Abstract
Aim
Based on reports of exaggerated blood pressure responses to whole-body exercise in patients with metabolic syndrome (MetSyn), we tested the hypothesis that MetSyn adults would exhibit augmented sympathetic and pressor responses to mechanoreflex and metaboreflex activation when compared with healthy, age-matched control subjects.
Methods
We studied 12 adults with MetSyn (34±3 years) and 12 healthy control subjects (34±3 years). Heart rate (HR; ECG), blood pressure (BP; finger photoplethysmography), and MSNA (microneurography of the peroneal nerve) were measured during: (1) Static handgrip exercise at 15% of maximal voluntary contraction (MVC), and (2) Static handgrip exercise at 30% MVC to fatigue, followed by post-exercise ischemia (PEI). Increases in MSNA, HR, and BP were assessed.
Results
During static exercise at both 15 and 30% MVC, increases in MSNA, HR, and BP were not different between groups. MSNA remained significantly elevated from baseline during PEI and responses were not different between groups.
Conclusion
Sympathetic and pressor responses to mechanoreflex and metaboreflex activation are not augmented in younger adults with MetSyn.
Keywords: blood pressure, MSNA, obesity, static exercise, exercise pressor reflex
INTRODUCTION
Increases in heart rate and blood pressure during exercise are the result of an integration of central command and the exercise pressor reflex (Alam et al., 1937; Coote et al., 1971; Goodwin et al., 1972; Mark et al., 1985; Mitchell et al., 1983). Central command plays an important role in decreased parasympathetic efferent activity and increased heart rate and blood pressure responses to exercise, but has little effect on muscle sympathetic nerve activity (MSNA) during mild exercise intensities (Goodwin et al., 1972; Mitchell et al., 1983; Rowell et al., 1990). At the same time, mechanical and chemical stimuli within the contracting muscles activate afferents that reflexively increase sympathetic outflow (mechanoreflex and metaboreflex, respectively) in an attempt to maintain adequate perfusion of the exercising muscle (Coote et al., 1971; Mark et al., 1985; Mitchell et al., 1983; Stebbins et al., 1988).
Based on reports of exaggerated blood pressure responses to exercise in patients with metabolic syndrome (MetSyn) (Miyai et al., 2013; Tsioufis et al., 2012), it is reasonable to speculate sympathetic nervous system activation during metabo- and mechanoreflex stimulation might be exaggerated as well. This augmented pressor response, if present, could negate the beneficial effects of exercise in this population, for whom exercise is recommended as a non-pharmacological strategy for improving fitness and reducing cardiovascular risk. However, research regarding metaboreflex sensitivity in persons demonstrating individual components of the MetSyn (i.e. obesity, hypertension, dyslipidemia) has yielded conflicting results. For example, metaboreflex responsiveness in hypertensive humans has been shown to be blunted (Rondon et al., 2006) or increased (Delaney et al., 2010; Sausen et al., 2009). Similar controversies exist when examining human obesity (Dipla et al., 2010; Negrao et al., 2001; Trombetta et al., 2003). These discrepancies may be due, at least in part, to the presence and/or absence of overt MetSyn. Consistent with this concept, the individual components of the MetSyn fail to predict exaggerated blood pressure responses to whole-body exercise independent of the presence of MetSyn (Miyai et al., 2013; Tsioufis et al., 2012). To investigate potential mechanisms underlying exaggerated blood pressure responses to whole-body exercise in MetSyn, we tested the hypothesis that young adults with MetSyn would exhibit augmented sympathetic and pressor responses to mechanoreflex and metaboreflex activation when compared with healthy, age-matched control subjects.
MATERIALS AND METHODS
Subjects
Twelve adults with MetSyn and 12 age-matched, healthy control subjects completed the current study. Adults were characterized as having MetSyn if they met at least three of the following National Cholesterol Education Program Adult Treatment Panel III criteria as modified by the American Diabetes Association: central obesity (waist circumference >102 cm males, >88 cm females), pre-hypertension (resting BP ≥130/≥85 mmHg), hypertriglyceridemia (triglycerides ≥150 mg.dL-1), hyperglycemia (fasting glucose ≥100 mg.dL-1) and/or dyslipidemia (HDL<40 mg.dL-1 males, <50 mg.dL-1 females) (Grundy et al., 2004). Of the adults with MetSyn, each subject met exactly 3 of the 5 aforementioned criteria; specifically, 11 met the criterion for waist circumference, 10 for blood pressure, 10 for HDL, 5 for triglycerides, and 0 for glucose. Healthy control subjects did not meet any of the criteria for MetSyn. All subjects were sedentary, non-smokers, free from neurological disorders, and were not taking any cardiovascular or lipid-lowering medications, as determined by self-report. Female subjects were not pregnant and were studied during the early follicular phase (days 1-5) of the menstrual cycle to avoid potential confounding effects of female hormone levels (Jarvis et al., 2011). Hormonal contraception was allowed and women on contraception were studied during the placebo phase. Subjects were instructed to refrain from exercise, non-steroidal anti-inflammatory drugs, alcohol, and caffeine for 24 -hours prior to the study day and all studies were completed after a 10-hour fast. Written informed consent was obtained from all subjects. Procedures were approved by the Institutional Review Board at the University of Wisconsin – Madison and conformed to the standards set by the Declaration of Helsinki.
Measurements
Height, weight, and waist circumference were measured. Whole-body DEXA scans (dual-energy x-ray absorptiometry; GE Lunar Prodigy; Milwaukee, WI) provided measures of total body fat and lean forearm mass (using anatomical landmarks). Venous blood was collected after a 10-hour fast and analyzed for levels of high density lipoprotein (HDL), triglycerides, and glucose (CardioChek; PTS Panels; Indianapolis, IN, USA). Additional plasma samples were frozen at -80°C for subsequent analysis of insulin and leptin (Wisconsin National Primate Research Center; Madison, WI). To examine physical activity levels, subjects completed the Paffenbarger questionnaire (Paffenbarger et al., 1993).
Cardiorespiratory Variables
Heart rate and blood pressure were measured continuously. Heart rate was measured from the electrocardiogram (Datex-Ohmeda; Helsinki, Finland). Beat-by-beat arterial pressure was measured by finger pulse photoplethysmography (Finapres model 2300; Ohmeda, Englewood, CO, USA) and corrected using automated arm cuff sphygmomanometry (Datex-Ohmeda; Helsinki, Finland). Subjects were instructed to avoid extraneous sympathoexcitatory maneuvers including Valsalvas and prolonged expirations and compliance was verified using a respiratory belt transducer positioned at the midchest level (Piezo Respiratory Belt Transducer; ADinstruments, Colorado Springs, CO). Data were sampled in real time with signal-processing software (PowerLab, ADinstruments, Colorado Springs, CO), were digitized, stored on a computer at 400 Hz, and analyzed off-line using PowerLab. Post-processing was completed using PowerLab’s Chart7 application package for mean blood pressures, heart rates, and respiratory rates.
Static Forearm Exercise
Maximal Voluntary Contraction (MVC, kg) of the non-dominant arm was determined as the average of the two highest measurements from 5 trials using a hand dynamometer. MVCs were then repeated using a force transducer and the highest voltage achieved from 3 maximum contractions was calibrated as 100% MVC. Static exercise consisted of squeezing the transducer forcefully enough to consistently maintain a low (15% MVC; primarily mechanoreflex stimulation) or moderate (30% MVC; a combination of central command, mechanoreflex, and metaboreflex stimulation) workload (Rowell, 1992; Seals, 1988). Visual feedback of force production was provided to the subject (LabChart 7; ADinstruments).
Microneurography
The technique of Vallbo et al. (Vallbo et al., 1979) was used to record postganglionic MSNA as described previously (Limberg et al., 2012). A unipolar tungsten microelectrode was inserted percutaneously into a muscle fascicle of the right fibular nerve with the subject in the supine position. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch resulted in the appearance of afferent activity, and no afferent neural response was evoked by skin stimulation. Once an acceptable nerve recording was obtained, the subject was instructed to maintain a relaxed position for the duration of the study.
Neural signals were passed to a differential preamplifier, an amplifier (total gain=100,000), a band-pass filter (700-2,000 Hz), and an integrator (time constant =100 msec) to obtain mean voltage neurograms (Rys Systems; Milwaukee, WI, USA). Data were sampled in real time with signal-processing software (PowerLab, ADinstruments; Colorado Springs, CO). Sympathetic bursts in the mean voltage neurogram were identified off-line using a semi-automatic analysis program (LabChart, Adinstruments; Colorado Springs, CO) and burst identification was confirmed visually by a single investigator. Nerve activity was reported as burst frequency (bursts per minute), burst incidence (bursts per 100 heart beats), and total activity [bursts per minute × burst amplitude; reported as % of rest = (Exercise÷Rest) × 100].
Study Protocol
Subjects were supine with the non-dominant arm extended to the side at heart level. Room temperature was controlled at 22°C. Data collection commenced approximately 30 minutes after achieving a clear nerve recording. Following 2 minutes of quiet rest, subjects completed 2 trials: 1) Static handgrip exercise at 15% MVC for 2 minutes, 2) Static handgrip exercise at 30% MVC to fatigue, followed by 2 minutes of post exercise ischemia (PEI). PEI began after the subject could no longer maintain 30% MVC and consisted of rapid inflation of an occlusion cuff on the exercising arm proximal to the antecubital fossa to suprasystolic pressure (~220 mmHg); the cuff remained inflated for 2 minutes. PEI traps local metabolites and preserves activation of metabosensitive afferent nerve endings, thus separating the metaboreflex from central command and the mechanoreflex (Alam et al., 1937; Mark et al., 1985; Saito et al., 1989; Seals et al., 1989; Stebbins et al., 1985). Participants were asked to rate their perceived exertion (RPE) at the end of the fatiguing exercise (Borg scale; range 6 to 20). Trials were separated by a minimum of 10 minutes of quiet rest to ensure all main outcome variables returned to baseline levels. Trials were conducted in random order. MSNA, heart rate, and blood pressure values are reported as an average of 1-minute sections of data during key timepoints (Delaney et al., 2010; Jarvis et al., 2011) (See Figure 1). We have shown 1-minute sections of resting MSNA measurements to be repeatable [CV = 11±2% Present study; CV = 10±2% (Limberg et al., 2012)].
Figure 1. Study Timeline.
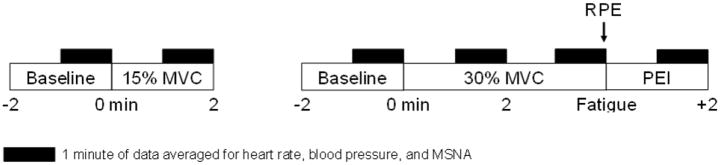
Subjects completed 2 trials: 1) Static handgrip exercise at 15% MVC for 2 minutes, 2) Static handgrip exercise at 30% MVC to fatigue, followed by 2 minutes of post-exercise ischemia (PEI). Participants completed a Borg scale to rate perceived exertion (RPE) at the end of the fatiguing exercise. MSNA, heart rate, and blood pressure values are reported as an average of 1-minute sections of data during key timepoints (black boxes). Trials were separated by a minimum of 10 minutes of quiet rest and were conducted in random order.
Data Analysis
The main dependent variables were MSNA, heart rate, and blood pressure. The primary analysis was to test whether absolute changes [Δ: Exercise – Rest; Fatigue – Rest; PEI - Rest] in key variables were greater in MetSyn when compared with controls (See Figure 1). Statistical analysis was done using SigmaPlot Version 12.0 (Systat Software, Inc.; San Jose, CA). Subject characteristics were compared using an independent samples t-test. All other responses were analyzed using a two way repeated measures analysis of variance approach to determine the significance of the fixed effect of group (Control/MetSyn) and/or condition (Rest/Exercise; Rest/Fatigue/PEI) on parameters of interest. Bonferroni post hoc comparisons were performed when significant effects were observed. All data are presented as mean±standard error and significance was determined a priori at p<0.05.
RESULTS
Subject Characteristics
Subject characteristics are summarized in Table 1. Twelve adults with MetSyn and 12 healthy control subjects participated in the current study. Adults with MetSyn were clinically obese – exhibiting significantly higher weight, waist circumference, BMI, and body fat when compared with healthy controls (p<0.001). In addition, adults with MetSyn displayed higher blood pressure, insulin, leptin, and lower HDL cholesterol (p<0.05). There was a trend for greater triglyceride levels in adults with MetSyn (p=0.06). Groups were not significantly different in regard to age (p=0.82), physical activity (p=0.78), lean forearm size (p=0.30), forearm MVC (p=0.25), or average resting MSNA (Burst Frequency, p=0.33; Burst Incidence, p=0.29).
Table 1. Subject Demographics.
Data are presented as Mean±SE. N=12 per group unless otherwise noted. Leptin, Insulin (Control n=11, MetSyn n=7); PAQ (MetSyn n=11); Body Fat, Lean Forearm Mass (MetSyn n=10).
| Control (n=12) | MetSyn (n=12) | |
|---|---|---|
| Sex (M/F) | 8/4 | 9/3 |
| Age (yrs) | 34±3 | 34±3 |
| Weight (kg) | 70±3 | 105±6* |
| BMI (kg/m2) | 23±1 | 34±2* |
| Body fat (%) | 19±2 | 36±2* |
| Waist (cm) | 78±3 | 108±3* |
| Glucose (mg/dL) | 79±4 | 76±5 |
| HDL (mg/dL) | 66±5 | 39±2* |
| Triglycerides (mg/dL) | 76±10 | 130±22 |
| SBP (mmHg) | 124±2 | 138±3* |
| DBP (mmHg) | 70±2 | 83±3* |
| MBP (mmHg) | 88±2 | 103±3* |
| Insulin (uU/mL) | 10±1 | 17±4* |
| Leptin (ng/mL) | 3±1 | 9±1* |
| MSNA Burst Frequency (burst/min) | 21±3 | 29±6 |
| MSNA Burst Incidence (burst/100 heart beat) | 36±5 | 46±8 |
| MVC (kg) | 37±3 | 42±3 |
| Lean Forearm Mass (g) | 963±73 | 1084±89 |
| PAQ (kcal/wk) | 2161±572 | 1772±381 |
p<0.05 vs Control.
Abbreviations: BMI (body mass index), MVC (maximal voluntary contraction), HDL (high density lipoprotein), SBP (systolic blood pressure), DBP (diastolic blood pressure), MBP (mean blood pressure), PAQ (physical activity questionnaire), MSNA (muscle sympathetic nerve activity).
Static Exercise at 15% MVC
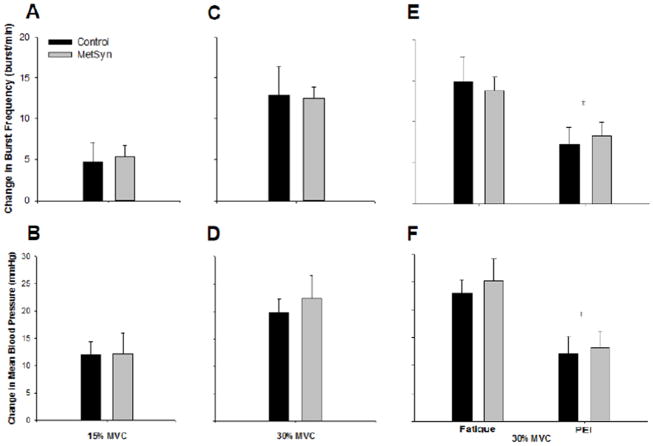
Results are summarized in Table 2 and Figure 2A-B. Burst frequency, total activity, heart rate, and blood pressure increased with 2 minutes of static exercise at 15% MVC (all p<0.01; Main effect of Condition). Increases (Δ) in MSNA burst frequency (Control 5±2, MetSyn 5±1 burst/min; p=0.83), burst incidence (2±4 vs 5±3 burst per 100 heart beats; p=0.68), total activity (26±15 vs 51±16 %Rest; p=0.17), heart rate (9±2 vs 5±1 beat/min; p=0.11), and blood pressure (12±2 vs 12±4 mmHg; p=0.98) were not different between groups. Breathing rate (breath/min) was not different between groups and did not change with exercise (all p>0.05; data not shown).
Table 2. Reflex responses to static handgrip exercise and post-exercise ischemia (PEI).
Data are presented as Mean±SE. 15% MVC: Control n=10, MetSyn n=11. 30% MVC: Control n=11, MetSyn n=12.
| Burst Frequency (burst/min) |
Burst Incidence (burst/100 heart beats) |
Total Activity (% Rest) |
Heart Rate (beat/min) |
Mean Arterial Blood Pressure (mmHg) |
||
|---|---|---|---|---|---|---|
| 15% MVC Static Handgrip Exercise (2 min) | ||||||
| Control | Rest | 22±3 | 36±5 | 100 | 60±3 | 88±3 |
| Exercise | 27±4† | 39±6 | 126±15† | 68±2† | 100±5† | |
| MetSyn | Rest | 28±6 | 42±8 | 100 | 64±3 | 96±3 |
| Exercise | 33±6† | 46±7 | 151±16† | 69±4† | 108±5† | |
| 30% MVC Static Handgrip Exercise (2 min) | ||||||
| Control | Rest | 21 ±3 | 36±5 | 100 | 60±3 | 87±2 |
| Exercise | 34±4† | 46±5† | 266±64† | 75±4† | 107±4† | |
| MetSyn | Rest | 30±5 | 46±8 | 100 | 64±3 | 97±3* |
| Exercise | 42±5† | 54±6† | 205±21† | 77±3† | 119±6*† | |
| 30% MVC Static Handgrip Exercise (Fatigue) + Post-Exercise Ischemia | ||||||
| Control | Rest | 21±3 | 36±5 | 100 | 60±3 | 87±2 |
| Fatigue | 36±4† | 47±5 | 276±62† | 78±4† | 110±4† | |
| PEI | 28±3† | 49±5† | 205±23† | 60±4‡ | 99±3†‡ | |
| MetSyn | Rest | 30±5 | 46±8 | 100 | 64±3 | 97±3 |
| Fatigue | 43±5† | 54±5 | 230±31† | 79±3† | 122±5† | |
| PEI | 38±5† | 58±6† | 207±30† | 66±4‡ | 110±5†‡ | |
p<0.05 vs Control.
p<0.05 vs Rest.
p<0.05 vs Fatigue.
MVC (maximal voluntary contraction).
Figure 2. Reflex responses to metaboreflex and mechanoreflex activation.
Sympathetic (A, p=0.83) and blood pressure (B, p=0.98) responses to mechanoreflex activation (15% MVC Exercise, 2 min) were not different between groups. Sympathetic (C, p=0.78) and blood pressure (D, p=0.69) responses to combined central command, mechanoreflex, and metaboreflex activation (30% MVC Exercise, 2 min) were not different between groups. Fatiguing exercise (30% MVC Exercise, Fatigue) followed by post-exercise ischemia (PEI) resulted in a persistent rise in muscle sympathetic nerve activity (E) and blood pressure (F) which were blunted from fatigue levels (‡p<0.01 vs Fatigue); differences in group responses were not detected.
Static Exercise at 30% MVC
Results are summarized in Table 2 and Figure 2C-D. Burst frequency, burst incidence, total activity, heart rate, and blood pressure increased with 2 minutes of static exercise at 30% MVC (all p<0.01; Main effect of Condition). Absolute changes (Δ) in MSNA burst frequency (Control 13±4, MetSyn 13±1 burst/min; p=0.78), incidence (9±4 vs 7±3 bursts per 100 heart beats; p=0.65), total activity (166±64 vs 105±21 %Rest; p=0.76), heart rate (14±2 vs 14±2 beat/min; p=0.91), and blood pressure (20±3 vs 22±4 mmHg; p=0.69) were not different between groups. Breathing rate was not different between groups and the increase with exercise was not different between groups (all p>0.05; data not shown).
30% MVC Post-Exercise Ischemia
Results are summarized in Table 2 and Figure 2E-F. When 30% MVC static handgrip exercise was completed to fatigue, burst frequency, total activity, heart rate, and blood pressure increased from resting levels (all p<0.01; Main effect of Condition). PEI resulted in a decrease in heart rate (p<0.01) and blood pressure (p<0.01) from fatigue levels (Main effect of Condition); differences in group responses were not detected. Importantly, time to fatigue (Control 3.4±0.5 min; MetSyn 3.0±0.3 min; p=0.48) and the rate of perceived exertion (RPE; Control 15±1; MetSyn 15±1; p=0.70) were not different between groups. Additionally, breathing rate was not different between groups and responses to exercise and PEI were not different between groups (all p>0.05; data not shown).
DISCUSSION
Given the importance of the sympathetic nervous system to blood pressure control, we used an isometric forearm exercise model and direct sympathetic neural recordings to systematically examine the impact of MetSyn on autonomic regulation of the circulation during exercise. Based on reports of exaggerated blood pressure responses to whole-body exercise in patients with metabolic syndrome (Miyai et al., 2013; Tsioufis et al., 2012), we tested the hypothesis that MetSyn adults would exhibit augmented sympathetic and pressor responses to mechanoreflex and metaboreflex activation when compared with healthy, age-matched control subjects. Contrary to our hypothesis, sympathetic and pressor responses to mechanoreflex and metaboreflex activation are not augmented in younger adults with MetSyn.
Central Command and the Muscle Mechanoreflex
The immediate increases in heart rate and blood pressure observed at exercise onset are the result of both central command and the muscle mechanoreflex. Experimentally, the observed increase in MSNA at a static exercise workload of approximately 15% of maximum (15% MVC) is primarily a result of mechanoreflex stimulation (Rowell, 1992; Seals, 1988), with little contribution from the metaboreflex and/or central command. Thus we examined MSNA, heart rate, and blood pressure responses to 2 minutes of static handgrip exercise at 15% MVC in adults with MetSyn and age-matched healthy controls. Importantly, subject cohorts did not differ in lean forearm mass or MVC (Table 1). This is essential, given greater forearm mass has been previously linked to higher forces and greater mechanical compression of feed arteries (Rowell et al., 1981; Seals, 1989). Our results suggest mechanoreflex responses are not augmented in adults with MetSyn (Table 2, Figure 2A-B). These results are consistent with previous research showing blood pressure responses to isometric handgrip exercise to be similar between older hypertensive adults with and without MetSyn (Aldo Ferrara et al., 2007).
Isolating the Muscle Metaboreflex
Chemical changes in contracting muscles reflexively increase MSNA, heart rate, and blood pressure during exercise (Saito et al., 1989; Seals et al., 1989; Stebbins et al., 1985). However, at higher intensities (i.e. 30% MVC), the increase in MSNA during exercise may be the result of central command, mechanoreflex, and metaboreflex stimulation (Rowell, 1992; Seals, 1988). When these mechanisms are studied in combination (after 2 minutes of 30% MVC exercise), our results suggest responses are not augmented in adults with MetSyn (Table 2, Figure 2C-D); however, we cannot distinguish between the contribution of central command, the mechanoreflex, and/or the metaboreflex. With the addition of PEI, we were able to examine the contribution of the metaboreflex to observed responses, mostly independent from mechanoreflex and central command (Alam et al., 1937; Mark et al., 1985). In the present study, MSNA and blood pressure remained elevated above resting levels during PEI in both groups (Table 2, Figure 2E-F), although blood pressure fell from levels observed at fatigue [identifying a partial contribution of central command and the mechanoreflex to blood pressure responses during exercise in both groups (Alam et al., 1937; Mark et al., 1985)]. Heart rate, however, did not remain elevated during PEI in either group and instead returned to resting levels (Table 2) – confirming previous work showing none of the rise in heart rate with static handgrip exercise can be attributed to the metaboreflex.
Given the important contribution of the muscle metaboreflex to blood pressure during exercise, and previous research showing exaggerated blood pressure responses to whole-body exercise in adults with MetSyn (Gaudreault et al., 2013; Miyai et al., 2013; Tsioufis et al., 2012), we hypothesized sympathetic nervous system activation during metaboreflex stimulation would be exaggerated as well. The mechanisms underlying this exaggerated blood pressure response to exercise in MetSyn were previously unexamined and studies reporting on the effect of individual components of MetSyn (i.e. hypertension, obesity, dyslipidemia) were conflicting (Delaney et al., 2010; Dipla et al., 2010; Minami et al., 1999; Negrao et al., 2001; Rondon et al., 2006; Sausen et al., 2009; Trombetta et al., 2003). Contrary to our hypothesis, reflex responses to PEI were preserved in younger adults with MetSyn (Table 2, Figure 2E-F). Given the study was carefully designed to independently examine potential mechanisms underlying neurocirculatory responses to exercise, we conclude metaboreflex and/or mechanoreflex activation of the sympathetic nervous system are not exaggerated in human MetSyn. Taken together, these results suggest alternative mechanisms, such as endothelial dysfunction, arterial stiffness, impaired diastolic function, and/or altered baroreflex sensitivity (i.e. impaired baroreflex buffering and inability to restrain blood pressure during exercise) likely play a more important role in exaggerated blood pressure responses to whole-body exercise in MetSyn adults than the exercise pressor reflex per se.
Experimental Considerations
Static handgrip exercise was performed to fatigue prior to PEI and the time to fatigue and rates of perceived exertion (RPE) were not different between groups; thus, we can assume all subjects reached a common metabolic end-point (Seals, 1993). However, the concentration of metabolic byproducts was not assessed. If the stimulus (metabolic byproducts) behind the elicited response (increase in heart rate, blood pressure, and MSNA) was different between groups, this could complicate interpretation of our findings. For example, insulin resistance has been shown to reduce glycolysis (Bjorntorp, 1997), and thus the presence of metabolic byproducts; therefore, if the similar rise in MSNA (Table 2) in adults with MetSyn was achieved at a lesser metabolic end-point when compared with controls, we may have underestimated their metaboreflex response. Additionally, stimulation of Type II skeletal muscle fibers has been shown to result in a greater pressor response when compared to Type I fibers (Saito, 1995). If adults with MetSyn exhibit a smaller proportion of Type II fibers (Bassett, 1994; He et al., 1995), this could also affect interpretation of our results.
Adults with MetSyn have been shown previously to exhibit higher MSNA when compared with healthy controls (Grassi et al., 2005). The exact mechanisms behind this increase are unknown; however a rise in basal MSNA may occur in response to changes in body composition, altered insulin signaling, and/or changes in the peripheral vasculature. Interestingly, resting MSNA was not significantly higher in the current research cohort (Main effects and Interaction effects, Range p=0.18-0.48) despite increases in central adiposity, blood pressure, and plasma insulin and leptin concentrations (Table 1). The lack of significant group differences in baseline MSNA may be the result of the heterogeneous nature of MetSyn and between-subject variability in baseline measures of MSNA. Alternatively, given the relatively young age of the current subjects [~30 years vs ~50 years; (Grassi et al., 2005; Miyai et al., 2013; Tsioufis et al., 2012)], and early disease status, it is reasonable to speculate with increasing age and disease severity, the trend for group differences in resting MSNA would reach statistical significance. Furthermore, whereas metaboreflex and/or mechanoreflex activation of the sympathetic nervous system is not exaggerated in younger adults with MetSyn, this does not suggest increasing age and duration of disease would not result in such adaptations. This idea is supported by previous research showing increased metabosensitive activation of the sympathetic nervous system in older heart failure patients (Notarius et al., 2001) and highlights the potential confounding effect of increasing age and/or overt cardiovascular disease on research results and the importance of controlling for such confounders in the present study.
CONCLUSION
Exercise is a common and effective non-pharmacological way to combat MetSyn; however, recent research has shown adults with MetSyn exhibit an exaggerated blood pressure response to whole-body exercise (Miyai et al., 2013; Tsioufis et al., 2012). The mechanisms behind this exaggerated response in MetSyn adults are not completely understood. However, the current results suggest augmented responses to mechanoreflex and metaboreflex activation are not present in younger MetSyn subjects. Future research will be necessary to explore the potential effects of advancing age, in addition to endothelial dysfunction, arterial stiffness, impaired diastolic function, and/or altered baroreflex sensitivity on blood pressure responses to exercise in human MetSyn.
Acknowledgments
Thank you to Edward McKenna, Keelin O’Neil, Ann Bolgert, and Meghan Crain for technical assistance. Thank you to Drs. John Dopp and Andrea Mason for equipment use. In addition, we would like to thank the involvement of WNPRC Assay Services and the partial support of NIH grant RR000167. This study was supported by the American Heart Association pre-doctoral awards #0815622G (JKL) and 10PRE3870000 (JKL), the University of Wisconsin Madison Virginia Horne Henry Foundation (JKL, WGS), and grants HL105820 (WGS) and RR000167 (WGS) from the National Institutes of Health.
Glossary
- BMI
Body mass index
- BP
Blood pressure
- DBP
Diastolic blood pressure
- DEXA
Dual-energy x-ray absorptiometry
- ECG
Electrocardiogram
- Ex
Exercise
- HDL
High density lipoprotein
- HR
Heart rate
- MBP
Mean blood pressure
- MetSyn
Metabolic syndrome
- MSNA
Muscle sympathetic nerve activity
- MVC
Maximal voluntary contraction
- PAQ
Physical activity questionnaire
- PEI
Post exercise ischemia
- RPE
Rate of perceived exertion
- SBP
Systolic blood pressure
Footnotes
Author contributions: Concept and design (JKL), Data collection and analysis (JKL, BJM), Data interpretation and manuscript preparation (JKL, BJM, WGS). All authors approved of the final manuscript.
CONFLICT OF INTEREST
There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldo Ferrara L, Guida L, Ferrara F, De Luca G, Castaldo R, Viola S, Russo R. Blood pressure at rest, during 24 h monitoring and in response to sympathetic stimulation in hypertensive patients with metabolic syndrome. Int J Cardiol. 2007;117:312–316. doi: 10.1016/j.ijcard.2006.04.085. [DOI] [PubMed] [Google Scholar]
- Bassett DR., Jr Skeletal muscle characteristics: relationships to cardiovascular risk factors. Med Sci Sports Exerc. 1994;26:957–966. [PubMed] [Google Scholar]
- Bjorntorp P. Neuroendocrine factors in obesity. J Endocrinol. 1997;155:193–195. doi: 10.1677/joe.0.1550193. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299:H1318–1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipla K, Zafeiridis A, Koidou I, Geladas N, Vrabas IS. Altered hemodynamic regulation and reflex control during exercise and recovery in obese boys. Am J Physiol Heart Circ Physiol. 2010;299:H2090–2096. doi: 10.1152/ajpheart.00087.2010. [DOI] [PubMed] [Google Scholar]
- Gaudreault V, Despres JP, Rheaume C, Almeras N, Bergeron J, Tremblay A, Poirier P. Exercise-induced hypertension in men with metabolic syndrome: anthropometric, metabolic, and hemodynamic features. Metab Syndr Relat Disord. 2013;11:7–14. doi: 10.1089/met.2012.0071. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- He D, Bolstad G, Brubakk A, Medbo JI. Muscle fibre type and dimension in genetically obese and lean Zucker rats. Acta Physiol Scand. 1995;155:1–7. doi: 10.1111/j.1748-1716.1995.tb09938.x. [DOI] [PubMed] [Google Scholar]
- Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R193–200. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Morgan BJ, Sebranek JJ, Proctor LT, Walker BJ, Eldridge MW, Schrage WG. Altered neurovascular control of the resting circulation in human metabolic syndrome. J Physiol. 2012;590:6109–6119. doi: 10.1113/jphysiol.2012.239780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Minami M, Atarashi K, Ishiyama A, Hirata Y, Goto A, Omata M. Pressor hyperreactivity to mental and hand-grip stresses in patients with hypercholesterolemia. J Hypertens. 1999;17:185–192. doi: 10.1097/00004872-199917020-00002. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Miyai N, Shiozaki M, Yabu M, Utsumi M, Morioka I, Miyashita K, Arita M. Increased mean arterial pressure response to dynamic exercise in normotensive subjects with multiple metabolic risk factors. Hypertens Res. 2013 doi: 10.1038/hr.2012.215. [DOI] [PubMed] [Google Scholar]
- Negrao CE, Trombetta IC, Batalha LT, Ribeiro MM, Rondon MU, Tinucci T, Forjaz CL, Barretto AC, Halpern A, Villares SM. Muscle metaboreflex control is diminished in normotensive obese women. Am J Physiol Heart Circ Physiol. 2001;281:H469–475. doi: 10.1152/ajpheart.2001.281.2.H469. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens. 2006;19:951–957. doi: 10.1016/j.amjhyper.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of the circulation during exercise. Int J Sports Med. 1992;13(Suppl 1):S25–27. doi: 10.1055/s-2007-1024583. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Freund PR, Hobbs SF. Cardiovascular responses to muscle ischemia in humans. Circ Res. 1981;48:I37–47. [PubMed] [Google Scholar]
- Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Saito M. Differences in muscle sympathetic nerve response to isometric exercise in different muscle groups. Eur J Appl Physiol Occup Physiol. 1995;70:26–35. doi: 10.1007/BF00601805. [DOI] [PubMed] [Google Scholar]
- Saito M, Mano T, Iwase S. Sympathetic nerve activity related to local fatigue sensation during static contraction. J Appl Physiol. 1989;67:980–984. doi: 10.1152/jappl.1989.67.3.980. [DOI] [PubMed] [Google Scholar]
- Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol. 2009;105:351–356. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol. 1988;64:2197–2203. doi: 10.1152/jappl.1988.64.5.2197. [DOI] [PubMed] [Google Scholar]
- Seals DR. Influence of muscle mass on sympathetic neural activation during isometric exercise. J Appl Physiol. 1989;67:1801–1806. doi: 10.1152/jappl.1989.67.5.1801. [DOI] [PubMed] [Google Scholar]
- Seals DR. Influence of active muscle size on sympathetic nerve discharge during isometric contractions in humans. J Appl Physiol. 1993;75:1426–1431. doi: 10.1152/jappl.1993.75.3.1426. [DOI] [PubMed] [Google Scholar]
- Seals DR, Enoka RM. Sympathetic activation is associated with increases in EMG during fatiguing exercise. J Appl Physiol. 1989;66:88–95. doi: 10.1152/jappl.1989.66.1.88. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol. 1988;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Longhurst JC. Bradykinin-induced chemoreflexes from skeletal muscle: implications for the exercise reflex. J Appl Physiol. 1985;59:56–63. doi: 10.1152/jappl.1985.59.1.56. [DOI] [PubMed] [Google Scholar]
- Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrao CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003;285:H974–982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- Tsioufis C, Kasiakogias A, Tsiachris D, Kordalis A, Thomopoulos C, Giakoumis M, Bounas P, Pittaras A, Michaelides A, Stefanadis C. Metabolic syndrome and exaggerated blood pressure response to exercise in newly diagnosed hypertensive patients. Eur J Prev Cardiol. 2012;19:467–473. doi: 10.1177/1741826711410819. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]