prospective hospital-based cohort studies at the participating institutions. All aspects of this study have been approved by the Institutional Review Board.
Biological Samples
Plasma samples were collected twice by the SPOTRIAS research staff in the acute phase (less than 9 hours after stroke onset) and at 48 hours (36 - 60 hours after stroke onset), and analyzed by an investigator blinded to the clinical information.
Quantification of F2-isoprostane
To assay F2-isoP, acute phase and 48-hour plasma samples were frozen at -80 degrees Celsius prior to processing. F2-isoP was quantified using an 8-Isoprostane Enzyme Immunoassay Kit (Cayman Chemical, Ann Arbor, MI) by the Antioxidants Research Laboratory at the Jean Mayer USDA Human Nutrition Research Center on Aging (HNRCA) at Tufts University.
Quantification of the MMPs
To assay MMP-9 and MMP-2, samples were drawn in ethylene-diaminetetraacetic acid (EDTA) tubes, using a butterfly needle to reduce shear stress, placed on ice and immediately centrifuged at 3000 revolutions per minutes for 15 minutes. Plasma was subsequently isolated and frozen at -80 °C for later measurement. Levels of MMPs were analyzed using a commercially available enzyme-linked immunosorbent assay (ELISA) (R&D systems) and expressed in nanograms per milliliter (ng/mL). ELISA was performed according to the manufacturer’s instruction.
Statistical Analysis
Statistical analysis was performed using SAS statistical software (SAS Institute, Cary, NC) and PASW (SPSS Inc., Chicago, IL). WMHv was adjusted for head size and natural log-transformed (lnWMHv) prior to analysis. Univariate analyses to evaluate the relation between lnWMHv and dichotomous variables were tested by Student’s t test or Mann-Whitney test. Because age, oxidative stress biomarker values, and MMP levels were not normally distributed, Spearman correlation coefficients were obtained between lnWMHv and age, F2-isoP, MMP-2, and MMP-9. Wilcoxon signed rank test was used to assess for significant differences between median values.
To account for strong effects of age on WMH, each variable was examined in bivariate, age-adjusted analysis prior to consideration for multivariable analysis. Those variables associated with lnWMHv at p<0.2 in age-adjusted analyses were included into two multivariable linear regression models constructed to examine the association between lnWMHv and MMP-2 levels. Multicollinearity was tested in the multiple linear regression analyses to confirm adequate model fit. We determined that NIHSS score was a strong predictor of acute infarct size (DWI) volume; thus, Model 1 included acute AIS phase variables: gender, prior stroke, HTN, HL, IV tPA, and admission NIHSS. Model 2 included 48-hour phase variables: gender, prior stroke, HTN, HL, IV tPA, and 48h NIHSS.
RESULTS
Table 1 summarizes the clinical characteristics of the study cohort with univariate and age-adjusted associations with lnWMHv. There were 405 participants (mean age 70 years, 42.5% female, and 92% white). Of these, 72% presented with hypertension, 47% with hyperlipidemia, 41% with reported current alcohol use, and 29% with obesity. Mean lnWMHv was 1.34 (±1.32) (Figure 1). As expected, lnWMHv strongly correlated with age (ρ=0.6, p<0.0001). History of HTN(p<0.001), AF(p<0.001), prior stroke (p=0.001), CAD (p=0.008), NIHSS at baseline (p=0.01) and 48 hours (p<0.001) were also correlated with lnWMHv. In age-adjusted analysis, only prior stroke remained significantly associated with lnWMHv (p=0.006).
Table 1.
Clinical characteristics and univariate associations with WMH severity in acute ischemic stroke subjects (n=405).
| Variable | Subjects | P# | Age-Adjusted p |
|---|---|---|---|
| Demographics and baseline measures | |||
| Age (years), mean±SD | 69.9±15.2 | <0.001 | … |
| BMI, mean±SD | 27.8±6.0 | 0.12 | 0.90 |
| Race (Caucasian), n (%) | 371 (91.6) | 0.55 | 0.40 |
| Female sex, n (%) | 172 (42.5) | 0.05 | 0.13 |
| NIHSS, median (IQR) | 5 (3-12) | 0.01 | 0.93 |
| WMHv, median (IQR) | 3.4 (1.4-9.5) | ⋯ | ⋯ |
| Risk factors, n (%) | |||
| Hypertension | 291 (71.9) | <0.001 | 0.06 |
| Current alcohol use | 165 (40.7) | 0.24 | 0.72 |
| Hyperlipidemia | 191 (47.2) | 0.25 | 0.18 |
| Obese (BMI ≥ 30) | 103 (29.1) | 0.47 | 0.96 |
| Atrial fibrillation | 120 (29.6) | <0.001 | 0.95 |
| Current smoking | 80 (19.8) | 0.32 | 0.95 |
| Diabetes mellitus | 87 (21.5) | 0.28 | 0.63 |
| Prior stroke | 76 (18.8) | 0.001 | 0.006 |
| Coronary artery disease | 105 (25.9) | 0.008 | 0.82 |
| Prior TIA | 37 (9.1) | 0.18 | 0.35 |
| Treatments and outcomes | |||
| IV t-PA, n (%) | 158 (39.1) | 0.28 | 0.17 |
| 48h NIHSS, median (IQR) | 3 (1-9) | <0.001 | 0.78 |
Spearman or Pearson correlations, and t-test or Mann Whitney test were used based on the distribution of the variables.
Abbreviations: BMI: body mass index; IQR: the 25th to 75th percentile interquartile range; IV tPA: intravenous tissue plasminogen activator; NIHSS: National Institutes of Health Stroke Scale; TIA: transient ischemic attack; WMH(v): white matter hyperintensity (volume)
Figure 1.
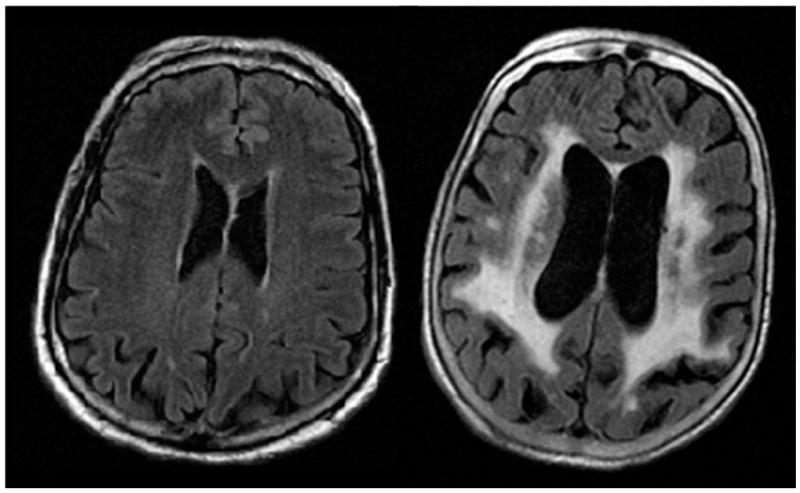
Burden of white matter hyperintensity (WMH) identified on T2 fluid attenuated inversion recovery (FLAIR) MRI varies from mild (left) to severe (right) in patients with acute ischemic stroke
Distributions of the study biomarkers measured after stroke are specified in Table 2. Data for each biomarker were available for subsets of the total population, ranging from 92% available for MMPs measured at baseline to 60% available for F2-isoP measured at 48 hours. Statistically significant differences were found between the levels of the MMPs (p<0.0001 for both MMP-2 and MMP-9) and F2-isoP (p=0.001) measured at baseline and those measured at 48 hours.
Table 2.
Distribution of plasma MMP and F2-isoP levels in subjects with acute ischemic stroke.
| Baseline | 48 hours | p | |||||
|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | ||
| F2-isoP (pg/mL) | 346 | 55.5 | 36.8-78.1 | 241 | 45.1 | 28.7-71.4 | 0.001 |
| MMP-2 (ng/mL) | 374 | 296.8 | 239.0-376.7 | 258 | 266.3 | 219.5-338.8 | <0.0001 |
| MMP-9 (ng/mL) | 374 | 176.3 | 91.6-309.0 | 258 | 144.8 | 77.4-229.6 | <0.0001 |
IQR = the 25th to 75th percentile interquartile range
Correlations between lnWMHv, MMPs, and F2-isoP measured at baseline and 48 hours are listed in Table 3. In this analysis, lnWMHv correlated with baseline (ρ=0.23, p=<0.0001) and 48 hour (ρ=0.19, p=0.002) plasma MMP-2 but not with MMP-9 concentrations (Figure 2). There was an inverse relationship between lnWMHv and the change in MMP-2 levels from baseline to 48h (ρ =-0.18, p=0.005). In multivariable analysis, 48-hour but not baseline plasma MMP-2 levels were independently associated with lnWMHv (β=0.12, p=0.047) (Table 4).
Table 3.
Correlations between lnWMHv, MMP and F2-isoP levels measured at baseline and 48 hours in subjects with acute ischemic stroke.
| Baseline | 48 hours | |||||
|---|---|---|---|---|---|---|
| n | Correlation (ρ)* | p | n | Correlation (ρ)* | p | |
| F2-isoP | 346 | -0.06 | 0.30 | 241 | -0.04 | 0.53 |
| MMP-2 | 374 | 0.23 | <0.0001 | 258 | 0.19 | 0.002 |
| MMP-9 | 374 | 0.006 | 0.91 | 258 | 0.05 | 0.45 |
Spearman’s correlation coefficient
Abbreviations: F2-isoP: F2-isioprostane; IQR: the 25thto 75th percentile interquartile range; MMP: matrix metalloproteinase
Figure 2.
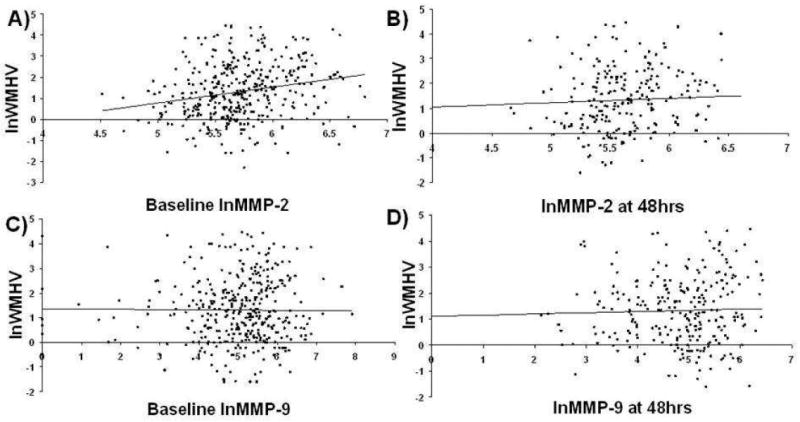
Correlations between WMH volume and plasma MMP-2 and MMP-9 levels measured at baseline and at 48 hours after acute ischemic stroke.
Table 4.
Multivariable analysis of WMHv in subjects with acute ischemic stroke at baseline and 48 hours post-stroke.
| Model 1 | |||||
|---|---|---|---|---|---|
| B | 95%CI for B | β | p | ||
| Low | High | ||||
| Gender | 0.238 | -0.025 | 0.501 | 0.090 | 0.08 |
| Prior stroke | 0.368 | 0.042 | 0.694 | 0.110 | 0.03 |
| Hypertension | 0.768 | 0.473 | 1.064 | 0.266 | <0.001 |
| Hyperlipidemia | -0.030 | -0.304 | 0.244 | -0.218 | 0.83 |
| IV t-PA | -0.267 | -0.539 | 0.004 | -0.100 | 0.05 |
| NIHSS at baseline | 0.028 | 0.008 | 0.049 | 0.143 | 0.007 |
| MMP-2 at baseline | 0.0002 | -0.0001 | 0.001 | 0.055 | 0.27 |
| Model 2 | |||||
| Gender | 0.362 | 0.052 | 0.671 | 0.138 | 0.02 |
| Prior stroke | 0.219 | -0.184 | 0.621 | 0.064 | 0.29 |
| Hypertension | 0.640 | 0.294 | 0.985 | 0.231 | <0.001 |
| Hyperlipidemia | 0.193 | -0.127 | 0.514 | 0.075 | 0.24 |
| IV t-PA | -0.160 | -0.470 | 0.151 | -0.061 | 0.31 |
| NIHSS at 48 h | 0.003 | 0.011 | 0.059 | 0.173 | 0.005 |
| MMP-2 at 48 h | 0.002 | 0.0001 | 0.003 | 0.121 | 0.047 |
Model 1 included all baseline variables associated with lnWMHv at p<0.2 in age-adjusted analyses
Model 2 includes all 48h variables associated with lnWMHv at p<0.2 in age-adjusted analyses
Abbreviations: IV tPA: intravenous tissue plasminogen activator; NIHSS: National Institutes of Health Stroke Scale; MMP: matrix metalloproteinase; lnWMHv: natural log of white matter hyperintensity volume
DISCUSSION
This is the first study to examine the association between WMH severity quantitatively assessed on MRI using a validated volumetric protocol and the plasma levels of MMP-2 in patients with AIS. Because MMP-2 is believed to play a role in chronic remodeling of the BBB by increasing its permeability, it may be increased in the plasma of patients with increased severity of WMH.16 Our findings demonstrate that in patients with ischemic stroke, higher MMP-2 plasma levels are associated with larger volumes of WMH on MRI, likely reflecting increased capillary remodeling and angiogenesis in the region of chronically ischemic white matter. MMP-2 activity is likely to increase BBB permeability that contributes to the progression of WMH and evolution of AIS.4, 8, 14 Evidence from animal and human studies suggests a link between MMP-2 and white matter disease. In a rat model of hypoperfusion, MMP-2, but not MMP-9 was associated with optic nerve and corpus callosum demyelination with increased MMP-2 expression seen in the microglia and later in the capillary endothelium.21 In a rat model using a selective MMP-2 inhibitor, and a murine MMP-2 knockout MMP-2 was shown to mediate white matter injury in cerebral hypoperfusion.16 A study in humans reported higher levels of MMP-2 in patients with small vessel stroke subtype.22
In this analysis, pre-existing cerebrovascular disease burden detected as WMHv on brain MRI of AIS subjects was associated with plasma levels of MMP-2 drawn at baseline and 48 hours after stroke onset. However, only the 48-hour plasma MMP-2 levels were independently associated with WMH severity. MMP-2 CSF levels rise shortly after the onset of hypoxia-ischemia in AIS and peak by 24 hours.23 Thus, the baseline (hyperacute) values may be confounded by the chronic level of MMP-2 activation coupled with the MMP-2 response to acute ischemia. Therefore, the 48-hour value may be a better reflection of the relationship between MMP-2 and WMHv. Furthermore, the association of MMP-2 levels at 48 hours post-stroke with WMH burden independent of NIHSS score (as a measure of stroke severity) implies that plasma MMP-2 may also be specific for chronic BBB injury, even in the setting of acute ischemic injury.
One must interpret these findings with caution and in the context of limitations inherent to this study’s design. Firstly, acute ischemic stroke is a complex and highly dynamic condition; thus, multiple confounders related to stroke severity, patient’s premorbid condition, and therapeutic interventions may affect validity of plasma biomarker levels, and consequently limit their utility. Statistical models including these confounders in a relatively small patient sample have limited power to uncover significant associations between the independent and the outcome variables; however, this conservative approach is unlikely to increase the chance of type I error.
Secondly, without pre-stroke biomarker levels as baseline and in the absence of longterm clinical post-stroke outcome measures – which are both limitations of many AIS studies – the relationship between WMHv and plasma MMP-2 levels is not fully elucidated. However, the major strength of this analysis, i.e. using a validated, quantitative, and reliable method of WMHv assessment, provides an outcome measure that reflects a pre-existing burden of cerebrovascular disease unlikely to be affected by the variables that arise acutely in the setting of cerebral ischemia.
Furthermore, although the relationship between systemic biomarkers and WMH would be least confounded by the effects of acute cerebral injury if measured prior to AIS, such design is impractical outside of the large, prospective population-based studies. If measured in convalescent subjects, chronic oxidative stress would most likely be more appropriate to assess at least 30-90 days post-stroke; however, variability due to major life style changes and medication-related effects post-stroke could independently alter the estimate of pre-stroke associations.
Finally, measuring systemic oxidative stress, as opposed to CSF biomarker level, may not be sensitive enough to reflect the degree of oxidative stress in the brain. This might in part explain why in our study of AIS subjects, WMHv did not correlate with F2-isoprostane, a known marker of oxidative stress. In addition to limitations related to timing of the biomarker assessment with respect to the acute versus chronic brain injury, limitations monitoring CNS processes using peripheral biomarkers are relevant, because altered access of the CNS biomarkers into systemic circulation proportional to the degree of injury and variability related to measurement of endothelial dysfunction in WMH may exist.24
Despite the limitations, this study serves as an essential proof-of-principle that the link exists between plasma MMP-2 levels and WMH burden detected on brain MRI of AIS subjects. The future research steps must include validation of these findings in a prospective cohort of stroke patients with convalescent plasma MMP-2 levels and, in parallel, testing of these biomarkers in a cohort of healthy adults with volumetric WMH assessment. If validated, plasma MMP-2 levels may provide a sensitive and specific estimate of pre-existing cerebrovascular disease burden in patients with stroke.
CONCLUSION
In patients with stroke, plasma MMP-2 levels correlate with pre-existing WMH burden. If validated, these findings may further elucidate the role of MMP-2 in pathophysiology of chronic cerebrovascular injury such as WMH and its role in susceptibility of the brain to acute ischemia.
Acknowledgments
We thank the research fellows, coordinators, technical, and administrative support of the NIH SPOTRIAS study at Massachusetts General Hospital and Brigham and Women’s Hospital, and the staff of the Antioxidants Research Laboratory at Tufts University for biomarker quantification.
Sources of Funding
Funding provided by the Sarnoff Cardiovascular Research Foundation (Z.A.C.); NIH SPOTRIAS grant P50NS051343 (K.L.F.); NIH NINDS K23NS064052 and R01NS082285 (N.S.R.); the American Stroke Association-Bugher Foundation (K.L.F., E.H.L., N.S.R.); and The Deane Institute for Integrative Study of Atrial Fibrillation and Stroke at MGH (K.A., K.L.F.).
Footnotes
Conflicts of Interest / Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 2.Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40(2):530–6. doi: 10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 5.Ovbiagele B, Saver J. Cerebral white matter hyperintensities on MRI: Current concepts and therapeutic implications. Cerebrovasc Dis. 2005;22:83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- 6.Helenius J, Tatlisumak T. Treatment of leukoaraiosis: A futuristic view. Curr Drug Targets. 2007;8:839–845. doi: 10.2174/138945007781077436. [DOI] [PubMed] [Google Scholar]
- 7.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leukoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw JM, Sandercock PAG, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, et al. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtype. Stroke. 2008;39:1327–1332. doi: 10.1161/STROKEAHA.107.500124. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- 11.Shibata H, Nabika T, Moriyama H, Masuda J, Kobayashi S. Correlation of NO metabolites and 8-iso prostaglandin F2a with periventricular hyperintensity severity. Arterioscler Thromb Vasc Biol. 2004;24:1659–1663. doi: 10.1161/01.ATV.0000137415.67349.3c. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt R, Hayn M, Fazekas F, Kapeller P, Esterbauer H. Magnetic resonance imaging white matter hyperintensities in clinically normal elderly individuals: Correlations with plasma concentrations of naturally occurring antioxidants. Stroke. 1996;27:2043–2047. doi: 10.1161/01.str.27.11.2043. [DOI] [PubMed] [Google Scholar]
- 13.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;27:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 14.Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: Considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs. 2009;10:644–654. [PubMed] [Google Scholar]
- 15.Cho KO, La HO, Cho YJ, Sung KKW, Kim SY. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res. 2006;83:285–291. doi: 10.1002/jnr.20727. [DOI] [PubMed] [Google Scholar]
- 16.Nakaji K, Ihara M, Takahashi C, Itohara S, Noda M, Takahashi R, et al. Matrix metalloproteinase-2 plays a critical role in the pathogenesis of white matter lesions after chronic cerebral hypoperfusion in rodents. Stroke. 2006;37:2816–2823. doi: 10.1161/01.STR.0000244808.17972.55. [DOI] [PubMed] [Google Scholar]
- 17.Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, et al. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furie KL. Oxidative stress and MMPs. 2006 http://projectreporter.nih.gov/project_info_description.cfm?aid=7138842&icde=130559652006.
- 19.Gurol ME, Bottiglieri T, Diaz-Arrastia R, Smith EE, Engel CR, Greenberg SM, et al. Homocysteine, brain atrophy, and white matter disease in Alzheimer’s disease and cerebral amyloid angiopathy. American Neurological Association Annual Meeting; Toronto Canada. 2004. [Google Scholar]
- 20.Rost NS, Rahman RM, Biffi A, Smith EE, Kanakis A, Fitzpatrick K, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75:1670–1677. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihara M, Tomimoto H, Kinoshita M, Oh J, Noda M, Wakita H, et al. Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab. 2001;21(7):828–34. doi: 10.1097/00004647-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lucivero V, Prontera M, Mezzapesa DM, Petruzzellis M, Sancilio M, Tinelli A, et al. Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol Sci. 2007;28(4):165–70. doi: 10.1007/s10072-007-0814-0. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szolnoki Z. Chemical events behind leukoaraiosis: Medicinal chemistry offers new insight into a specific microcirculation disturbance in the brain (a chemical approach to a frequent cerebral phenotype) Curr Med Chem. 2007;14:1027–1036. doi: 10.2174/092986707780362907. [DOI] [PubMed] [Google Scholar]


