Abstract
The alteration in expression of B cell lymphoma-2 (Bcl-2) family of protein members in cancer is involved mainly in the regulation of apoptosis. Bcl-2 family proteins are currently used as major targets in the development of methods to improve treatment outcomes for cancer patients that underwent clinical trials. Although many agents have been developed for targeting Bcl-2 in the past decade, some previous attempts to target Bcl-2 have not resulted in beneficial clinical outcome for reasons unknown. Here, we propose that this was due in part for not considering the cellular level of a different antiapoptotic protein, i.e., galectin-3 (Gal-3). Gal-3 is a member of the β-galactoside binding protein family and a multifunctional oncogenic protein which regulates cell growth, cell adhesion, cell proliferation, angiogenesis, and apoptosis. Gal-3 is the sole protein that contains the NWGR anti-death motif of the Bcl-2 family and inhibits cell apoptosis induced by chemotherapeutic agents through phosphorylation, translocation and regulation of survival signaling pathways. It is now established that Gal-3 is a candidate target protein to suppress antiapoptotic activity and anticancer drug resistance. In this review, we describe the role and relevance of Gal-3 and Bcl-2 protein family in the regulation of apoptosis and propose a novel combination therapy modality. Combination therapy that targets Gal-3 could be essential for improvement of the efficacy of Bcl-2 targeting therapy in cancers and should be studied in future clinical trials. Otherwise, not considering Gal-3 cellular level could lead to trial failure.
Keywords: Apoptosis, Bcl-2, Galectin-3, Therapy
1 Introduction
Apoptosis was first formally described and named in 1972 as a stereotypic morphological response to many different types of cellular insult different from necrosis. Apoptosis is one of the major mechanisms of cell death in response to cancer therapies [1, 2]. Inhibiting apoptosis is widely accepted as a crucial step in the transition from normal to cancer cells. Alterations in susceptibility to apoptosis not only contribute to neoplastic development but also can enhance resistance to conventional anticancer therapies, such as radiation and cytotoxic agents [3].
One of the suggested mechanisms of resistance to cytotoxic antineoplastic drugs is the alteration in expression of B cell lymphoma-2 (Bcl-2) family of protein members. The Bcl-2 family of proteins consists of 25 pro- and antiapoptotic members, which interact to maintain a balance between newly forming cells and old dying cells. The phosphorylation at Ser70 of Bcl-2 by protein kinase C may activate or inactivate its antiapoptotic function, depending on cell type and death-signaling molecules [4–11]. Accumulating evidence points to a crucial role for the Bcl-2 family in regulating apoptosis in cancer cells, and it is clear that exploiting this relationship is an attractive approach for novel anticancer agents [12]. However, some attempts to target Bcl-2 clinically have not demonstrated major antitumor activity [13].
Galectin-3 (Gal-3) is a member of animal lectins which are a family of carbohydrate-binding proteins characterized by their affinity for β-galactoside and a sequence of the carbohydrate recognition domain (CRD). Gal-3, a 31-kDa unique chimeric gene product, consists of three structural domains: an NH2-terminal domain, repeated collagen-like sequence, and COOH-terminal containing a single CRD [14, 15]. Gal-3 is widely expressed in cancer cells as well as in epithelial and immune cells [16–20]. The expression of Gal-3 is related with tumor invasion and metastatic potential of several types of cancer [17, 19–22]. It has been shown that Gal-3 is a multi-functional oncogenic protein and regulates cell growth, cell adhesion, cell proliferation, angiogenesis, and apoptosis [14, 23–30]. Of note, Gal-3, which contains the Asp-Trp-Gly-Arg (NWGR) anti-death motif of the Bcl-2 family, inhibits cell apoptosis induced by some chemotherapeutic agents in cancer cells [31–35]. In addition, nuclear export of Gal-3 phosphorylated at Ser6 regulates its antiapoptotic activity in response to chemotherapeutic drugs [36–39]. These apparent similarities between Bcl-2 and Gal-3 in their antiapoptotic functions and the posttranslational modification of serine phosphorylation lead us to surmise the possibility that targeting Gal-3 with anti-Bcl-2 treatment might be therapeutically valuable.
This review presents the current knowledge of roles of Bcl-2 family and Gal-3 in the regulation of apoptosis. Furthermore, we will discuss the clinical significances and future perspectives of Gal-3 as improvement of the efficacy of anti-cancer drug chemotherapy.
2 The classification of the Bcl-2 family of proteins
To date, 25 members of the Bcl-2 family of proteins have been identified [40]. These proteins are localized to mitochondria, smooth endoplasmic reticulum, and perinuclear membranes. They are divided into three subfamilies according to their pro-and antiapoptotic effects and the presence of Bcl-2 homology (BH) domains [41]. The proapoptotic Bcl-2 family members are subdivided into two classes: the multidomain effector proteins and the BH3-only proteins. The multidomain members include Bcl-2-associated x protein (Bax) and Bcl-2 homologous agonist killer (Bak). They contain structural features of BH 1–3 domains and are critically important because when they are activated, they change from monomers to oligomers that disrupt the integrity of the mitochondrial outer membrane—a process called mitochondrial outer membrane permeabilization (MOMP) [12, 42, 43]. MOMP causes the leakage of components such as cytochrome c from the inter-membrane space of mitochondria into the cytoplasm. Other proapoptotic proteins, such as Bcl-2 interacting mediator of cell death (Bim), BH3 interacting domain death agonist (Bid), Bcl-2-associated death promoter (Bad), p53 upregulated modulator of apoptosis (Puma), and Noxa (also known as PMAIP1) contain only the BH3 domain. BH3-only proteins can bind to and regulate the antiapoptotic Bcl-2 proteins to promote apoptosis. BH3-only protein signaling is essential for the initiation of the mitochondrial apoptotic pathway. On the other hand, the antiapoptotic subfamily contains the Bcl-2, Bcl-2-related gene long isoform (Bcl-XL), B cell lymphoma-w (Bcl-w), myeloid cell leukemia-1 (Mcl-1), and so on, which suppress apoptosis and possess three or four BH domains [44]. These antiapoptotic proteins all promote cell survival by inactivating their proapoptotic Bcl-2 family counterparts and preserving mitochondrial outer membrane integrity [41].
3 The interaction among the Bcl-2 family of proteins
Generally, two major pathways lead to caspase activation and apoptosis in cells: the extrinsic pathway and the intrinsic pathway. The extrinsic cell death pathway can function independently of mitochondria and is triggered by Fas ligand or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), subsequently activating caspase 8. Caspase 8 transforms Bid into truncated Bid (tBid), which activates the intrinsic apoptotic pathway and initiates a cascade of caspase activation [45]. By contrast, the intrinsic cell death pathway, also known as the mitochondrial apoptotic pathway, is activated by various signals including radiation, cytotoxic drugs, cellular stress, and growth factor withdrawal and involves the release of components such as cytochrome c from the mitochondrial membrane space.
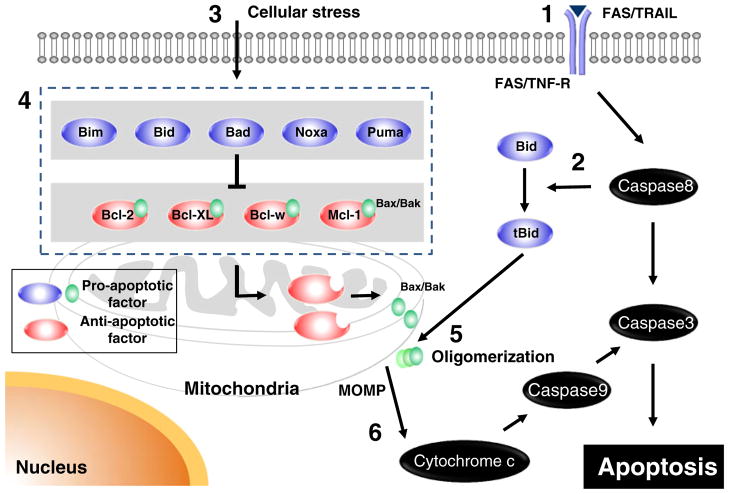
Most cells express a variety of both anti- and proapoptotic Bcl-2 proteins, and the interaction between proteins within this family dictates whether a cell survives or dies [46]. Here, we provide an overview of the role of Bcl-2 family in apoptosis (Fig. 1). However, the exact mechanisms of how Bcl-2 proteins interconnect to regulate MOMP and apoptosis after these signals have been controversially discussed [12, 43, 44].
Fig. 1.
The role of the Bcl-2 family of proteins in apoptosis. 1 The extrinsic pathway is activated by Fas ligand or TRAIL, subsequently activating caspase 8. 2 Caspase 8 transforms Bid into truncated Bid (tBid). In addition, caspase 8 initiates a cascade of caspase activation. 3 Diverse forms of extracellular stress, (DNA damage, cytotoxic drugs, and cytokine withdrawal) initiate the intrinsic pathway. 4 BH3-only proteins (Bim, Bid, Bad, Noxa, and Puma) engage with antiapoptotic Bcl-2 family proteins (Bcl-2, Bcl-XL, Bcl-w, and Mcl-1) to relieve their inhibition of Bax and Bak to activate them. 5 Bax and Bak are oligomerized and activated, leading to mitochondrial outer membrane permeabilization (MOMP). 6 Once mitochondrial membranes are permeabilized, cytochrome c is released into the cytoplasm, resulting in activation of caspase 9, subsequently caspase 3, which is the initiation step for the cascade of caspase activation
The “direct activation” model divides BH3-only proteins into two groups, which are activators (such as Bim, tBid, and maybe Puma) and sensitizers (such as Bad or Noxa). Activators directly bind to Bax/Bak and induce their oligomerization, resulting in Bax/Bak pore formation and MOMP. Antiapoptotic Bcl-2 proteins can sequester the BH3-only activators and prevent Bax/Bak oligomerization. On the other hand, sensitizers act as decoys and inhibit engaging of antiapoptotic Bcl-2 proteins with activators or Bax/Bak [47, 48].
The second “derepression” model suggests that Bax/Bak is always active, and the antiapoptotic Bcl-2 proteins prevent cell death by binding to Bax/Bak. The role of the BH3-only proteins is to bind to the antiapoptotic Bcl-2 family proteins to release active Bax/Bak. The active Bax/Bak is then able to integrate into the mitochondrial outer membrane, create oligomerization, and form pores resulting in MOMP [49, 50].
Recently, Leber et al. proposed the third “embedded together” model, which combines features of both models [12, 51]. In this model, antiapoptotic Bcl-2 proteins sequester both active Bax and BH3-only activators, and BH3-only sensitizers displace Bax and BH3-only activators from the antiapoptotic proteins.
These significant interactions occur at the mitochondrial outer membrane. However, further investigation is needed to fully elucidate the mechanisms of how Bcl-2 family proteins bind to each other. It has been shown that antiapoptotic Bcl-2 family members contain a hydrophobic binding pocket formed by the folding of their BH1, BH2, and BH3 domains, and BH3-only proteins can bind into this groove via their BH3 domain [12, 41, 46]. Bcl-2/Bax heterodimerization, which prevents Bax oligomerization, is also very important for antiapoptotic function. Interestingly, the NWGR motif in Bcl-2 has been shown to be critical for Bcl-2/Bax heterodimerization [52].
4 Antiapoptotic Bcl-2 family members as targets for cancer treatment
Pathologic overexpression of the antiapoptotic Bcl-2 family proteins subverts the natural apoptotic response and contributes to tumor initiation and progression as well as to resistance to chemotherapy. Consequently, evidence about a crucial role for the Bcl-2 family in regulating apoptosis in cancer suggests that these family members are attractive targets for the treatment of cancers [53]. Although many agents have been developed, they have some problems individually.
The first agent targeting Bcl-2 that entered clinical trials was a Bcl-2 antisense, oblimersen sodium, an 18 mer anti-sense oligonucleotide designed to target the first six codons of Bcl-2 mRNA [54]. The combination treatment of oblimersen with an anticancer drug increased the chemotherapeutic effect in phase I studies [55–57]. In phase II and III clinical trials [44], the addition of oblimersen improved clinical outcomes in combination with other anticancer chemotherapeutic agents in patients with melanoma and relapse or refractory CLL [58, 59], while it failed to improve outcomes in patients with small cell lung cancer (SCLC) [60].
Gossypol, a polyphenol derived from the cottonseed plant, was the first natural compound discovered that demonstrated inhibition of Bcl-2, Bcl-XL, and Mcl-1 [61]. In preclinical studies, many groups have shown gossypol’s potent proapoptotic activity [62]. Further phase I and II trials are ongoing to evaluate L -gossypol (AT-101) in combination with conventional chemotherapeutics including SCLC and non-SCLC, CLL, prostate cancers, and glioblastomas (AT-101, http://clinicaltrials.gov). However, gossypol has toxicity problems, most likely due to two reactive aldehyde groups [63] and is also known to cause male infertility [64].
The two Bcl-2 inhibitor drugs furthest in clinical development are GX15-070 (obatoclax) and ABT-737. Obatoclax was discovered as a result of a high-throughput screen of natural compounds that disrupted protein interactions in the Bcl-2 family and was the first pan antiapoptotic Bcl-2 protein inhibitor to be described [65]. This small molecule indole bipyrrole compound has been shown to bind to all antiapoptotic Bcl-2 family and disrupt protein–protein interactions in the family in vitro [66]. Obatoclax has been tested in phase I clinical trials in patients with hematological and myeloid malignancies and was well tolerated [67, 68]. However, in more recent phase II studies in patients with relapsed or refractory classical Hodgkin lymphoma and relapsed SCLC, obatoclax displayed limited clinical activity [69, 70]. Interestingly, Vogler et al. suggested that the mechanism of cell killing by obatoclax in vitro is not exclusively via the Bcl-2 family-regulated mitochondrial apoptosis pathway [64], and limited clinical activity may be because of inadequate inhibition of Bcl-2 family proteins [13]. Obatoclax had also been reported to induce both neurological symptoms in early clinical trials of patients with CLL as well as neuronal toxicity in mice [67, 71], which might be because of targets outside the Bcl-2 family.
Perhaps, the most advanced compounds are the ABT-737 and ABT-263 molecules. ABT-737 was developed as a rational Bcl-2 inhibitor using nuclear magnetic resonance structure-based design with the BH3 region of Bad [72]. ABT-737 binds to and inhibits Bcl-2, Bcl-XL, and Bcl-w with higher affinities than any of other Bcl-2 inhibitors. This drug exhibits a dependence on key components of the intrinsic apoptotic pathway, including Bax, Bak, and caspase 9 [64] and is extremely effective at enhancing the cytotoxicity of a variety of chemotherapy agents in acute lymphoblastic leukemia in vitro and in vitro [73]. However, ABT-737 has problems for drug delivery and does not bind to Mcl-1, with resistance observed in cells that express Mcl-1 [74, 75]. To overcome the delivery problems, ABT-263 (Navitoclax) was developed for use in the clinic. Navitoclax is an oral version of ABT-737 and shares a similar binding profile and affinities to Bcl-2, Bcl-XL, and Bcl-w proteins. It is active as a single agent in SCLC xenografts and enhanced the activity of chemotherapy agents in preclinical studies or phase I studies of B cell lymphoma, multiple myeloma, and SCLC [76–78]. Although many clinical trials are currently underway, Bcl-2 targeting by Navitoclax showed limited single-agent activity against advanced and recurrent SCLC in phase II study, which has recently been completed [79]. Some strategies are also being developed to complement the activity by neutralizing Mcl-1 [80–82].
Efforts to target the Bcl-2 family for cancer therapy have yielded remarkable advances in the past decade. However, a single BH3 mimetic to Bcl-2 family may not be sufficient as monotherapeutic to cure cancer patients. Furthermore, accumulating clinical trial failures lead us to predict other factor related with the regulation of apoptosis or anticancer drug resistance by Bcl-2 family proteins.
5 Regulation of apoptosis by Gal-3 in cancer cells
The galectins are a family of mammalian β-galactoside binding proteins that share highly conserved CRDs. To date, 15 galectin members have been identified, and they are classified into three subgroups depending on their structural differences and the number of CRDs within their polypeptide chains [14, 83]. Gal-3 is the exclusive member of the chimera-type galectin subgroup and contains one CRD. Gal-3 is expressed broadly in many tumor cells, and clinical evidences have shown that the expression of Gal-3 is associated with the carcinogenesis and malignant potential in melanoma; lymphoma; and thyroid, gastric, colon, uterine, and renal cancers [16–22, 84, 85].
The roles of Gal-3 in human cancer are well documented [86]. Gal-3 has been shown to be involved in cell growth, cell proliferation, cell differentiation, cell adhesion, angiogenesis, apoptosis, tumor progression and metastasis mainly through binding to glycoproteins. In particular, Gal-3 is shown to be involved in the regulation of apoptosis [30]. The process of the antiapoptotic action includes phosphorylation, translocation, and regulation of survival signaling and the caspase pathway (Fig. 2) [87].
Fig. 2.
Model for the regulation of apoptosis by Gal-3. Anticancer drugs can induce DNA damage, which causes Gal-3 phosphorylated by Casein Kinase 1 (CK1) translocate from the nuclear to cytoplasm. Gal-3 upregulates the ERK pathway and induces Bad phosphorylation, leading to mitochondrial stabilization. Akt activated by Gal-3 inhibits apoptosis by blocking transformation of Bid into tBid, which is essential for cytochrome c release from the mitochondrial intermembrane space. After treatment of proapoptotic agents, Gal-3 in cytoplasm decreases Bad expression and attenuates the depolarization of the mitochondrial membrane. Consequently, the stabilization of the mitochondrial membrane prevents cytochrome c release and subsequent caspase activation, resulting in suppression of apoptosis and resistance to chemotherapeutic agents
Phosphorylation of Gal-3 plays the role of an “on–off” switch for protein–carbohydrate interactions. The major site of phosphorylation is Ser6 and a minor site is Ser12, as identified by mass spectrophotometric analysis [88, 89]. Overexpression of wild-type Gal-3 inhibits apoptosis induced by stimuli, while substitution of Ser6 of Gal-3 by the non-phosphorylatable alanine inhibits its antiapoptotic function [34]. These results demonstrate that Gal-3 phosphorylation is critical for the regulation of its antiapoptotic signaling activity.
Recent evidence has shown that Gal-3 translocates either from the cytosol or the nucleus to the mitochondria following exposure to apoptotic stimuli such as anticancer drugs and block changes in the mitochondrial membrane potential, thereby preventing apoptosis [39, 90]. Phosphorylated wild-type Gal-3 exported from the nucleus to the cytoplasm and protected cancer cells from drug-induced apoptosis while non-phosphorylated Ser6 mutant Gal-3 is neither exported from the nucleus nor protected cancer cells from drug-induced apoptosis [36]. Other groups have also reported that Gal-3 is found in the cytoplasm and perinuclear mitochondrial membranes, where it is involved in the control of apoptosis [38, 91]. The translocation of Gal-3 is carried out via at least two independent nuclear pathways: a passive diffusion and an active transport [92]. Synexin, a calcium-dependent and phospholipid-binding protein, contributes to the translocation of Gal-3 to mitochondria [39]. The translocation of Gal-3 is necessary for its effect on apoptosis regulation.
Several reports suggest that intracellular Gal-3 may directly affect the mitochondrial integrity, leading to the inhibition of both cytochrome c release from mitochondria and the downstream effector caspase-3 [39, 87]. Gal-3 can also attenuate the depolarization of the mitochondrial membrane through decrease of Bad expression [35]. In addition, Gal-3 has been shown to regulate the expression of several molecules in the survival signaling pathway. Firstly, phosphorylated Gal-3 increases the activity of the mitogen-activated protein kinase pathway, such as pERK, which is known to be involved in the regulation of mitochondrial stability and apoptosis, subsequently induces Bad phosphorylation [36]. Akt activated by Gal-3 also inhibits apoptosis by blocking transformation of Bid into tBid, which is essential for cytochrome c release from the mitochondrial intermembrane space [93]. Accumulating data strongly suggest that Gal-3 has a significant relevance to Bcl-2 family proteins in the regulation of apoptosis and is a candidate target protein to suppress antiapoptotic activity.
6 Clinical perspective of Gal-3
Many researchers, who consider Gal-3 as a therapeutic target for several cancers, have invented and developed the inhibitor for it [94]. The thiodigalactoside diester Td131_1 has been reported as a highly specific small molecule inhibitor of Gal-3 [95]. This small molecule has a high affinity and specificity for Gal-3 due to the specific interactions of its two ester moieties with Arg144 and Arg186 of Gal-3. Td131_1 was shown to promote apoptosis, chemosensitivity, and radiosensitivity in papillary thyroid cancer (PTC) cell lines and ex vivo PTC [96]. Moreover, as one of the candidates for the treatment of cancers by the application of a Gal-3 inhibitory approach, a novel therapy has been reported using modified citrus pectin (MCP). GCS-100, which is a MCP in the clinical development, binds to Gal-3 as an antagonist by targeting the CRD. Activity has been observed in animal melanoma and breast, colon, and prostate cancer models [97–99] and in phase I studies in patients with relapsing or refractory colorectal cancer and CLL [100]. Studies of the safety of GCS-100 in patients with CLL and multiple myeloma are ongoing (GCS-100, http://clinicaltrials.gov). It has also been reported to show anti-myeloma activity with synergy in combination with dexamethasone, bortezomib, or PI11195 without affecting normal lymphocyte viability in preclinical studies [101].
As mentioned above, Mcl-1 has been recognized as an important determinant of chemoresistance. Interestingly, GCS-100 is shown to induce dose- and time-dependent decreases in Mcl-1 and Bcl-XL levels via caspase-dependent pathway accompanied by a rapid induction of Noxa protein [100]. Thus, GCS-100 not only suppresses Gal-3 multiple role in the regulation of apoptosis but also complements the effect of Bcl-2 targeting therapy by neutralizing Mcl-1. Furthermore, we suggest another mechanism of how targeting the CRD of Gal-3 relates with Bcl-2 family in the regulation of apoptosis. Although Gal-3 is not a member of the Bcl-2 family, it is possible that Gal-3 can sequester Bax like antiapoptotic Bcl-2 family proteins and regulate apoptosis because Gal-3 shares significant structural properties with Bcl-2. Both proteins are rich in proline, glycine, and alanine amino acid residues in their N-terminal and contain the NWGR motif in the C-terminal domain. This NWGR motif designated as the anti-death motif is found in the BH-1 domain of Bcl-2 [102]. Intriguingly, the NWGR motif in Bcl-2 is significant for Bcl-2/Bax heterodimerization, which prevents Bax oligomerization [52]. It was also reported that an amino acid substitution of Gly to Ala in the NWGR motif of Gal-3 abrogated its apoptosis-resistance properties [31, 91]. Thus, it is not unreasonable to expect that Gal-3 can replace or mimic Bcl-2 by binding to Bax using this motif and regulate antiapoptosis. Since sugar-binding antagonists of Gal-3 such as GCS-100 targets the CRD domain, they may occupy the NWGR motif in the CRD, subsequently resulting in release of Bax from Gal-3 and Bax oligomerization. Gal-3 might be a mitochondrial associated apoptotic regulator through interaction with Bcl-2 family proteins.
The best results for patients with cancer may be achieved by appropriate drug combinations [43]. Finally, we propose a novel combination therapy model in Fig. 3 based on the following: (a) Gal-3 has multiple role in the regulation of apoptosis; (b) GCS-100, an antagonist of Gal-3, decreases Mcl-1 expression, which has been recognized as a determinant of chemoresistance; and (c) it is possible that Gal-3 might replace or mimic antiapoptotic Bcl-2 and regulate antiapoptosis by binding to Bax using the NWGR motif. For example, ABT-263 binds to and inhibits Bcl-2, Bcl-XL, and Bcl-w, resulting in effective enhancement of the cytotoxicity of several chemotherapy agents. However, in the instance where cancer cells highly express Mcl-1, ABT-263 may not result in substantial antitumor activity clinically. The suppression of Mcl-1 via the caspase-dependent pathway by Gal-3 inhibitor is useful for cancers that highly express Mcl-1 and show resistance to ABT-263. Moreover, the inhibition of Gal-3 roles in other apoptotic and survival signaling pathways against cancers that express elevated levels of Gal-3 will help Bcl-2 targeting therapy efficiency. In addition, targeting the CRD of Gal-3 may induce the release of Bax from Gal-3, subsequently resulting in MOMP.
Fig. 3.
A novel combination therapy model. a ABT-263 (Navitoclax) is developed for use in the clinic and an oral version of ABT-737. It binds to and inhibits Bcl-2, Bcl-XL, and Bcl-w with higher affinities than any of other Bcl-2 inhibitors, resulting in effective enhancement of the cytotoxicity of several chemotherapy agents. b However, resistance against ABT-263 is observed in cancer cells that express Mcl-1 because ABT-263 does not bind to Mcl-1. On the other hand, Gal-3 that highly expresses in diverse cancers have multiple roles in the regulation of apoptosis (as shown in Fig. 2). Gal-3 may replace or mimic Bcl-2 by binding to Bax through the NWGR motif, which is critical for the antiapoptotic function of both Bcl-2 and Gal-3 proteins. c We propose a novel combination therapy model. Combination therapy targeting Gal-3 using sugar-binding antagonist with the conventional anti-Bcl-2 treatment leads to suppression of antiapoptotic function of Gal-3 itself. Interestingly, GCS-100, which binds to Gal-3 as an antagonist, is shown to reduce Mcl-1 expression and regulate the other bcl-2 family proteins. In addition, targeting the CRD of Gal-3 containing the NWGR motif may induce release of Bax from Gal-3, subsequently resulting in MOMP
7 Conclusions
Gal-3 can provide the theoretical foundation for a new therapeutic target for improving chemotherapy of cancers. Although much remains to be explored, the apparent similarity between Bcl-2 and Gal-3 in their antiapoptotic functions leads us to surmise that targeting Gal-3 with anti-Bcl-2 treatment is therapeutically valuable. Combination therapy targeting Gal-3 may lead to the improvement of efficacy in anti-Bcl-2 treatment in cancers and may result in one of the best approaches targeting Bcl-2 family proteins.
Based on the limited success of anti-Bcl-2 drugs to improve clinical outcome of cancer patients, we propose that prior to initiation of clinical trials, the level of Gal-3 in the cancer cells should be determined. If the cells are positive for Gal-3, the anti-Bcl-2 treatment should include a Gal-3 antagonist to ensure that it is silenced as well.
Acknowledgments
The work was supported by the National Institute of Health grant R37CA46120 (to A.R.).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Y. Harazono, Department of Oncology, School of Medicine, Wayne State University, Detroit, MI, USA. Department of Pathology, School of Medicine, Wayne State University, Detroit, MI, USA. Karmanos Cancer Institute, Detroit, MI 48201, USA
K. Nakajima, Department of Oncology, School of Medicine, Wayne State University, Detroit, MI, USA. Department of Pathology, School of Medicine, Wayne State University, Detroit, MI, USA. Karmanos Cancer Institute, Detroit, MI 48201, USA
A. Raz, Email: raza@karmanos.org, Department of Oncology, School of Medicine, Wayne State University, Detroit, MI, USA. Department of Pathology, School of Medicine, Wayne State University, Detroit, MI, USA. Karmanos Cancer Institute, Detroit, MI 48201, USA
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nature reviews. Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 4.Haldar S, Basu A, Croce CM. Serine-70 is one of the critical sites for drug-induced Bcl2 phosphorylation in cancer cells. Cancer Research. 1998;58:1609–1615. [PubMed] [Google Scholar]
- 5.Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for anti-apoptosis function. The Journal of Biological Chemistry. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 6.Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin- CDK6 complex phosphorylates and inactivates Bcl-2. Nature Cell Biology. 2000;2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 7.Pratesi G, Perego P, Zunino F. Role of Bcl-2 and its post-transcriptional modification in response to antitumor therapy. Biochemical Pharmacology. 2001;61:381–386. doi: 10.1016/s0006-2952(00)00538-4. [DOI] [PubMed] [Google Scholar]
- 8.Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. The Journal of Biological Chemistry. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 9.Salah-Eldin AE, Inoue S, Tsukamoto S, Aoi H, Tsuda M. An association of Bcl-2 phosphorylation and Bax localization with their functions after hyperthermia and paclitaxel treatment. International journal of cancer Journal International du Cancer. 2003;103:53–60. doi: 10.1002/ijc.10782. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Molecular and Cellular Biology. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokote H, Terada T, Matsumoto H, Kakishita K, Kinoshita Y, Nakao N, et al. Dephosphorylation-induced decrease of anti-apoptotic function of Bcl-2 in neuronally differentiated P19 cells following ischemic insults. Brain Research. 2000;857:78–86. doi: 10.1016/s0006-8993(99)02414-2. [DOI] [PubMed] [Google Scholar]
- 12.Leber B, Geng F, Kale J, Andrews DW. Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Reviews in Molecular Medicine. 2010;12:e28. doi: 10.1017/S1462399410001572. [DOI] [PubMed] [Google Scholar]
- 13.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. The Journal of Biological Chemistry. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 15.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 16.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. The American Journal of Pathology. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. The American journal of Pathology. 1995;147:815–822. [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu DK, Hammes SR, Kuwabara I, Greene WC, Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. The American Journal of Pathology. 1996;148:1661–1670. [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinov KN, Robbins BA, Liu FT. Galectin-3, a beta-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. The American Journal of Pathology. 1996;148:25–30. [PMC free article] [PubMed] [Google Scholar]
- 20.van den Brule FA, Buicu C, Berchuck A, Bast RC, Deprez M, Liu FT, et al. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Human Pathology. 1996;27:1185–1191. doi: 10.1016/s0046-8177(96)90313-5. [DOI] [PubMed] [Google Scholar]
- 21.Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. International journal of cancer Journal International du Cancer. 1994;56:474–480. doi: 10.1002/ijc.2910560404. [DOI] [PubMed] [Google Scholar]
- 22.Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995;75:2818–2826. doi: 10.1002/1097-0142(19950615)75:12<2818::aid-cncr2820751206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 24.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. Journal of Molecular Medicine. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 25.Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Experimental Cell Research. 1998;245:294–302. doi: 10.1006/excr.1998.4253. [DOI] [PubMed] [Google Scholar]
- 26.Inohara H, Raz A. Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Research. 1995;55:3267–3271. [PubMed] [Google Scholar]
- 27.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. The American journal of Pathology. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang RY, Liu FT. Galectins in cell growth and apoptosis. Cellular and Molecular Life Sciences: CMLS. 2003;60:267–276. doi: 10.1007/s000180300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, et al. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Research. 2004;64:3376–3379. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- 30.Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. Journal of Bioenergetics and Biomembranes. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Research. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 32.Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Research. 1999;59:4148–4154. [PubMed] [Google Scholar]
- 33.Lin HM, Moon BK, Yu F, Kim HR. Galectin-3 mediates genistein-induced G(2)/M arrest and inhibits apoptosis. Carcinogenesis. 2000;21:1941–1945. doi: 10.1093/carcin/21.11.1941. [DOI] [PubMed] [Google Scholar]
- 34.Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. The Journal of Biological Chemistry. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 35.Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T, et al. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anti-cancer drug in prostate cancer. Cancer Research. 2006;66:3114–3119. doi: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- 36.Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Molecular and Cellular Biology. 2004;24:4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakahara S, Hogan V, Inohara H, Raz A. Importin-mediated nuclear translocation of galectin-3. The Journal of Biological Chemistry. 2006;281:39649–39659. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- 38.van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. International journal of cancer Journal International du Cancer. 2000;89:361–367. doi: 10.1002/1097-0215(20000720)89:4<361::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. The Journal of Biological Chemistry. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 40.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 41.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular Cell Biology. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 42.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3330. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weyhenmeyer B, Murphy AC, Prehn JH, Murphy BM. Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer. Experimental Oncology. 2012;34:192–199. [PubMed] [Google Scholar]
- 44.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 46.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biology. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 48.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Molecular Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 50.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 51.Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis: An International Journal on Programmed Cell Death. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 53.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2012;30:3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense & Nucleic Acid Drug Development. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 55.Chi KN, Gleave ME, Klasa R, Murray N, Bryce C, Lopes de Menezes DE, et al. A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2001;7:3920–3927. [PubMed] [Google Scholar]
- 56.Marcucci G, Byrd JC, Dai G, Klisovic MI, Kourlas PJ, Young DC, et al. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 2003;101:425–432. doi: 10.1182/blood-2002-06-1899. [DOI] [PubMed] [Google Scholar]
- 57.Tolcher AW, Kuhn J, Schwartz G, Patnaik A, Hammond LA, Thompson I, et al. A Phase I pharmacokinetic and biological correlative study of oblimersen sodium (genasense, g3139), an antisense oligonucleotide to the bcl-2 mRNA, and of docetaxel in patients with hormone-refractory prostate cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2004;10:5048–5057. doi: 10.1158/1078-0432.CCR-03-0701. [DOI] [PubMed] [Google Scholar]
- 58.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2009;27:5208–5212. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudin CM, Salgia R, Wang X, Hodgson LD, Masters GA, Green M, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26:870–876. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei J, Kitada S, Rega MF, Emdadi A, Yuan H, Cellitti J, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Molecular Cancer Therapeutics. 2009;8:904–913. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammad RM, Wang S, Aboukameel A, Chen B, Wu X, Chen J, et al. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(−)-gossypol] against diffuse large cell lymphoma. Molecular Cancer Therapeutics. 2005;4:13–21. [PubMed] [Google Scholar]
- 63.Shelley MD, Hartley L, Groundwater PW, Fish RG. Structure-activity studies on gossypol in tumor cell lines. Anti-Cancer Drugs. 2000;11:209–216. doi: 10.1097/00001813-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death and Differentiation. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 65.Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2005:226–230. doi: 10.1182/asheducation-2005.1.226. [DOI] [PubMed] [Google Scholar]
- 66.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death And Differentiation. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 67.O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 69.Oki Y, Copeland A, Hagemeister F, Fayad LE, Fanale M, Romaguera J, et al. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood. 2012;119:2171–2172. doi: 10.1182/blood-2011-11-391037. [DOI] [PubMed] [Google Scholar]
- 70.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74:481–485. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 72.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 73.Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned TM, et al. Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood. 2007;110:2057–2066. doi: 10.1182/blood-2007-03-080325. [DOI] [PubMed] [Google Scholar]
- 74.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 75.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Research. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 77.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. The lancet. Oncology. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazumder S, Choudhary GS, Al-Harbi S, Almasan A. Mcl-1 Phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Research. 2012;72:3069–3079. doi: 10.1158/0008-5472.CAN-11-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu H, Krystal GW. Actinomycin D decreases Mcl-1 expression and acts synergistically with ABT-737 against small cell lung cancer cell lines. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2010;16:4392–4400. doi: 10.1158/1078-0432.CCR-10-0640. [DOI] [PubMed] [Google Scholar]
- 82.Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Molecular Cancer Therapeutics. 2011;10:1264–1275. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- 83.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Reviews in Molecular Medicine. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 84.Prieto VG, Mourad-Zeidan AA, Melnikova V, Johnson MM, Lopez A, Diwan AH, et al. Galectin-3 expression is associated with tumor progression and pattern of sun exposure in melanoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2006;12:6709– 6715. doi: 10.1158/1078-0432.CCR-06-0758. [DOI] [PubMed] [Google Scholar]
- 85.Kopper L, Timar J. Genomics of renal cell cancer—does it provide breakthrough? Pathology Oncology Research: POR. 2006;12:5–11. doi: 10.1007/BF02893425. [DOI] [PubMed] [Google Scholar]
- 86.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review) International Journal of Oncology. 2004;25:983–992. [PubMed] [Google Scholar]
- 87.Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy. 2007;10:101–108. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. The Journal of Biological Chemistry. 1993;268:26712–26718. [PubMed] [Google Scholar]
- 89.Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. The Journal of Biological Chemistry. 2000;275:36311–36315. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- 90.Matarrese P, Fusco O, Tinari N, Natoli C, Liu FT, Semeraro ML, et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. International journal of cancer Journal International du Cancer. 2000;85:545–554. [PubMed] [Google Scholar]
- 91.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Research. 2006;66:9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- 93.Oka N, Nakahara S, Takenaka Y, Fukumori T, Hogan V, Kanayama HO, et al. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Research. 2005;65:7546–7553. doi: 10.1158/0008-5472.CAN-05-1197. [DOI] [PubMed] [Google Scholar]
- 94.Pieters RJ. Inhibition and detection of galectins. Chembiochem: A European journal of Chemical Biology. 2006;7:721–728. doi: 10.1002/cbic.200600011. [DOI] [PubMed] [Google Scholar]
- 95.Delaine T, Cumpstey I, Ingrassia L, Le Mercier M, Okechukwu P, Leffler H, et al. Galectin-inhibitory thiodigalactoside ester derivatives have antimigratory effects in cultured lung and prostate cancer cells. Journal of Medicinal Chemistry. 2008;51:8109–8114. doi: 10.1021/jm801077j. [DOI] [PubMed] [Google Scholar]
- 96.Lin CI, Whang EE, Donner DB, Jiang X, Price BD, Carothers AM, et al. Galectin-3 targeted therapy with a small molecule inhibitor activates apoptosis and enhances both chemosensitivity and radiosensitivity in papillary thyroid cancer. Molecular Cancer Research: MCR. 2009;7:1655–1662. doi: 10.1158/1541-7786.MCR-09-0274. [DOI] [PubMed] [Google Scholar]
- 97.Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconjugate Journal. 1994;11:527–532. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]
- 98.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. Journal of the National Cancer Institute. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 99.Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. Journal of the National Cancer Institute. 1995;87:348–353. doi: 10.1093/jnci/87.5.348. [DOI] [PubMed] [Google Scholar]
- 100.Streetly MJ, Maharaj L, Joel S, Schey SA, Gribben JG, Cotter FE. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chauhan D, Li G, Podar K, Hideshima T, Neri P, He D, et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Research. 2005;65:8350–8358. doi: 10.1158/0008-5472.CAN-05-0163. [DOI] [PubMed] [Google Scholar]
- 102.Hanada M, Aime-Sempe C, Sato T, Reed JC. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. The Journal of Biological Chemistry. 1995;270:11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]