Abstract
Objectives
To examine whether grandmothers’ smoking behavior during pregnancy was associated with birth weights in their grandchildren, considering possible birth cohort effects in the grandmothers’ generation.
Methods
The birth weights of 935 singleton children were compared by grandmothers’ and mothers’ smoking status during pregnancy. In 2008, women (n=397) from the Michigan Bone Health and Metabolism Study were interviewed about their own birth history, including whether their own mother smoked while pregnant with them, and the birth histories of their offspring. While also accounting for family clustering, linear mixed models were used to evaluate whether birth weight differences in the grandchildren were associated with grandmothers’ and mothers’ smoking behavior during pregnancy. Associations were compared among grandmothers born from 1904–1928 versus grandmothers born from 1929–1945 to determine potential birth cohort effects.
Results
Forty-six (5%) grandchildren had grandmothers and mothers who smoked while pregnant, while 455 (49%) had grandmothers and mothers who did not smoke during pregnancy. After adjustment, birth weight was an average of 346 (95% confidence interval: 64 to 628) grams higher in grandchildren whose grandmother and mother both smoked during pregnancy relative to grandchildren whose grandmother and mother both did not smoke during pregnancy, but only among grandmothers who were born from 1929–1945. For grandmothers born from 1904–1928, grandchildren birth weights did not differ by grandmother and mother smoking status.
Conclusions
Birth weight may be associated with grandmother and mother smoking behaviors during pregnancy, but birth cohort effects should be considered.
Keywords: smoking, birth weight, intergenerational, cohort effects
Most intergenerational studies describe relationships from two generations, specifically between parents and their children. Yet, infant birth weight may also be influenced by grandparental factors. Studies with data from three generations of families have reported higher birth weights in grandchildren with taller grandmothers (1) and in grandmothers with Type-2 diabetes (2). In addition, grandfathers with lower social class status had grandchildren with lower birth weights relative to grandchildren whose grandfathers were from a higher social class (1). The Dutch Famine Birth Cohort study showed that mothers with in-utero exposure to famine during the first or second trimester of their own gestation had children with lower birth weights compared to mothers without in-utero famine exposure (3).
Maternal smoking during pregnancy is a well-established risk factor for low birth weight in offspring (4–10). However, a women’s in-utero exposure to tobacco may affect subsequent generations via heritable mutations in the germ cells or alterations in gene expression (11, 12). From a social perspective, knowledge about smoking use during pregnancy in past generations might be informative for a family’s historical socioeconomic status (13). Understanding whether the deleterious impacts of smoking on birth weight endure in subsequent generations may be informative from a family planning and public health perspective.
To our knowledge, only two studies have examined the relationship between grandmothers’ smoking behavior during pregnancy and grandchildren’s birth weight (13, 14). A British study found that grandmothers’ smoking during pregnancy did not impact grandchildren’s birth weight after controlling for the mothers’ own smoking behavior during pregnancy (14). In contrast, a study in Baltimore reported lower birth weights among grandchildren whose grandmothers and mothers both smoked during pregnancy compared to grandchildren whose grandmothers smoked, but whose mothers did not smoke during pregnancy (13).
Women’s smoking behaviors are influenced by society, economics, and health policy (15–17), so the concentration and amount of tobacco exposure may differ by birth cohort. In the early 1900s, cigarette use was socially unacceptable among women, but by the 1940s and continuing into the 1960s, smoking became a symbol of independence and liberation for women (15, 16). Thus, the relationship between grandmothers’ smoking behavior during pregnancy and grandchildren’s birth weight might vary by grandmothers’ time of birth and reproduction.
The Michigan Bone Health and Metabolism Study provided a unique opportunity to evaluate the relationship of grandmothers’ smoking during pregnancy on grandchildren’s birth weight by grandmother birth cohorts using data collected in three generations of white families from the upper Midwestern United States. This sample comprised 935 offspring (G3) who were born to 397 mothers (G2). Smoking behavior during pregnancy was available for 320 grandmothers (G1), the G2 women’s mothers. These G1 grandmothers were born between 1904 and 1945 and gave birth to G2 women from 1942 to 1967 - time periods when fluctuations in women’s smoking patterns and behaviors occurred (17, 18). With these data, this study examined the relationship between grandmothers’ smoking behavior during pregnancy and grandchildren’s birth weight, and examined whether associations varied by the mother’s own smoking behavior and by the birth cohort of the grandmother.
METHODS
Study Population
The Michigan Bone Health and Metabolism Study (MBHMS) is a women’s health study that has been described (19). In 1988, 543 female residents of Tecumseh, Michigan who were daughters of participants of the Tecumseh Community Health Study (TCHS) were enrolled in the MBHMS. The TCHS began in 1959 and was a longitudinal study of health and chronic disease in an entire community of semi-rural, middle-class white families (20). In 1992, MBHMS expanded recruitment to include age-eligible women whose families were not TCHS participants. When enrollment was completed, the MBHMS comprised 664 women aged 24–50 years.
Data Collection and Measures
At baseline, MBHMS participants completed family pedigrees, including birthdates of their parents and siblings. In 2008, MBHMS participants who were still active (n=561, 85%) were invited to participate in a supplemental telephone-based interview that more fully characterized their own birth history and the birth histories of their children. The University of Michigan Institutional Review Board approved this data collection. Informed consent was obtained from all MBHMS participants at baseline and during the supplemental telephone interviews.
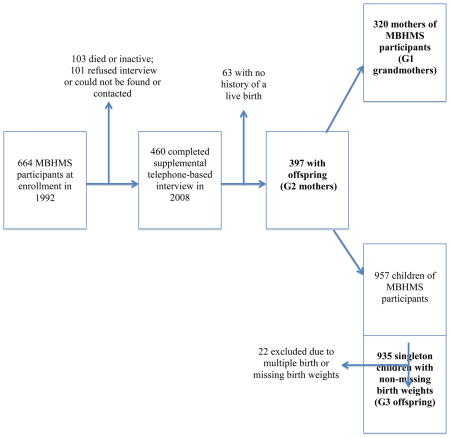
A total of 460 (82%) women completed the 2008 supplemental telephone interview; 101 women refused or could not be found or contacted. Among those interviewed, 397 (86%) were mothers (G2) who provided birth weights, sex, and birthdates on 957 offspring (G3). Data from 22 G3 offspring were excluded due to multiple birth status or missing birth weights. The family pedigree information identified 320 grandmothers (G1). A description of the study sample within each generation is shown in the Appendix.
In the 2008 supplemental interview, G2 women self-reported their own birth weights, birthdate, singleton status, and whether their own mother (G1) smoked while pregnant with them (response options: Yes/No/Don’t Know). Mothers’ (G2) smoking status during pregnancy with G3 offspring was constructed based on self-reported responses from their MBHMS annual follow-up visits about whether they ever or currently smoked (Yes/No). For both G1 (grandmothers) and G2 (mothers), smoking behavior was dichotomized into smoked versus did not smoke during pregnancy, which was combined into a single four-category variable to capture smoking behavior across two generations of pregnancies: 1) both G1 and G2 smoked; 2) G1 smoked and G2 did not smoke; 3) G1 did not smoke and G2 smoked; and 4) neither G1 nor G2 smoked.
For all G2 women, self-reported marital status and highest level of education and clinically-collected measures of height and weight were obtained at baseline. Education was dichotomized by any college experience, since all G2 women completed high school. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Birth weights were converted to grams (g) from pounds and ounces.
Statistical Analysis
Characteristics of G3 offspring were compared by G1 grandmother smoking status during their pregnancy with G2 mother. Cochran-Mantel-Haenszel and t-statistic tests were used to evaluate whether frequency distributions and mean differences in the characteristics were statistically significant by G1 grandmother smoking status. Due to the nested structure of the data, linear mixed regression models with random intercepts for both G1 clustering (G2 women may be sisters with the same G1 mother) and the G2 clustering (G3 offspring may be siblings with the same G2 mother) were fit (21). These regression models estimated the mean difference and 95% confidence intervals (CI) in G3 birth weight associated with G1 and G2 smoking behavior, while simultaneously allowing adjustment for other covariates and accounting for correlated variances (21). Mean differences in G3 birth weight associated with G1 and G2 smoking behavior were estimated by including indicator variables in the regression models, using the group of G3 offspring whose G1 grandmother and G2 mother both did not smoking during pregnancy as the referent group. Models included adjustment for G3 first born status and sex, G2 first born status, singleton status, birth weight, birth year, marital status, education, and adult body mass index and height, and G1 birth year. To examine birth cohort effects, G1 birth year was dichotomized along the median birth year of 1929 and the data were re-examined. Analyses were completed using SAS v9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Mean (SD) birth weight for the G3 offspring was 3483 (559) g. Seventy-six (24%) G1 grandmothers smoked while pregnant; thus, 190 (20%) of G3 offspring were born to 82 (21%) G2 mothers with in-utero smoking exposure. The mean (SD) birth weight of G3 offspring whose G1 grandmother smoked during pregnancy was 3561 (568) g compared to a mean (SD) birth weight of 3458 (545) g among G3 offspring whose G1 grandmother did not smoke during pregnancy (p=0.02, data not shown).
Across two generations, 46 (5%) G3 offspring had G1 grandmothers and G2 mothers who both smoked during pregnancy. In contrast, 455 (49%) G3 offspring had G1 grandmothers and G2 mothers who both did not smoke during pregnancy. A total of 130 (14%) G3 offspring had G1 grandmothers who smoked during pregnancy, but whose G2 mothers did not and 160 (17%) G3 offspring had G1 grandmothers who did not smoke during pregnancy, but whose G2 mothers did.
By G1 Grandmother Birth Cohort
The birth year range of G1 grandmothers was 1904 to 1945 and the median birth year was 1929. There were 457 G3 offspring with G1 grandmothers born from 1904 to 1928 (early birth cohort) and 469 G3 offspring with G1 grandmothers born from 1929 to 1945 (late birth cohort). Characteristics observed in the G3 offspring and G2 mothers by G1 grandmothers’ smoking behavior during pregnancy and birth cohort are described in Table 1.
Table 1.
Characteristics in G3 Offspring and G2 Mother by G1 Grandmother Smoking Status and Birth Cohort
| G1 Early Birth Cohort (1904 – 1928) | Total Sample | G1 Grandmother Smoking Status
|
||
|---|---|---|---|---|
| Non-Smoker | Smoker | |||
|
| ||||
| n = 457 | n = 333 | n = 92 | P-value | |
| G3 Offspring | ||||
| First Born, n (%) | 192 (42) | 142 (43) | 39 (42) | 0.97 |
| Male Sex, n (%) | 254 (56) | 180 (54) | 59 (64) | 0.08 |
| Birth Weight, g, mean (SD) | 3481 (566) | 3463 (546) | 3481 (600) | 0.79 |
| G2 Mother | ||||
| Married, n (%) | 259 (87) | 190 (90) | 56 (85) | 0.29 |
| Some College, n (%) | 333 (73) | 233 (70) | 70 (76) | 0.25 |
| Smoked during G3 Pregnancy, n (%) | 112 (26) | 78 (25) | 16 (18) | 0.23 |
| BMI at Enrollment, kg/m2, mean (SD) | 26.1 (5.6) | 25.9 (5.3) | 26.8 (6.6) | 0.35 |
| Height at Enrollment, cm, mean (SD) | 163.1 (6.0) | 163.2 (6.4) | 162.3 (4.6) | 0.31 |
| Age at G3 Birth, years, mean (SD) | 27.0 (5.5) | 26.8 (5.3) | 27.3 (5.6) | 0.36 |
| Singleton Birth, n (%) | 435 (95) | 321 (96) | 82 (89) | <0.01 |
| First Born, n (%) | 111 (24) | 79 (24) | 27 (29) | 0.27 |
| Birth Weight, g, mean (SD) | 3316 (644) | 3372 (639) | 3145 (653) | 0.05 |
| G1 Late Birth Cohort (1929 – 1945) | Total Sample | G1 Grandmother Smoking Status
|
||
|---|---|---|---|---|
| Non-Smoker | Smoker | |||
|
| ||||
| n = 469 | n = 353 | n= 95 | P-value | |
| G3 Offspring | ||||
| First Born, n (%) | 196 (42) | 144 (41) | 41 (43) | 0.68 |
| Male Sex, n (%) | 255 (54) | 183 (52) | 59 (62) | 0.07 |
| Birth Weight, g, mean (SD) | 3486 (547) | 3453 (547) | 3630 (527) | <0.01 |
| G2 Mother | ||||
| Married, n (%) | 340 (91) | 265 (93) | 57 (81) | <0.01 |
| Some College, n (%) | 333 (71) | 246 (70) | 72 (76) | 0.24 |
| Smoked during G3 Pregnancy, n (%) | 115 (29) | 82 (28) | 30 (35) | 0.19 |
| BMI at Enrollment, kg/m2, mean (SD) | 24.7 (4.8) | 24.4 (4.5) | 26.0 (6.1) | 0.13 |
| Height at Enrollment, cm, mean (SD) | 162.9 (5.9) | 163.1 (5.8) | 162.6 (5.5) | 0.58 |
| Age at G3 Birth, years, mean (SD) | 26.2 (5.0) | 26.4 (5.1) | 25.5 (4.7) | 0.12 |
| Singleton Birth, n (%) | 465 (99) | 349 (99) | 95 (100) | 0.30 |
| First Born, n (%) | 145 (31) | 111 (31) | 30 (32) | 0.98 |
| Birth Weight, g, mean (SD) | 3277 (540) | 3321 (510) | 3185 (590) | 0.15 |
BMI, Body Mass Index. Sums by G1 grandmother smoking status do not equal value in total sample because of missing data.
In the early birth cohort, 36 (23%) G1 grandmothers smoked during pregnancy; thus, there were 40 (21%) G2 mothers with in-utero smoke exposure who gave birth to 92 (20%) G3 offspring. Mean birth weights among G3 offspring whose G1 grandmothers were in the early birth cohort did not vary by G1 smoking behavior during pregnancy (p=0.79).
In the late birth cohort, 39 (24%) G1 grandmothers smoked during pregnancy; thus, 41 (21%) G2 mothers had in-utero smoke exposure, which affected 95 (20%) G3 offspring. The mean (SD) birth weight was 3630 (527) g for G3 offspring whose late birth cohort G1 grandmothers smoked while pregnant, but was 3453 (547) g for G3 offspring whose G1 grandmother did not smoke (p<0.01).
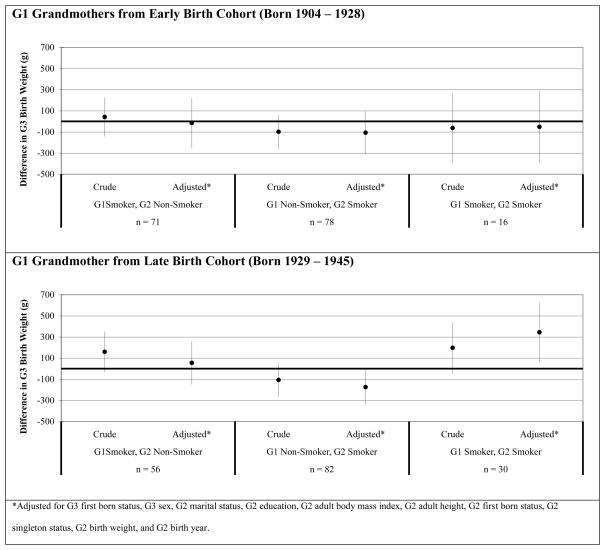
Figure 1 shows the results of the linear regression models by G1 birth cohort. Among the G1 early birth cohort (top graph), no mean differences in G3 birth weight were observed by the G1 and G2 smoking behavior categories relative to the referent group. However, among the G1 late birth cohort (bottom graph), two statistically significant findings were observed. When both G1 and G2 smoked during pregnancy (n=30), the adjusted mean difference in G3 offspring birth weight was 346 g (95% CI: 64 to 628) higher compared to G3 offspring birth weights when both G1 and G2 did not smoke (referent). In addition, when G1 did not smoke and G2 smoked during pregnancy (n=82), the adjusted mean difference in G3 birth weight was 174 g (95% CI: −339 to −9) lower relative to the G3 offspring birth weights from the referent group.
Figure 1. Difference in G3 Offspring Birth Weight Associated with G1 Grandmother and G2 Mother Smoking Behavior among G1 Grandmothers Born from 1904 to 1928 (Top Graph) and G1 Grandmothers Born from 1929 to 1945 (Bottom Graph).
Three distinct categories representing grandmothers’ and mothers’ smoking behavior during pregnancy were compared to the referent group (neither grandmother nor mother smoked during pregnancy). Lines represent 95% confidence intervals.
DISCUSSION
Using data collected over three generations of white families from the upper Midwestern United States, this study found that the relationship between grandmothers’ smoking behavior during pregnancy and grandchildren’s birth weight varied by the grandmother’s time of birth. For grandmothers born from 1904–1928, grandchildren’s birth weight was not associated with grandmothers’ smoking behavior during pregnancy. However, grandmothers born from 1929–1945 and who smoked during pregnancy had heavier grandchildren if the mother also smoked during pregnancy. In both grandmother birth cohorts, grandchildren’s birth weights were lowest when mothers smoked during pregnancy and grandmothers did not – a finding consistent with the existing literature on the impacts of maternal smoking on infant birth weight.
In the early 1900s, cigarette use was not socially accepted and was associated with poor morals and improper behavior (15, 16). By the 1930s, in conjunction with women’s suffrage and new employment roles of women during the Second World War, smoking became a symbol of independence and liberation (15, 16). National health data showed that the prevalence of smoking rose steeply from the mid-1930s to the mid-1960s (18, 22). In addition, the design of cigarettes, including the concentration of toxins, such as tar, carbon monoxide, and nicotine, and the use of filters, began in the 1950s and has evolved ever since (18, 23). Research suggests birth weight is negatively associated with a higher numbers of cigarettes smoked and with higher levels of chemical toxicity (8).
In this study, grandmothers’ smoking prevalence was similar across the birth cohorts. However, based on the historical context of smoking use in women, we speculate that the concentration and toxicity levels of tobacco exposure among smokers differed by birth cohort. These differences might explain the birth weight disparity that was observed. One explanation is residual confounding; specifically, smoking behavior among the grandmothers in the late birth cohort – and subsequently in their daughters – may be associated with unmeasured characteristics that resulted in higher offspring birth weights. For example, smokers from the late birth cohort may have smoked more frequently and for a longer duration when pregnant compared to smokers from the early birth cohort. Alternatively, women’s smoking as a symbol of independence in the 1930–1960s, which aligns more closely with the women from the late birth cohort, may have occurred primarily among those with a higher socioeconomic status. As a consequence, unmeasured variations in socioeconomic status among families from the late birth cohort group, but not the early birth cohort group, may have contributed to higher birth weights in the grandchildren.
Another explanation is that the higher birth weights observed in the grandchildren was a chance finding. We identified 46 grandchildren whose grandmother and mother smoked during pregnancy. This number was reduced in the examination by grandmother birth cohort, which further decreased statistical power. However, lack of power is typically associated with type-2 error - a failure to reject the null hypothesis (24). Since we reported a significant positive association, this explanation is less likely, but repeating this analysis in a larger dataset of families would be informative.
Finally, these findings might be suggestive of developmental plasticity, in which the phenotype changes in response to environmental cues provided by the mother that occur during fetal development (25–30). Theories about the mechanism by which these phenotypic changes might occur include inherited mutations in the germ cells or alterations in gene expression (11, 12). Although these theories primarily stem from the literature on the generational effects of in-utero diethylstilbestrol exposure, parallels to the generational impacts of in-utero smoking exposure can be applied. Fortunately, progress in genetic technology and epigenetic research make investigation of these theories possible in future research studies.
It is our understanding that only two studies have examined grandmother’s smoking behavior in pregnancy and its influence on grandchildren’s birth weights. Data from the Baltimore site of the U.S. Collaborative Perinatal Project (CPP) reported lower birth weights among offspring whose grandmother and mother smoked while pregnant compared to offspring whose grandmother smoked while pregnant but whose mother did not (13). Most grandmothers in the CPP were likely born from 1920–1950 (31), overlapping with grandmothers from the late birth cohort in our study. Yet, for MBHMS families from the late birth cohort, grandmothers and mothers who smoked during pregnancy had offspring with the highest average birth weights. Characteristic differences between the study populations might explain the disparity. For example, the CPP was racially diverse compared to the MBHMS (31) and smoking behaviors and birth weight varies by race (32, 33). Misra et al identified a three-way interaction between grandmother’s smoking, mother’s smoking, and mother’s parity on offspring birth weight (13). In this interaction, the authors observed the largest reduction in birth weight among those whose grandmothers smoked and whose mothers also smoked and were parous (13). The relationship between grandmother’s smoking and mother’s smoking and parity was not an objective of our study, but these interactions were analyzed to allow comparisons. We found no evidence of a three-way interaction; however, since parity may confound our results, the models adjusted for parity.
Data from the British 1958 National Child Development Study reported a positive but small and non-significant association between grandmother smoking during pregnancy and grandchildren birth weight (14). While the 1958 British cohort study used a mainly homogenous study population of white families that included grandmothers born from 1909–1943 (34), their analysis adjusted for maternal characteristics, including mother’s smoking status during pregnancy. Thus, although seemingly in agreement with our results, it is not known whether larger birth weight differences in the grandchildren would be observed in an analysis that stratified by mothers smoking behavior during pregnancy.
The observation of higher birth weights in grandchildren whose grandmother and mother smoked during pregnancy is contrary to the current knowledge about the negative health effects of smoking during pregnancy. However, this observation may provide insight about possible biological mechanisms for certain birth outcomes. A similar contradictory finding has been described between smoking behavior before and during pregnancy and a lower the risk of preeclampsia (35–37), and the possibility that both phenomena may share a similar biological pathway is worthy of further investigation.
Another important finding to note was that, regardless of the time of grandmother’s birth, the lowest birth weights were in offspring whose mother smoked, but whose grandmother did not. Furthermore, women who reported that their own mother smoked while pregnant with them had lower birth weights compared to women without this exposure. This finding is consistent with the generally accepted belief that maternal smoking causes low birth weight, a relationship that has been described in numerous other studies (4–10, 13, 14).
Recall and reporting bias is a study limitation. The MBHMS participant self-reported most information, including their smoking behavior during pregnancy, their own in-utero tobacco exposure, and the birth weights in their offspring. Studies indicate that women can reliably self-report their own in-utero smoking exposure (38–40) and their children’s birth weights (40–43). However, self-reporting tends to underestimate women’s own smoking behavior during pregnancy (44). It is unclear how this bias would affect our results, since we showed offspring birth weights to also be dependent on grandmother’s smoking behavior; however, we suspect that this bias is likely non-differential. We did not have information about gestational length, nor did we have specific details about smoking behaviors, including number of cigarettes smoked, toxicity of cigarettes, and duration and timing of smoking during pregnancy. Availability of these data would improve precision in our measures and permit analysis of the specific impacts of birth outcomes by tobacco concentration and volume. Finally, statistical power was further reduced when stratified by smoking status across generations, although significant birth weight differences in the grandchildren were still observed.
In this intergenerational study, birth weights in children were higher when grandmothers and mothers both smoked while pregnant, but only among those whose grandmothers were born in the years after 1928. This observation is in opposition to the current thinking about birth weight and in-utero tobacco exposure and requires replication in a larger sample of families. If, however, these findings were replicated, they could provide insight about possible biological mechanisms of various birth outcomes, be informative for future family planning over multiple generations, and should prompt research on its possible health impacts from a life-course and genetics perspective.
Acknowledgments
The authors thank Mary Crutchfield, Cora Alcock, Angela Crippen, and Megan McFarland for their work on the 2008 collection of the birth history data. This study was supported by the National Institutes of Health (grants AR051384, AR040888, and AR-20557). Salary support for the corresponding author was provided by National Institutes of Health grant T32 AG027677.
Appendix. Depiction of how the study samples in each generation were identified
Footnotes
Conflicts of Interest: None declared
References
- 1.Emanuel I, Filakti H, Alberman E, Evans SJ. Intergenerational studies of human birthweight from the 1958 birth cohort. 1. Evidence for a multigenerational effect. Br J Obstet Gynaecol. 1992;99(1):67–74. doi: 10.1111/j.1471-0528.1992.tb14396.x. [DOI] [PubMed] [Google Scholar]
- 2.McCarron P, Davey Smith G, Hattersley AT. Type 2 diabetes in grandparents and birth weight in offspring and grandchildren in the ALSPAC study. J Epidemiol Community Health. 2004;58(6):517–22. doi: 10.1136/jech.2003.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumey LH. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944–1945. Paediatr Perinat Epidemiol. 1992;6(2):240–53. doi: 10.1111/j.1365-3016.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 4.Aagaard-Tillery KM, Porter TF, Lane RH, Varner MW, Lacoursiere DY. In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am J Obstet Gynecol. 2008;198(1):66, e1–6. doi: 10.1016/j.ajog.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 5.Cnattingius S, Axelsson O, Eklund G, Lindmark G. Smoking, maternal age, and fetal growth. Obstet Gynecol. 1985;66(4):449–52. [PubMed] [Google Scholar]
- 6.Horta BL, Victora CG, Menezes AM, Halpern R, Barros FC. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol. 1997;11(2):140–51. doi: 10.1046/j.1365-3016.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 7.Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr Perinat Epidemiol. 2008;22(2):162–71. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 8.Peacock JL, Bland JM, Anderson HR, Brooke OG. Cigarette smoking and birthweight: type of cigarette smoked and a possible threshold effect. Int J Epidemiol. 1991;20(2):405–12. doi: 10.1093/ije/20.2.405. [DOI] [PubMed] [Google Scholar]
- 9.Steyn K, de Wet T, Saloojee Y, Nel H, Yach D. The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth To Ten Study. Paediatr Perinat Epidemiol. 2006;20(2):90–9. doi: 10.1111/j.1365-3016.2006.00707.x. [DOI] [PubMed] [Google Scholar]
- 10.Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11(4):427–33. doi: 10.1097/00001648-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman RH, Adam E. Findings in female offspring of women exposed in utero to diethylstilbestrol. Obstet Gynecol. 2002;99(2):197–200. doi: 10.1016/s0029-7844(01)01682-9. [DOI] [PubMed] [Google Scholar]
- 12.Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES) Int J Epidemiol. 2006;35(4):862–8. doi: 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- 13.Misra DP, Astone N, Lynch CD. Maternal smoking and birth weight: interaction with parity and mother’s own in utero exposure to smoking. Epidemiology. 2005;16(3):288–93. doi: 10.1097/01.ede.0000158198.59544.cf. [DOI] [PubMed] [Google Scholar]
- 14.Hypponen E, Smith GD, Power C. Effects of grandmothers’ smoking in pregnancy on birth weight: intergenerational cohort study. BMJ. 2003;327(7420):898. doi: 10.1136/bmj.327.7420.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amos A, Haglund M. From social taboo to “torch of freedom”: the marketing of cigarettes to women. Tob Control. 2000;9(1):3–8. doi: 10.1136/tc.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britton GA. A review of women and tobacco: have we come such a long way? J Obstet Gynecol Neonatal Nurs. 1998;27(3):241–9. doi: 10.1111/j.1552-6909.1998.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 17.Waldron I. Patterns and causes of gender differences in smoking. Soc Sci Med. 1991;32(9):989–1005. doi: 10.1016/0277-9536(91)90157-8. [DOI] [PubMed] [Google Scholar]
- 18.Garfinkel L. Trends in cigarette smoking in the United States. Prev Med. 1997;26(4):447–50. doi: 10.1006/pmed.1997.0191. [DOI] [PubMed] [Google Scholar]
- 19.Sowers MF, Zheng H, McConnell D, Nan B, Karvonen-Gutierrez CA, Randolph JF., Jr Testosterone, sex hormone-binding globulin and free androgen index among adult women: chronological and ovarian aging. Hum Reprod. 2009;24(9):2276–85. doi: 10.1093/humrep/dep209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napier JA. Field methods and response rates in the Tecumseh community health study. Am J Public Health Nations Health. 1962;52:208–16. doi: 10.2105/ajph.52.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer JD. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. Journal of Educational and Behavioral Statistics. 1998;24(4):323–355. [Google Scholar]
- 22.Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health. 1996;86(2):231–6. doi: 10.2105/ajph.86.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of The Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. pp. 13–26. [PubMed] [Google Scholar]
- 24.Banerjee A, Chitnis UB, Jadhav SL, Bhawalkar JS, Chaudhury S. Hypothesis testing, type I and type II errors. Ind Psychiatry J. 2009;18(2):127–31. doi: 10.4103/0972-6748.62274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bateson P. Developmental plasticity and evolutionary biology. J Nutr. 2007;137(4):1060–2. doi: 10.1093/jn/137.4.1060. [DOI] [PubMed] [Google Scholar]
- 27.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430(6998):419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 28.Gluckman PD, Hanson M. The fetal matrix: evolution, development, and disease/Peter Gluckman, Mark Hanson. New York: Cambridge University Press; 2005. [Google Scholar]
- 29.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 30.Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, et al. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet. 2009;373(9675):1654–7. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- 31.Niswander KR, Gordon MJ. The women and their pregnancies: the Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke. U.S. Department of Health, Education, and Welfarre, Public Health Service, National Institute of Health; 1972. [Google Scholar]
- 32.Geronimus AT, Neidert LJ, Bound J. Age patterns of smoking in US black and white women of childbearing age. Am J Public Health. 1993;83(9):1258–64. doi: 10.2105/ajph.83.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessol NA, Fuentes-Afflick E, Bacchetti P. Risk of low birth weight infants among black and white parents. Obstet Gynecol. 1998;92(5):814–22. doi: 10.1016/s0029-7844(98)00310-x. [DOI] [PubMed] [Google Scholar]
- 34.Hypponen E, Davey Smith G, Shepherd P, Power C. An intergenerational and lifecourse study of health and mortality risk in parents of the 1958 birth cohort: (II) mortality rates and study representativeness. Public Health. 2005;119(7):608–15. doi: 10.1016/j.puhe.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181(4):1026–35. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 36.England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12:2471–83. doi: 10.2741/2248. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Klebanoff MA, Levine RJ, Puri M, Moyer P. The puzzling association between smoking and hypertension during pregnancy. Am J Obstet Gynecol. 1999;181(6):1407–13. doi: 10.1016/s0002-9378(99)70384-4. [DOI] [PubMed] [Google Scholar]
- 38.Simard JF, Rosner BA, Michels KB. Exposure to cigarette smoke in utero: comparison of reports from mother and daughter. Epidemiology. 2008;19(4):628–33. doi: 10.1097/EDE.0b013e3181761cbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cupul-Uicab LA, Ye X, Skjaerven R, Haug K, Longnecker MP. Reproducibility of reported in utero exposure to tobacco smoke. Ann Epidemiol. 2011;21(1):48–52. doi: 10.1016/j.annepidem.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson M, Williams MA, White E, Daling JR, Holt VL, Malone KE, et al. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147(2):136–40. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- 41.Adegboye AR, Heitmann B. Accuracy and correlates of maternal recall of birthweight and gestational age. BJOG. 2008;115(7):886–93. doi: 10.1111/j.1471-0528.2008.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lumey LH, Stein AD, Ravelli AC. Maternal recall of birthweights of adult children: validation by hospital and well baby clinic records. Int J Epidemiol. 1994;23(5):1006–12. doi: 10.1093/ije/23.5.1006. [DOI] [PubMed] [Google Scholar]
- 43.Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers’ recall of birthweight and gestational age. Br J Obstet Gynaecol. 1987;94(8):731–5. doi: 10.1111/j.1471-0528.1987.tb03717.x. [DOI] [PubMed] [Google Scholar]
- 44.Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, et al. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173(3):355–9. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]