Abstract
BLyS (B lymphocyte stimulator) family cytokines and receptors play key roles in B-2 cell maturation and survival, but their importance for B-1 cells remains less clear. Here we use knockout mice to show that APRIL (A proliferation-inducing ligand), but not BLyS, plays a role in peritoneal B-1 cell maintenance. APRIL likely exerts its effects on peritoneal B-1 cells through binding to HSPG (heparan sulfate proteoglycans) rather than to the TACI (transmembrane activator and cyclophilin ligand interactor) receptor. Finally, we show that peritoneal macrophages express high levels of APRIL message, and are a likely local source of the cytokine in this anatomic locale.
Keywords: BLyS, APRIL, TACI, HSPG, B-1 cell
1. Introduction
Cells of the two primary mammalian B cell lineages, B-1 and B-2, play distinct roles in humoral immunity, reflecting intrinsic differences in their generation, BCR repertoire, and anatomic distribution (reviewed in [1–3]). In accord with their barrier and housekeeping roles, the B-1 receptor repertoire is less diverse and more similar to the germline compared with the highly diversified B-2 repertoire [4,5]. Found primarily in serous fluids and membranes, B-1 cells are constitutively activated [3,6–8] and produce so-called natural antibodies, including some that recognize self-molecules, as well as those with specificities for commensal and opportunistic bacteria [6,9]. In contrast, the numerically larger B-2 pool is comprised of quiescent, recirculating cells that populate secondary lymphoid organs and mediate inducible immune responses that entail the generation of long-lived plasma and memory B cells [10]. Together, these differences suggest that B-1 and B-2 pools likely occupy different homeostatic niches.
Members of the B lymphocyte stimulator (BLyS) family of cytokines and receptors play key roles in the selection and homeostatic control of B-2 subsets including follicular (FO), marginal zone (MZ) and germinal center B cells [11–18]. This family consists of two cytokines, BLyS [a.k.a. B cell activating factor of the tumor-necrosis-factor family, or BAFF] and a proliferation-inducing ligand (APRIL), as well as three receptors, BCMA, TACI, and BR3.
Members of the BLyS family may also be important for homeostasis of the B-1 lineage, though they may play more subtle roles. For example, peritoneal cavity (PerC) B-1 cells express two of the three BLyS family receptors, as do PerC B-2 cells [19,20]. Nevertheless, while mature pre-immune B-2 subsets are exquisitely sensitive to BLyS levels and require BLyS-BLyS receptor 3 (BR3) signals in order to survive [14,16,21–24], splenic B-1 cell numbers are modestly reduced upon BLyS neutralization, and PerC B-1 cells are unaffected [25]. Conversely, BLyS transgenic mice have significantly increased numbers of mature naïve B-2 cells, but normal [26] or modestly altered [27] numbers of peritoneal B-1 cells. Together, these and other results argue against a required role for BLyS in PerC B-1 cell homeostasis.
In contrast to this relative BLyS independence, increasing but incomplete evidence suggests a major role for APRIL (A proliferation-inducing ligand) in PerC B-1 cell homeostasis. For example, APRIL transgenic mice show an accumulation of B-1 cells in the PerC and mediastinal lymph nodes [28], and anti-APRIL antibody treatment inhibits PerC B-1 cell expansion in these animals [29]. However, one study reported comparable percentages of PerC B-1a cells (B220+ CD5+) in wild-type and APRIL knockout mice [30]. Another study involving a different APRIL knockout strain reported normal numbers of B cell subsets in bone marrow, spleen, and lymph nodes, but did not examine peritoneal B-1 cells [31]. In addition, there are conflicting reports on the effect of TACI fusion proteins, which should neutralize both BLyS and APRIL, on B-1 subsets. Gross et al. found that the percentage of PerC B-1 cells was moderately decreased in BALB/c mice treated with TACI-Ig, and absolute numbers were significantly decreased in TACI-Ig transgenics (C57BL/6 background) [14]. In contrast, Schneider et al. observed no significant difference in the percentage of peritoneal B-1a and B-1b subsets in TACI-Fc transgenic mice compared to control littermates [32]. Taken together, these observations imply roles for both BLyS and APRIL in B-1 cell homeostasis, with the relative importance of each cytokine dictated in part by the local microenvironment. Alternatively, both cytokines may be required, but at different points in B-1 cell maturation.
We undertook studies of PerC B-1 cells in order to clarify roles for BLyS and APRIL in B-1 B cell persistence in this anatomic compartment. Our findings suggest that APRIL, but not BLyS, is important for PerC B-1 cell development or maintenance; but that the effects of APRIL are not mediated by classical BLyS family receptors per se, but likely act via APRIL binding to heparan sulfate proteoglycans (HSPG).
2. Materials and Methods
2.1 Mice and peritoneal lavage
C57BL/6J mice were purchased from The Jackson Laboratory or the National Cancer Institute. BLyS knockout [16], APRIL knockout [30], and BLyS/APRIL double knockout strains are on a C57BL/6 background (≥10th generation) [33]. TACI knockout mice have been previously described [34]. All animal handling procedures were approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee. Peritoneal cells were isolated by lavage as described [35]; typical yields were in the range of 1.0 × 106 to 3.0 × 106 cells per adult wild-type mouse.
2.2 Flow cytometry
Antibodies or reagents reactive to the following antigens were used for flow cytometry: B220 (clone RA3-6B2), IgM (clone 11/41), CD23 (clone B3B4), CD21/35 (clone 4E3), BR3 (clone 7H22-E16), CD3ε (clone 145-2C11), all from eBioscience; CD19 (clone 1D3), CD5 (clone 53-7.3), CD11b (clone M1-70), all from BD Pharmingen; TACI (clone 166010) and BCMA (clone 161616) from R&D Systems); APRIL (clone A3D8) and Armenian hamster isotype control (clone HTK888) from BioLegend. Data were collected on a BD LSR II flow cytometer and analyzed with FlowJo software (TreeStar). Doublet exclusion was performed by FSC/SSC width vs. height, and live/dead cell discrimination was assessed with DAPI (Invitrogen). Peritoneal B-1a (B220+ IgM+ CD11b+ CD5+), B-1b (B220+ IgM+ CD11b+ CD5−), and B-2P (B220+ IgM+ CD11b− CD5−) cells were sorted with a FACS Aria II™ (BD).
2.3 Quantitative polymerase chain reaction (qPCR) analysis
For gene expression analyses, RNA was extracted with the RNeasy kit (Qiagen) and reverse-transcribed using SuperScript II Reverse Transcriptase (Invitrogen) following manufacturer’s protocols. cDNA was amplified using TaqMan Universal Master Mix and Taqman probes for various genes (Applied Biosystems). Real-time PCR was performed with an ABI 7300 (Applied Biosystems). Relative expression was calculated using GAPDH expression as an endogenous control for samples that were FACS sorted.
2.4 Heparinase-III treatment
Peritoneal cells were washed and resuspended in PBS at a density of 2–3×106/400 μl in 1.5-mL Eppendorf tubes. 25 mU/ml Heparinase III (Sigma-Aldrich) was added and cells were subsequently incubated with gentle shaking for 3 hours at 37°C. Control samples were treated identically, but without the addition of enzyme. Cells were washed with PBS and used for the APRIL binding assay.
2.5 APRIL binding assay
To detect APRIL binding on the surface of peritoneal B cell subsets, untreated or Heparinase III-treated cells were incubated with 500 ng/ml of mouse MegaAPRIL (H-APRIL), delta HSPG-APRIL (ΔH-APRIL), or human MegaAPRIL (ENZO Life Sciences) at 4°C for 30 minutes. When indicated, H-APRIL was pre-incubated with heparin (1ug/ml) for 15 minutes at room temperature prior to the cell-binding assay. Cells were washed with PBS twice, stained with anti-APRIL antibody or isotype control, collected on a BD LSR II flow cytometer, and analyzed with FlowJo software (Tree Star).
3. Results
3.1 APRIL, but not BLyS, is a mediator of B-1 cell development or maintenance in the peritoneal cavity
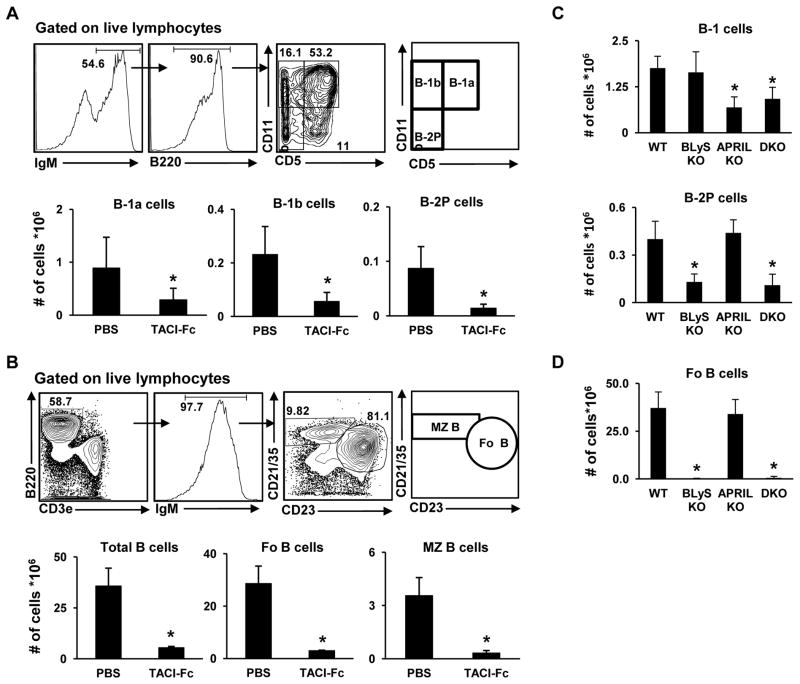
Because of conflicting reports regarding B-1 persistence in transgenics for a fusion protein that should neutralize both BLyS and APRIL [14,32], we began by treating 3–4 month old C57BL/6 mice with 100 μg of human TACI-Fc [36] twice (days 0 and 4), and assessing the effect on PerC and splenic B cell subsets at day 8 (Figure 1). We observed a significant (p ≤ 0.05) decrease in PerC B-1a, B-1b and B-2 subsets in TACI-Fc-treated mice in comparison to PBS-injected mice (Fig. 1A). As expected [14,32,36], there was a significant decrease in splenic total, FO, and MZ B cells following TACI-Fc treatment due to its BLyS neutralizing capacity (Fig. 1B).
Figure 1. APRIL, rather than BLyS, is a key mediator of B-1 development or persistence in the murine peritoneal cavity.
(A) C57BL/6 mice were treated with PBS or TACI-Fc (100 μg/mouse i.p.) on days 0 and 4 and analyzed on day 8. Upper panel shows flow cytometry gating strategy for identification of peritoneal B-1a (IgM+B220+CD11b+CD5+), B-1b (IgM+B220+CD11b+CD5−), and B-2 (B-2P) (IgM+B220+CD11b−CD5−) subsets. Lower panels show absolute numbers of each subset in the peritoneal cavity. (B) Gating strategy for identification of splenic B-2 cell subsets (upper panel) and absolute numbers (lower panel) of total (B220+IgM+), follicular (Fo) (B220+IgM+CD23+CD21lo/−), and marginal zone (MZ) (B220+IgM+CD23−CD21hi) B cells in mice treated with PBS or TACI-Fc. (C) Absolute numbers of peritoneal cavity B-1 and B-2P cells in wild-type (WT) mice, BLyS or APRIL single knockout (KO) mice, and BLyS-APRIL double knockouts (DKO). Gating strategy was as shown in A. (D) Absolute numbers of FO B cells (B220+IgM+AA4.1−CD23+CD21lo/−) in spleens of mice indicated. * = p < 0.05 by Student’s t-test.
To address the individual roles of BLyS and APRIL, we assessed B cells in BLyS knockout (KO), APRIL knockout, and BLyS/APRIL double knockout (DKO) mice – all backcrossed for > 10 generations to C57BL/6 [37]. Compared to wild-type (WT) mice, the number of B-1 cells in the peritoneal cavity is significantly lower in APRIL KO and BLyS-APRIL DKOs, but not the BLyS KOs (Fig. 1C, upper panel). Conversely, peritoneal B-2 (B-2P) cells are significantly reduced in only the BLyS KO and BLyS-APRIL DKO mice (Fig. 1C, lower panel), as are splenic FO (Fig. 1D) and MZ B cells (not shown). Together, these results implicate APRIL, rather than BLyS, as a mediator of PerC B-1 B cell development or maintenance.
3.2 TACI expression by peritoneal B-1a cells is elevated
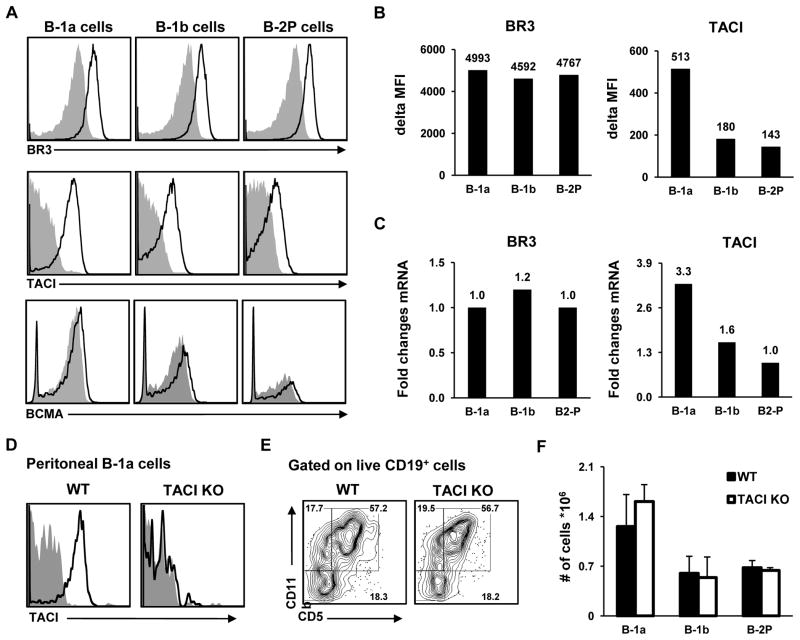
We next examined BLyS family receptor expression on PerC B cell subsets to determine whether the pattern is consistent with a role for APRIL in PerC B-1 cell homeostasis. BLyS binds to all three of the BLyS family receptors, whereas APRIL binds to TACI and BCMA, but not to BR3 (reviewed in [13]). PerC B-1 cells have been reported to express BR3 and TACI, but not BCMA [19]; however, there are conflicting observations regarding the level of TACI expression on peritoneal B-1 compared to splenic B-2 cells [19,20], and BLyS receptor expression in different perC B cell subsets has neither been reported nor compared. Accordingly, we examined expression of all three receptors by cell surface staining as well as mRNA expression (Figure 2). BR3 is expressed on the surface at similar levels among these three peritoneal subsets, whereas BCMA expression is barely detectable (Fig. 2A). TACI expression by B-1a cells is significantly higher compared with B-1b or B-2P cells, both in terms of surface protein (Fig. 2A, 2B) and mRNA transcript levels (Fig. 2C). Real-time PCR analysis of BR3 and TACI expression in the three sorted subsets confirmed surface receptor expression patterns (Fig. 2C). These data show that of the three BLyS receptors, only TACI levels differ between peritoneal B-1a and B-1b subsets, and are somewhat higher on B-1 cells than on B-2P cells. This is consistent with a role for APRIL in erC B-1a homeostasis, but does not rule out a role for B-1b or B-2P cells, and further suggests the possibility of subtle differences between B-1a and B-1b subsets.
Figure 2. Peritoneal B-1a cells show elevated TACI expression compared to B-1b and B-2P cells.
Subsets were gated as shown in Figure 1A and enriched by FACS. Surface stain histograms (A) and mean fluorescence intensity (MFI) (B) for BLyS receptors on peritoneal B cell subsets show similar expression levels for BR3, but elevated TACI expression on B-1a cells. (B) shows average difference in MFI compared to isotype control. (C) Fold change compared to universal murine RNA (= 1.0) for BR3 and TACI transcripts in sorted peritoneal B cell subsets. Compared with wild-type (WT) C57BL/6 mice, TACI KO mice show no surface TACI expression (open histograms) over isotype control (grey histograms) for peritoneal B-1a (D), B-1b, or B-2P cells (not shown). Percentages (E) and absolute numbers (F) of peritoneal B-1a, B-1b, and B-2P subsets are similar in WT and TACI KO mice.
3.3 TACI is not required for peritoneal B-1 cell persistence
We next investigated whether APRIL may work through TACI to mediate homeostasis of the peritoneal B-1 pool. TACI KO mice show increased activation and accumulation of peripheral B cells as well as autoimmune phenomena, suggesting a negative regulatory role for TACI among B-2 subsets [38,39], and there is one report of similar proportions of PerC B-1 cells between TACI KO and WT littermates [34]. We compared absolute numbers of peritoneal B-1 cell subsets between WT (C57BL/6) and TACI KO mice (C57BL/6 background) (Figure 2D) and found no differences for B-1a, B-1b, or B-2P subsets (Fig. 2E, F). Consistent with previous findings [34,39], we found almost twice the WT numbers of splenic FO and MZ subsets in TACI KO mice (not shown). These data show that despite elevated TACI expression on B-1a cells, this receptor is dispensable for PerC B-1 cell homeostasis.
3.4 HSPGs are a major binding partner for APRIL on all peritoneal B cell subsets
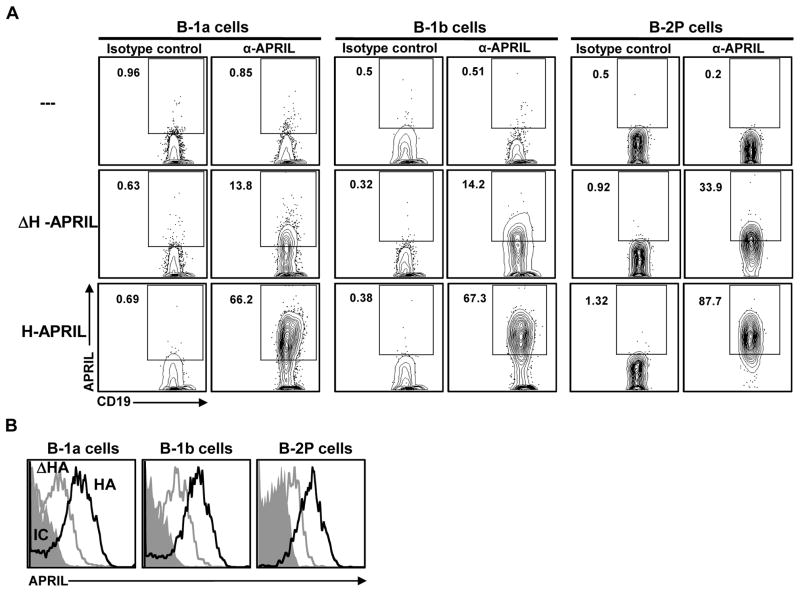
While our results indicate that APRIL is important for PerC B-1 cell development or maintenance, it appears to depend on neither TACI nor BCMA to exert its effects. However, heparan sulfate proteoglycans (HSPGs) are APRIL-specific binding partners, independent of the TACI and BCMA receptors [40,41]. Furthermore, HSPG-mediated binding of APRIL is essential for IgA production in collaboration with TACI [42], and plays a key role in plasma cell survival [43]. The crystal structure of APRIL reveals that its proteoglycan-binding domain is distinct from the TACI/BCMA binding site [41,44]. Accordingly, we employed delta HSPG-APRIL (ΔH-APRIL), which lacks the HSPG binding domain but retains the TACI/BCMA binding site, to assess APRIL binding among peritoneal B cell subsets (Figure 3). ΔH-APRIL binds to ~14% of peritoneal B-1a and B-1b cells and ~34% of B-2P cells (Fig 3A). In contrast, H-APRIL, which contains binding sites for HSPG, TACI and BCMA, binds to ~67% of peritoneal B-1a and B-1b cells and ~88% of B-2P cells (Fig 3A). H-APRIL not only binds to a greater proportion of peritoneal B cells, but also shows increased per-cell binding compared with ΔH-APRIL (Fig 3B).
Figure 3. APRIL binds HSPG on all peritoneal B cell subsets.
Peritoneal B cell subsets were gated as shown in Figure 1A, and cultured with either H-APRIL, which contains binding sites for both HSPG and TACI/BCMA; or ΔH-APRIL, which lacks the HSPG binding site. (A) Peritoneal B cells were incubated in medium alone (---), medium plus ΔH-APRIL, or medium plus H-APRIL for 30 minutes at 4°C, then washed and stained with anti-APRIL antibody. (B) Representative surface stain histograms for subsets shown in (A), and stained with isotype control antibody (grey histogram) or anti-APRIL antibody and previously incubated with ΔH-APRIL (grey line) or H-APRIL (black line).
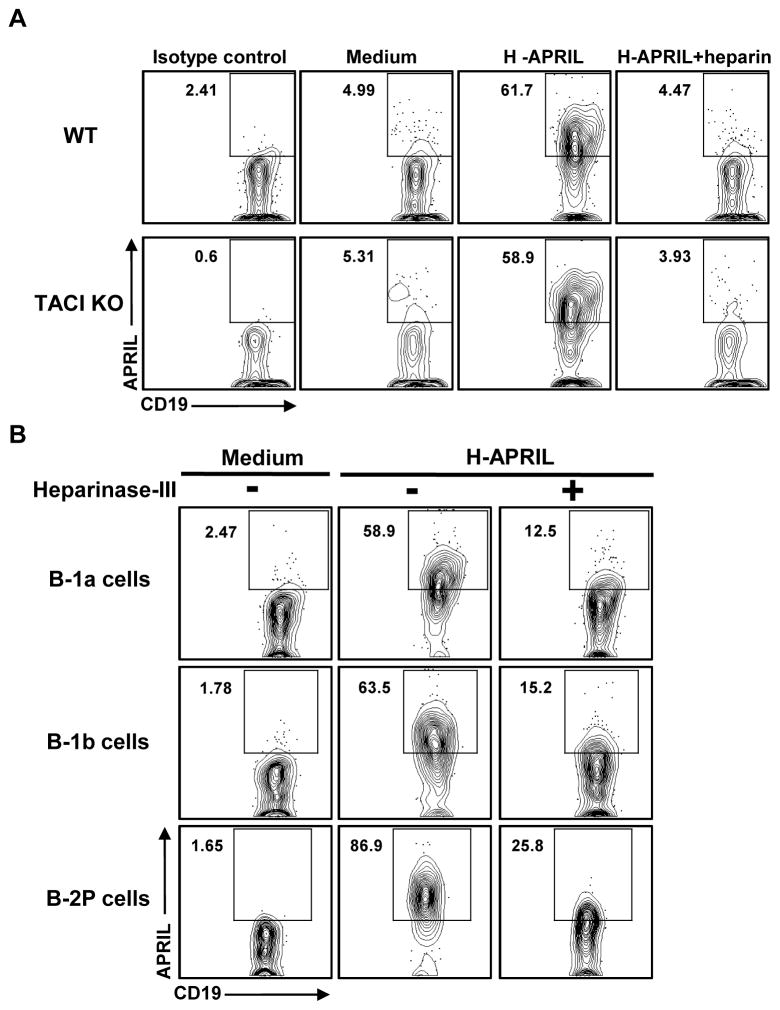
To further examine the APRIL-HSPG interaction on peritoneal B cells, we used two approaches to abrogate binding (Figure 4). First, we treated H-APRIL with heparin in order to mask the N-terminal HSPG recognition domain [40,41] prior to incubation with peritoneal B cells from WT and TACI KO mice. H-APRIL binding to B-1a cells of both strains is markedly reduced when H-APRIL is pre-incubated with heparin (Fig. 4A). Similar results were observed for peritoneal B-1b and B-2P cells from WT and TACI KO mice (not shown). Our second approach was to pre-treat cells with Heparinase-III, which cleaves the heparan sulfate side chains of HSPG [40,41], prior to incubation with H-APRIL. Binding is again markedly decreased in all peritoneal subsets (Fig. 4B). Taken together, these results indicate that HSPG, rather than TACI receptors, are a key binding partner for APRIL on peritoneal B cell subsets.
Figure 4. HSPG, but not TACI receptors, are required for APRIL binding to peritoneal B cell subsets.
(A) Peritoneal B-1 cell from wild type (WT) C57BL/6 mice or TACI KO mice were incubated in medium alone, medium plus H-APRIL, or heparin treated H-APRIL for 30 minutes at 4°C, then washed and stained with anti-APRIL antibody. B) Untreated or heparinase-III treated WT peritoneal B-1 cells were incubated in medium alone or medium plus H-APRIL for 30 minutes at 4°C, then washed and stained with anti-APRIL antibody.
3.5 Macrophages are a major source of APRIL in the peritoneum
Both APRIL and BLyS re found as circulating soluble trimers and oligomers (for example, [45]), but the question arises as to potential sources of these cytokines in the peritoneal cavity. Macrophages and B cells comprise a significant proportion (≥ 80%) of peritoneal cells [35]. Moreover, monocytes/macrophages are the principal source of APRIL in lymph nodes [46]. We therefore sorted B-1a, B-1b, B-2P, and macrophages from the peritoneum and examined BLyS and APRIL mRNA expression in each subset (Table I). Our results suggest that macrophages are an abundant source of APRIL, but not BLyS, in the peritoneal cavity. This is consistent with a role for APRIL, rather than BLyS, in modulating B cell homeostasis in the peritoneum.
Table I.
| BLyS | APRIL | |
|---|---|---|
| Universal murine RNA | 1.00 | 1.00 |
| Peritoneal B-1a cellsa | 0.02b | 0.03b |
| Peritoneal B-1b cellsa | 0.01b | 0.04b |
| Peritoneal B-2P cellsa | 0.03b | 0.02b |
| Peritoneal macrophagesa | 1.64b | 8.26b |
Peritoneal B cell subsets were gated as shown in Figure 1A, with peritoneal macrophages gated as B220− IgM− CD11bhi and enriched by FACS.
Numbers show fold change compared to universal murine RNA (= 1.0) for BLyS and APRIL transcripts in sorted peritoneal cell subsets.
4. Discussion
These studies have employed genetic and biochemical approaches to analyze the roles of BLyS family ligands and receptors in PerC B-1 cell homeostasis. The results indicate that PerC B-1 cell numbers are maintained in large part by APRIL, rather than BLyS. However, the effects of APRIL appear to be independent of either TACI or BCMA, and the bulk of APRIL-binding to PerC B-1 cells depends on HSPGs. Finally, we show that macrophages are likely a major source of APRIL in the PerC microenvironment. Together, these findings indicate that the homeostatic control of B2 and PerC B1 cells are mediated by distinct BLyS family members. Further, these findings help to reconcile the seemingly discrepant findings that PerC B-1 cells are reduced in APRIL KO but not TACI KO mice.
Despite their critical roles in B2 lineage homeostasis, neither BLyS nor BR3 plays a major role in PerC B1 production or maintenance. This has been evidenced by prior studies that show no changes in B1 B cell numbers or turnover in BR3 mutant mice [21], as well as insubstantial changes in peritoneal B1 cell numbers in BLyS transgenics, BLyS knockout mice, or during BLyS-specific neutralization [25–27]. Conversely, previous studies of APRIL-deficient mice [30,31,47] demonstrate that the development, maturation, proportions, and numbers of all B2 subsets are normal. Thus, the BLyS-BR3 axis appears to be unique and non-redundant in the control of primary B-2 cell numbers, selection, and turnover.
In contrast, there is mounting evidence that B-1 cells occupy a separate and independently controlled homeostatic niche, distinct from the B-2 compartment. This is consistent with the different developmental pathways, BCR repertoires, and anatomic locations of cells of the two lineages [48,49]. Here we provide evidence that APRIL-HSPG interactions play a partial but significant role in peritoneal B-1 cell persistence. Regarding two prior reports of APRIL knockouts, Varfolomeev et al. did not examine B-1 cells [31], whereas Castigli et al. reported similar percentages of PerC B-1 cells in APRIL KO and wild-type littermate mice [30]. One possible explanation for the discrepancy with our results is the typically low cellularity of peritoneal lavage (see section 2.1), raising the possibility that differences in absolute numbers of peritoneal B-1 cells would not be reflected in frequency plots. In addition, the genetic background of the mice used for the earlier study was mixed (C57BL/6 and 129/Sv) [30], raising the possibility of variation in peritoneal B-1 numbers for both knockouts and wild-type littermates. Castigli et al. [30] generously provided APRIL KO mice to one of the authors of this paper (W.S.), and the mice used here resulted from at least 10 backcrosses to strain C57BL/6 [37]. Our APRIL KO mice showed a significant reduction in PerC B-1 numbers, and there was no further reduction in the BLyS/APRIL DKO mice, indicating a major and non-redundant role for APRIL in PerC B-1 maintenance.
The apparent discrepancy between reduced B-1 numbers in our APRIL knockout mice, yet normal B-1 numbers in TACI knockouts, is explained in part by our biochemical data indicating that HSPG are the key APRIL binding partner on PerC B-1 cells (discussed further below). Although B-2 cells can bind APRIL through both TACI and HSPG [40,41], these associations do not appear to be critical to primary B cell homeostasis, since developing and mature B-2 subsets remain normal in APRIL knockout mice (our results and [30,31]).
Here we show for the first time that APRIL, likely through HSPG dependent events, plays a role in peritoneal B-1 cell homeostasis. APRIL can bind to TACI, BCMA and HSPG [36,41,50–52]. Of these, we find that BCMA is not expressed by peritoneal B-1 cells, and TACI is neither required for B-1 cell maintenance nor for APRIL binding to B-1 cells. Instead, HSPG are likely responsible for most APRIL binding. This suggests that HSPG expression is important for peritoneal B-1 cell maintenance. Indeed, many previously unappreciated roles for HSPG in B cell development and function are emerging. HSPG expression is regulated during BM B cell development, and syndecan-1 (CD138) appears to be a co-receptor for APRIL-mediated survival of normal and multiple myeloma plasma cells (reviewed in [53]). In mice, there is evidence that the pro- to pre-B cell transition is regulated by the HSPG sulfation pattern [54]; moreover, syndecan-1 expression distinguishes BM progenitors for B-1a, B-1b, and B-2 lineages [43,55,56].
Our APRIL-deficient mice still have some B-1 cells in the peritoneal cavity, indicating that alternative survival factors may act during peritoneal B-1 development, or are required for persistence of an APRIL-independent feeder pool within this particular locale. Indeed, a recent report shows that FcγRIIb regulates PerC B-1 survival, while BLyS rescues FcRIIb-mediated apoptosis of peritoneal B-1 cells [57]. A subtle role for BLyS is indicated by our prior study where BLyS neutralization led to a moderate (non-significant) reduction in splenic but not peritoneal cavity B-1 cells [25]. Additional studies show that although BLyS is not absolutely required for B-1 cell survival, it may somewhat augment B-1a cell numbers in some anatomic locations, including spleen and lymph nodes as well as the peritoneal cavity [16,26,27]. Although further work will be required to determine definitively whether and how BLyS affects B-1 cell development or survival in various anatomic locales, there is evidence that BLyS modulates peritoneal B-1 cell function: it acts via BR3 as a costimulator of proliferation and activation in response to TLR9 ligands [19], and abrogates IL10-induced downregulation of responses to TLR4 ligands [58].
If APRIL is important for PerC B-1 persistence, the question arises as to potential sources of this cytokine. The APRIL knockouts previously mentioned have normal overall percentages and numbers of T cells, as well as neutrophils, NK cells, and monocytes [30,31], with increased effector/memory T cell numbers in one case [30]. All of these cell types have been reported to express APRIL, with particularly high levels by macrophages [47,59,60]. Our work identifies macrophages as the likely source of most APRIL in the peritoneal cavity. There is mounting evidence in both mice and humans that local, myeloid cell sources of APRIL are key to plasma cell persistence in bone marrow, lymph nodes, and mucosal tissues [11,46,61–63]. This suggests the possibility that antibody-secreting B-1 cells may have similar requisites for establishment of a persistent survival niche.
Highlights.
APRIL, but not BLyS, mediates peritoneal B-1 cell maintenance
TACI expression is elevated on B-1a cells, but is not required for B-1 persistence
HSPG are a key binding partner for APRIL on all peritoneal B cell subsets
Peritoneal macrophages may be a local source of APRIL
Acknowledgments
The authors thank Thi-Sau Migone and Human Genome Sciences, Inc. for providing the TACI-Fc reagent [36], Patrick O’Neill for expert technical assistance, and Laurie Baker for careful reading of the manuscript. This work was supported in part by a Sponsored Research Agreement (Human Genome Sciences, Inc.) and awards PR094034 (Dept. of the Army) to MPC; T32-AI055428 (NIH) to VJS; and AR050193 (NIH) to WS.
Abbreviations
- APRIL
A proliferation-inducing ligand
- BCMA
B cell maturation antigen
- B-2P
Cells of the B-2 lineage/phenotype from the peritoneal cavity
- BLyS (BAFF)
B lymphocyte stimulator (B-cell activating factor belonging to the TNF family)
- BR3
BLyS Receptor 3
- DKO
double knockout
- HSPG
heparan sulfate proteoglycans
- KO
knockout
- PerC
peritoneal cavity
- TACI
transmembrane activator and cyclophilin ligand interactor
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 2.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli KR, Gerstein RM. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23:1–2. doi: 10.1016/j.immuni.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 5.Herzenberg LA, Baumgarth N, Wilshire JA. B-1 cell origins and VH repertoire determination. Curr Top Microbiol Immunol. 2000;252:3–13. doi: 10.1007/978-3-642-57284-5_1. [DOI] [PubMed] [Google Scholar]
- 6.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 8.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Bos NA, Bun JC, Popma SH, Cebra ER, Deenen GJ, van der Cammen MJ, Kroese FG, Cebra JJ. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996;64:616–623. doi: 10.1128/iai.64.2.616-623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 11.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 12.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–891. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley JE, Scholz JL, Quinn WJ, 3rd, Stadanlick JE, Treml JF, Treml LS, Hao Y, Goenka R, O’Neill PJ, Matthews AH, Parsons RF, Cancro MP. Homeostatic control of B lymphocyte subsets. Immunol Res. 2008;42:75–83. doi: 10.1007/s12026-008-8036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 15.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 16.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 17.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009;53:1–16. doi: 10.1007/s12013-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, Mackay F, Lam KP. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur J Immunol. 2006;36:1837–1846. doi: 10.1002/eji.200635956. [DOI] [PubMed] [Google Scholar]
- 20.Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP, Kalled SL, Scott ML. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol. 2004;173:2331–2341. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- 21.Lentz VM, Cancro MP, Nashold FE, Hayes CE. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J Immunol. 1996;157:598–606. [PubMed] [Google Scholar]
- 22.Miller DJ, Hayes CE. Phenotypic and genetic characterization of a unique B lymphocyte deficiency in strain A/WySnJ mice. Eur J Immunol. 1991;21:1123–1130. doi: 10.1002/eji.1830210506. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 24.Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 25.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone TS, Shlomchik MJ, Cancro MP. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavin AL, Duong B, Skog P, Ait-Azzouzene D, Greaves DR, Scott ML, Nemazee D. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 28.Planelles L, Carvalho-Pinto CE, Hardenberg G, Smaniotto S, Savino W, Gomez-Caro R, Alvarez-Mon M, de Jong J, Eldering E, Martinez AC, Medema JP, Hahne M. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell. 2004;6:399–408. doi: 10.1016/j.ccr.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Guadagnoli M, Kimberley FC, Phan U, Cameron K, Vink PM, Rodermond H, Eldering E, Kater AP, van Eenennaam H, Medema JP. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood. 2011;117:6856–6865. doi: 10.1182/blood-2011-01-330852. [DOI] [PubMed] [Google Scholar]
- 30.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varfolomeev E, Kischkel F, Martin F, Seshasayee D, Wang H, Lawrence D, Olsson C, Tom L, Erickson S, French D, Schow P, Grewal IS, Ashkenazi A. APRIL-deficient mice have normal immune system development. Mol Cell Biol. 2004;24:997–1006. doi: 10.1128/MCB.24.3.997-1006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, Cachero TG, Finke D, Beermann F, Tschopp J. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–1697. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah HB, Joshi SK, Rampuria P, Devera TS, Lang GA, Stohl W, Lang ML. BAFF- and APRIL-dependent maintenance of antibody titers after immunization with T-dependent antigen and CD1d-binding ligand. J Immunol. 2013;191:1154–1163. doi: 10.4049/jimmunol.1300263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 35.Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010 doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, Gan Y, Cho YH, Garcia AD, Gollatz E, Dimke D, LaFleur D, Migone TS, Nardelli B, Wei P, Ruben SM, Ullrich SJ, Olsen HS, Kanakaraj P, Moore PA, Baker KP. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 37.Jacob CO, Guo S, Jacob N, Pawar RD, Putterman C, Quinn WJ, 3rd, Cancro MP, Migone TS, Stohl W. Dispensability of APRIL to the development of systemic lupus erythematosus in NZM 2328 mice. Arthritis Rheum. 2012;64:1610–1619. doi: 10.1002/art.33458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 39.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, Tumas D, Grewal IS, Dixit VM. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2:638–643. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 40.Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals ST, Hahne M, Spaargaren M, Medema JP. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–648. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- 41.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, Qiang F, Gorelik L, Kalled SL, Acha-Orbea H, Rennert PD, Tschopp J, Schneider P. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurai D, Hase H, Kanno Y, Kojima H, Okumura K, Kobata T. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood. 2007;109:2961–2967. doi: 10.1182/blood-2006-08-041772. [DOI] [PubMed] [Google Scholar]
- 43.Reijmers RM, Groen RW, Kuil A, Weijer K, Kimberley FC, Medema JP, van Kuppevelt TH, Li JP, Spaargaren M, Pals ST. Disruption of heparan sulfate proteoglycan conformation perturbs B-cell maturation and APRIL-mediated plasma cell survival. Blood. 2011;117:6162–6171. doi: 10.1182/blood-2010-12-325522. [DOI] [PubMed] [Google Scholar]
- 44.Wallweber HJ, Compaan DM, Starovasnik MA, Hymowitz SG. The crystal structure of a proliferation-inducing ligand, APRIL. J Mol Biol. 2004;343:283–290. doi: 10.1016/j.jmb.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 45.Stohl W, Metyas S, Tan SM, Cheema GS, Oamar B, Roschke V, Wu Y, Baker KP, Hilbert DM. Inverse association between circulating APRIL levels and serological and clinical disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2004;63:1096–1103. doi: 10.1136/ard.2003.018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan IC. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 47.Stein JV, Lopez-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodriguez D, Gomez-Caro R, De Jong J, Martinez AC, Medema JP, Hahne M. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sindhava VJ, Scholz JL, Cancro MP. Roles for BLyS family members in meeting the distinct homeostatic demands of innate and adaptive B cells. Front Immunol. 2013;4:37. doi: 10.3389/fimmu.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 51.Kimberley FC, van Bostelen L, Cameron K, Hardenberg G, Marquart JA, Hahne M, Medema JP. The proteoglycan (heparan sulfate proteoglycan) binding domain of APRIL serves as a platform for ligand multimerization and cross-linking. FASEB J. 2009;23:1584–1595. doi: 10.1096/fj.08-124669. [DOI] [PubMed] [Google Scholar]
- 52.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, Hsu YM, Whitty A. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–1931. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 53.Reijmers RM, Spaargaren M, Pals ST. Heparan sulfate proteoglycans in the control of B cell development and the pathogenesis of multiple myeloma. FEBS J. 2013;280:2180–2193. doi: 10.1111/febs.12180. [DOI] [PubMed] [Google Scholar]
- 54.Buono M, Visigalli I, Bergamasco R, Biffi A, Cosma MP. Sulfatase modifying factor 1-mediated fibroblast growth factor signaling primes hematopoietic multilineage development. J Exp Med. 2010;207:1647–1660. doi: 10.1084/jem.20091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tung JW, Mrazek MD, Yang Y, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci U S A. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amezcua Vesely MC, Schwartz M, Bermejo DA, Montes CL, Cautivo KM, Kalergis AM, Rawlings DJ, Acosta-Rodriguez EV, Gruppi A. FcgammaRIIb and BAFF differentially regulate peritoneal B1 cell survival. J Immunol. 2012;188:4792–4800. doi: 10.4049/jimmunol.1102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sindhava V, Woodman ME, Stevenson B, Bondada S. Interleukin-10 mediated autoregulation of murine B-1 B-cells and its role in Borrelia hermsii infection. PLoS One. 2010;5:e11445. doi: 10.1371/journal.pone.0011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 60.Pradet-Balade B, Medema JP, Lopez-Fraga M, Lozano JC, Kolfschoten GM, Picard A, Martinez AC, Garcia-Sanz JA, Hahne M. An endogenous hybrid mRNA encodes TWE-PRIL, a functional cell surface TWEAK-APRIL fusion protein. EMBO J. 2002;21:5711–5720. doi: 10.1093/emboj/cdf565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf AI, Mozdzanowska K, Quinn WJ, 3rd, Metzgar M, Williams KL, Caton AJ, Meffre E, Bram RJ, Erickson LD, Allman D, Cancro MP, Erikson J. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J Clin Invest. 2011;121:3954–3964. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]