Abstract
Bone is a preferred site for breast cancer metastasis and leads to pathological bone loss due to increased osteoclast-induced bone resorption. The homing of tumor cells to the bone depends on the support of the bone microenvironment in which the tumor cells prime the pre-metastatic niche. The colonization and growth of tumor cells then depends on adaptations in the invading tumor cells to take advantage of normal physiological responses by mimicking bone marrow cells. This concerted effort by tumor cells leads to uncoupled bone remodeling in which the balance of osteoclast-driven bone resorption and osteoblast-driven bone deposition is lost. Breast cancer bone metastases often lead to osteolytic lesions due to hyperactive bone resorption. Release of growth factors from bone matrix during resorption then feeds a ‘vicious cycle’ of bone destruction leading to many skeletal related events. In addition to activity in bone, some of the factors released during bone resorption are also known to be involved in skeletal muscle regeneration and contraction. In this review, we discuss the mechanisms that lead to osteolytic breast cancer bone metastases and the potential for cancer-induced bone-muscle cross-talk leading to skeletal muscle weakness.
Introduction
Bone metastases are common in patients with advanced malignancy. Primary tumors exhibit metastatic tropism to particular organs and the skeleton is a preferred site for breast cancer metastasis. Breast cancer that is metastatic to bone causes a significant imbalance in normal bone remodeling through perturbation of osteoclast-mediated bone resorption and osteoblast-mediated bone formation (1). Bone metastases are classified based on radiographic appearance as either osteolytic or osteoblastic (osteosclerotic). Breast cancer is typically associated with osteolytic lesions but most cases involve uncoupled components of both bone destruction and new bone formation. Bone metastases from breast cancer affect 65-80% of patients with advanced malignancy (2). Bone metastases cause severe bone pain, increased risk of pathological fracture, hypercalcemia and nerve compression syndromes that significantly reduce the quality of life (1). Perhaps most devastating is the fact that once the primary tumor has spread to the bone it is incurable. The current standard of care for patients with bone loss due to osteolytic bone metastases includes anti-resorptive therapy aimed at reducing skeletal related events but is not curative with regard to tumor burden (1, 2).
A significant co-morbidity of osteolytic bone metastases is muscle weakness and fatigue that is often associated with cancer cachexia. Cachexia is a common paraneoplastic syndrome that is characterized by severe wasting due to loss of both fat and lean body mass (3, 4). Although the age and chemotherapeutic treatment regimens of patients with advanced disease and bone metastases makes it is difficult to assess the true incidence of malignancy-induced muscle weakness (5), a clinical perspective suggests that many patients do experience severe muscle weakness and fatigue. Improving muscle function and mobility of cancer patients would have a positive impact on adherence to treatment regimens and overall health (5). Therefore, a better understanding of the mechanism(s) of muscle weakness associated with bone metastases and cancer cachexia will lead to targeted therapeutics. Moreover, refocusing attention to determine muscle quality in addition to improving muscle mass will likely provide the most beneficial treatment options for this devastating complication of malignancy.
Molecular mechanisms of bone metastasis
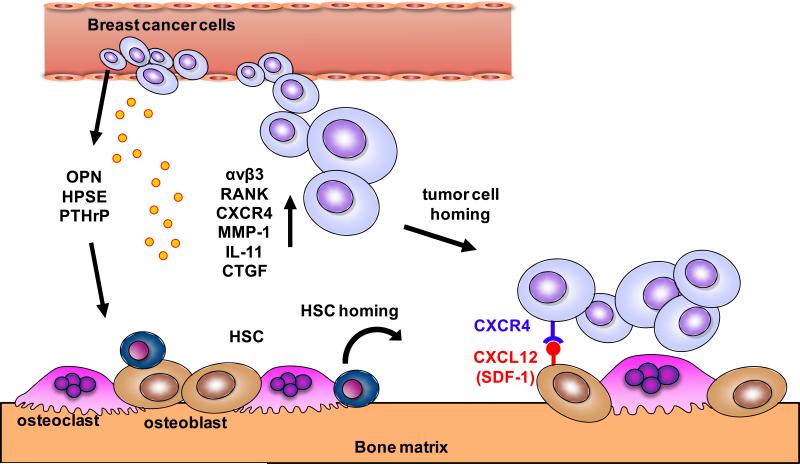
The initiation and progression of bone metastasis is a complex multistep process. Tumor cells must detach from the primary tumor and enter the systemic circulation (intravasation), evade detection by the immune system and adhere to capillaries in the bone marrow leading to extravasation into the bone marrow space (6). Tumor cells in the bone first form micro-metastases that can either develop into overt metastatic lesions or lay dormant for long periods before reactivating in the bone microenvironment. In either case it is believed that the invading tumor cells prime the bone microenvironment by enriching the pre-metastatic niche (local environment) for further colonization and growth of tumor cells (Figure 1) (2, 7-9).
Figure 1. Pre-metastatic niche and bone homing.
Modulation of the bone microenvironment by circulating breast cancer cells results in priming of the bone marrow as a pre-metastatic niche through tumor cell secretion of osteopontin (OPN), heparanase (HPSE) and parathyroid hormone related protein (PTHrP). Colonization of the bone and recruitment of hematopoietic stem cells (HSCs) occurs by tumor cell expression of integrins (αvβ3), receptor activator of NF-kB (RANK), CXCR4, matrix metalloproteinase (MMP-1), IL-11 and connective tissue growth factor (CTGF). CXCL12 (SDF-1) expression on osteoblasts facilitates homing of tumor cells to bone.
The hematopoietic system plays an important role in development of the pre-metastatic niche. The bone marrow may serve as a protective milieu for dormant tumor cells to resist chemotherapeutic attack and tumor cells may use the same physiological mechanisms as those used by hematopoietic stem cells (HSCs) homing to bone (10, 11). In the pre-metastatic niche, the invading tumor cells prime the stroma by production of factors that elicit responses in cells of the bone microenvironment and make it conducive to tumor colonization and growth (2). In addition, bone resorption also regulates HSC homing (12). Factors derived from tumor cells include osteopontin (OPN) which promotes bone marrow cell migration and tumor cell proliferation (13, 14); heparanase (HPSE) which acts in the extracellular matrix to reduce heparin sulfate chain length leading to increased bone resorption (15); and parathyroid hormone-related protein (PTHrP) that promotes bone resorption (16) and may also enhance production of bone marrow chemokines such as C-C motif ligand 2 (CCL2) (17). Recently it has also been shown that the sympathetic nervous system is also capable of stimulating stromal cells thus promoting breast cancer bone metastasis (18).
Tumor cells invading the bone also express factors that facilitate further recruitment to the bone microenvironment, a process called osteotropism (19). αvβ3 integrin promotes adhesion of breast cancer cells in bone and is associated with bone metastasis (20). αvβ3 integrin also cooperates with bone sialoprotein (BSP) and matrix metalloproteinase-2 (MMP-2) to promote tumor cell colonization in bone (21, 22). Receptor activator of nuclear factor k-B (RANK) mediates osteoclast induced bone resorption and supports tumor cell colonization (23). The chemokine CXC ligand 12 (CXCL12; also known as stromal cell-derived factor 1 [SDF-1]) is a potent chemo-attractant for HSCs and is highly expressed on osteoblasts and bone marrow stromal cells. Expression of its receptor, CXC receptor 4 (CXCR4) on cancer cells plays an important role in bringing tumor cells to bone (24). In addition, interactions between CXCL12 and CXCR4 in the bone microenvironment lead to an up-regulation of αvβ3 integrin, facilitating additional cell adhesion. CXCR4 was identified as one of a set of proteins highly overexpressed in breast cancer cells (MDA-MB-231) of high bone metastatic potential by serial selection in vivo (10). Kang et al., also found that matrix metalloproteinase 1 (MMP-1), IL-11 and connective tissue growth factor (CTGF) were highly expressed in in vivo serially selected tumor cells that exhibited increased homing to bone compared to parental cells. IL-11 and MMP-1 stimulate bone resorption by increasing osteoblast production of RANK ligand (RANKL). Increased expression of MMP-1 and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS1) suppresses osteoprotegerin (OPG) expression in osteoblasts, which leads to osteoclast differentiation (25). CTGF stimulates osteoblast proliferation which leads to further osteoclast activation and increased osteolysis (2).
Following osteotropism the tumor cells adapt to the bone marrow space by expressing factors that allow growth in their new microenvironment. Osteolytic lesions are the most common type observed from breast cancers metastatic to bone. PTHrP secreted by breast cancer cells was the first characterized tumor-derived mediator of bone destruction (16). In mice without detectable circulating PTHrP or hypercalcemia, neutralizing antibodies to PTHrP blocked breast cancer bone metastases-associated bone loss and tumor growth. It was then shown that TGFβ in the bone microenvironment induced the expression of PTHrP by metastatic breast cancer cells that led to increased bone destruction (26). Breast cancer cells in bone also express cyclooxygenase-2 (COX-2) which supports the development and progression of bone metastases controlled by prostaglandin (PGE2) leading to bone resorption (27). The osteolytic factors IL-8 and IL-11 are also expressed by tumor cells in the bone microenvironment and directly support osteoclast maturation (28, 29). In addition to secreted factors, tumor cells express transcription factors that support growth in bone. The transcription factors, GLI2, runt related transcription factor 2 (RUNX2) and hypoxia-induced growth factor 1 α (HIF1α) promote osteolysis. GLI2, part of the Hedgehog signaling network, induces PTHrP expression leading to bone destruction (30). RUNX2 regulates MMP-9 transcription leading to increased tumor cell invasion by breaking down the extracellular matrix (31). HIF1α expression inhibits osteoblast differentiation and promotes osteoclastogenesis thus supporting bone resorption and tumor growth (32, 33). Tumor cells also express Jagged1 (Jag1) that activates the Notch pathway, which activates osteoclast differentiation (34).
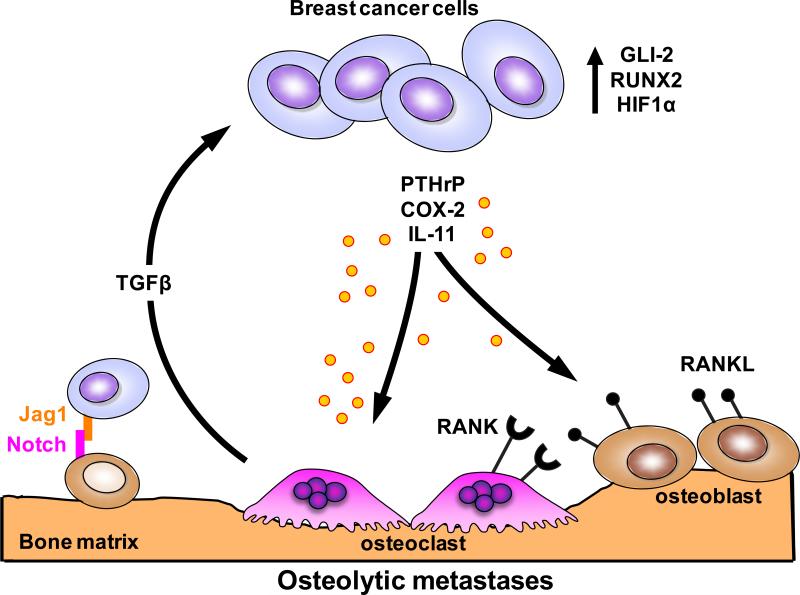
In the normal adult setting bone is constantly remodeled to adjust for functional demands or to repair microfractures that occur as a part of normal activity. This process is driven by the coupled activity of osteoclasts that resorb mineralized matrix and osteoblast that lay down new bone (35, 36). Ultimately tumor cells in the bone microenvironment disrupt this normal physiological process and skew balance either toward bone destruction or bone formation. In the case of most breast cancers metastatic to bone, the tumor cells produce factors that directly or indirectly induce the formation of osteoclasts. In turn, bone resorption releases growth factors from bone matrix (e.g. TGFβ) that stimulate tumor growth and further osteolysis. This reciprocal interaction between breast cancer cells and the bone microenvironment results in a ‘vicious cycle’ that increases both bone destruction and the tumor burden (Figure 2)(2).
Figure 2. Vicious cycle of osteolytic bone metastasis.
Osteolytic bone destruction due to dysregulation of normal bone remodeling is predominant in breast cancer metastasis. Breast cancer cells colonizing the bone secrete osteolytic factors: parathyroid hormone related protein (PTHrP), cyclooxygenase-2 (COX-2) and IL-11. Tumor cells also express transcription factors GLI2, runt related transcription factor 2 (RUNX2) and hypoxia-induced growth factor 1α (HIF1α) that promote osteolysis. Jagged1 (Jag1) expressed on tumor cells activates osteoclast differentiation by inducing Notch signaling in pre-osteoclasts. Bone resorption releases TGFβ from the bone matrix, which enhances tumor cell proliferation and survival thus feeding a vicious cycle leading to further bone destruction.
Pre-clinical data suggests that reducing bone resorption prevents the development of bone metastases. Osteoclast inhibitors are useful agents to slow or reverse bone loss (2) while anti-resorptive therapy (bisphosphonates), osteoprotegerin (OPG) and other RANKL antagonists reduce growth of bone metastases (37, 38). TGFβ antagonism is another mechanism for reducing tumor growth in bone. TGFβ is abundant in the mineralized bone matrix and is released from the matrix during osteoclastic bone resorption (39). Blocking the TGFβ pathway reduces bone metastasis and tumor burden (40-43). Blocking bone resorption especially through modulating TGFβ signaling offers a promising area for therapeutic intervention in bone metastasis and potentially its comorbidities.
Muscle dysfunction associated with breast cancer bone metastasis
Muscle and bone anabolism are tightly coupled during growth and development. Conversely, muscle and bone catabolism occur during aging. Yet the cellular and molecular mechanisms linking these two tissues are not well understood.
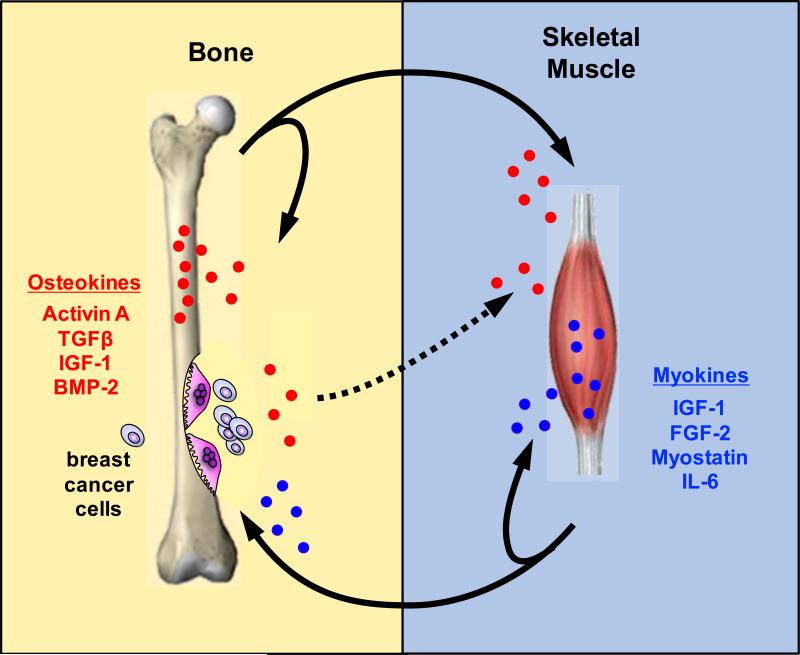
Muscle is known to secrete many factors capable of affecting other tissues. These factors, collectively termed myokines, include the bone active molecules insulin-like growth factor 1 (IGF-1), fibroblast growth factor 2 (FGF-2), Myostatin (also called growth and differentiation factor 8 [GDF8]) and IL-6 (44). Our current understanding of bone and muscle cross-talk seems to show a predominant role of signaling in the direction of muscle to bone. Yet bone derived factors are also known to modulate muscle. For example, Indian hedgehog (Ihh) promotes myoblast survival and myogenesis in both mouse and chick embryos (45) thus indicating bidirectional bone-muscle cross-talk. It seems likely that in cases of abnormal physiology, such as osteolytic bone metastases, that the signals are co-opted and lead to a shift in the homeostatic signaling balance (Figure 3).
Figure 3. Bone-muscle cross-talk.
Bone and muscle are physically and functionally tightly coupled. Insulin-like growth factor 1 (IGF-1), fibroblast growth factor 2 (FGF-2), Myostatin and IL-6 are factor released from muscle that have functions in muscle as well as bone. These factors have been collectively termed myokines. Likewise Activin A, TGFβ, IGF-1 and bone morphogenic protein 2 (BMP-2) released from bone affect bone as well as muscle and thus we have called these osteokines. During osteolytic bone resorption due to breast cancer bone metastases osteokines are released and may be responsible for systemic skeletal muscle weakness. In certain settings tumor-derived factors could also lead to modulation of muscle activity (dotted line).
Data from our laboratory using a pre-clinical model of breast cancer bone metastases (MDAMB-231 cells) shows significant reduction in forelimb grip strength and ex vivo maximum specific force generation of the extensor digitorum longus (EDL) muscle that cannot be explained by reduction in muscle mass. Ex vivo specific force calculations compensate for differences in size and weight of individual muscles. Further muscle dysfunction is systemic and dependent on tumor-induced osteolytic bone resorption without tumor cell involvement in the muscle. Primary MDA-MB-231 tumors (mammary fat pad injection site) do not elicit muscle dysfunction (46). Our investigation into muscle function in mice with breast cancer bone metastases was borne out of the observation that these mice develop cachexia with advancing bone destruction. Cancer cachexia is one of the most common paraneoplastic conditions in advanced malignancy occurring in approximately 80 percent of patients. There is no effective treatment for cancer cachexia and it has been estimated to be responsible for 20 percent of cancer related deaths (3, 47). However there is a large heterogeneity in clinical presentation of cachexia that can vary according to tumor type, site and individual patient factors. In fact the true incidence of cancer cachexia is likely greatly underestimated (5).
Many well established models of cancer cachexia have used reduction of muscle size to imply muscle dysfunction. However this does not take into account the loss of muscle quality. Our lab has shown that mice with bone metastases exhibited a primary defect (in addition to loss of muscle weight) that is independent of cachexia, although mechanisms of cancer cachexia may also be at work (48). In a mouse model of multiple myeloma that leads to osteolytic bone lesions but without measurable cachexia we observed systemic muscle dysfunction (49). In both of these mouse models of osteolytic bone loss, the severity of muscle dysfunction correlated with an increase in bone destruction.
The salient question therefore is what factor(s) derived from bone matrix during resorption is capable of inducing systemic muscle dysfunction? Bone matrix is a rich storehouse of growth factors that have known effects on muscle, such as Activin A, TGFβ, IGF-1 and bone morphogenic protein 2 (BMP-2) (50, 51). It is useful to begin by considering these as potentiators of muscle dysfunction due to bone destruction.
The high affinity Activin type 2 receptor, ActRIIB, mediates signaling of a small group of TGFβ family members (Activin A, Myostatin, GDF-11) and is important in regulating muscle mass (52). Pharmacological blockade of ActRIIB prevents muscle wasting, induces muscle satellite cell mobilization and differentiation and significantly prolongs survival in murine models of cachexia (53). In addition, blockade of ActRIIB dramatically improves muscle function in a Duchenne muscular dystrophy model (mdx mice) (54). However in these studies it is not possible to determine if the effect is due to blocking Activin A, Myostatin or GDF-11 signaling due to receptor usage overlap. Myostatin signaling antagonism has been investigated as a way to improve muscle wasting due to cachexia since Myostatin is a potent inhibitor of skeletal muscle differentiation and growth (55). Activin A has also been shown to function with Myostatin to reduce muscle size (56). GDF-11 shares 90% sequence homology with Myostatin and in skeletal muscle inhibits myoblast differentiation (57) suggesting that GDF-11 may act in a very similar manner as Myostatin.
TGFβ is a potent regulator of wound healing in muscle and persistent exposure leads to altered extracellular matrix architecture and formation of fibrotic tissue in muscle (58). Increased TGFβ signaling in muscle also inhibits satellite cell activation and impairs myocyte differentiation (59, 60). Increased TGFβ signaling is also associated with skeletal muscle dysfunction in many of the muscular dystrophies (61, 62). In a direct assessment of the effect of TGFβ on muscle function the contractile properties of the extensor digitorum longus muscle (EDL) were examined from limbs exposed to recombinant TGFβ. Muscle function from limbs receiving TGFβ treatment exhibited a significant reduction in specific force (63). These experiments suggest that TGFβ is a capable factor in reducing muscle function independent of changes in muscle mass.
In contrast to the negative effects possible from Activin A, Myostatin and TGFβ signaling in muscle, IGF-1 and BMP-2 signaling results in muscle hypertrophy (58, 64, 65). IGF-1 is a major regulator of muscle mass due to its effect on myogenic cell proliferation and differentiation (66). Likewise, BMP signaling leads to muscle hypertrophy but interestingly specific force (corrected for muscle mass) is significantly lower when BMP signaling is constitutively activated (64). This result demonstrates the importance of interpreting muscle specific function not merely muscle mass in murine models of skeletal muscle weakness.
In addition to factors released from bone matrix during osteoclast-driven resorption, other factors present in patients with malignancy involving bone may play important roles in muscle weakness. Serum vitamin D levels are low among breast cancer patients with either osteoporosis or metastatic bone disease and receiving bisphosphonate therapy (67). Vitamin D deficiency has been studied in rodent models using vitamin D receptor knock-out (VDRKO) mice. Functional muscle tests in VDRKO mice exhibited and increase in sinking episodes in a forced swim test, reduced ‘time on’ in a rotarod test (68, 69) and reduced time before falling from a vertical screen test (70). These results indicate an overall defect in motor performance in mice lacking proper vitamin D metabolism. In human studies, Rickets and osteomalacia are associated with muscle weakness. In addition to general weakness, more specific muscle deficits are also commonly reported, including reduced timed up and go (TUG), 6-minute walk, stair climbing and object lifting (71, 72). It should be noted that myopathies reported with vitamin D deficiency might also involve calcium and phosphate deficiencies thus complicating the assessment of individual factors. Fibroblast growth factor-23 (FGF-23) neutralizing antibody, which increases serum phosphate and vitamin D levels, has been shown to improve murine grip strength in a model of rickets/osteomalacia (X-linked hypohosphatemic rickets/osteomalacia [XLH]) suggesting that vitamin D levels could influence muscle function (73).
MicroRNA (miRNA) profiling of tumors has identified signatures associated with diagnosis and progression.. Human miRNA Let-7 was recently shown to be elevated in serum of mice harboring breast cancer bone metastases (74). miRNA Let-7 is also elevated in serum of elderly patients with muscle weakness and has been suggested to reduce regenerative capacity in aging (75).
Another intriguing possibility is the role of the sympathetic nervous system in muscle weakness due to bone metastases. The sympathetic nervous system modulates skeletal muscle metabolism, ion transport and contractility. Recent evidence has shown that the sympathetic nervous system is capable of promoting breast cancer bone metastasis through stimulation of marrow stromal cells (18), yet a connection to muscle weakness has not been investigated.
Summary
Bone and muscle functions are tightly coupled in normal physiology. Recent studies have focused on muscle as an endocrine organ with a predominant role over bone in bone-muscle cross-talk. Osteolytic bone metastases from breast cancer represent a severe divergence from normal bone physiology by tipping the balance of remodeling. Bone is a rich storehouse of growth factors that have activity in bone (as a part of normal remodeling) and in other organs, including muscle. It is therefore possible that during hyperactive bone resorption, bone might have a predominant role over muscle in bone-muscle cross-talk and become a source of ‘osteokines’ that affect muscle function. Likewise, factors released from muscle may play an important role in bone metabolism that could further exacerbate the role of bone in muscle dysfunction. Identification and characterization of such factors would provide new possibilities for therapeutic intervention in muscle weakness associated with malignancy and perhaps cancer cachexia.
Acknowledgments
This work was supported by NIH grants (U01CA143057 from the NCI Tumor Microenvironment Network; R01CA69158), the Susan G. Komen Foundation, the Indiana Economic Development Grant, the Jerry W. and Peggy S. Throgmartin Endowment of the IU Simon Cancer Center, the IU Simon Cancer Center Breast Cancer Program, and a generous donation from the Withycombe family (T.A. Guise).
Footnotes
Disclosure of Potential Conflicts of Interest
T.A. Guise reports receiving commercial research grants from AstraZeneca and Exelexis and is a consultant/advisory board member for Novartis. No potential conflicts of interest were disclosed by the other author.
References
- 1.Roodman GD. Mechanisms of bone metastasis. The New England journal of medicine. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nature reviews Cancer. 2011;11:411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell metabolism. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Mechanisms of cancer cachexia. Physiological reviews. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 5.Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of Cachexia among Cancer Patients Based on Four Definitions. Journal of oncology. 2009;2009:693458. doi: 10.1155/2009/693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer cell. 2011;20:701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. The American journal of pathology. 1998;153:865–73. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MJ, Weilbaecher KN, et al. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5001–9. doi: 10.1158/1078-0432.CCR-07-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda T. Cellular and molecular basis of preferential metastasis of breast cancer to bone. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2000;5:75–81. doi: 10.1007/s007760050012. [DOI] [PubMed] [Google Scholar]
- 12.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature medicine. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 13.Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. Journal of cellular and molecular medicine. 2010;14:2037–44. doi: 10.1111/j.1582-4934.2010.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly T, Suva LJ, Huang Y, Macleod V, Miao HQ, Walker RC, et al. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer research. 2005;65:5778–84. doi: 10.1158/0008-5472.CAN-05-0749. [DOI] [PubMed] [Google Scholar]
- 16.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. The Journal of clinical investigation. 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer research. 2009;69:1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS biology. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clinical & experimental metastasis. 2007;24:599–608. doi: 10.1007/s10585-007-9112-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer research. 2007;67:5821–30. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 21.Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Bone sialoprotein, matrix metalloproteinase 2, and alpha(v)beta3 integrin in osteotropic cancer cell invasion. Journal of the National Cancer Institute. 2004;96:956–65. doi: 10.1093/jnci/djh169. [DOI] [PubMed] [Google Scholar]
- 22.Waltregny D, Bellahcene A, de Leval X, Florkin B, Weidle U, Castronovo V. Increased expression of bone sialoprotein in bone metastases compared with visceral metastases in human breast and prostate cancers. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:834–43. doi: 10.1359/jbmr.2000.15.5.834. [DOI] [PubMed] [Google Scholar]
- 23.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 24.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer research. 2004;64:4302–8. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes & development. 2009;23:1882–94. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. The Journal of clinical investigation. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–96. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Fujita N, Oh-hara T, Morinaga Y, Nakagawa T, Yamada M, et al. Production of interleukin-11 in bone-derived endothelial cells and its role in the formation of osteolytic bone metastasis. Oncogene. 1998;16:693–703. doi: 10.1038/sj.onc.1201581. [DOI] [PubMed] [Google Scholar]
- 29.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 30.Sterling JA, Oyajobi BO, Grubbs B, Padalecki SS, Munoz SA, Gupta A, et al. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer research. 2006;66:7548–53. doi: 10.1158/0008-5472.CAN-06-0452. [DOI] [PubMed] [Google Scholar]
- 31.Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Molecular and cellular biology. 2005;25:8581–91. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PloS one. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer research. 2007;67:4157–63. doi: 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- 34.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. The Journal of biological chemistry. 2010;285:25103–8. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 37.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer research. 2000;60:2949–54. [PubMed] [Google Scholar]
- 38.Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–40. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- 39.Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nature medicine. 2009;15:960–6. doi: 10.1038/nm.1943. [DOI] [PubMed] [Google Scholar]
- 40.Biswas S, Nyman JS, Alvarez J, Chakrabarti A, Ayres A, Sterling J, et al. Anti-transforming growth factor ss antibody treatment rescues bone loss and prevents breast cancer metastasis to bone. PloS one. 2011;6:e27090. doi: 10.1371/journal.pone.0027090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buijs JT, Stayrook KR, Guise TA. The role of TGF-beta in bone metastasis: novel therapeutic perspectives. BoneKEy reports. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PloS one. 2009;4:e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright LE, Frye JB, Lukefahr AL, Timmermann BN, Mohammad KS, Guise TA, et al. Curcuminoids block TGF-beta signaling in human breast cancer cells and limit osteolysis in a murine model of breast cancer bone metastasis. Journal of natural products. 2013;76:316–21. doi: 10.1021/np300663v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiGirolamo DJ, Kiel DP, Esser KA. Bone and skeletal muscle: neighbors with close ties. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28:1509–18. doi: 10.1002/jbmr.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bren-Mattison Y, Hausburg M, Olwin BB. Growth of limb muscle is dependent on skeletal-derived Indian hedgehog. Developmental biology. 2011;356:486–95. doi: 10.1016/j.ydbio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waning DL, Mohammad KS, Andersson DC, John SK, Rieken S, Xie W, et al. Cancer-Induced Bone Disease. IBMS; Miami, FL USA: 2013. Role of the tumor-bone microenvironment in muscle weakness and cachexia. [Google Scholar]
- 47.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. The lancet oncology. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 48.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nature reviews Clinical oncology. 2013;10:90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 49.Waning DL, Mohammad KS, Reiken S, Marks AR, Roodman GD, Guise TA. Cancer-Induced Bone Disease. IBMS; Miami, FL USA: 2013. Muscle weakness is associated with osteolysis in multiple myeloma. [Google Scholar]
- 50.Sakai R, Eto Y. Involvement of activin in the regulation of bone metabolism. Molecular and cellular endocrinology. 2001;180:183–8. doi: 10.1016/s0303-7207(01)00496-8. [DOI] [PubMed] [Google Scholar]
- 51.Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. Journal of biomedical materials research Part A. 2007;81:437–42. doi: 10.1002/jbm.a.31085. [DOI] [PubMed] [Google Scholar]
- 52.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18117–22. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Pistilli EE, Bogdanovich S, Goncalves MD, Ahima RS, Lachey J, Seehra J, et al. Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. The American journal of pathology. 2011;178:1287–97. doi: 10.1016/j.ajpath.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–8. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 56.Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol. 2010;24:1998–2008. doi: 10.1210/me.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeanplong F, Falconer SJ, Thomas M, Matthews KG, Oldham AM, Watson T, et al. Growth and differentiation factor-11 is developmentally regulated in skeletal muscle and inhibits myoblast differentiation. Open Journal of Molecular and Integrative Physiology. 2012;2:127–38. [Google Scholar]
- 58.Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Experimental cell research. 2010;316:3050–8. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 59.Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. Journal of cellular physiology. 1987;133:567–72. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- 60.Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. Journal of cellular physiology. 1989;138:311–5. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- 61.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–34. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 62.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. Journal of applied physiology. 2008;104:579–87. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 63.Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle & nerve. 2012;45:55–9. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, et al. BMP signaling controls muscle mass. Nature genetics. 2013;45:1309–18. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 65.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skeletal muscle. 2011;1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocrine reviews. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 67.Wang-Gillam A, Miles DA, Hutchins LF. Evaluation of vitamin D deficiency in breast cancer patients on bisphosphonates. The oncologist. 2008;13:821–7. doi: 10.1634/theoncologist.2008-0013. [DOI] [PubMed] [Google Scholar]
- 68.Burne TH, Johnston AN, McGrath JJ, Mackay-Sim A. Swimming behaviour and post-swimming activity in Vitamin D receptor knockout mice. Brain research bulletin. 2006;69:74–8. doi: 10.1016/j.brainresbull.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Minasyan A, Keisala T, Zou J, Zhang Y, Toppila E, Syvala H, et al. Vestibular dysfunction in vitamin D receptor mutant mice. The Journal of steroid biochemistry and molecular biology. 2009;114:161–6. doi: 10.1016/j.jsbmb.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 70.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Impaired motor performance in mice lacking neurosteroid vitamin D receptors. Brain research bulletin. 2004;64:25–9. doi: 10.1016/j.brainresbull.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Russell JA. Osteomalacic myopathy. Muscle & nerve. 1994;17:578–80. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 72.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1:626–9. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 73.Aono Y, Hasegawa H, Yamazaki Y, Shimada T, Fujita T, Yamashita T, et al. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:803–10. doi: 10.1002/jbmr.275. [DOI] [PubMed] [Google Scholar]
- 74.Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer cell. 2013;24:542–56. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, et al. Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiological genomics. 2011;43:595–603. doi: 10.1152/physiolgenomics.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]