Abstract
Rationale
The noncompetitive NMDA antagonist ketamine produces rapid antidepressant effects in treatment-resistant patients suffering from major depressive and bipolar disorders. However, abuse liability is a concern.
Objectives
This study examined abuse-related effects of keta-mine using intracranial self-stimulation (ICSS) in rats. The higher-affinity NMDA antagonist MK-801 and the monoamine reuptake inhibitor cocaine were examined for comparison.
Methods
Male Sprague Dawley rats were implanted with electrodes targeting the medial forebrain bundle and trained to respond to brain stimulation under a frequency–rate ICSS procedure. The first experiment compared the potency and time course of ketamine (3.2–10.0 mg/kg) and MK-801 (0.032–0.32 mg/kg). The second experiment examined effects of repeated dosing with ketamine (3.2–20.0 mg/kg/day) and acute cocaine (10.0 mg/kg).
Results
Following acute administration, ketamine (3.2–10 mg/kg) produced only dose- and time-dependent depressions of ICSS and failed to produce an abuse-related facilitation of ICSS at any dose or pretreatment time. In contrast, MK-801 (0.032–0.32 mg/kg) produced a mixed profile of rate-increasing and rate-decreasing effects; ICSS facilitation was especially prominent at an intermediate dose of 0.18 mg/kg. Repeated dosing with ketamine produced dose-dependent tolerance to the rate-decreasing effects of ketamine (10.0 and 18.0 mg/kg) but failed to unmask expression of ICSS facilitation. Termination of ketamine treatment failed to produce withdrawal-associated decreases in ICSS. As reported previously, 10.0 mg/kg cocaine facilitated ICSS.
Conclusions
The dissociable effects of ketamine and MK-801 suggest differences in the pharmacology of these nominally similar NMDA antagonists. Failure of ketamine to facilitate ICSS contrasts with other evidence for the abuse liability of ketamine.
Keywords: Ketamine, MK-801, Intracranial self-stimulation, Depression, Bipolar, Cocaine, Rats
Introduction
The noncompetitive N-methyl-d-aspartate (NMDA) antagonist ketamine produces rapid and robust antidepressant effects in treatment-resistant patients suffering from major depressive disorder (Berman et al. 2000; Murrough et al. 2012; Zarate et al. 2006) and bipolar disorder (Diazgranados et al. 2010; Zarate et al. 2012). Preclinical research is currently focused on elucidating mechanisms responsible for these rapid antidepressant effects. However, use of ketamine to treat depression is likely to be constrained by concerns over abuse liability. For example, ketamine is second only to heroin as the most abused illicit substance in Hong Kong (Shek 2007). In the USA, ketamine is classified as a Schedule III drug by the Drug Enforcement Agency. Illicit use is rare compared to other abused drugs (Substance Abuse and Mental Health Services Administration 2013) and appears to occur most commonly among young adults in social contexts that also involve music and dancing (McCambridge et al. 2007; Winstock et al. 2012).
Intracranial self-stimulation (ICSS) is one approach used to assess and compare abuse liability of drugs (Carlezon and Chartoff 2007; Kornetsky et al. 1979; Wise et al. 1992). For ICSS in general, animals are trained to lever press for brain stimulation delivered via electrodes implanted in brain regions such as the medial forebrain bundle. In “frequency–rate” ICSS procedures, different frequencies of brain stimulation are used to engender frequency-dependent increases in ICSS rates and thereby establish a wide range of baseline ICSS rates. This type of procedure is useful in part because of its sensitivity to detection of drug effects on both low and high rates of operant responding. Many drugs of abuse, and especially stimulants like cocaine and amphetamine, increase low ICSS rates maintained by low brain-stimulation frequencies, and this “facilitation” of ICSS is often interpreted as an abuse-related effect (Vlachou and Markou 2011; Bauer et al. 2013a, 2013b). The noncompetitive NMDA receptor antagonists phencyclidine and MK-801 have been evaluated under a number of ICSS parameters, and both compounds facilitated ICSS in frequency–rate procedures (Carlezon and Wise 1993; Corbett 1989; Sundstrom et al. 2002; Wise et al. 1992). However, ketamine effects have not been reported in subjects responding under a frequency–rate ICSS procedure. To address this issue, the present study compared effects of ketamine and MK-801 on ICSS using a frequency-rate procedure that has been used previously to evaluate effects of opioids (Altarifi and Negus 2011; Altarifi et al. 2013; Negus et al. 2010, 2012b), monoamine releasers and uptake inhibitors (Bauer et al. 2013a, 2013b; Bonano et al. 2013; Negus et al. 2012a; Rosenberg et al. 2013), and cannabinoids (Kwilasz and Negus 2012).
Materials and methods
Subjects
Experiments were conducted in 14 adult male Sprague Dawley rats (Harlan Laboratories Inc, Frederick, MD) weighing between 300 and 350 g at the time of surgery. All rats were housed individually in plastic cages in the vivarium with a 12-h/12-h light/dark cycle (lights on 0600–1800 hours) and had free access to food and water except during experimental sessions. Procedures were approved by the Institutional Animal Care and Use Committee and complied with federal guidelines (Institute of Laboratory Animal Resources 2011).
Surgery
Rats were anesthetized with isoflurane gas (2.5–3 % in oxygen; Webster Veterinary, Phoenix, Arizona, USA) for implantation of stainless steel electrodes. The cathode of each electrode (Plastics One, Roanoke, Virginia, USA) was 0.25 mm in diameter and covered with polyamide insulation, except at the flattened tip. The anode was 0.124 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior and 1.7 mm lateral from the bregma, and 8.8 mm below the skull) (Pereira Do Carmo et al. 2009). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured with orthodontic resin. Rats received ketoprofen (5 mg/kg IP for 2 days) as a postoperative analgesic and were allowed to recover for at least 7 days before commencing ICSS training. Behavioral criteria were used to confirm efficacy of probe placement for maintaining ICSS. Rats were used only if they responded at criterion levels for brain stimulation (see training).
Apparatus
ICSS experiments were conducted in 12 sound-attenuating chambers that contained operant conditioning chambers (29.2×30.5×24.1 cm3) equipped with a single response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow, and green positioned 7.6 cm directly above the lever), a house light, and an ICSS stimulator (Med Associates, St Albans, Vermont, USA). Electrodes were connected to the stimulator through bipolar cables and a commutator (Model SL2C, Plastics One). Programming of behavioral sessions and data collection were computer controlled by Med-State software (Med PC, Version 4.1, Med Associates).
Training
Rats were trained under a fixed-ratio (FR) 1 schedule of brain stimulation using procedures similar to those described previously to study effects of mu/kappa/delta opioid receptor agonists (Altarifi and Negus 2011; Altarifi et al. 2013; Negus et al. 2010), dopamine/norepinephrine/serotonin releasers and uptake inhibitors (Bauer et al. 2013a, 2013b; Bonano et al. 2013; Rosenberg et al. 2013), and cannabinoids (Kwilasz and Negus 2012). The terminal schedule consisted of sequential 10-min components. During each component, a descending series of 10 brain-stimulation frequencies was presented, with a 60-s trial at each frequency. The frequency range extended from 158 to 56 Hz in 0.05-log increments, and stimulation intensity was individually determined during training and remained constant for each rat (range 120–260 μA). Each frequency trial began with a 10-s timeout, during which the house light was off and responding had no scheduled consequences. During the last 5 s of this timeout, five noncontingent stimulations were delivered once per second at the frequency available during that trial, and the lever lights were illuminated during each stimulation. This noncontingent stimulation was then followed by a 50-s “response” period, during which the house light was illuminated, and each lever press produced electrical stimulation (0.5-s train of 0.1 ms square-wave cathodal pulses) and illumination for 0.5 s of the colored stimulus lights over the lever. Training continued until rats reliably responded at rates ≥50 % maximum control rates (see “Data analysis” in the “Materials and methods”) for at least three and no more than six trials of all components for at least three consecutive days. Additionally, rats were habituated to saline injections until these injections had significant no effect on ICSS frequency–rate curves as determined by two-way analysis of variance (see “Data analysis” in the “Materials and methods”).
Dose effect and time course experiments
A group of eight drug-naïve rats at the start of the experiment were used for dose effect and time course studies of ketamine and MK-801. Surgery was performed on 12 rats. Eight of the 12 rats successfully met ICSS training criteria and were included in the ICSS dose effect and time course experiments. ICSS test sessions for dose effect testing consisted of five sequential components. The first component of each test session was considered an acclimation component, and data from this component were discarded. Data from the second and third “baseline” components were used to calculate control parameters of frequency-rate curves for that test session in that rat (see “Data analysis” in the “Materials and methods”). Immediately after completion of the baseline components, a dose of test drug was administered intraperitoneally (IP), and after the designated pretreatment time, ICSS was evaluated during two test components (10 min each, 20 min total). Testing was conducted twice per week (typically Tuesday and Friday). Drugs, doses, and pretreatment times were as follows: keta-mine (3.2–10.0 mg/kg; 10 min) and MK-801 (0.032–0.32 mg/kg; 15 min). Dose order across rats was counterbalanced using a Latin-square design. Additional studies were also conducted to investigate the time course of effects produced by 5.6 and 10 mg/kg ketamine and 0.18 and 0.32 mg/kg MK-801. For these studies, baseline components were conducted as above. Rats were then immediately injected with a dose of test drug, and pairs of ICSS test components was initiated 10, 30, 100, and 300 min after the injection. Testing was initiated with different drugs in different rats. Saline has been tested previously using this time course procedure (e.g. Altarifi et al. 2012) and was not tested in the present study. If ICSS performance remained stable in a given rat after completion of testing with the initial drug, then the rat was advanced to testing with the other drug. Testing continued until each drug had been tested in groups of five to seven rats. Six rats received both ketamine and MK-801 treatment and two rats received only ketamine treatment.
Repeated ketamine experiment
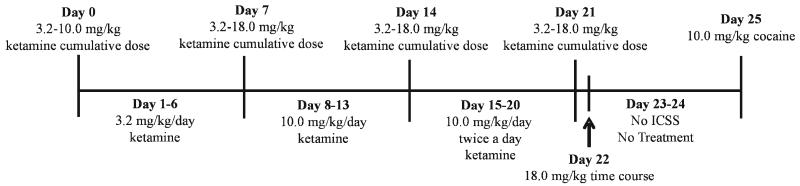
Initial dose effect and time course studies with acute ketamine failed to reveal ketamine-induced increases in rates of ICSS maintained by any brain stimulation frequency at any time. Consequently, follow-up studies were conducted in a drug-naïve group of six rats to examine effects of repeated dosing using a testing strategy that produced tolerance to ICSS rate-decreasing effects and enhanced expression of ICSS rate-increasing effects with mu opioid receptor agonists (Altarifi and Negus 2011; Altarifi et al. 2013). The principle goal of this experiment was to evaluate the degree to which ketamine might produce ICSS facilitation under conditions that produced tolerance to rate-decreasing effects of ketamine. Surgery was performed on eight rats. Six of the eight rats successfully met ICSS training criteria and were included in the repeated ketamine experiments. Training was conducted as described above, and once training and habituation to saline injections were completed, “predrug baseline” sessions were conducted over a period of three consecutive days to establish baseline ICSS performance before administration of ketamine. Each predrug baseline session consisted of three components as described above. Testing proceeded over a period of 26 days, with ICSS assessment beginning at 1600 hours each day (Fig. 4). On days 0, 7, 14 and 21, ketamine was administered using a cumulative dosing regimen. Specifically, test sessions consisted of three “baseline” ICSS components as described above, followed by cumulative administration of ketamine at 30 min intervals, such that each sequential ketamine dose increased the total cumulative dose by 0.25 log units. A pair of ICSS test components was initiated 10 min after each sequential ketamine dose. The ketamine dose range was 3.2–10 mg/kg on day 0 and 3.2–18 mg/kg on days 7, 14, and 21. Ketamine was also administered on intervening days as follows: days 1–6, 3.2 mg/kg/day; days 8–13, 10 mg/kg/day; days 15–20, 10 mg/kg twice per day (0900 and 1600 hours). On these intervening days, three baseline ICSS components were conducted, the daily ketamine dose was administered, and two test ICSS components were conducted beginning 10 min after ketamine. On days 15–20, when 10 mg/kg ketamine was administered twice per day, one ketamine dose was administered at 0900 hours without ICSS, and the second ketamine dose was administered at 1600 hours in the context of ICSS. On day 22, ketamine was not administered in the morning, and the time course of effects produced by 18 mg/kg/day ketamine was determined at 0900 hours using the time course testing procedure described above. Ketamine treatment and ICSS testing were omitted on days 23 and 24. On day 25, effects of 10 mg/kg cocaine were tested as a positive control for drug-induced increases in ICSS. Specifically, three baseline components were conducted as described above, followed first by IP administration of 10 mg/kg cocaine and then 10 min later by two ICSS test components.
Fig. 4.
Shows the timeline of events for the repeated ketamine experiment. On days 0, 7, 14, and 21, ketamine was administered using a cumulative dosing regimen. For cumulative dosing, three baseline ICSS components were conducted, followed by cumulative administration of ketamine (0.25 log unit increases), and a pair of ICSS test components were conducted 10 min after each sequential ketamine dose. Ketamine was also administered on intervening days in the same manner as dose effect test days. On day 22, the time course of effects for ketamine 18.0 mg/kg was determined. On day 23 and 24, ketamine treatment and ICSS testing were omitted. On day 25, the effects of 10.0 mg/kg cocaine was determined as a positive control
Data analysis
The primary dependent variable was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these raw data, reinforcement rates (i.e. stimulations) from each trial were converted into the percent maximum control rate (% MCR). For dose effect and time course testing, the MCR was determined during the baseline components of each daily test session and was defined as the mean of the maximal stimulations observed in any frequency trial during the second and third baseline components. Thus, % MCR for each trial was calculated as (stimulations during a frequency trial/MCR)×100. Normalized data from the frequency trials of consecutive test components were then averaged across rats for display and for statistical analysis using two-way repeated measures analysis of variance (ANOVA), with drug dose or time as one factor and ICSS frequency as the other factor. A significant ANOVA was followed by a Holm–Sidak post hoc test, and the criterion for significance was set at P<0.05. To provide an additional summary of ICSS performance, the total number of stimulations delivered across all 10 frequency trials was determined for each component. The average number of total stimulations per test component was expressed as a percentage of the average number of total stimulations per component during the second and third baseline components (% baseline). Data from the study of repeated ketamine experiment were analyzed using a similar approach, with the exception that baseline MCR and total stimulations were calculated from the three-day predrug baseline components conducted before any ketamine administration (six total predrug baseline components). Frequency–rate curves for each cumulative dose effect were examined by two-way repeated measures ANOVA with dose and frequency as the two factors. In addition, baseline frequency–rate curves collected on Day 0 before initiation of ketamine dosing were compared to baseline frequency-rate curves from days 7, 14, and 21 to assess effects of repeated ketamine exposure and ketamine withdrawal. Specifically, baseline ICSS determinations on days 7 and 14 represented 24 h withdrawal periods from the most recent dose of 6-day treatment with 3.2, and 10 mg/kg/day ketamine, respectively; baseline ICSS determination on day 21 represented a 7-h withdrawal period from the most recent dose of 6-day treatment with 20 mg/kg/day.
Drugs
(±) Ketamine HCl (Sigma-Aldrich, St. Louis, MO), (+) MK-801 hydrogen maleate (dizocilpine) (Sigma-Aldrich), and cocaine HCl (provided by National Institute on Drug Abuse, National Institute of Health, Bethesda, MD) were dissolved in 0.9 % physiological saline. All drugs were administered intraperitoneally at a volume of 1.0 ml/kg. Doses and pretreatment times were based on preliminary studies and previous studies in the literature (Carlezon and Wise 1993; Corbett 1989; Herberg and Rose 1989; Páleníček et al. 2011a, b).
Results
Effects of ketamine and MK-801 on ICSS
For the eight rats used in dose effect and time course studies, the mean±SEM maximum control rate (MCR) was 55.88±2.01 stimulations per trial, and the mean total stimulations per component delivered across all frequencies was 253.07±10.79. Figure 1 shows the effects of ketamine and MK-801 on ICSS. After saline treatment, increasing frequencies of brain stimulation maintained increasing rates of ICSS. Ketamine dose dependently decreased ICSS. Ketamine produced a significant main effect of frequency (F9,54=75.06, P<0.001), dose (F3,18= 15.40, P<0.001), and significant interaction (F27,162=3.88, P<0.001). The 1.0 and 3.2 mg/kg doses of ketamine exerted no effect on ICSS, but 5.6 mg/kg ketamine decreased ICSS at three intermediate frequencies (112-141 Hz), and 10.0 mg/kg ketamine significantly decreased ICSS at the six highest frequencies (79-158 Hz) (Fig. 1a, b). Conversely, MK-801 produced mixed rate-increasing and rate-decreasing effects that depended on dose and brain stimulation frequency. MK-801 produced a significant main effect of frequency (F9,45=39.45, P<0.001), dose (F4,20=19.78, P<0.001), and significant interaction (F36,180=7.58, P<0.001). Thus, 0.032 mg/kg MK-801 did not alter ICSS, 0.1 mg/kg MK-801 increased ICSS at one intermediate frequency (89 Hz), and 0.18 mg/kg MK-801 produced a biphasic effect, increasing ICSS at low and intermediate frequencies (63-100 Hz) but decreasing ICSS at the highest frequencies (141-158 Hz). The high dose of MK-801, 0.32 mg/kg, significantly depressed ICSS at the five highest frequencies (100–158 Hz) (Fig. 1c, d).
Fig. 1.
Dose-dependent effect of ketamine and MK-801 on ICSS. Left panels a, c show drug effects on full ICSS frequency-rate curves. Abscissae indicate frequency of electrical brain stimulation (Hz) (log scale). Ordinates indicate percent maximum control reinforcement rate (%MCR). Drug name and doses are indicated in legends. Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from vehicle rates as determined by a two-way ANOVA followed by a Holm-Sidak post hoc test, P<0.05. Right panels b, d show summary ICSS data expressed as percent baseline total stimulations delivered across all frequencies of brain stimulation per test component. Abscissae indicate drug dose (mg/kg). Ordinates indicate percent baseline stimulations per test component. Upward/downward arrows indicate significant drug-induced increase/decrease in ICSS relative to vehicle for at least one brain stimulation frequency as determined by analysis of full frequency-rate curves in the left panels. All data show mean±SEM for six to seven rats (ketamine, n=7; MK-801, n=6), except for 1.0 mg/kg ketamine data shown for five rats
Figure 2 shows the time course of effects produced by 5.6 and 10 mg/kg ketamine. Ketamine (5.6 mg/kg) produced a significant main effect of frequency (F9,45=42.43, P<0.001), time (F4,20=5.15, P<0.01),and no significant interaction (F36,180= 1.28, NS). Ketamine (10.0 mg/kg) produced a significant main effect of frequency (F9,54=97.14, P<0.001), time (F4,24=5.15, P<0.01), and significant interaction (F36,216= 3.27, P<0.001). Treatment with 5.6 mg/kg ketamine had modest effects manifested as small but significant decreases in ICSS after 10 min at 71 Hz, 30 min at 71 and 126 Hz, and 100 min at 71–79 Hz (Fig. 2a, b). Treatment with 10.0 mg/kg ketamine produced greater decreases in ICSS across a broader range of frequencies at 10, 30, and 100 min (Fig. 2c, d). Neither dose produced significant effects on ICSS after 300 min. ICSS was not increased at any frequency or any time for either ketamine dose. Figure 3 shows the time course of effects produced by 0.18 and 0.32 mg/kg MK-801. MK-801 (0.18 mg/kg) produced a significant main effect of frequency (F9,36 = 38.33, P <0.001), time (F4,16=11.50, P<0.001), and no significant interaction (F36,144=1.64, P<0.05). MK-801 (0.32 mg/kg) produced a significant main effect of frequency (F9,45=17.47, P<0.001), time (F4,20=3.90, P<0.05), and significant interaction (F36,180 =7.20, P<0.001). Treatment with 0.18 mg/kg MK-801 significantly increased reinforcement rates at 10, 30, and 100 min across a broad range of brain stimulation frequencies (63–126 Hz) (Fig. 3a, b); this MK-801 dose did not produce rate-decreasing effects at any time in the time course study. Conversely, a higher dose of 0.32 mg/kg MK-801 depressed ICSS after 10 and 30 min at high frequencies (100–156 Hz). At 100 min, 0.32 mg/kg MK-801 produced a biphasic effect,increasing reinforcement rats at 63 Hz, but decreasing reinforcement rate at the highest frequencies (126–158). At 300 min, ICSS was still significantly depressed at 124–141 Hz (Fig. 3c, d).
Fig. 2.
Time course of effects produced by 5.6 and 10.0 mg/kg ketamine. Left panels a, c show drug effects on full ICSS frequency-rate curves. Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from baseline rates as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, P<0.05. Right panels b, d show summary ICSS data expressed as percent baseline total stimulations delivered across all frequencies of brain stimulation per test component. Other details as in Fig. 1. All data show mean±SEM for six to seven rats (5.6 mg/kg, n=6; 10.0 mg/kg, n=7)
Fig. 3.
Time course of effects produced by 0.18 and 0.32 mg/kg MK-801. Left panels a, c show drug effects on full ICSS frequency-rate curves. Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from baseline rates as determined by a two-way ANOVA followed by a Holm-Sidak post hoc test, P<0.05. Right panels b, d show summary ICSS data expressed as percent baseline total stimulations delivered across all frequencies of brain stimulation per test component. Other details as in Figs. 1 and 2. All data show mean±SEM for five to six rats (0.18 mg/kg, n=5; 0.32 mg/kg, n=6)
Effects of repeated ketamine on cumulative dose effect curves
For the six rats used in the repeated dosing ketamine experiment (Fig. 4), the mean±SEM maximum control rate (MCR) during predrug baseline sessions was 57.00±3.33 stimulations per trial, and the mean total stimulations per component delivered across all frequencies was 276.75± 14.59. Under predrug baseline conditions (i.e., before any ketamine administration), brain stimulation maintained a frequency-dependent increase in ICSS rates. Figure 5 shows mean frequency-rate ICSS curves for the predrug baseline determination before initiation of repeated ketamine treatment and before ketamine testing on days 0, 7, 14, and 21 of repeated ketamine treatment. Daily baseline ICSS frequency-rate curves were not significantly affected by exposure to and withdrawal from repeated ketamine: significant main effect of frequency (F9,45=75.73, P<0.001), no significant effect of day (F4,20=1.76, NS), and no significant interaction (F36,180=1.29, NS).
Fig. 5.
Baseline ICSS performance before initiation of repeated ketamine (predrug baseline) and on days 0, 7, 14, and 21 of repeated ketamine treatment. Data for days 7 and 14 were determined 23 h after the most recent ketamine dose. Data for day 21 were collected 7 h after the most recent ketamine dose. Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from baseline rates as determined by a two-way ANOVA followed by a Holm-Sidak post hoc test, P<0.05. Other details as in Fig. 1. All data show mean±SEM for six rats
Figure 6 shows the effects of cumulative ketamine on ICSS on days 0, 7, 14, and 21 of repeated daily ketamine treatment. On day 0, before initiation of repeated ketamine, cumulative ketamine (3.2-10.0 mg/kg) produced a dose-dependent decrease in ICSS (Fig. 6a, b). Specifically, 3.2 mg/kg ketamine did not alter ICSS, 5.6 mg/kg ketamine decreased ICSS at frequencies of 89, 100, and 126 Hz, and 10.0 mg/kg ketamine decreased ICSS at 89–141 Hz. Following repeated 3.2 mg/kg/day ketamine, cumulative doses of 5.6 and 10 mg/kg ketamine decreased ICSS at fewer frequencies than initially, and a higher dose of 18 mg/kg ketamine was introduced, which decreased ICSS at 100-158 Hz (Fig. 6c, d). Following repeated 10 mg/kg/day ketamine (Fig. 6e, f) and 20 mg/kg/day ketamine (Fig. 6g, h), ICSS was not altered by any cumulative dose of ketamine (3.2-18 mg/kg). Thus, during repeated ketamine treatment, tolerance developed to the rate-decreasing effects of ketamine, but ICSS was not increased by any ketamine dose at any frequency at any time. Statistical results for repeated ketamine are as follows: drug naïve (day 0): significant main effect of frequency (F9,45=70.40, P<0.001), dose (F3,15=3.41, P<0.05), and no significant interaction (F27,135=1.36, NS). Repeated 3.2 mg/kg/day (day 7): significant main effect of frequency (F9,45=52.31, P<0.001), dose (F4,20=12.51, P<0.001), and significant interaction (F36,180=2.38, P<0.001). Repeated 10.0 mg/kg/day (day 14): significant main effect of frequency (F9,45=72.65, P<0.001), no significant effect of dose (F4,20=2.31, NS), and no significant interaction (F36,180 =1.25, NS). Repeated 10.0 mg/kg/day×2 (day 21): significant main effect of frequency (F9,45=106.30, P<0.001), no significant effect of dose (F4,20=0.79, NS), and no significant interaction (F36,180= 0.85, NS).
Fig. 6.
Effects of repeated ketamine on cumulative ketamine dose effect curves. Cumulative ketamine dose effect curves were determined before repeated daily ketamine administration (a, b), following 6-days of 3.2 mg/kg/day ketamine (c, d), following 6 days of 10.0 mg/kg/day (e, f), and following 6 days of 10.0 mg/kg/day×2 (twice a day, totaling 20.0 mg/kg/day) (g, h). Left panels a, c, e, g show drug effects on full ICSS frequency-rate curves. Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from baseline rates as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, P<0.05. Right panels b, d, f, h show summary ICSS data expressed as percent baseline total stimulations delivered across all frequencies of brain stimulation per test component. Other details as in Fig. 1. All data show mean±SEM for six rats
On day 22, the time course of effects produced by a bolus dose of 18.0 mg/kg ketamine was determined. Significant decreases in ICSS were observed at 100–112 Hz (P<0.05), but ICSS was not increased at any frequency at any time (data not shown). On day 25, the effects of 10 mg/kg cocaine were examined as a positive control, and cocaine significantly facilitated ICSS at brain stimulation frequencies of 63–100 Hz (Fig. 7): Significant main effect of frequency (F9,45=48.69, P<0.001), dose (F1,5=15.59, P<0.01), and interaction (F9,45=4.00, P<0.001).
Fig. 7.
Effects of 10.0 mg/kg cocaine following the termination of repeated ketamine on day 25. The figure shows drug effects on full ICSS frequency-rate curves. Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from baseline rates as determined by a two-way ANOVA followed by a Holm-Sidak post hoc test, P<0.05. Other details as in Fig. 1. All data show mean±SEM for six rats
Discussion
This study used a frequency-rate ICSS procedure to compare abuse-related effects of the noncompetitive NMDA antagonists ketamine and MK-801. There were two main findings. First, the two compounds produced dissociable behavioral effects. Specifically, ketamine produced only rate-decreasing effects, whereas MK-801 produced a mixed profile of both rate-increasing and rate-decreasing effects. Second, repeated ketamine treatment produced tolerance to the rate-decreasing effects of ketamine but failed to unmask abuse-related facilitation of ICSS. Taken together, these findings suggest that effects of ketamine in ICSS may be mediated by mechanisms other than or in addition to NMDA receptor antagonism. These results also suggest that ketamine may be less likely than MK-801 to produce a stimulant-like profile of abuse-related effects, although failure of ketamine to facilitate ICSS contrasts with other evidence for abuse liability of ketamine (e.g. Rocha et al. 1996; Suzuki et al. 2000).
Effects of MK-801 and ketamine on ICSS
The present results are consistent with previous studies showing that MK-801 facilitated ICSS in rats across a variety of reinforcement schedules and testing procedures. For example, MK-801 increased rates of ICSS maintained by fixed brain-stimulation frequencies and intensities under FR 1 and variable-interval 10-s schedules (Herberg and Rose 1989; Olds 1996). MK-801 also decreased brain stimulation thresholds required to maintain ICSS in procedures that manipulated either frequency of stimulation (Carlezon and Wise 1993; Corbett 1989; Sundstrom et al. 2002) or intensity of stimulation (Kenny et al. 2003; Bespalov et al. 1999). The present study extends these earlier results by showing that MK-801 facilitated low ICSS rates maintained by low brain-stimulation frequencies only at doses similar to or just below those that also decreased higher ICSS rates maintained by higher brain-stimulation frequencies. This mixed profile of rate-increasing and rate-decreasing effects distinguishes MK-801 from effects of some other drugs, such as cocaine or amphetamine, that exclusively facilitate ICSS across a broad dose range (Bauer et al. 2013b; Negus et al. 2012a). In contrast to MK-801, ketamine only depressed ICSS. This agrees with a previous study that found more robust rate-increasing effects with MK-801 than with ketamine in rats trained to respond for a fixed frequency and intensity of brain stimulation under a variable-interval 10-s schedule (Herberg and Rose 1989). That study evaluated ICSS rates in 10-min bins for 60 min. Rates were stable after vehicle treatment and 3.0 mg/kg ketamine increased mean ICSS rates in the 10-20 and 40-50 min time bins. However, these increases were small relative to effects of MK-801, ICSS rates were not affected at other time points by 3.0 mg/kg ketamine, and higher ketamine doses (10-100 mg/kg) only depressed ICSS as in the present study. Taken together with the present results, these findings provide little evidence for facilitation of ICSS by acute treatment with ketamine.
Effects of acute ketamine in the present study were superficially similar to effects of acute treatment with mu opioid receptor agonists like morphine in opioid-naïve rats (Altarifi and Negus 2011; Altarifi et al. 2013). Thus, mu agonists also produced primarily ICSS depression at early times after their administration. However, as initial rate-decreasing effects of mu agonists dissipated in time course studies, rate-increasing effects often emerged and predominated at later times. Moreover, repeated morphine administration produced tolerance to rate-decreasing effects of mu agonists and enhanced expression of rate-increasing effects. This dependence of mu agonist ICSS effects on parameters of acute or repeated mu agonist exposure suggested that ketamine effects may also depend on parameters of acute or repeated exposure. However, in contrast to morphine, ketamine failed to facilitate ICSS at any time after its acute administration in the present study. Repeated ketamine produced tolerance to ketamine’s rate-decreasing effects, but again unlike morphine, repeated ketamine failed to unmask rate-increasing effects of ketamine, although cocaine did facilitate ICSS in these rats. Overall, then, ketamine failed to facilitate ICSS under conditions that were sensitive to ICSS facilitation by other drugs of abuse.
Relationship of MK-801 and ketamine effects on ICSS with their effects on other types of schedule-controlled behavior or on locomotor activity
The frequency-rate ICSS procedure used in the present study engendered a wide range of baseline behavioral rates maintained by a wide range of brain-stimulation frequencies. Similarly broad ranges of baseline behavioral rates can be maintained by food or other reinforcers by using fixed-interval (FI) schedules of reinforcement, and one application of FI schedules in behavioral pharmacology has been to study the degree to which drug effects on rates of operant responding correlate with baseline response rates (Kelleher and Morse 1968; Sanger and Blackman 1976; Branch 1984). Given the potential role of rate-dependency as a determinant of drug effects, it is notable that effects of MK-801 and ketamine on varying rates of ICSS in the present study are consistent with their effects on varying response rates maintained by other reinforcers under FI schedules. For example, in rats responding for food under a multiple FR 30 FI 300-s schedule, MK-801 increased low rates of responding maintained during early segments of the fixed intervals but decreased high rates of responding maintained during later segments (McMillan et al. 1992). This effect is analogous to MK-801 facilitation of low ICSS rates maintained by low brain-stimulation frequencies but depression of high rates maintained by high frequencies in the present study. In contrast to MK-801, McMillan et al. (1992) also found that ketamine only decreased responding across all segments of the FI, similar to ketamine’s exclusive rate-decreasing effects on ICSS in the present study. Ketamine also produced exclusively rate-decreasing effects in squirrel monkeys responding under a FI 8-min schedule of shock presentation (Byrd 1982).
Another operant conditioning schedule that engenders low rates of behavior is the differential reinforcement of low rates (DRL) of responding procedure, which requires animals to wait a specific time between operant responses to receive a reinforcer. MK-801 increases low rates of behavior in rats responding under DRL 15-s and 72-s operant schedules (Ardayfio et al. 2008; Hillhouse and Porter 2014; Sanger 1992; Stephens and Cole 1996), whereas ketamine decreases rates of behavior in rats responding on DRL 72-s operant schedule (Hillhouse and Porter 2014). Taken together, these studies demonstrate that low rates of operant responding are increased by MK-801 but decreased by ketamine across a broad range of conditions. Similar results have been obtained in procedures that measure locomotor activity. For example, at doses used in this study, MK-801 significantly increases relatively low rates of locomotor activity in rats, whereas ketamine does not (Engin et al. 2009; Gilmour et al. 2009; Koike et al. 2011; Réus et al. 2011; Wegener et al. 2011).
Implications for pharmacological mechanisms of action
Ketamine and MK-801 have long been considered to share a common mechanism of action as noncompetitive NMDA antagonists that vary primarily in terms of their potency. Drug discrimination procedures have provided one source of behavioral data supportive of a shared mechanism of action, and ketamine produces cross substitution with MK-801 and the other noncompetitive NMDA antagonist phencyclidine in drug discrimination procedures (De Vry and Jentzsch 2003; Grant et al. 1996; Killinger et al. 2010; Overton et al. 1989; Rocha et al. 1996). However, MK-801 and ketamine have remarkably different affinities at the non-competitive NMDA binding site. For example, one study found that MK-801 (Ki=2.5 nM) was nearly 500-fold more potent for the noncompetitive NMDA receptor site than keta-mine (Ki=1,190 nM) (Bresink et al. 1995). Moreover, ketamine’s selectivity for NMDA receptors over other receptor types is modest (Hirota et al. 2002; Nishimura et al. 1998; Seeman et al. 2005; Smith et al. 1987), suggesting that keta-mine may produce effects at targets other than NMDA receptors. The differential behavioral effects of MK-801 and keta-mine in the present study are consistent with different mechanisms of action of these drugs to alter low rates of responding maintained by low frequencies of brain stimulation in ICSS procedures or by other reinforcers in FI or DRL schedules of reinforcement. The identity of these distinct mechanisms remains to be determined.
Implications for abuse liability assessment
ICSS is often described as one behavioral procedure for preclinical assessment of abuse liability, and drug-induced facilitation of ICSS is often interpreted as an abuse-related effect (Bauer et al. 2013b; Carlezon and Chartoff 2007; Kornetsky et al. 1979; Vlachou and Markou 2011; Wise et al. 1992; Wise 1996). The effectiveness of MK-801 to facilitate ICSS in this and other studies is consistent with its effectiveness to produce abuse-related effects in other preclinical procedures, such as drug self-administration in nonhuman primates (Beardsley et al. 1990; Koek et al. 1988) and rats (Carlezon and Wise 1996). Moreover, MK-801 produced a profile of mixed rate-increasing and rate-decreasing effects, and we have suggested previously that such a profile may be indicative of lower reinforcing effects relative to drugs like cocaine or amphetamine, which produce exclusive facilitation of ICSS across a broad dose range (Bauer et al. 2013b; Negus et al. 2012a). In agreement with this possibility, drug self-administration data suggest that NMDA antagonists may have weaker or less reliable reinforcing effects than stimulants like cocaine (Beardsley et al. 1990; French 1994; Marquis and Moreton 1987). However, the failure of ketamine to facilitate ICSS in the present study is not consistent with the efficacy of keta-mine to maintain self-administration in rats (de la Peña et al. 2012; Rocha et al. 1996) and rhesus monkeys (Broadbear et al. 2004; Moreton et al. 1977; Young and Woods 1981) or with epidemiological evidence for ketamine abuse cited in the introduction. Ketamine sometimes fails to maintain self-administration in animals (De Luca and Badiani 2011; Rocha et al. 1996; Woolverton et al. 2001), and its abuse by humans appears to be confined to a small fraction of drug users (Substance Abuse and Mental Health Services Administration 2013) and a narrow range of contexts (McCambridge et al. 2007; Winstock et al. 2012). Nonetheless, of the more than 60 drugs from multiple drug classes that we have studied in this frequency-rate ICSS procedure, ketamine is comparable only to Δ9-tetrahydrocannabinol and other cannabinoid-1 receptor agonists in its failure to facilitate ICSS despite evidence for reinforcing effects in assays of drug self-administration (Kwilasz and Negus 2012). It is possible that ketamine could facilitate ICSS under other circumstances that have not yet been identified. For example, Δ9-tetrahydrocannabinol effects on ICSS were reported to be strain- (Lepore et al. 1996) and dose- (Katsidoni et al. 2013) dependent in rats. However, the present results with ketamine illustrate the need for caution in extrapolating from ICSS studies to other preclinical assays of abuse liability or to abuse potential in humans.
Acknowledgement
This research was supported by NIH grant R01 NS070715.
Footnotes
Conflict of interest None declared.
Contributor Information
Todd M. Hillhouse, Department of Psychology, Virginia Commonwealth University, PO Box 842018, 806 West Franklin Street, Richmond 23284, VA, USA
Joseph H. Porter, Department of Psychology, Virginia Commonwealth University, PO Box 842018, 806 West Franklin Street, Richmond 23284, VA, USA
S. Stevens Negus, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 410 North 12th Street, PO Box 980613, Richmond VA 23298, USA.
References
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. doi:10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Miller LL, Negus SS. Role of μ-opioid receptor reserve and μ-agonist efficacy as determinants of the effects of μ-agonists on intracranial self-stimulation in rats. Behav Pharmacol. 2012;23:678–692. doi: 10.1097/FBP.0b013e328358593c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. Abuse-related effects of μ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol. 2013;24:459–470. doi: 10.1097/FBP.0b013e328364c0bd. doi:10.1097/FBP.0b013e328364c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, Marek GJ. The 5-hydroxytryptamine2a receptor antagonist R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J Pharmacol Exp Ther. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: implications for abuse liability assessment. Behav Pharmacol. 2013a;24:448–458. doi: 10.1097/FBP.0b013e328363d1a4. doi:10.1097/FBP.0b013e328363d1a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013b;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hayes BA, Balster RL. The self-administration of MK-801 can depend upon drug-reinforcement history, and its discriminative stimulus properties are phencyclidine-like in rhesus monkeys. J Pharmacol Exp Ther. 1990;252:953–959. [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. Eur Neuropsychopharmacol. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3223-5. doi:10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch MN. Rate dependency, behavioral mechanisms, and behavioral pharmacology. J Exp Anal Behav. 1984;42:511–522. doi: 10.1901/jeab.1984.42-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresink I, Danysz W, Parsons CG, Mutschler E. Different binding affinities of NMDA receptor channel blockers in various brain regions—indication of NMDA receptor heterogeneity. Neuropharmacology. 1995;34:533–540. doi: 10.1016/0028-3908(95)00017-z. [DOI] [PubMed] [Google Scholar]
- Broadbear J, Winger G, Woods J. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary—adrenal axis in rhesus monkeys. Psychopharmacology (Berl) 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Byrd LD. Comparison of the behavioral effects of phencyclidine, ketamine, d-amphetamine and morphine in the squirrel monkey. J Pharmacol Exp Ther. 1982;220:139–144. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by MK-801. Brain Res. 1993;620:339–342. doi: 10.1016/0006-8993(93)90177-o. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D. Possible abuse potential of the NMDA antagonist MK-801. Behav Brain Res. 1989;34:239–246. doi: 10.1016/s0166-4328(89)80105-6. [DOI] [PubMed] [Google Scholar]
- de la Peña JBI, Lee HC, de la Peña IC, Woo TS, Yoon SY, Lee HL, et al. Rewarding and reinforcing effects of the NMDA receptor antagonist—benzodiazepine combination, Zoletil®: difference between acute and repeated exposure. Behav Brain Res. 2012;233:434–442. doi: 10.1016/j.bbr.2012.05.038. doi:10.1016/j.bbr.2012.05.038. [DOI] [PubMed] [Google Scholar]
- De Luca M, Badiani A. Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology (Berl) 2011;214:549–556. doi: 10.1007/s00213-010-2062-x. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Role of the NMDA receptor NR2B subunit in the discriminative stimulus effects of ketamine. Behav Pharmacol. 2003;14:229–235. doi: 10.1097/00008877-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized addon trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- French ED. Phencyclidine and the midbrain dopamine system: electrophysiology and behavior. Neurotoxicol Teratol. 1994;16:355–362. doi: 10.1016/0892-0362(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Pioli E, Dix S, Smith J, Conway M, Jones W, Loomis S, Mason R, Shahabi S, Tricklebank M. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology (Berl) 2009;205:203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G, Grant J, Rogawski MA. Dizocilpine-like discriminative stimulus effects of low-affinity uncompetitive NMDA antagonists. Neuropharmacology. 1996;35:1709–1719. doi: 10.1016/s0028-3908(96)00147-5. [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Rose IC. The effect of MK-801 and other antagonists of NMDA-type glutamate receptors on brain-stimulation reward. Psychopharmacology (Berl) 1989;99:87–90. doi: 10.1007/BF00634458. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH. Ketamine, but not MK-801, produces antidepressant-like effects in rats responding on a differential-reinforcement-of-low-rate operant schedule. Behav Pharmacol. 2014;25:80–91. doi: 10.1097/FBP.0000000000000014. doi:10.1097/FBP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Hirota K, Hashimoto Y, Lambert DG. Interaction of intravenous anesthetics with recombinant human M1-M3 muscarinic receptors expressed in Chinese hamster ovary cells. Anesth Analg. 2002;95:1607–1610. doi: 10.1097/00000539-200212000-00025. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the care and use of laboratory animals. 8th ed. Institute of Laboratory Animals Resources, Commission of Life Sciences, National Research Council; Washington DC: 2011. [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol. 2013;16:2273–2284. doi: 10.1017/S1461145713000709. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and α-Amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Killinger BA, Peet MM, Baker LE. Salvinorin A fails to substitute for the discriminative stimulus effects of LSD or ketamine in Sprague-Dawley rats. Pharmacol Biochem Behav. 2010;96:260–265. doi: 10.1016/j.pbb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Winger GD. MK-801, a proposed noncompetitive antagonist of excitatory amino acid neurotransmission, produces phencyclidine-like behavioral effects in pigeons, rats and rhesus monkeys. J Pharmacol Exp Ther. 1988;245:969–974. [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343:389–400. doi: 10.1124/jpet.112.197780. doi:10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in Δ9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci. 1996;58:365–372. doi: 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Moreton JE. Animal models of intravenous phencyclinoid self-administration. Pharmacol Biochem Behav. 1987;27:385–389. doi: 10.1016/0091-3057(87)90587-9. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Winstock A, Hunt N, Mitcheson L. 5-Year trends in use of hallucinogens and other adjunct drugs among UK dance drug users. Eur Addict Res. 2007;13:57–64. doi: 10.1159/000095816. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Wright DW, Wenger GR. Effects of phencyclidine-like drugs on responding under multiple fixed ratio, fixed interval schedules. Behav Pharmacol. 1992;3:143–147. [PubMed] [Google Scholar]
- Moreton JE, Meisch RA, Stark L, Thompson T. Ketamine self-administration by the rhesus monkey. J Pharmacol Exp Ther. 1977;203:303–309. [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2012;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. doi:10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey E, Rosenberg M, Cheng K, Rice K. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012a;340:501–509. doi: 10.1124/jpet.111.186783. doi:10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC. Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain. 2012b;13:317–327. doi: 10.1016/j.jpain.2011.12.003. doi:10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88:768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- Olds ME. Dopaminergic basis for the facilitation of brain stimulation reward by the NMDA receptor antagonist, MK-801. Eur J Pharmacol. 1996;306:23–32. doi: 10.1016/0014-2999(96)00217-8. [DOI] [PubMed] [Google Scholar]
- Overton D, Shen CF, Ke G, Gazdick L. Discriminable effects of phencyclidine analogs evaluated by multiple drug (PCP versus other) discrimination training. Psychopharmacology (Berl) 1989;97:514–520. doi: 10.1007/BF00439557. [DOI] [PubMed] [Google Scholar]
- Páleníček T, Fujáková M, Brunovský M, Balíková M, Horáček J, Gorman I, et al. Electroencephalographic spectral and coherence analysis of ketamine in rats: correlation with behavioral effects and pharmacokinetics. Neuropsychobiology. 2011;63:202–218. doi: 10.1159/000321803. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, Souza CT, Quevedo J. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res. 2011;221:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Ward AS, Egilmez Y, Lytle DA, Emmett-Oglesby MW. Tolerance to the discriminative stimulus and reinforcing effects of ketamine. Behav Pharmacol. 1996;7:160–168. [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013;14:246–259. doi: 10.1016/j.jpain.2012.11.006. doi:10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ. NMDA antagonists disrupt timing behaviour in rats. Behav Pharmacol. 1992;3:593–600. [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Tallerico T. Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry. 2005;10:877–883. doi: 10.1038/sj.mp.4001682. [DOI] [PubMed] [Google Scholar]
- Shek DTL. Tackling adolescent substance abuse in Hong Kong: where we should and should not go. Sci World J. 2007;7:2021–2030. doi: 10.1100/tsw.2007.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Bouchal RL, DeSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, Crisp T. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26:1253–1260. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Cole BJ. AMPA antagonists differ from NMDA antagonists in their effects on operant DRL and delayed matching to position tasks. Psychopharmacology (Berl) 1996;126:249–259. doi: 10.1007/BF02246455. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: summary of national findings, NSDUH Series H-46. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. HHS Publication No. (SMA) 13-4795. [Google Scholar]
- Sundstrom JM, Hall FS, Stellar JR, Waugh EJ. Effects of isolation-rearing on intracranial self-stimulation reward of the lateral hypothalamus: baseline assessment and drug challenges. Life Sci. 2002;70:2799–2810. doi: 10.1016/s0024-3205(02)01509-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kato H, Aoki T, Tsuda M, Narita M, Misawa M. Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice. Life Sci. 2000;67:383–389. doi: 10.1016/s0024-3205(00)00639-1. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. In: Olmstead MC, editor. Animal models of drug addiction. Humana; Totowa: 2011. pp. 3–56. [Google Scholar]
- Wegener N, Nagel J, Gross R, Chambon C, Greco S, Pietraszek M, Gravius A, Danysz W. Evaluation of brain pharmacokinetics of (+)MK-801 in relation to behaviour. Neurosci Lett. 2011;503:68–72. doi: 10.1016/j.neulet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012;110:1762–1766. doi: 10.1111/j.1464-410X.2012.11028.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annual Review of Neuroscience. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, Hauck Newman A. Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology (Berl) 2001;154:375–382. doi: 10.1007/s002130000616. [DOI] [PubMed] [Google Scholar]
- Young AM, Woods JH. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther. 1981;218:720–727. [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]