Abstract
The tumor suppressor p53 and microRNAs (miRNAs) are linked through a complex network. Several miRNAs modulate p53 expression, while p53 regulates the transcription and/or biogenesis of several other miRNAs. Here we report the development of a cell-based assay used with a library of human miRNA mimics in a high-throughput screen for miRNAs that modulate p53 expression. Overexpression of miR-542-3p in cancer cells elevated p53 expression, stimulated the expression of p53 targets and inhibited cell proliferation. Mechanistically, miR-542-3p increased p53 protein stability by weakening interactions between p53 and its negative regulator MDM2. Further, miR-542-3p suppressed ribosome biogenesis by downregulating a subset of ribosomal proteins such as RPS23, leading to upregulation of RPL11 and stabilization of p53. The 3'UTR in the RPS23 transcript contained a miR-542-3p binding site, suggesting that RPS23 is a direct target of miR-542-3p. Our results define miR-542-3p as an important new positive regulator of p53 with potential applications in cancer treatment.
Keywords: microRNA, p53, ribosome, MDM2, protein stability, RPS23
Introduction
The p53 tumor suppressor, encoded by the TP53 gene, functions mainly by transcriptionally regulating genes that govern many cellular events such as cell cycle, apoptosis, autophagy, senescence, metabolism and angiogenesis. Genetic deletion or germ-line mutation of TP53 leads to high incidence and early onset of cancer in both mice and human (1, 2). The TP53 gene is mutated in about half of all human tumors, while tumors retaining wild-type TP53 often have abnormal p53 function as a result of alterations occurring in regulators of p53 (reviewed in (3, 4)). Restoration of p53 function is thereby an attractive strategy for tumor management, and fully understanding the regulation of p53 is of particular interest in the field of cancer research (5).
The p53 protein level remains very low in unstressed cells, but is rapidly induced upon exposure to stimuli, such as DNA damage, hypoxia, nutrient deprivation, or oncogenic activation (6). Ribosomal stress, which can be induced by serum deprivation, growth contact inhibition or actinomycin D treatment, also triggers the activation of p53. Up-regulation of p53 protein in response to ribosomal stress is largely due to the disruption of interaction between p53 and MDM2, an oncogenic E3 ligase that not only targets p53 for proteasome-mediated degradation but also inhibits the transactivation activity of p53 (7, 8). Mechanistically, ribosomal stress reduces the expression of PICT1, which leads to the release of the ribosomal subunit RPL11 from its anchored sites in the nucleolus (9). The liberated RPL11 then interacts and sequestrates MDM2 in the nucleus, thereby stabilizing and activating p53 (10). Furthermore, depletion of various ribosome subunit proteins by siRNAs can disrupt ribosome maturation and activate p53 due to sequestration of MDM2 by certain ribosome subunit proteins including RPL11, whose translation is increased upon depletion of RPS6 or RPS23 (11, 12). Thus, targeting the integrity of ribosome biogenesis may be a practical way to activate p53 for cancer treatment for TP53 wild-type cancers (13, 14).
MicroRNAs (miRNAs) are small non-coding RNAs that act as regulators of gene expression. Aberrant expression of miRNAs is often seen in cancer. MiRNAs can function as tumor suppressors or oncogenes and modulate many aspects of carcinogenesis, such as cell proliferation, cell cycle control, DNA repair, apoptosis, metastasis and angiogenesis (15, 16). P53 modulates the expression of miRNAs by either activating the transcription of some miRNA-coding genes or modulating the biogenesis of a subset of miRNAs (17, 18). The expression of p53 is also under the control of several miRNAs. For example, miR-125b directly targets the 3’UTR of p53 (19). Using a luciferase reporter driven by p53-binding motif, Park and colleagues screened a number of cancer-related miRNAs and identified miR-29 miRNAs as positive albeit indirect regulators of p53 expression through targeting p85-alpha and CDC42 (20). However, comprehensive analysis of microRNAs that regulate p53 expression has not been reported.
In the present study, we identify several miRNAs that regulate p53 expression. Among them, we describe miR-542-3p as a novel inducer of ribosomal stress and a potent positive regulator of p53 tumor suppressor.
Materials and methods
Cell lines
U2OS, T98G, U118, LN229 (American Type Culture Collections), HCT116, HCT116 p53−/− (Dr Bert Vogelstein) and normal human foreskin fibroblasts (Dr. Denise Galloway) were all grown in DMEM supplemented with 10% FBS and 2 mM L-glutamine. JHOC7 and OVISE cells (Dr. Hiroaki Itamochi) were cultured in RPMI medium with 10% FBS and 2 mM L-glutamine. Cells were authenticated by short tandem repeat (STR) DNA profiling at Bio-Synthesis (Lewisville, TX) for U2OS, HCT116, T98G, U118 and LN229 and at CTAG (Vancouver, BC) for JHOC7 and OVISE. All cells were used for this study within 6 months of resuscitation.
Plasmids, siRNAs, miRNA mimics and transfection
3’-UTR of human RPS23 was amplified by PCR and cloned into pGL3-control (Promega) to obtain pGL3-RPS23 3’UTR plasmid. Putative binding site of miR-542-3p in RPS23 3’-UTR was mutated using the QuikChange site-direct mutagenesis kit (Stratagene). RPS23 coding region was amplified by PCR and cloned into pLenti4-V5 vector (Invitrogen). MiR-542-3p and the spanning sequences (150 bp on each end) were amplified and inserted into pSM30-GFP vector (a gift of Dr. Guangwei Du) to generate miR-542-3p precursor. Specific siRNAs included siRPL11 (SMARTpool siGenome, Dharmacon), siRPS23 (5’-GCCATTAGGAAGTGTGTAA-3’), siRPS28 (5’-GTAACTGAGATGCTCCTTT-3’), siRPL22 (5’-CAAAGAGAGTTACGAATTA-3’) (Sigma) and luciferase (siLuc, 5’-AACGTACGCGGAATACTTCGA-3’, Qiagen). Delivery of plasmids, siRNAs and miRNA mimics (Dharmacon) was done as described (21).
MiRNA mimic library screening
Human miRIDIAN miRNA mimic library (v10.1, Dharmacon) was reversely transfected into U2OS cells followed by immunostaining with anti-p53 (DO-1, sc-126, Santa Cruz, 1:500) and Alexa594-conjugated secondary antibody (Invitrogen). Images were captured and processed as described (22, 23). Average intensity of p53 in nucleus was determined for calculation of z-score values with the formula Z= (X-μnc)/σ, where X was the score of individual sample, μnc was the mean of negative controls and σ was the standard deviation of the whole population. Average Z-scores from three independent screens were calculated.
Western blot analysis
Whole-cell extracts were obtained for SDS-PAGE electrophoresis as described (22). Primary antibodies included mouse anti-p53 (sc-126, Santa Cruz), RPS23 (sc-100837, Santa Cruz), RPL22 (sc-373993, Santa Cruz), RPS28 (14796-1-AP, Proteintech Group), MDM2 (04–1530, Millipore), MDM4 (sc-74468, Santa Cruz), PIRH2 (sc-166293, Santa Cruz), p21 (554228, BD Pharmigen), Bax (H00000581-M01, Abnova), RPL11 (37–3000, Invitrogen), ARF (#2407, Cell Signaling) and rabbit anti-PA28γ (38–3800, Zymed), cleaved caspase-3 (#9661, Cell signaling) and Actin (sc-1616-R, Santa Cruz). Ponceau staining or actin was used as loading controls.
Immunoprecipitation
Cells were lysed in NP-40 lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40). Pre-cleared lysates (~300 μg of protein) were incubated for 2 h at 4°C with 2 μg antibody of interest and then precipitated with 40 μl of fresh Protein-A/G plus agarose beads (Santa Cruz) at 50% slurry overnight at 4°C. The beads were then pelleted, washed and boiled in 2 × Tris–glycine SDS sample buffer for western blotting.
Real-time PCR
Total RNAs were extracted using Trizol reagent (Invitrogen) and reverse-transcribed using the Taqman microRNA or cDNA Reverse Transcription Kit (Applied Biosystem). The Taqman MiRNA Assay or Gene Expression Kit was used for quantitative PCR. Ct values were employed for quantification of transcripts. MiRNA or p53 expression was normalized to the values of RNU24 or GAPDH.
RNA quality control and Illumina gene expression analysis
Total RNA was extracted using RNAeasy kit (Qiagen). The quality of RNA was analyzed using a bioanalyzer (Agilent) with the RNA Nano kit. RNA was labeled and hybridized to the HT-12 v4 expression beadchip (Illumina), followed by analysis performed with annotations found in the lumiHumanAll.db package. Data from three independent transfections are available in the NCBI GEO database, Accession No. GSE47363.
Luciferase assay
Cells were co-transfected with miRNA mimics and pGL3-control firefly luciferase vectors containing empty or RPS23 3’-UTR. pRL-TK Renilla plasmid was co-transfected as an internal control. Luciferase assays were performed two days post-transfection (21). Relative luciferase activity was calculated by normalizing the ratio of Firefly/Renilla luciferase to that of negative control-transfected cells.
Cell cycle analysis
Cells were fixed with 70% ice-cold ethanol overnight, followed by staining for DNA content with 10 μg/ml propidium iodide in PBS containing 10 μg/ml RNase A. Flow cytometry analysis was performed to determine the distribution of cell cycle on a FACSCanto system (BD Sciences).
Statistical analysis
All the statistic analyses were performed with student t-test (paired, 2-tail). All results were expressed as mean ± standard deviation. P-value < 0.05 was considered significant.
Results
Identification of miR-542-3p as a positive regulator of p53 expression
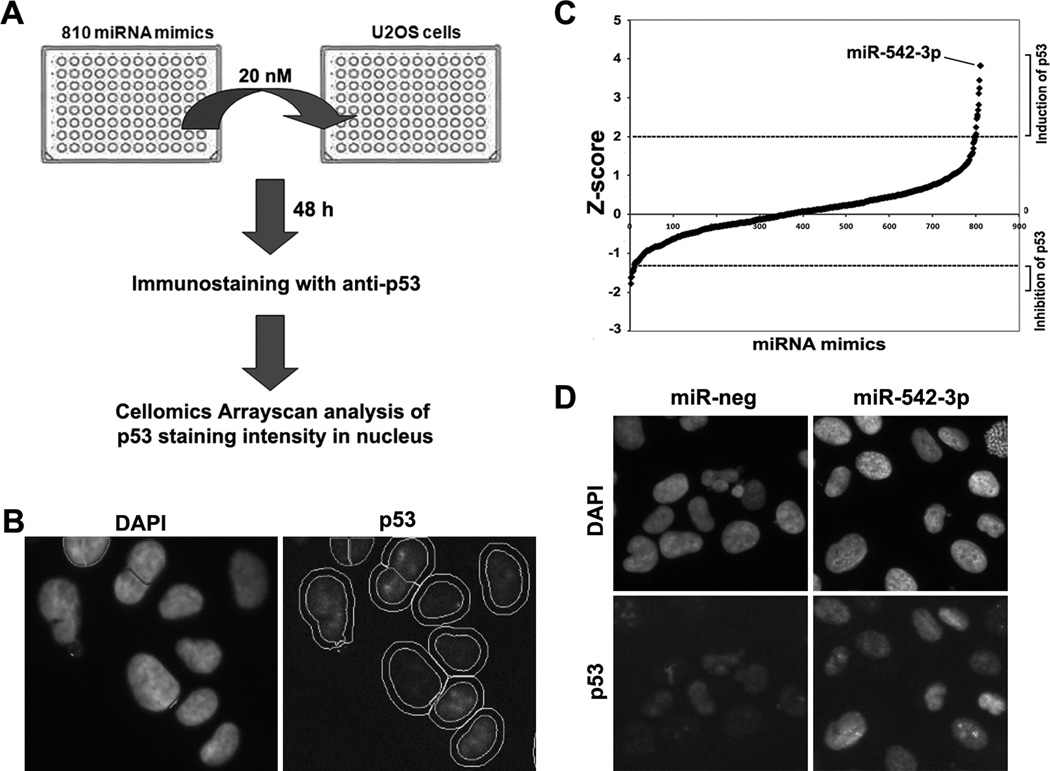
To search for miRNAs that directly or indirectly modulate the expression of p53, we developed a high-throughput fluorescence microscopy cell-based assay. The average immunofluorescent staining intensity of p53 in the nucleus after transfection of miRNA mimics was used as a readout. The screening was performed using U2OS cells, an osteosarcoma cell line with wild-type TP53. We screened a human miRNA mimics library containing 810 miRNAs (Fig. 1A and 1B). As the basal level of p53 is low in U2OS cells, p53 siRNA caused a moderate reduction of nuclear p53 fluorescence intensity (z=-1.94). We set a threshold of z<-1.2 for miRNAs that significantly reduced the expression of p53 (Fig. 1C, Supplementary Table 1), which included fifteen potential novel negative regulators of p53 and two known negative regulators of p53, miR-125b (z=-1.22) and miR-504 (z=-1.27) (19, 24). In contrast, our immunofluorescence intensity-based screen more sensitively identified potential positive regulators of p53. Our positive control, the topoisomerase II inhibitor etoposide strongly induced p53 fluorescent intensity (z>5). We set a cutoff of z>2 for miRNAs that significantly enhanced p53 expression, which included 13 potential novel positive regulators of p53 (Fig. 1C, Supplementary Table S1). As reactivation of p53 is therapeutically important for the treatment of cancers, we focused on miR-542-3p, which was the strongest p53-upregulating miRNA identified in the screen (Fig. 1C and 1D) and is downregulated in many cancer types such as colon, prostate and lung cancers (25).
Figure 1. Identification of miRNAs that regulate p53 expression.
(A) Scheme of miRNA gain-of-function screening for p53 immunofluorescence staining intensity in U2OS cells. (B) Representative images of p53 immunofluorescence staining. Nuclear regions were marked with circles (inner circle) based on DAPI staining. The outer circles were used to determine the intensity of p53 staining in cytoplasm (signal in ring region). (C) Z-score plot of miRNA mimics in the regulation of nuclear p53 immunofluorescent intensity. Several miRNA mimics were identified to positively or negatively affect p53-staining intensity (Z-score >2 or <-1.2)(Supplemental Table S1). MiR-542-3p is the top hit that modulated p53-staining intensity in U2OS. (D) Representative images of p53 immunofluorescence staining in miR-neg or miR-542-3p transfected cells.
MiR-542-3p disrupts p53-MDM2 interaction and increases the stability of p53 protein
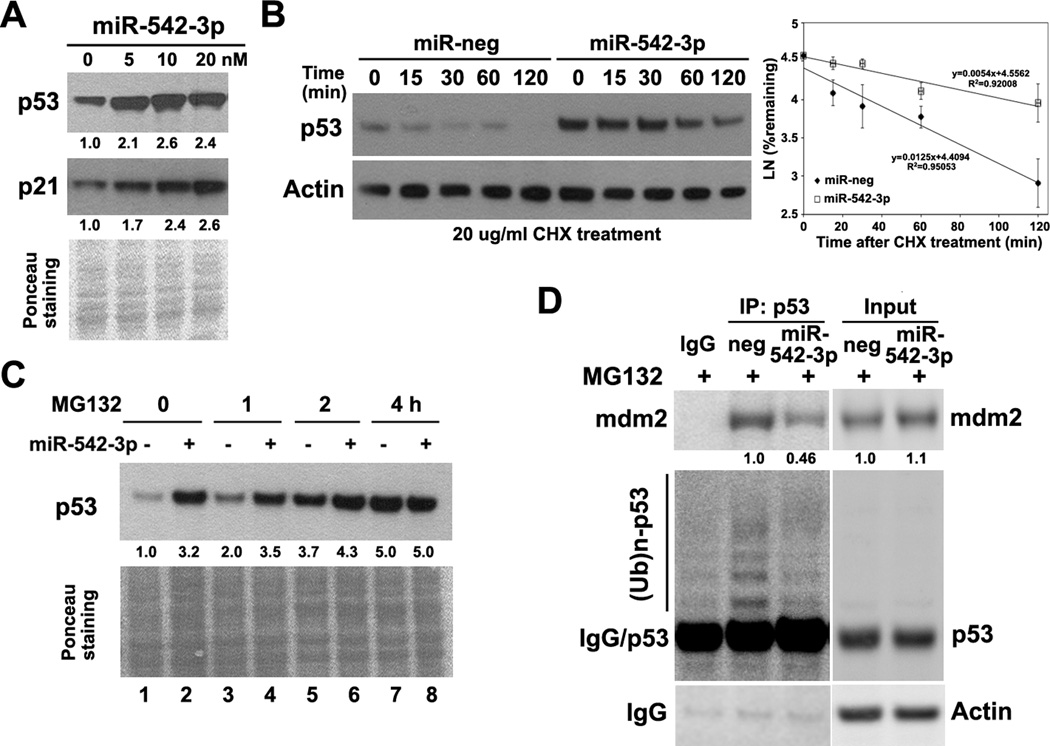
In agreement with the screening result, overexpression of miR-542-3p by synthetic mimics markedly increased expression of p53 protein as detected by western blotting in U2OS cells (Fig. 2A). In addition, transfection of a plasmid containing miR-542-3p primary sequence (pri-miR-542) efficiently produced miR-542-3p in U2OS cells and increased p53 expression (Fig.S1). However, miR-542-3p did not increase p53 expression at the transcript level (Fig. S2). To test whether miR-542-3p leads to stabilization of wild-type p53 protein, we treated U2OS cells with the protein synthesis inhibitor cycloheximide and evaluated the half-life of p53 protein. Overexpression of miR-542-3p increased the half-life of p53 from ~50 min to 130 min in U2OS cells (Fig. 2B). Overexpression of miR-542-3p failed to further increase p53 level in U2OS cells treated with a proteasome inhibitor, MG132 (Fig. 2C, lanes 7 and 8). Furthermore, miR-542-3p induces p53 expression in both normal human fibroblast (HF) and HCT116 (wild-type p53, colon cancer), but not in MDA-MB-231 (breast cancer), T98G (glioma) and U118 (glioma) cells that harbor mutant p53 (26) with stabilized p53 expression (Fig. S3). These data suggest that miR-542-3p leads to inhibition of proteasome-mediated degradation of wild-type p53 protein.
Figure 2. MiR-542-3p increases the stability of p53 protein.
(A) MiR-542-3p induces p53 at protein level. U2OS cells were transfected with negative or miR-542-3p mimics at indicated concentration. Cells were pelleted for western blotting of p53 and p21. (B) MiR-542-3p increases the stability of p53 protein. U2OS cells transfected with either negative or miR-542-3p mimics at 10 nM were reseeded and treated with protein synthesis inhibitor cycloheximide (CHX, 20μg/ml) to block de novo protein synthesis. Cells were harvested at indicated time for western blot analysis. Half-life of p53 was determined by densitometric analysis of p53 bands from three independent experiments using the formula t1/2=−Ln2/S, where S represents the slope from each linear regression. (C) MiR-542-3p failed to further increase p53 level in the presence of MG132. U2OS cells transfected with either negative or miR-542-3p mimics at 10 nM were reseeded and treated with MG132 (2μM) before western blot analysis of p53. (D) MiR-542-3p reduced MDM2-p53 interaction and p53 poly-ubiquitination. HCT116 cells transfected with either negative or miR-542-3p mimics were treated with MG132 (2μM) for 4hr. Cells were then lysed for immunoprecipitation with anti-p53. Immunoprecipitated proteins were subjected to western blot analysis of MDM2 and polyubiquitinated p53 ((Ub)n-p53). Quantitation of protein levels was labeled below the corresponding blots (same for Fig.3–5).
Since miR-542-3p increases the stability of p53, next we analyzed the integrity of p53 degradation pathway. Overexpression of miR-542-3p had no significant effect on the expression levels of either MDM2, the major E3 ligase targeting p53 for ubiquitin-proteasome-mediated degradation (7), or PIRH2, another E3 ligase which can target p53 for degradation (27) (Fig. S4). Moreover, miR-542-3p had no effect on the expression of MDM4 and PA28γ (Fig.S4), two known positive regulators of p53-MDM2 interaction (4, 28). However, the interaction between p53 and MDM2 as well as the poly-ubiquitination of p53 were attenuated after overexpressing miR-542-3p in HCT116 cells (Fig. 2D), suggesting that miR-542-3p may stabilize p53 by affecting other regulators of p53-MDM2 interaction.
MiR-542-3p induces ribosomal p53 response
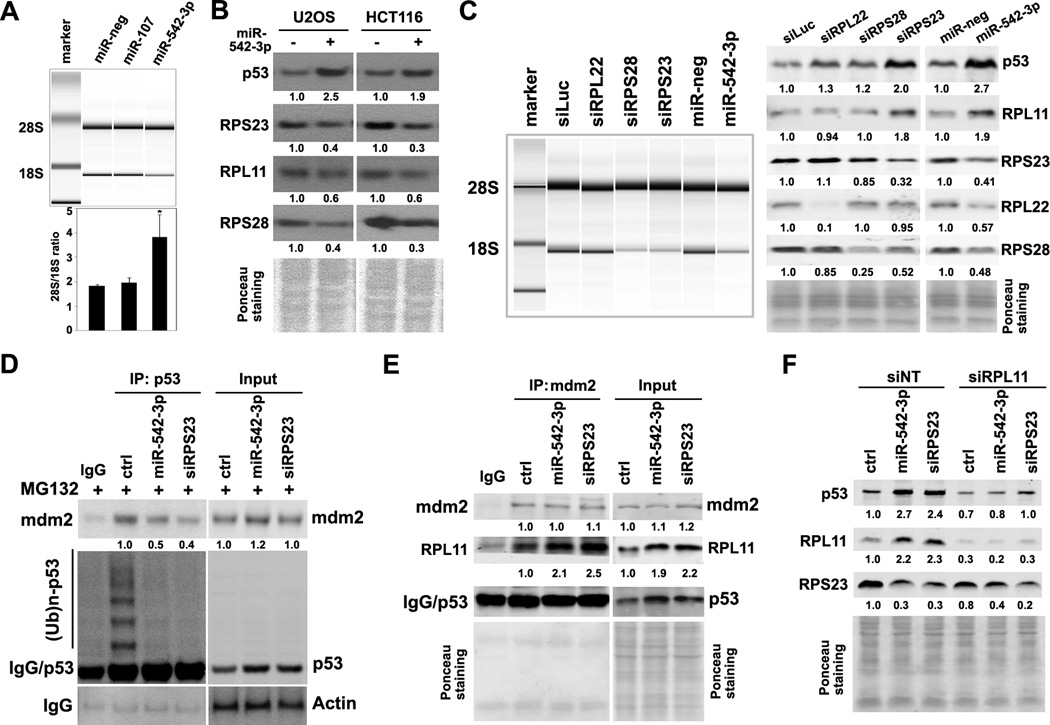
To identify targets of miR-542-3p that lead to the disruption of p53-MDM2 interaction, we performed microarray-based expression profiling in control or miR-542-3p mimic-transfected U2OS cells using Illumina whole genome gene expression platform. This analysis identified p53 signaling as the most altered pathway in miR-542-3p-overexpressing cells (Table S2 and Table S3). Noticeably, miR-542-3p overexpression reduced the level of 18S rRNA, the core of ribosome 40S rRNA subunit, by 2-fold without affecting the level of 28S rRNA, the core of ribosome 60S rRNA (Fig. 3A). Treatment with low dose of actinomycin D, known to induce ribosome stress (29), strongly upregulated p53 level in U2OS cells (Fig S5). Therefore, miR-542-3p may suppress the maturation of 18S rRNA to induce the ribosomal p53 response.
Figure 3. MiR-542-3p induces ribosomal p53 response.
(A) MiR-542-3p induces ribosome abnormality. U2OS cells were transfected as indicated. Forty-eight hours later, RNA was extracted and run on Agilent Bioanalyser. The 28S/18S ratio was calculated from electropherogram data. (B) MiR-542-3p suppresses the expression of RPS23, RPS28 and RPL22. U2OS and HCT116 cells transfected with negative or miR-542-3p mimics were analyzed for the expression of ribosome subunits by western blotting. (C) Effect of RPL22, RPS23 and RPS28 depletion by siRNA on 18S rRNA, RPL11 and p53 levels. U2OS cells transfected as indicated were collected for RNA quality analysis by Agilent Bioanlyser (left panel) or western blot analysis (right panel). (D) Depletion of RPS23 decreases MDM2-p53 interaction and p53 polyubiquitination. U2OS cells transfected as indicated were treated with MG132 (2μM) for 4hr and then lyzed for immunoprecipitation with anti-p53 followed by western blot analysis. (E) miR-542-3p and siRPS23 increase RPL11 and its association with MDM2. Cells transfected as indicated were lyzed for immunoprecipitation with anti-MDM2 followed by western blot analysis. (F) Depletion of siRPL11 reverses miR-542-3p and siRPS23-mediated p53 induction. U2OS cells were co-transfected as indicated were lyzed for western blot analysis. *, P < 0.01.
Since ribosome subunits, especially the small ribosome subunit proteins (RPS), play important roles in 18S rRNA maturation (30), we first determined the effect of miR-542-3p overexpression on protein levels of ribosomal subunits RPS23, RPS28 and RPL22, whose transcript levels were robustly downregulated in miR-542-3p-overexpressing cells (Table S4). The protein levels of RPS23, RPS28, and RPL22 were reduced in miR-542-3p-overexpressing U2OS and HCT116 cells (Fig. 3B). Next, we examined whether down-regulation of these ribosome subunits can cause ribosome biogenesis defects in U2OS cells. In agreement with the essential roles of RPS23 and RPS28 in small ribosome subunit maturation, depletion of RPS23 and RPS28 by siRNAs robustly reduced 18S rRNA level without affecting 28S rRNA, while knockdown of RPL22 has no effect on production of both 28S and 18S rRNA (Fig. 3C, left panel). Importantly, RPS23 depletion induced p53 by 2-fold, while depletion of RPS28 or RPL22 had very mild effect on p53 level (Fig. 3C, right panel). Similar to miR-542-3p overexpression, depletion of RPS23 weakened p53-MDM2 interaction and reduced p53 poly-ubiquitination (Fig. 3D). In addition, miR-542-3p overexpression or RPS23-depletion induced RPL11 (Fig. 3C and Fig. 3E), a ribosomal protein known to sequester MDM2 and mediate p53 induction following RPS23 depletion (11), and the association between MDM2 and RPL11 (Fig. 3E). Depletion of RPL11 by pooled siRNAs almost completely blocked p53 induction upon miR-542-3p overexpression or RPS23 knockdown (Fig. 3F). Thus, our data suggest that miR-542-3p induces p53 mainly through suppression of RPS23, which leads to upregulation of RPL11 and sequesteration of MDM2 by RPL11.
RPS23 is a direct target of miR-542-3p
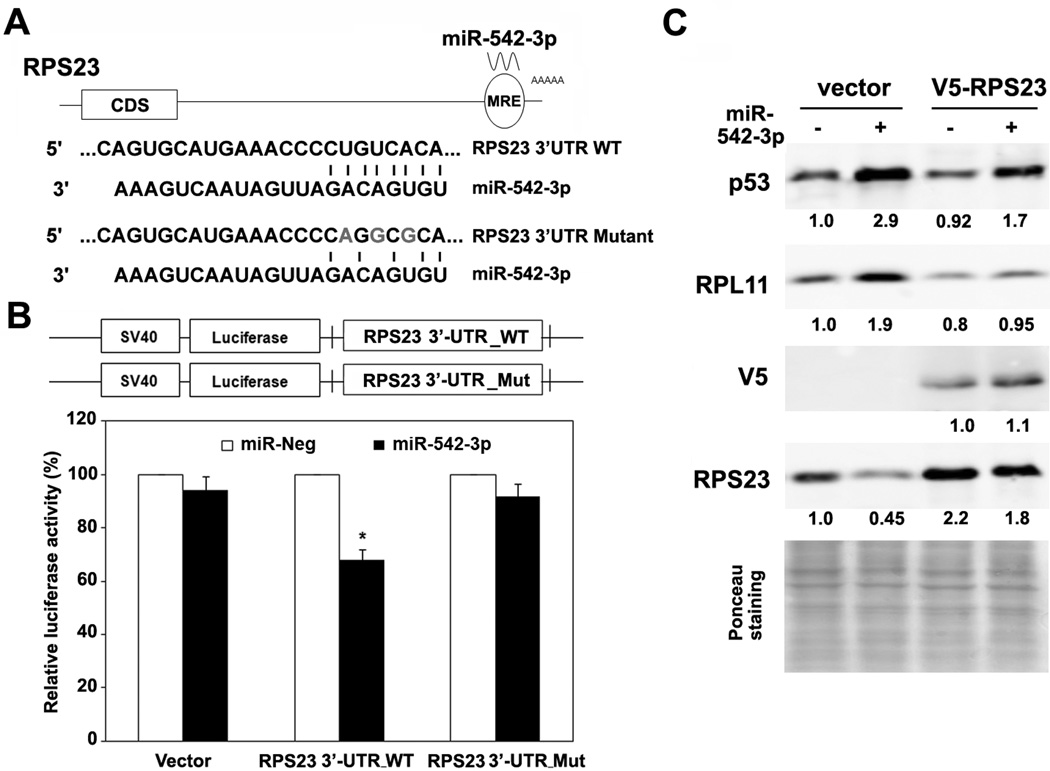
The 3’-UTR of the RPS23 transcript contains a predicted binding site of miR-542-3p by prediction algorithms MiRanda and TargetScan. To demonstrate whether RPS23 mRNA is a direct target of miR-542-3p, we first amplified fragments of RPS23 cDNA with PCR using multiple primer pairs (Fig. S6A). As shown in supplemental Fig. S6B, U2OS cells does express the 3.3-kb long mRNA of RPS23 with a predicted miR-542-3p binding site at C-terminus of 3’UTR that was confirmed by Sanger sequencing (Fig. S6C). We then cloned 3’-UTR of RPS23 mRNA downstream of the open-reading frame of the luciferase gene of pGL3 vector (RPS23 3’UTR, Fig. 4A) and transfected the construct together with miR-542-3p or negative control mimics into U2OS cells. MiR-542-3p significantly downregulated luciferase activity of the construct fused with RPS23 3’UTR, while it did not affect that of the empty vector control (Fig. 4B). Mutation of the potential miR-542-3p binding site in RPS23 3’UTR (RPS23 3’UTR mutant, Fig. 4A) completely abolished the inhibitory effect of miR-542-3p on luciferase activity (Fig. 4B), implying that RPS23 mRNA is a direct target of miR-542-3p.
Figure 4. RPS23 is a direct target of miR-542-3p.
(A) The putative miR-542-3p binding site (wild-type or mutant) in the transcript of RPS23. (B) MiR-542-3p targets 3’UTR of RPS23. Wild-type or mutant RPS23 3′-UTR were cloned into the pGL3 vector, as 3′ fusions to the luciferase gene. U2OS were co-transfected with the indicated miRNA mimics and luciferase vectors. Luciferase activity was assayed 48 h later and normalized to that of negative control-transfected cells from three independent experiments. (C) Ectopic RPS23 partially suppresses p53 induction by miR-542-3p. U2OS cells expressing vector or V5-tagged RPS23 coding region were transfected with negative or miR-542-3p mimics followed by western blot analysis. *, P < 0.01.
Next, we expressed the V5-tagged RPS23 transcript lacking its 3’UTR in U2OS cells. The ectopic V5-tagged RPS23 was not affected by miR-542-3p expression as expected (Fig 4C). MiR-542-3p-mediated induction of RPL11 was largely diminished and p53 induction by miR-542-3p was partially reverted in cells expressing V5-RPS23 (Fig. 4C), suggesting that direct targeting of the 3’UTR of RPS23 by miR-542-3p is at least partially responsible for miR-542-3p-mediated RPL11 and p53 induction.
MiR-542-3p activates p53 pathway in MDM2-overexpressing or ARF-deficient cells
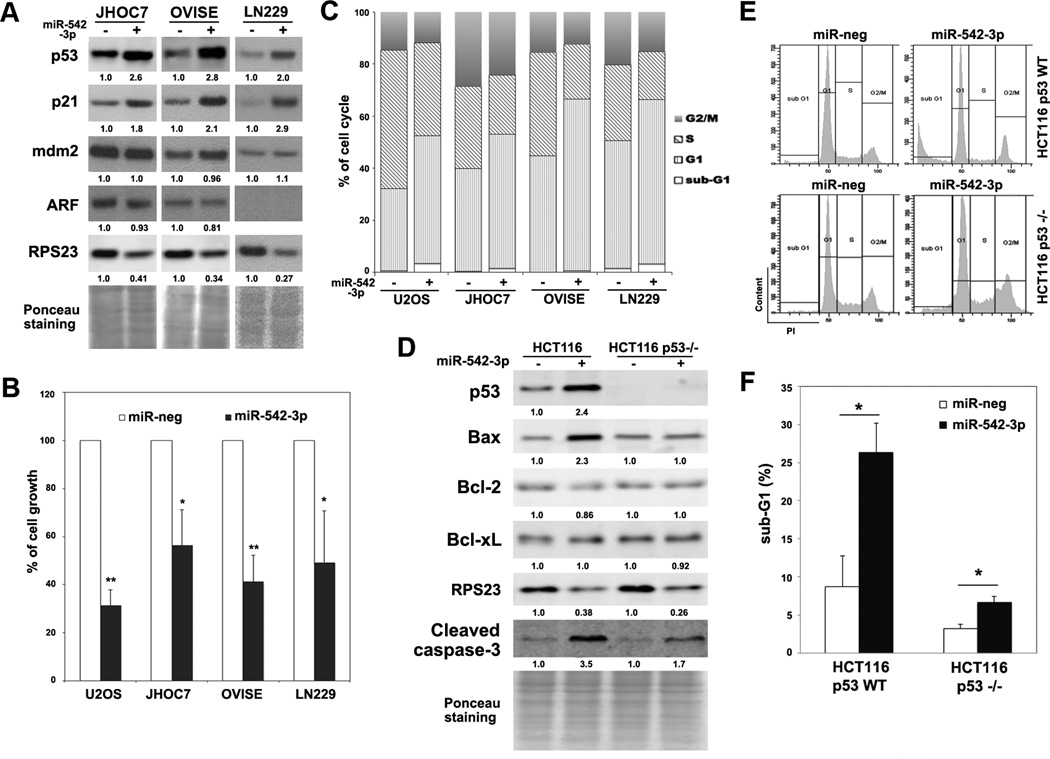
MDM2 amplification is an important mechanism leading to suppression of p53 function in tumors with wild-type TP53, such as melanoma and ovarian clear cell carcinoma (31, 32). Stabilization of p53 by disrupting the MDM2-p53 interaction is an important therapeutic approach to target tumor cells with wild-type TP53 and MDM2 amplification (5). In addition, ARF-deficiency is responsible for MDM2-mediated proteasomal degradation of p53 in a subset of cancer cells with wild-type TP53 (33). To determine whether miR-542-3p can rescue p53 protein expression in MDM2-overexpressing or ARF-deficient cells, we tested two ovarian clear cell carcinoma lines, JHOC7 and OVISE and one glioblastoma cell line, LN229. JHOC7 and OVISE expressed high levels of MDM2 and low amounts of wild-type p53 (unpublished data), while LN229 cells are ARF-deficient and express wild-type p53 (34). Overexpression of miR-542-3p in JHOC7, OVISE and LN229 cells significantly increased p53 expression and reduced RPS23 levels (Fig. 5A). Furthermore, both HCT116 and U2OS cells are indeed ARF-silenced cell lines (35, 36). These data indicate that miR-542-3p may be useful in restoring the function of p53 in MDM2-overexpressing or ARF-deficient tumors.
Figure 5. MiR-542-3p activates p53 pathway in MDM2-overexpressing or ARF-deficient cells.
(A) MiR-542-3p increases p53 in two ovarian clear cell carcinoma lines (JHOC7 and OVISE) and one glioma cell line (LN229). Cells were transfected with negative or miR-542-3p mimics followed by western blot analysis. (B) MiR-542-3p inhibits cell growth. Cells were reseeded at 1x104 cells/well in 12-well plates, cultured for 5 days and then fixed for crystal violet staining. Relative cell growth was calculated after re-solubilizing the plates. (C) MiR-542-3p induces G1 cell cycle arrest. Control or miR-542-3p-overexpressing cells were fixed and stained for cell cycle profiling by FACS. (D-F) p53 is crucial for miR-542-3p-induced cell death. HCT116 p53 WT and p53™/™ cells were transfected as indicated followed by western blot (D) or cell cycle analysis (E). Percentage of cells in sub-G1 was quantitated (F). *, P < 0.05. **, P < 0.01.
Since p53 plays important roles in cell growth arrest and depletion of RPS23 also robustly inhibited cell growth of U2OS cells (Fig. S7), we then determined if miR-542-3p affected cell growth. Consistent with the activation of the p53 pathway (Table S3), overexpression of miR-542-3p suppressed cell growth of U2OS, LN229, OVISE and JHOC7 cells significantly (Fig. 5B), which may be due to cell cycle arrest at G1-phase (Fig. 5C) mediated by p21 induction (Fig. 2A and Fig. 5A) and a mild induction of cell death (accumulation of cells at sub-G1 phase, Fig. 5C). However, miR-542-3p still robustly inhibited growth of U2OS cells after re-expression of RPS23 (Fig. S8) or p53 depletion by shRNA (Fig. S9A), suggesting that the inhibition of growth by miR-542-3p can be mediated through multiple pathways. Interestingly, in HCT116 cells, overexpression of miR-542-3p caused a dramatic increase of cell death (subG1, 26.4% vs. 8.7%, Fig. 5E and 5F) and an induction of Bax and cleaved Caspase-3 levels (Fig 5D). Although overexpression of miR-542-3p still inhibited the growth of p53™/ ™ HCT116 cells (Fig.S9B), they only mildly induced cell death in p53™/ ™ HCT116 cells (subG1, 3.2% vs. 6.7%, Fig. 5E and 5F), implying that miR-542-3p-induced cell death operates largely through the p53 signaling pathway.
Discussion
The tumor suppressor p53 is an important therapeutic target as it is mutated or functionally inactivated in most human tumors. Multiple strategies have been developed to reactivate p53. For example, small molecules have been identified or designed to correct the folding of mutant p53, thus activating the function of p53, or to stabilize the wild-type p53 by protecting p53 from MDM2-mediated degradation (reviewed in (37)). Our current study demonstrates that miRNAs may represent another type of therapeutic agent that can stimulate p53 function. In particular, miR-542-3p stabilized wild-type p53 through disrupting MDM2-mediated degradation and thus may be useful in the treatment of tumors harboring wild-type p53, in particular those with either MDM2 amplification or ARF-deficiency.
Accumulated evidence has linked miRNAs to the p53 pathway (reviewed in (38)). In particular, several studies identified a few miRNAs that serve as either negative regulators (miR-125b and miR-504) or positive regulators (miR-29, miR-34a, miR-122, miR-335 and miR-192/194/215) of p53 expression (19, 20, 24, 39–42). Using a miRNA library-screening platform, we have identified several miRNAs that can regulate the expression of p53. Although two known negative regulators of p53 (miR-125b and miR-504) showed a mild inhibitory role on p53 expression (Z<-1.2) due to limited sensitivity of picking up negative regulators of p53 by immunofluorescence, it is surprising that our screening did not identify any miRNAs that were previously reported to induce p53 expression and activation. This discrepancy may be due to relatively low sensitivity of our screen. However, most of those miRNAs are not identified even when we lowered the cutoff to z>1, suggesting that these miRNAs may not regulate p53 expression in U2OS cells. In contrast, miR-542-3p induced p53 expression in a wide range of p53-wild-type cell lines.
The ribosome is a ribonucleoprotein, which is made of a complex of RNAs and proteins and serves as the site of protein synthesis. Accelerated ribosome biogenesis, reflected by enlarged nucleoli, is an important marker of aggressive tumor cells (43, 44). Tumor cells may be more dependent on their ability to produce ribosomes and are therefore potentially more vulnerable to compromised ribosomal function (14). Concordantly, targeting ribosome integrity by a specific RNA pol I inhibitor may have selective tumor-killing effect through induction of p53 (13, 45). Our study suggests that targeting ribosome integrity by a miRNA may be an alternatively attractive way to enhance p53 function. Consistent with a previous report that depletion of the ribosome subunit RPS23 by a siRNA activates p53 in an RPL11-dependent manner in A549 cells (11), we demonstrate that depletion of RPS23 by either siRNA or miR-542-3p strongly stimulated the function of p53 by increasing RPL11 level and enhancing RPL11-MDM2 association to disrupt MDM2-mediated p53 ubiquitination and degradation in U2OS cells. Interestingly, although RPS28 is essential for 18S rRNA biogenesis (Fig. 3C), depletion of RPS28 had no significant effect on the levels of p53 and RPL11 (Fig. 3C). This finding may reflect the differential roles of RPSs in controlling the biogenesis of small ribosome subunit (30) and raise the possibility that p53 may be triggered only if certain steps of 18S rRNA maturation is interrupted during small ribosome subunit biogenesis, which needs future investigation. It is also noteworthy that although miR-542-3p and RPS23 siRNA showed similar effects on RPS23 and 18S rRNA suppression, miR-542-3p had a stronger effect on p53 induction in U2OS cells (2.7 fold vs. 2 fold, Fig. 3C) and ectopic expression of RPS23 transcript lacking 3’UTR did not completely block miR-542-3p-mediated p53 induction, indicating that additional mechanism(s) will exist to activate p53 in miR-542-3p-overexpressing cells. Furthermore, miR-542-3p may have a selective effect on tumor cells as ectopic expression of miR-542-3p produced a much weaker effect on p53 expression in normal human foreskin fibroblasts (Fig. S3). Future studies will be necessary to test this therapeutic selectivity.
The precursor of miR-542-3p, pre-miR-542, is co-transcribed with pre-miR-424 and pre-miR-503 as one transcript, which produces mature miRNAs, miR-542-5p, miR-542-3p, miR-424 and miR-503. It is predicted that the expression of this miRNA cluster can be controlled by transcriptional factors such as MAPKs (46). Accordingly, miR-542-3p is repressed by c-Src-related signaling molecules, such as EGFR, Ras and MAPKs, in HCT116 cells (25). It is thus not surprising that miR-542-3p is generally underexpressed in cancers such as colon, prostate, and lung cancers (25). Furthermore, silencing of this cluster can be mediated by promoter methylation, such as in prostate cancer (47).
Overexpression of miR-542-3p can inhibit cell growth and prevent tumor formation in vivo (25), which is consistent with our observation that the p53 pathway is robustly induced by overexpression of miR-542-3p. However, although miR-542-3p-mediated cell death is largely dependent on p53 (Fig. 5E and 5F), miR-542-3p can also suppress the cell growth of p53-deficient tumor cells, such as p53-depleted U2OS cells, p53™/ ™ HCT116 cells (Fig. S9) and SW480 cells (25), possibly because i) ribosomal disruption can suppress cell growth in a p53-independent pathway (48) and ii) miR-542-3p also inhibits survivin (BIRC5) (49), ILK (25) and many components of the ERK/MAPKs pathway revealed by our gene expression analysis (Table S2). Thus, it will be interesting to test whether delivery of miR-542-3p through nanoparticles or other technology is therapeutically applicable for treating tumors in a broad genetic background, regardless of p53 mutational status.
Interestingly, miR-542-5p, which shares the same precursor with miR-542-3p, also has tumor suppressive functions in neuroblastoma (50). Lower expression of miR-542-3p is correlated with poor survival in patients with neuroblastoma (50). Ectopic expression of synthetic miR-542-5p decreases invasion of neuroblastoma cells in vitro and suppresses tumor growth and metastases in an orthotopic mouse xenograft model (46). Therefore, simultaneously rescuing the expression of miR-542-5p and miR-542-3p by lentiviral-based delivery of pre-miR-542 may have better tumor suppressive effect in cancer treatment than expressing either miRNA alone.
Taken together, our studies identified miR-542-3p as a novel regulator of the p53 tumor suppressor via disruption of ribosome biogenesis. Our studies combined with other studies, provide support for an exploration of ectopic expression of miR-542-3p as a treatment strategy for cancers with wild-type TP53.
Supplementary Material
Acknowledgments
Funding: This work was supported by Howard Hughes Medical Institute, the National Institutes of Health (NIH)/ NHLBI [R21 HL092978 to T.T.], the NIH/ NCI [R01 CA125636 to T.T.] and Fanconi Anemia Research Fund (to T.T.). Y.W. is a research fellow supported by Canadian Institute of Health Research and Michael Smith Foundation for Health Research. J.W. was supported by PHS NRSA 2T32 GM007270 from NIGMS.
We thank members of FHCRC imaging facility, genomics facility, Taniguchi lab and Huntsman lab for technical assistance and discussions. We thank Drs. Muneesh Tewari, Guangwei Du, Denise Galloway, Bert Vogelstein and Hiroaki Itamochi for reagents. We thank Drs. Chris Kemp and Akiko Shimamura for critical reading of the manuscript.
Footnotes
Potential conflicts of interest: none.
References
- 1.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava S, Tong YA, Devadas K, Zou ZQ, Sykes VW, Chen Y, et al. Detection of both mutant and wild-type p53 protein in normal skin fibroblasts and demonstration of a shared 'second hit' on p53 in diverse tumors from a cancer-prone family with Li- Fraumeni syndrome. Oncogene. 1992;7:987–991. [PubMed] [Google Scholar]
- 3.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 4.Marine JC, Francoz S, Maetens M, Wahl G, Toledo F, Lozano G. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 2006;13:927–934. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]
- 5.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 6.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 7.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 8.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki M, Kawahara K, Nishio M, Mimori K, Kogo R, Hamada K, et al. Regulation of the MDM2-P53 pathway and tumor growth by PICT1 via nucleolar RPL11. Nat Med. 2011;17:944–951. doi: 10.1038/nm.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 11.Fumagalli S, Di Cara A, Neb-Gulati A, Natt F, Schwemberger S, Hall J, et al. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nat Cell Biol. 2009;11:501–508. doi: 10.1038/ncb1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fumagalli S, Ivanenkov VV, Teng T, Thomas G. Suprainduction of p53 by disruption of 40S and 60S ribosome biogenesis leads to the activation of a novel G2/M checkpoint. Genes Dev. 2012;26:1028–1040. doi: 10.1101/gad.189951.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drygin D, Rice WG, Grummt I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol. 2010;50:131–156. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 15.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Taniguchi T. MicroRNAs and DNA damage response: implications for cancer therapy. Cell Cycle. 2013;12:32–42. doi: 10.4161/cc.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 19.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Huang JW, Calses P, Kemp CJ, Taniguchi T. MiR-96 downregulates REV1 and RAD51 to promote cellular sensitivity to cisplatin and PARP inhibition. Cancer Res. 2012;72:4037–4046. doi: 10.1158/0008-5472.CAN-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Huang JW, Li M, Cavenee WK, Mitchell PS, Zhou X, et al. MicroRNA-138 modulates DNA damage response by repressing histone H2AX expression. Mol Cancer Res. 2011;9:1100–1111. doi: 10.1158/1541-7786.MCR-11-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang JW, Wang Y, Dhillon KK, Calses P, Villegas E, Mitchell PS, et al. Systematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivity. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-13-0292. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song JS, et al. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell. 2010;38:689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oneyama C, Morii E, Okuzaki D, Takahashi Y, Ikeda J, Wakabayashi N, et al. MicroRNA-mediated upregulation of integrin-linked kinase promotes Src-induced tumor progression. Oncogene. 2012;31:1623–1635. doi: 10.1038/onc.2011.367. [DOI] [PubMed] [Google Scholar]
- 26.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 27.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Zhang R. Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation. Embo J. 2008;27:852–864. doi: 10.1038/emboj.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashcroft M, Taya Y, Vousden KH. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira-Cerca S, Poll G, Gleizes PE, Tschochner H, Milkereit P. Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function. Mol Cell. 2005;20:263–275. doi: 10.1016/j.molcel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Polsky D, Melzer K, Hazan C, Panageas KS, Busam K, Drobnjak M, et al. HDM2 protein overexpression and prognosis in primary malignant melanoma. J Natl Cancer Inst. 2002;94:1803–1806. doi: 10.1093/jnci/94.23.1803. [DOI] [PubMed] [Google Scholar]
- 32.Kalloger SE, Kobel M, Leung S, Mehl E, Gao D, Marcon KM, et al. Calculator for ovarian carcinoma subtype prediction. Mod Pathol. 2011;24:512–521. doi: 10.1038/modpathol.2010.215. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 34.Robinson JP, Vanbrocklin MW, Lastwika KJ, McKinney AJ, Brandner S, Holmen SL. Activated MEK cooperates with Ink4a/Arf loss or Akt activation to induce gliomas in vivo. Oncogene. 2011;30:1341–1350. doi: 10.1038/onc.2010.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paliwal S, Pande S, Kovi RC, Sharpless NE, Bardeesy N, Grossman SR. Targeting of C-terminal binding protein (CtBP) by ARF results in p53-independent apoptosis. Mol Cell Biol. 2006;26:2360–2372. doi: 10.1128/MCB.26.6.2360-2372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korgaonkar C, Zhao L, Modestou M, Quelle DE. ARF function does not require p53 stabilization or Mdm2 relocalization. Mol Cell Biol. 2002;22:196–206. doi: 10.1128/MCB.22.1.196-206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selivanova G. Therapeutic targeting of p53 by small molecules. Semin Cancer Biol. 2010;20:46–56. doi: 10.1016/j.semcancer.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Feng Z, Zhang C, Wu R, Hu W. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3:44–50. doi: 10.1093/jmcb/mjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 41.Pichiorri F, Suh SS, Rocci A, De Luca L, Taccioli C, Santhanam R, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Scarola M, Schoeftner S, Schneider C, Benetti R. miR-335 directly targets Rb1 (pRb/p105) in a proximal connection to p53-dependent stress response. Cancer Res. 2010;70:6925–6933. doi: 10.1158/0008-5472.CAN-10-0141. [DOI] [PubMed] [Google Scholar]
- 43.Derenzini M, Trere D, Pession A, Govoni M, Sirri V, Chieco P. Nucleolar size indicates the rapidity of cell proliferation in cancer tissues. J Pathol. 2000;191:181–186. doi: 10.1002/(SICI)1096-9896(200006)191:2<181::AID-PATH607>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Chan JC, Hannan KM, Riddell K, Ng PY, Peck A, Lee RS, et al. AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci Signal. 2011;4:ra56. doi: 10.1126/scisignal.2001754. [DOI] [PubMed] [Google Scholar]
- 45.Drygin D, Lin A, Bliesath J, Ho CB, O'Brien SE, Proffitt C, et al. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011;71:1418–1430. doi: 10.1158/0008-5472.CAN-10-1728. [DOI] [PubMed] [Google Scholar]
- 46.Schmeier S, MacPherson CR, Essack M, Kaur M, Schaefer U, Suzuki H, et al. Deciphering the transcriptional circuitry of microRNA genes expressed during human monocytic differentiation. BMC Genomics. 2009;10:595. doi: 10.1186/1471-2164-10-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Formosa A, Lena AM, Markert EK, Cortelli S, Miano R, Mauriello A, et al. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2013;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- 48.Donati G, Montanaro L, Derenzini M. Ribosome biogenesis and control of cell proliferation: p53 is not alone. Cancer Res. 2012;72:1602–1607. doi: 10.1158/0008-5472.CAN-11-3992. [DOI] [PubMed] [Google Scholar]
- 49.Yoon S, Choi YC, Lee S, Jeong Y, Yoon J, Baek K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010;584:4048–4052. doi: 10.1016/j.febslet.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 50.Schulte JH, Marschall T, Martin M, Rosenstiel P, Mestdagh P, Schlierf S, et al. Deep sequencing reveals differential expression of microRNAs in favorable versus unfavorable neuroblastoma. Nucleic Acids Res. 2010;38:5919–5928. doi: 10.1093/nar/gkq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.