Abstract
Hantaviruses predominantly replicate in primary human endothelial cells and cause 2 diseases characterized by altered barrier functions of vascular endothelium. Most hantaviruses restrict the early induction of interferon-β (IFNβ) and interferon stimulated genes (ISGs) within human endothelial cells to permit their successful replication. PHV fails to regulate IFN induction within human endothelial cells which self-limits PHV replication and its potential as a human pathogen. These findings, and the altered regulation of endothelial cell barrier functions by pathogenic hantaviruses, suggest that virulence is determined by the ability of hantaviruses to alter key signaling pathways within human endothelial cells. Our findings indicate that the Gn protein from ANDV, but not PHV, inhibits TBK1 directed ISRE, kB and IFNβ induction through virulence determinants in the Gn cytoplasmic tail (GnT) that inhibit TBK1 directed IRF3 phosphorylation. Further studies indicate that in response to hypoxia induced VEGF, ANDV infection enhances the permeability and adherens junction internalization of microvascular and lymphatic endothelial cells. These hypoxia/VEGF directed responses are rapamycin sensitive and directed by mTOR signaling pathways. These results demonstrate the presence of at least two hantavirus virulence determinants that act on endothelial cell signaling pathways: one that regulates antiviral IFN signaling responses, and a second that enhances normal hypoxia-VEGF-mTOR signaling pathways to facilitate endothelial cell permeability. These findings suggest signaling pathways as potential targets for therapeutic regulation of vascular deficits that contribute to hantavirus diseases and viral protein targets for attenuating pathogenic hantaviruses.
Introduction
Hantaviruses predominantly infect the endothelial cell lining of vessels and nonlytically cause two diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (Duchin et al., 1994; Lahdevirta et al., 1982; Lee, 1982; Nichol et al., 1993; Schmaljohn, 2001; Yanagihara and Silverman, 1990; Zaki et al., 1995). HFRS results from infection by Eurasian hantaviruses (Hantaan virus, HTNV; Dobrava virus, DOBV; Puumala virus, PUUV) (Lahdevirta et al., 1982; Lee et al., 1982; Schmaljohn, 2001) while hantaviruses identified throughout the Americas (ie. Andes virus, ANDV; Sin Nombre virus, SNV; New York virus, NYV) are associated with HPS (Duchin et al., 1994; Enria et al., 1996; Lopez et al., 1996; Nichol et al., 1993; Schmaljohn, 2001). In contrast, Tula virus (TULV) and Prospect Hill virus (PHV) are hantaviruses that are not associated with any human disease (Plyusnin et al., 1994; Yanagihara et al., 1987). While TULV and PHV differ from pathogenic hantaviruses by their use of discrete integrin receptors (Gavrilovskaya et al., 1999; Gavrilovskaya et al., 1998; Raymond et al., 2005), PHV also fails to regulate early IFN induction which restricts its replication in endothelial cells and likely contributes to its inability to be a human pathogen (Alff et al., 2006; Alff et al., 2008; Geimonen et al., 2002; Spiropoulou et al., 2007). These findings suggest that hantaviruses contain virulence determinants that restrict antiviral IFN pathway signaling responses and alter normal endothelial cell signaling pathways that control vascular permeability.
Only a few viruses specifically target the endothelial cell (EC) lining of vessels and cause acute edematous or hemorrhagic disease. Mechanisms by which hantaviruses disrupt fluid barrier integrity and clearance functions of the endothelium are just beginning to be disclosed. Vascular permeability induced by hantaviruses is likely to be multifactorial in nature and result from virally altered EC responses and signaling pathways, tissue hypoxia and immune cell and platelet functions (Gavrilovskaya et al., 2012a; Gavrilovskaya et al., 2012b; Gavrilovskaya et al., 2010, 2012c, 2013; Gorbunova et al., 2010; Gorbunova et al., 2013; Gorbunova et al., 2011; Hammerbeck and Hooper, 2011; Kilpatrick et al., 2004; Koster and Mackow, 2012; Mori et al., 1999; Raymond et al., 2005; Taylor et al., 2013; Terajima et al., 1999; Vaheri et al., 2013). This is likely to occur through a collaboration of interactions which bypass redundant vascular systems that control critical fluid barrier functions. Failure of the endothelium to regulate hemorrhage or edematous fluid accumulation in tissues has severe pathologic consequences. Deficits in the regulation of vascular permeability are dramatically illustrated by findings in HPS patients which result in localized acute pulmonary edema, unprecedented pulmonary fluid accumulation rates (up to 1 liter/hour) and a ~40% mortality rate (Duchin et al., 1994; Koster and Mackow, 2012; Zaki et al., 1995). As the multifactorial nature of vascular regulation is impacted by many systems, a variety of hypotheses have been expounded, but need to be prefaced by stating that there is currently no data demonstrating that any of these theories play a causal role in vascular permeability induced by hantaviruses.
The primary understanding of hantavirus induced vascular deficits, remains the viruses ability to infect the endothelial cell lining of the vasculature and nonlytically cause edematous or hemorrhagic disease (Duchin et al., 1994; Lahdevirta et al., 1982; Lee, 1982; Nichol et al., 1993; Schmaljohn, 2001; Yanagihara and Silverman, 1990; Zaki et al., 1995). Hantaviruses dysregulate microvascular and lymphatic endothelial cell (MEC and LEC) functions that normally restrict fluid leakage from vessels and clear fluid from tissues (Gavrilovskaya et al., 2010, 2012c, 2013; Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2013; Gorbunova et al., 2011; Koster and Mackow, 2012; Raymond et al., 2005; Shrivastava-Ranjan et al., 2010). The effects of hantavirus infection of endothelial cells remains enigmatic and the focus of our studies of altered endothelial cell signaling pathways (Gavrilovskaya et al., 2013; Gorbunova et al., 2011) that are fundamental to altered vascular permeability and hantavirus virulence.
Hantavirus entry into human endothelial cells initially discriminates between pathogenic hantaviruses, whose infection is fostered by human αvβ3 integrins, and nonpathogenic TULV and PHV which are unaffected by the presence of αvβ3 integrins (Gavrilovskaya et al., 1999; Gavrilovskaya et al., 1998; Raymond et al., 2005). Since αvβ3 is a known regulator of vascular permeability this finding ties hantavirus receptor usage to vascular permeability (Gavrilovskaya et al., 2008; Raymond et al., 2005). Yet, in vitro, pulmonary microvascular and lymphatic endothelial cells (MECs, LECs), and human umbilical vein endothelial cells (HUVECs) are not permeablized by hantavirus infection alone suggesting that receptor usage itself is not a cause of vascular permeability (Gavrilovskaya et al., 2012c, 2013; Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2013). Interestingly, studies indicating that cell-associated pathogenic hantaviruses bind inactive αvβ3 integrins, days after infection, tie αvβ3 integrin usage to the regulation of signaling pathways induced by a potent vascular permeability inducer, vascular endothelial growth factor (VEGF) (Gavrilovskaya et al., 1999; Gavrilovskaya et al., 2010; Raymond et al., 2005; Robinson et al., 2004). αvβ3 normally forms a complex with VEGF receptor 2 (VEGFR2), which tempers VEGFR2 directed permeability in response to localized VEGF release. Knocking out β3 or inhibiting αvβ3 promotes VEGFR2 directed endothelial cell permeability (Borges et al., 2000; Byzova et al., 2000; Hodivala-Dilke et al., 1999; Reynolds et al., 2002; Robinson et al., 2004). Furthering this association during hantavirus infection, the permeability of endothelial cells infected by ANDV, SNV and NY-1V, but not nonpathogenic TULV or PHV hantaviruses, is dramatically enhanced in response to VEGF (Gavrilovskaya et al., 2010, 2012c; Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2013; Gorbunova et al., 2011).
VEGF is a potent vascular permeability factor (VPF) that locally induces vascular permeability by binding endothelial cell VEGF receptors, within 1.5 mm of its release, and directing the disassembly of inter-endothelial cell adherens junctions (Dejana et al., 2008; Dvorak et al., 1995; Gavard, 2009; Gavard and Gutkind, 2006). VEGF is induced by hypoxia to facilitate repair, and increase gas exchange within the lung, and VEGF is inactivated by circulating soluble receptors that prevent systemic vascular permeability responses. VEGF induced pulmonary edema is known to be caused by hypoxia in high altitude settings (Berger et al., 2005; Hanaoka et al., 2003; Hopkins et al., 2005; Voelkel, 2002).
HPS patients are acutely hypoxic (Bustamante et al., 1997; Zaki et al., 1995) and a recent retrospective analysis of pulmonary edema fluids in a small number of HPS patients indicated the presence of high levels of VEGF (Gavrilovskaya et al., 2012a). Hantavirus infection of MECs and LECs may disengage one or more fluid barrier regulatory mechanisms, thereby increasing vascular leakage or fluid clearance resulting in tissue edema (Dehler et al., 2006; Schraufnagel et al., 2003). These findings suggest one of many mechanisms that may participate in HPS directed pulmonary edema and vascular deficits within hantavirus patients. However, although HPS patients are hypoxic there is as yet no causal evidence for this mechanism in hantavirus disease.
Consistent with roles for αvβ3 and hypoxia directed VEGF in hantavirus pathogenesis, hypoxia and VEGF tie into complex intracellular signaling pathways and feedback regulatory mechanisms that may be altered by virulence determinants within pathogenic hantaviruses. Hypoxia and VEGFR2 are tied to mTOR (mammalian target of rapamycin) directed cell division, control of cell size and feedback regulation of hypoxic responses (Kim et al., 2009; Xue et al., 2009). Studies presented below tie virulence determinants within hantavirus proteins to altered VEGF directed mTOR activation.
In addition to regulating cell receptor signaling, hantaviruses regulate IFN signaling pathways within human endothelial cells in order to successfully replicate and be human pathogens. Hantavirus replication is highly sensitive to the early addition of IFN or IFN pretreatment and hantaviruses grow to much lower titers in IFN competent cell lines than IFN locus defective Vero E6 cells (Alff et al., 2006). Interestingly, the effects of IFN addition are nearly absent when IFN is added 1 day post-infection (Alff et al., 2006), and consistent with hantaviruses inducing high level ISG responses at late times post-infection (Geimonen et al., 2002). In contrast to pathogenic hantaviruses, PHV rapidly induces IFNβ and IFN stimulated gene (ISG) responses that restrict its replication in human endothelial cells (Geimonen et al., 2002) and this response, in addition to receptor usage, are potential explanations for the absence of PHV associated human disease (Alff et al., 2006; Alff et al., 2008; Matthys et al., 2011; Matthys and Mackow, 2012). Our findings suggest that permissive hantavirus replication in human endothelial cells results from the selective restriction of early IFN induction (Alff et al., 2006; Geimonen et al., 2002; Matthys et al., 2011; Matthys and Mackow, 2012).
The ability of hantaviruses to regulate IFN induction and alter vascular and lymphatic endothelial cell signaling responses suggests the presence of encoded virulence determinants that permit viral replication and alter cellular responses which control fluid barrier functions of the endothelium. Here we show that hantaviruses contain virulence determinants that alter normal endothelial cell functions by regulating VEGF-mTOR signaling responses and permitting viral replication by inhibiting the early induction of Type 1 IFN. These findings suggest the presence of an IFN regulating virulence determinant in the Gn protein that is required for hantavirus replication in human endothelial cells and for subsequent vascular permeability deficits in HFRS and HPS patients. However, these clues to vascular dysfunction provide potential mechanisms by which hantaviruses induce vascular permeability and acute edema that remain to be defined in vivo.
Results
Hantavirus Regulation of Early IFN Responses Defines Virulence Determinants in the GnT
Replicating RNA viruses generate small amounts of dsRNA that are detected by cytoplasmic helicases which signal TBK1/IKKε complexes (Seth et al., 2006; Yoneyama and Fujita, 2007; Yoneyama et al., 2004) to activate NF-κB and cellular IFN response factors (IRFs3/5/7) (Hacker et al., 2011; Hiscott, 2007; Tu et al., 2013). Activated IRFs and NF-κB translocate to the nucleus and transcriptionally induce IFNβ and additional antiviral ISG responses from promoters containing IFN stimulated response elements (ISREs) (Charoenthongtrakul et al., 2013; Daffis et al., 2009; Delhase et al., 2011; Hacker et al., 2011; Lazear et al., 2013).
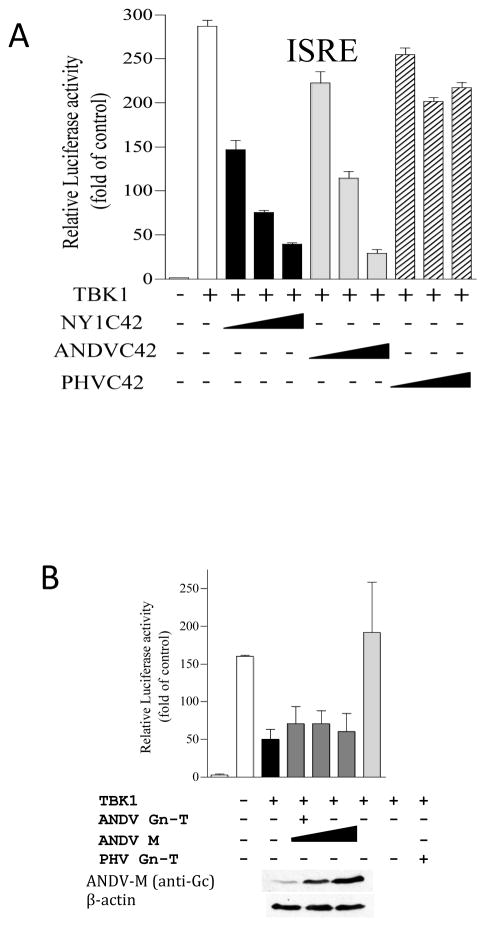
We have found that GnT proteins from NY-1V, ANDV, TULV and HTNV, but not PHV, regulate polyI:C, RIG-I, MDA5 and TBK1 directed ISRE, κB and IFNβ transcriptional responses upstream of constitutively active IRF3-5D, and at the level of the TBK1-TRAF3 complex (Alff et al., 2006; Alff et al., 2008; Geimonen et al., 2002; Matthys et al., 2013). Yet IFN signaling responses are cytoplasmic and only cytoplasmic elements of Gn are likely to affect regulation. To investigate this we truncated Gn to express only the C-terminal 42 residues (C42) within its cytoplasmic tail (GnT) and evaluated the ability of C42 domains to regulate ISRE and IFN transcriptional responses. Figure 1A demonstrates that GnT C42 domains from NYV or ANDV, but not PHV, inhibit TBK1 directed ISRE transcriptional responses in a dose dependent manner. Inhibition of IFNβ transcriptional responses by GnT constructs are similar to expressing GnGc proteins (Gc level monitored by Western) from ANDV M gene segments as indicated in Figure 1B. In both Figure 1A and 1B pathogenic hantavirus GnT, C42 or GnGc expression inhibited IFN signaling pathway responses. Additional studies indicate that the ANDV GnGc inhibits RIG-I, MDA5 and TBK1, but not IRF3-5D, directed transcription from an ISRE promoter and the IFNβ enhanceosome (Matthys et al., 2013; Matthys et al., 2011; Matthys and Mackow, 2012). In addition, GnGc expression also inhibited RIG-I directed IRF3 phosphorylation (Matthys et al., 2013). Collectively, these findings demonstrate that NY-1V, ANDV and TULV GnTs as well as the GnGc polyprotein inhibit RIG-I induced transcriptional responses by impacting TBK1 phosphorylation of IRF3 (Matthys et al., 2013). These findings indicate that the GnT domain contains an IFN regulating element with the potential to be a virulence determinant within hantaviruses that enhances viral replication and spread.
Figure 1. Gn and GnT Protein Expression Regulates TBK1 Directed ISRE Promoter Transcription.
A) HEK293T cells were transfected with ISRE promoter directed luciferase reporter and Renilla luciferase plasmids (Matthys et al., 2013). Cells were co-transfected with a TBK1 expressing plasmid and increasing amounts of ANDV, NYV or PHV GnT-C42 expression plasmids. Cells were harvested 1 day post-transfection and assayed for firefly luciferase activity. Results are presented as the percent induction compared to pcDNA3 induction control (100%) after standardization to Renilla luciferase levels as previously described (Matthys et al., 2013). B) HEK293T cells were transfected with IFNβ promoter directed luciferase reporter and Renilla luciferase plasmids and co-transfected with TBK1 plasmid and either ANDV or PHV Gn-T or the ANDV M segment expression plasmid. Cells were harvested and luciferase reporter responses analyzed as in A. ANDV Gc and β-actin (loading control) were detected by Western Blot analysis as described (Matthys et al., 2013).
VEGF and ANDV Infection Enhance VE-Cadherin Internalization and EC Permeability
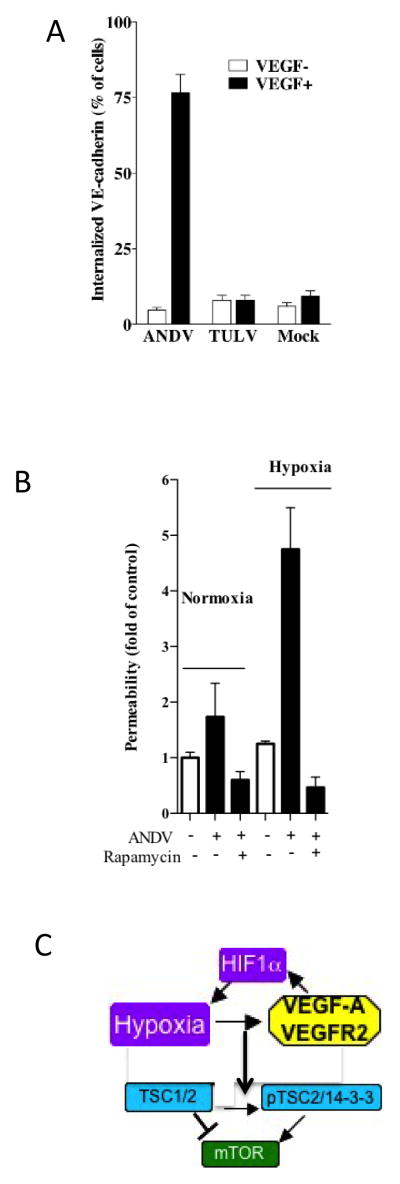
ANDV infects the endothelial cell lining of capillaries and results in patient hypoxia and acute pulmonary edema leading to respiratory distress (Bustamante et al., 1997; Duchin et al., 1994; Enria et al., 1996; Lopez et al., 1996; Zaki et al., 1995). Hypoxia itself induces VEGF which is a permeability factor that has the potential to cause edema during ANDV infection and contribute to HPS (Dvorak, 2006; Dvorak et al., 1995). VEGFR2 activation increases EC permeability by directing VE-cadherin internalization, but not degradation, which disassembles adherens junctions (AJs) and permits rapid reassembly of AJs (Corada et al., 1999; Corada et al., 2002; Gavard, 2009; Gavard and Gutkind, 2006; Gavard et al., 2008; Nawroth et al., 2002; Wallez et al., 2006; Zanetti et al., 2002). VE-cadherin internalization was monitored following the protocol of Gavard (Gavard, 2009; Gavard and Gutkind, 2006; Gavard et al., 2008). EC monolayers were infected with ANDV, TULV or mock infected, under hypoxic or normoxic conditions or following VEGF addition (shown). In order to assay VE-cadherin internalization, monolayers were incubated with FITC-labeled anti-VE-cadherin mAb (100 ng/ml; sc52751, Santa Cruz; 30 min, 4°C) and subsequently 1 hour at 37°C to synchronize internalization (Gavard, 2009). Cells were acid washed to remove extracellular VE-cadherin antibody and cells containing internalized VE-cadherin were quantitated by fluorescence microscopy (Gavard, 2009; Gavard and Gutkind, 2006; Gavard et al., 2008). The internalization of VE-cadherin was monitored following infection of primary pulmonary microvascular endothelial cells (MECs) by ANDV (HPS) or TULV (nonpathogenic). Figure 2A demonstrates the hyper-responsiveness of VE-cadherin internalization in ANDV infected MECs to VEGF addition and the absence of VEGF effects on TULV or mock infected MECs. These findings demonstrate that ANDV infection combined with VEGF addition dramatically mobilizes VE-cadherin from AJs to intracellular stores.
Figure 2. VEGF and Hypoxia Induced Permeability Responses are Rapamycin Sensitive.
MECs were mock, ANDV (CHI-7913), or TULV (Tula/Moravia/MA 5302V/94) infected in BSL3 at an MOI of 0.5. A) Analysis of ANDV induced VE-cadherin internalization was performed 3 days post-infection as previously described on pulmonary MECs (Gavrilovskaya et al., 2008; Gorbunova et al., 2010). B) Two days post infection, MECs were grown in normoxic or hypoxic conditions for 18 hours and treated with rapamycin (20 ng/ml) for 1 hour prior to evaluating permeability as previously described (Gavrilovskaya et al., 2012c; Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2013; Gorbunova et al., 2011). Monolayers were treated as indicated with VEGF (100 ng/ml) prior to assessing monolayer permeability to FITC-dextran (40,000; 0.5 mg/ml) as previously described (Gavrilovskaya et al., 2008; Gorbunova et al., 2010). C) Interrelationship of Hypoxia, HIF1a, VEGFA/VEGFR2 responses and the TSC1/2 regulated mTOR signaling pathway.
Hypoxia Induces EC Permeability via Rapamycin Sensitive mTOR Signaling
mTOR signaling is intimately tied to hypoxia directed VEGF induction and results from signaling pathways that are blocked by the mTOR inhibitor rapamycin (Gavrilovskaya et al., 2013). We recently evaluated responses of human pulmonary MECs and LECs in response to VEGF addition or hypoxic conditions (Gavrilovskaya et al., 2012c; Gorbunova et al., 2013). The permeability of MEC and lymphatic EC (LEC) monolayers was assayed by adding FITC-dextran to the upper chamber and monitoring levels in the lower chamber of confluent EC monolayers in response to VEGF addition (Gavrilovskaya et al., 2012c; Gorbunova et al., 2013). We observed little change in the permeability of ANDV infected MECs or LECs alone, but observed a dramatic increase in the permeability of VEGF or hypoxia treated ANDV infected MECs and LECs (Figure 2B). In contrast, neither VEGF nor hypoxia treatment of TULV infected cells resulted in an increase in MEC or LEC permeability. Interestingly, the hypoxia induced permeability was sensitive to the pathway specific mTOR inhibitor, rapamycin, indicating that permeability responses are mediated by mTOR directed HIF1α activation as well as HIF-1α, hypoxia and VEGF directed signaling responses during infection by pathogenic hantaviruses (Figure 2C)(Gavrilovskaya et al., 2013).
Consistent with this, we recently reported that ANDV infection dramatically increased HIF1α directed VEGF-A, ANG4 and EGLN3 mRNA levels within hypoxic MECs and LECs (Gavrilovskaya et al., 2013) and that human pulmonary edema fluids from HPS patients contains high levels of VEGF (Gavrilovskaya et al., 2012a). Hypoxia stabilizes the formation of HIF1α transcriptional complexes that induce VEGF, additional hypoxia responsive factors and stress regulators that impact the activation of mTOR signaling responses (Gavrilovskaya et al., 2013; Zhou et al., 2007) (Figure 2C). mTOR signaling also controls cell size and these findings support data demonstrating that VEGF and hypoxia direct the formation of giant LECs and MECs through a rapamycin sensitive mTOR dependent mechanism (Gavrilovskaya et al., 2012c, 2013). These findings suggest the presence of a second virulence determinant within pathogenic hantaviruses which, in addition to IFN pathway regulation, targets mTOR signaling pathways (Figure 2C).
Discussion
Our studies indicate that pathogenic hantaviruses contain virulence determinants that alter the normal regulation of endothelial cell signaling pathways to enhance viral replication and spread (IFN regulation) and foster the permeability of endothelial cell adherens junctions (aberrant hypoxia-VEGF-mTOR signaling) (Gavrilovskaya et al., 2012b; Matthys et al., 2013; Matthys and Mackow, 2012). Successful hantavirus replication within human endothelial cells is at least in part due to their ability to regulate the induction of IFNβ (Alff et al., 2006; Geimonen et al., 2002; Spiropoulou et al., 2007). Our findings indicate that GnTs from pathogenic ANDV, NYV, SNV as well as nonpathogenic TULV inhibit TBK1 directed ISRE, NF-κB or IFNβ transcriptional responses (Alff et al., 2006; Alff et al., 2008; Matthys et al., 2013; Matthys et al., 2011). GnTs fail to inhibit constitutively active IRF3-5D and block TBK1 directed IRF3 phosphorylation (Matthys et al., 2013). In contrast, the GnT of nonpathogenic PHV fails to regulate early IFN induction in human ECs and PHV fails to successfully replicate in human ECs or become a human pathogen (Alff et al., 2006; Alff et al., 2008; Matthys et al., 2011; Spiropoulou et al., 2007). Another virulence mechanism is suggested by failure of TULV and PHV to use αvβ3 integrins for entry or later cell association in comparison with pathogenic hantaviruses and the known role of αvβ3 in regulating vascular permeability (Coller and Shattil, 2008; Reynolds et al., 2002; Robinson et al., 2004).
GnT is an IFN Regulating Virulence Determinant
Our studies of GnGc and GnT C42 domains point out the ability of this expressed protein to inhibit the antiviral effects of IFN induction by blocking RIG-I/MDA5 directed TBK1/IKKε signaling responses(Alff et al., 2006; Alff et al., 2008; Matthys et al., 2013; Matthys et al., 2011; Matthys and Mackow, 2012). Findings presented here demonstrate the importance of the C42 GnT domain in regulation and differences between pathogenic and nonpathogenic PHV in GnT functions that foster its role as a determinant that facilitates viral replication in human ECs (Matthys et al., 2013; Matthys et al., 2011; Matthys and Mackow, 2012). Additional studies of the NYV GnT have recently established that only 1 change, Y627 to A, S or F, prevented GnT regulation of TBK1 directed ISRE, κB or IFNβ transcriptional responses (Matthys et al., 2013). Consistent with this, the Y627 residue was required for the NYV GnT to inhibit RIG-I directed IRF3 phosphorylation and cause a reduction in total IRF3 levels. Although these findings define a single tyrosine residue within the NYV GnT (Y627) required for inhibiting antiviral ISRE, κB and IFNβ transcriptional responses, residues within other hantavirus GnTs required for regulation have yet to be defined (Matthys et al., 2013). Nontheless these findings identify residue specific determinants that may be used for viral attenuation and define the GnT as an IFN regulating determinant of viral replication.
Hypoxia/VEGF Enhance ANDV Directed VE-Cadherin Internalization and EC Permeability
HPS is a highly lethal disease resulting in acute rapidly progressive pulmonary edema and shock. Hypoxia, thrombocytopenia and vascular permeability are hallmark findings of hantavirus patients and contribute to acute pulmonary edema in HPS disease (Duchin et al., 1994; Koster and Mackow, 2012; Nolte et al., 1995; Zaki et al., 1995). Pathogenic mechanisms accounting for the rapid rate of pulmonary fluid accumulation have yet to be demonstrated, but appear to be a consequence of the non-cytolytic hantavirus infection of endothelial cells (Gavrilovskaya et al., 2012b; Koster and Mackow, 2012; Taylor et al., 2013; Vaheri et al., 2013). Although, MECs and LECs are not permeabilized by hantavirus infection alone, hantavirus infection of the endothelium provides a means for the virus to alter EC responses that normally regulate capillary leakage and pulmonary fluid clearance (Gavrilovskaya et al., 2012a; Gavrilovskaya et al., 2012b; Gavrilovskaya et al., 2012c; Gavrilovskaya et al., 2008). Our findings indicate that hypoxia or VEGF addition are sufficient to induce hyper-permeability of ANDV, but not nonpathogenic TULV, infected MECs or LECs and that these responses are sensitive to the effects of the mTOR inhibitor rapamycin (Gavrilovskaya et al., 2013; Gavrilovskaya et al., 2008). These findings suggest that ANDV and other pathogenic hantaviruses encode virulence determinants which alter interrelated hypoxia-VEGF-mTOR responses (Figure 2C).
Hypoxia and VEGF Direct Permeability through Increased mTOR Signaling Responses
Constitutive mTOR activation results in the formation of giant cells, and mTOR signaling responses control HIF1α and VEGF directed permeability (Forsythe et al., 1996; Wolff et al., 2011). Genetic mutations in TSC1/TSC2 result in the formation of giant cells through the constitutive activation of mTOR and the downstream phosphorylation of p70-S6K (Laplante and Sabatini, 2012; Ruvinsky and Meyuhas, 2006). ANDV infection reportedly causes the formation of giant LECs in response to VEGF (Gavrilovskaya et al., 2012b). In fact, hypoxic conditions, 1–2% O2, or addition of CoCl2 (Kim et al., 2006), dramatically increased the number of ANDV infected giant MECs or LECs (80% or 70%, respectively) and their permeability (Gavrilovskaya et al., 2012a; Gavrilovskaya et al., 2012c; Gorbunova et al., 2013). Collectively these findings indicate that in the presence of hypoxia, ANDV directs the pathway specific activation of mTOR signaling responses that control lymphatic and vascular endothelial cell permeability and VE-cadherin internalization (Figure 2A–C).
Recent studies suggest that bradykinin may contribute to hantavirus directed vascular permeability (Taylor et al., 2013; Vaheri et al., 2013). Interestingly, hypoxia is linked to ANDV dysregulation of normal endothelial cell functions through effects on bradykinin, VEGF and thrombocytopenia, all of which regulate vascular permeability (Dehler et al., 2006; Gavard and Gutkind, 2006; Hanaoka et al., 2003; Liesmaa et al., 2009). Although not evaluated in their reports (Taylor et al., 2013; Vaheri et al., 2013), bradykinin and VEGF synergistically increase VEGFR2 phosphorylation (Thuringer et al., 2002) and secreted bradykinin induces VEGF (Knox et al., 2001). In fact, hypoxia itself induces bradykinin receptors on endothelial cells (Liesmaa et al., 2009), fostering the potential interrelationship of VEGF, bradykinin and hypoxia induced responses in hantavirus directed permeability (Gavrilovskaya et al., 2012b; Gavrilovskaya et al., 2013; Gorbunova et al., 2013; Liesmaa et al., 2009; Taylor et al., 2013; Thuringer et al., 2002; Vaheri et al., 2013).
Hypoxia induced VEGF causes high altitude-induced pulmonary edema (HAPE) (Berger et al., 2005; Dehler et al., 2006; Hanaoka et al., 2003; Scherrer et al., 2010), and the ability of hypoxia alone to induce edema and thrombocytopenia suggests that hypoxia may play a critical role in the HPS disease process (Berger et al., 2005; Christou et al., 1998; Dehler et al., 2006; Dvorak, 2006; Gavrilovskaya et al., 2010, 2013; Gorbunova et al., 2013; Koster and Mackow, 2012). HPS patients are clearly hypoxic and HPS patient pulmonary edema fluid contains elevated VEGF-A levels (Gavrilovskaya et al., 2012a). However, hypoxia directs a number of additional cellular responses which act on endothelial cell and platelet functions and which may participate in vascular leakage during ANDV infection (Irigoyen et al., 2007; Kulshreshtha et al., 2008; Liesmaa et al., 2009). Hypoxia increases endothelial NO synthase (eNOS) responsible for lymphatic vessel contraction and fluid clearance functions (Hagendoorn et al., 2004; Miao et al., 2008). Hypoxia also causes thrombocytopenia in mice and the production of the platelet inhibitor prostacyclin which renders platelets quiescent (Birks et al., 1975; Farmer et al., 2001).
Hypoxia-VEGF-mTOR Signaling Activation as a Determinant of Hantavirus Virulence
Hantavirus infected lymphatic EC are also hyper-responsive to the permeabilizing effects of VEGF (Gavrilovskaya et al., 2012b; Gavrilovskaya et al., 2012c, 2013; Gavrilovskaya et al., 2008). Although the role of LECs and lymphatic vessels in HPS have not been defined in HPS patients or animal models, there is also a compelling rationale for hantavirus infected LECs to impede fluid clearance functions of pulmonary lymphatic vessels that exacerbate pulmonary fluid accumulation (Alitalo, 2011). Hypoxic conditions and hantavirus infection of MECs or LECs induced permeability and giant cell formation via mTOR signaling responses that result in the phosphorylation of S6K (Laplante and Sabatini, 2012). Interestingly, we found that both hypoxia directed permeability and giant cell responses of ANDV infected MECs and LECs were inhibited by rapamycin, an mTOR inhibitor (Laplante and Sabatini, 2012). In fact, rapamycin is a known negative effector of hypoxia/VEGFA induced permeabilizing responses (Land and Tee, 2007; Wolff et al., 2011). Our findings indicate that hypoxia activates mTOR signaling responses within ANDV infected ECs (Gavrilovskaya et al., 2013; Gorbunova et al., 2013; Gorbunova et al., 2011; Robinson et al., 2004). This suggests that ANDV encodes a virulence determinant which activates mTOR pathways, although it is not clear how ANDV induces mTOR signaling responses.
Conclusions
At present there is little understanding of how hantaviruses alter redundant vascular barrier regulating systems to coordinately dysregulate pulmonary responses and cause acute pulmonary edema. Pathogenic hantaviruses appear to contain virulence determinants that facilitate viral replication and spread as well as alter normal MEC and LEC responses resulting in vascular hyper-permeability. Virulence determinants that selectively alter human endothelial cell functions are likely to be targets for therapeutics that resolve altered viral-cell responses at late stages of infection and for attenuating pathogenic hantaviruses.
Hantaviruses regulate early IFN responses to successfully negotiate human endothelial cells and cause disease. The Andes (ANDV) hantavirus causes hantavirus pulmonary syndrome, a highly lethal disease culminating in hypoxia, acute pulmonary edema and respiratory distress. This paper summarizes data on virulence determinants within hantaviruses that permit their replication in human microvascualr and lymphatic endothelial cells and their ability to alter normal signaling pathways that control vascular permeability. Our findings indicate that elements within the hantavirus GnTs regulate IRF3 phosphorylation by restricting total IRF3 levels. Further our findings indicate that hypoxic responses observed in HPS patients are sufficient to elicit permeabilizing endothelial cell responses that likely contribute to acute pulmonary edema and which are inhibited by rapamycin regulation of mTOR signaling pathways.
Hantaviruses regulate EC signaling pathways to successfully infect human ECs, alter normal EC functions and cause highly lethal diseases.
Virulence determinants permit hantavirus replication in ECs by altering signaling pathways that control vascular permeability.
Virulence elements within GnTs inhibit IRF3 phosphorylation required for the induction of antiviral IFNβ responses.
ANDV virulence determinants enhance mTOR signaling responses to hypoxic conditions that are blocked by rapamycin.
These findings suggest the potential for therapeutically targeting pathways altered by hantavirus infection.
Acknowledgments
We thank Eric Roth and Aleksandr Nasonov for technical support and Ken Marcu and Nancy Reich for insightful discussions. This work was supported by grants AI75022, AI055621, AI1092191 and AI097951 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alff PJ, Gavrilovskaya IN, Gorbunova E, Endriss K, Chong Y, Geimonen E, Sen N, Reich NC, Mackow ER. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. Journal of virology. 2006;80:9676–9686. doi: 10.1128/JVI.00508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alff PJ, Sen N, Gorbunova E, Gavrilovskaya IN, Mackow ER. The NY-1 hantavirus Gn cytoplasmic tail coprecipitates TRAF3 and inhibits cellular interferon responses by disrupting TBK1-TRAF3 complex formation. Journal of virology. 2008;82:9115–9122. doi: 10.1128/JVI.00290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Berger MM, Hesse C, Dehnert C, Siedler H, Kleinbongard P, Bardenheuer HJ, Kelm M, Bartsch P, Haefeli WE. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;172:763–767. doi: 10.1164/rccm.200504-654OC. [DOI] [PubMed] [Google Scholar]
- Birks JW, Klassen LW, Gurney CW. Hypoxia-induced thrombocytopenia in mice. J Lab Clin Med. 1975;86:230–238. [PubMed] [Google Scholar]
- Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. The Journal of biological chemistry. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- Bustamante EA, Levy H, Simpson SQ. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest. 1997;112:1133–1136. doi: 10.1378/chest.112.4.1133. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- Charoenthongtrakul S, Gao L, Parvatiyar K, Lee D, Harhaj EW. RING finger protein 11 targets TBK1/IKKi kinases to inhibit antiviral signaling. PloS one. 2013;8:e53717. doi: 10.1371/journal.pone.0053717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1998;18:768–776. doi: 10.1165/ajrcmb.18.6.2980. [DOI] [PubMed] [Google Scholar]
- Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Zanetta L, Orsenigo F, Breviario F, Lampugnani MG, Bernasconi S, Liao F, Hicklin DJ, Bohlen P, Dejana E. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood. 2002;100:905–911. doi: 10.1182/blood.v100.3.905. [DOI] [PubMed] [Google Scholar]
- Daffis S, Suthar MS, Szretter KJ, Gale M, Jr, Diamond MS. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS pathogens. 2009;5:e1000607. doi: 10.1371/journal.ppat.1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehler M, Zessin E, Bartsch P, Mairbaurl H. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur Respir J. 2006;27:600–606. doi: 10.1183/09031936.06.00061505. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Delhase M, Kim SY, Lee H, Naiki-Ito A, Chen Y, Ahn ER, Murata K, Kim SJ, Lautsch N, Kobayashi KS, Shirai T, Karin M, Nakanishi M. TANK-binding kinase 1 (TBK1) controls cell survival through PAI-2/serpinB2 and transglutaminase 2. Proceedings of the National Academy of Sciences of the United States of America. 2011;109:E177–186. doi: 10.1073/pnas.1119296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET, Moolenaar RL, Reef SE, Nolte KB, Gallaher MM, Butler JC, Breiman RF, Group HS. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group [see comments] The New England journal of medicine. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Discovery of vascular permeability factor (VPF) Exp Cell Res. 2006;312:522–526. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Enria D, Padula P, Segura EL, Pini N, Edelstein A, Posse CR, Weissenbacher MC. Hantavirus pulmonary syndrome in Argentina. Possibility of person to person transmission. Medicina. 1996;56:709–711. [PubMed] [Google Scholar]
- Farmer PJ, Bernier SG, Lepage A, Guillemette G, Regoli D, Sirois P. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. American journal of physiology Lung cellular and molecular physiology. 2001;280:L732–738. doi: 10.1152/ajplung.2001.280.4.L732. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J. Breaking the VE-cadherin bonds. FEBS Lett. 2009;583:1–6. doi: 10.1016/j.febslet.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Gavrilovskaya I, Gorbunova E, Koster F, Mackow E. Elevated VEGF Levels in Pulmonary Edema Fluid and PBMCs from Patients with Acute Hantavirus Pulmonary Syndrome. Adv Virol. 2012a;2012:674360. doi: 10.1155/2012/674360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya I, Gorbunova E, Matthys V, Dalrymple N, Mackow E. The Role of the Endothelium in HPS Pathogenesis and Potential Therapeutic Approaches. Adv Virol. 2012b;2012:467059. doi: 10.1155/2012/467059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Brown EJ, Ginsberg MH, Mackow ER. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. Journal of virology. 1999;73:3951–3959. doi: 10.1128/jvi.73.5.3951-3959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Pathogenic Hantaviruses Direct the Adherence of Quiescent Platelets to Infected Endothelial Cells. Journal of virology. 2010;84:4832–4839. doi: 10.1128/JVI.02405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Andes virus infection of lymphatic endothelial cells causes giant cell and enhanced permeability responses that are rapamycin and vascular endothelial growth factor C sensitive. Journal of virology. 2012c;86:8765–8772. doi: 10.1128/JVI.00817-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Hypoxia Induces Permeability and Giant Cell Responses of Andes Virus-Infected Pulmonary Endothelial Cells by Activating the mTOR-S6K Signaling Pathway. Journal of virology. 2013;87:12999–13008. doi: 10.1128/JVI.02103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. Journal of virology. 2008;82:5797–5806. doi: 10.1128/JVI.02397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geimonen E, Neff S, Raymond T, Kocer SS, Gavrilovskaya IN, Mackow ER. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13837–13842. doi: 10.1073/pnas.192298899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. Journal of virology. 2010;84:7405–7411. doi: 10.1128/JVI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova EE, Gavrilovskaya IN, Mackow ER. Slit2-Robo4 receptor responses inhibit ANDV directed permeability of human lung microvascular endothelial cells. Antiviral research. 2013;99:108–112. doi: 10.1016/j.antiviral.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. VEGFR2 and Src Kinase Inhibitors Suppress ANDV Induced Endothelial Cell Permeability. Journal of virology. 2011;85:2296–2303. doi: 10.1128/JVI.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nature reviews Immunology. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, Huang PL, Sessa WC, Fukumura D, Jain RK. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ Res. 2004;95:204–209. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- Hammerbeck CD, Hooper JW. T cells are not required for pathogenesis in the Syrian hamster model of hantavirus pulmonary syndrome. Journal of virology. 2011;85:9929–9944. doi: 10.1128/JVI.05356-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka M, Droma Y, Naramoto A, Honda T, Kobayashi T, Kubo K. Vascular endothelial growth factor in patients with high-altitude pulmonary edema. Journal of applied physiology. 2003;94:1836–1840. doi: 10.1152/japplphysiol.00575.2002. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. The Journal of biological chemistry. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;171:83–87. doi: 10.1164/rccm.200406-707OC. [DOI] [PubMed] [Google Scholar]
- Irigoyen M, Anso E, Martinez E, Garayoa M, Martinez-Irujo JJ, Rouzaut A. Hypoxia alters the adhesive properties of lymphatic endothelial cells. A transcriptional and functional study. Biochimica et biophysica acta. 2007;1773:880–890. doi: 10.1016/j.bbamcr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ED, Terajima M, Koster FT, Catalina MD, Cruz J, Ennis FA. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. Journal of immunology. 2004;172:3297–3304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- Kim DD, Kleinman DM, Kanetaka T, Gerritsen ME, Nivaggioli T, Weber D, Duran WN. Rapamycin inhibits VEGF-induced microvascular hyperpermeability in vivo. Microcirculation. 2009;17:128–136. doi: 10.1111/j.1549-8719.2009.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. Journal of immunology. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- Knox AJ, Corbett L, Stocks J, Holland E, Zhu YM, Pang L. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:2480–2488. doi: 10.1096/fj.01-0256com. [DOI] [PubMed] [Google Scholar]
- Koster F, Mackow E. Pathogenesis of the Hantavirus Pulmonary Syndrome. Future Virology. 2012;7:41–51. [Google Scholar]
- Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- Lahdevirta J, Enger E, Hunderi OH, Traavik T, Lee HW. Hantaan virus is related to hemorrhagic fever with renal syndrome in Norway. Lancet. 1982;2:606. doi: 10.1016/s0140-6736(82)90678-x. [DOI] [PubMed] [Google Scholar]
- Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. The Journal of biological chemistry. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, Thackray L, Brassil MM, Virgin HW, Nikolich-Zugich J, Moses AV, Gale M, Jr, Fruh K, Diamond MS. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS pathogens. 2013;9:e1003118. doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW. Hemorrhagic fever with renal syndrome (HFRS) Scand J Infect Dis Suppl. 1982;36:82–85. [PubMed] [Google Scholar]
- Lee HW, Baek LJ, Johnson KM. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever, from wild urban rats. J Infect Dis. 1982;146:638–644. doi: 10.1093/infdis/146.5.638. [DOI] [PubMed] [Google Scholar]
- Liesmaa I, Leskinen HK, Kokkonen JO, Ruskoaho H, Kovanen PT, Lindstedt KA. Hypoxia-induced expression of bradykinin type-2 receptors in endothelial cells triggers NO production, cell migration, and angiogenesis. Journal of cellular physiology. 2009;221:359–366. doi: 10.1002/jcp.21861. [DOI] [PubMed] [Google Scholar]
- Lopez N, Padula P, Rossi C, Lazaro ME, Franze-Fernandez MT. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology. 1996;220:223–226. doi: 10.1006/viro.1996.0305. [DOI] [PubMed] [Google Scholar]
- Matthys V, Cimica V, Dalrymple N, Glennon NB, Bianco C, Mackow ER. Hantavirus GnT elements mediate TRAF3 binding and inhibit RIG-I/TBK1 directed IFNb transcription by blocking IRF3 Phosphorylation. Journal of virology. 2013 doi: 10.1128/JVI.02647-13. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys V, Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. The C-Terminal 42 Residues of the TULV Gn Protein Regulate Interferon Induction. Journal of virology. 2011;85:4752–4760. doi: 10.1128/JVI.01945-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys V, Mackow ER. Hantavirus regulation of type I interferon responses. Adv Virol. 2012;2012:524024. doi: 10.1155/2012/524024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao RQ, Fontana J, Fulton D, Lin MI, Harrison KD, Sessa WC. Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:105–111. doi: 10.1161/ATVBAHA.107.155499. [DOI] [PubMed] [Google Scholar]
- Mori M, Rothman AL, Kurane I, Montoya JM, Nolte KB, Norman JE, Waite DC, Koster FT, Ennis FA. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J Infect Dis. 1999;179:295–302. doi: 10.1086/314597. [DOI] [PubMed] [Google Scholar]
- Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. Embo J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness [see comments] Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Nolte KB, Feddersen RM, Foucar K, Zaki SR, Koster FT, Madar D, Merlin TL, McFeeley PJ, Umland ET, Zumwalt RE. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Human Pathology. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Plyusnin A, Vapalahti O, Lankinen H, Lehvaslaiho H, Apekina N, Myasnikov Y, Kallio-Kokko H, Henttonen H, Lundkvist A, Brummer-Korvenkontio M, et al. Tula virus: a newly detected hantavirus carried by European common voles. Journal of virology. 1994;68:7833–7839. doi: 10.1128/jvi.68.12.7833-7839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond T, Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1163–1168. doi: 10.1073/pnas.0406743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24:2108–2114. doi: 10.1161/01.ATV.0000143857.27408.de. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends in biochemical sciences. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. New insights in the pathogenesis of high-altitude pulmonary edema. Progress in cardiovascular diseases. 2010;52:485–492. doi: 10.1016/j.pcad.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. Bunyaviridae and their Replication. In: Fields, editor. Virology. Lipppincott-Raven; Philadelphia: 2001. pp. 1581–1602. [Google Scholar]
- Schraufnagel DE, Agaram NP, Faruqui A, Jain S, Jain L, Ridge KM, Sznajder JI. Pulmonary lymphatics and edema accumulation after brief lung injury. American journal of physiology Lung cellular and molecular physiology. 2003;284:L891–897. doi: 10.1152/ajplung.00333.2002. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. Journal of virology. 2010;84:11227–11234. doi: 10.1128/JVI.01405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulou CF, Albarino CG, Ksiazek TG, Rollin PE. Andes and Prospect Hill hantaviruses differ in early induction of interferon although both can downregulate interferon signaling. Journal of virology. 2007;81:2769–2776. doi: 10.1128/JVI.02402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SL, Wahl-Jensen V, Copeland AM, Jahrling PB, Schmaljohn CS. Endothelial Cell Permeability during Hantavirus Infection Involves Factor XII-Dependent Increased Activation of the Kallikrein-Kinin System. PLoS pathogens. 2013;9:e1003470. doi: 10.1371/journal.ppat.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima M, Hendershot JD, 3rd, Kariwa H, Koster FT, Hjelle B, Goade D, DeFronzo MC, Ennis FA. High levels of viremia in patients with the Hantavirus pulmonary syndrome. J Infect Dis. 1999;180:2030–2034. doi: 10.1086/315153. [DOI] [PubMed] [Google Scholar]
- Thuringer D, Maulon L, Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. The Journal of biological chemistry. 2002;277:2028–2032. doi: 10.1074/jbc.M109493200. [DOI] [PubMed] [Google Scholar]
- Tu D, Zhu Z, Zhou AY, Yun CH, Lee KE, Toms AV, Li Y, Dunn GP, Chan E, Thai T, Yang S, Ficarro SB, Marto JA, Jeon H, Hahn WC, Barbie DA, Eck MJ. Structure and ubiquitination-dependent activation of TANK-binding kinase 1. Cell Rep. 2013;3:747–758. doi: 10.1016/j.celrep.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S, Mustonen J. Uncovering the mysteries of hantavirus infections. Nature reviews Microbiology. 2013;11:539–550. doi: 10.1038/nrmicro3066. [DOI] [PubMed] [Google Scholar]
- Voelkel NF. High-altitude pulmonary edema. The New England journal of medicine. 2002;346:1606–1607. doi: 10.1056/NEJM200205233462102. [DOI] [PubMed] [Google Scholar]
- Wallez Y, Vilgrain I, Huber P. Angiogenesis: the VE-cadherin switch. Trends Cardiovasc Med. 2006;16:55–59. doi: 10.1016/j.tcm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Wolff NC, Vega-Rubin-de-Celis S, Xie XJ, Castrillon DH, Kabbani W, Brugarolas J. Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Molecular and cellular biology. 2011;31:1870–1884. doi: 10.1128/MCB.01393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol. 2009;29:1172–1178. doi: 10.1161/ATVBAHA.109.185918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R, Daum CA, Lee PW, Baek LJ, Amyx HL, Gajdusek DC, Gibbs CJ. Serological survey of Prospect Hill virus infection in indigenous wild rodents in the USA. Trans R Soc Trop Med Hyg. 1987;81:42–45. doi: 10.1016/0035-9203(87)90275-6. [DOI] [PubMed] [Google Scholar]
- Yanagihara R, Silverman DJ. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch Virol. 1990;111:281–286. doi: 10.1007/BF01311063. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. RIG-I family RNA helicases: Cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007 doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zaki S, Greer P, Coffield L, Goldsmith C, Nolte K, Foucar K, Feddersen R, Zumwalt R, Miller G, Rollin P, Ksiazek T, Nichol S, Peters C. Hantavirus Pulmonary Syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- Zanetti A, Lampugnani MG, Balconi G, Breviario F, Corada M, Lanfrancone L, Dejana E. Vascular endothelial growth factor induces SHC association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling. Arterioscler Thromb Vasc Biol. 2002;22:617–622. doi: 10.1161/01.atv.0000012268.84961.ad. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis. 2007;28:28–37. doi: 10.1093/carcin/bgl085. [DOI] [PubMed] [Google Scholar]