Abstract
Young adults 18–24 years have the highest rates of problems associated with alcohol use among all age groups, and substance use is inversely related to engagement in substance-free activities. This pilot study investigated the promotion of one specific substance-free activity, exercise, on alcohol use in college students. Thirty-one sedentary college students who engaged in hazardous drinking (Alcohol Use Disorders Identification Test scores ≥ 8) were randomized to one of two conditions: (a) one 50-minute session of motivational enhancement therapy (MET) focused on increasing exercise, or (b) one 50-minute session of MET focused on increasing exercise plus 8 weeks of contingency management (CM) for adhering to specific exercise activities. All participants completed evaluations at baseline and post-treatment (2-months later) assessing exercise participation and alcohol use. Results of the pilot study suggest the interventions were well received by participants, the MET+CM condition showed an increased self-reported frequency of exercise in comparison to the MET alone condition, but other indices of exercise, physical fitness, and alcohol use did not differ between the interventions over time. These results suggest that a larger scale trial could better assess efficacy of this well received combined intervention. Investigation in other clinically relevant populations is also warranted.
Keywords: Motivational interviewing, contingency management, binge drinking, emerging adults, physical activity
Approximately 40% of college students engage in heavy episodic drinking, defined as drinking five or more drinks at least once in the past two weeks for men and drinking four or more drinks at least once in the past two weeks for women (5/4 criterion; Wechsler & Nelson, 2001). College students who engage in heavy episodic drinking experience a range of negative consequences and participate in other risky behaviors such as unprotected sex, other substance use, and driving after drinking (Wechsler, Lee, Kuo, & Lee, 2000; Johnston & McGovern, 2004). Although brief interventions such as Motivational Enhancement Therapy (MET) have been shown to reduce alcohol use in college students (Burke, Dunn, Atkins & Phelps, 2004; Cronce, & Larimer, 2011), few college students seek help for problems related to alcohol (Blanco et al., 2008; Knight et al., 2002) most likely because they do not perceive a need for treatment and treatment can be stigmatizing (Eisenberg, Hunt, Speer, & Zivin, 2011; Oleski, Mota, Cox, & Sareen, 2010). Therefore, interventions that do not stigmatize students and that do not focus directly on alcohol use may be better accepted.
Alcohol use is inversely related to participation in activities incompatible with drinking, such as exercise (i.e., moderate to vigorous intensity physical activity designed to improve physical fitness). Animal studies repeatedly demonstrate that rates of alcohol and drug self-administration vary inversely with the availability of substance-free reinforcers such as wheel-running (Ehringer, Hoft, & Zunhammer, 2009; Lynch, Peterson, Sanchez, Abel, & Smith, 2013). Within college students, heavy episodic drinking is inversely associated with engagement in exercise (Correia, Benson, & Carey, 2005; Correia, Carey, Simons, & Borsari, 2003), and prospective studies of regular exercise has been shown to have a negative relationship with substance use disorders in college students (e.g., Ströhle et al., 2007), although other studies, mainly cross-section in nature, find contradictory results (e.g., Musselman & Rutledge, 2010). A more nuanced understanding of the relationship between exercise and alcohol consumption comes from large a epidemiological study finding a curvilinear relationship with exercise being most associated with moderate drinking (Lisha, Sussman, & Leventhal, 2013).
Recently, interventions that promote alternative non-substance using activities have been found to be associated with reductions in heavy drinking and alcohol-related problems in college students (Murphy et al., 2012). Exercise is potentially a potent substance-free activity as it has well-established benefits on reducing symptoms of depression and anxiety (Asmundson et al., 2013; Eyre, & Baune, 2012; Salmon, 2001). Controlled trials find that exercise that is predominantly of moderate intensity and aerobic in type significantly reduces mood and anxiety symptoms (Bock, Marcus, King, Borrelli, & Roberts, 1999; Babyak, Blumenthal, Herman, Khatri, Doraiswamy, & Moore, 2000). This benefit of exercise may be especially relevant for alcohol use disorders, as mood and anxiety disorders frequently co-occur with alcohol use disorders (Kessler et al., 1997), and can be a source of motivation to drink heavily (Ham & Hope, 2003). Other potential mechanisms of action for why exercise could lead to reductions in drinking include improvements in self-esteem/life satisfaction (Boden, Fergusson, & Horwood, 2008; Maher et al., 2013), reduction in urges to drink (Taylor, Oh, & Cullin, 2013; Ussher, Sampuran, Doshi, West, & Drummond, 2004) and providing other means of social bonding in a non-drinking context (i.e., social place conditioned preference; Yates, Beckmann, Meyer, & Bardo, 2013).
Because exercise has many beneficial effects, it has been proposed as a potential intervention for alcohol use disorders and hazardous drinking (Read et al., 2001; Weinstock, 2010). Aerobic and resistance training that has been incorporated into substance use disorders treatment has been found to decrease symptoms of depression and anxiety (Frankel & Murphy, 1974; Palmer, Palmer, Michiels, & Thigpen, 1995). To our knowledge, only one prior study has investigated exercise as intervention in heavy drinking college students (Murphy, Pagano, & Marlatt, 1986). In that study, participants were randomly assigned to running, meditation, or a no-treatment control condition. Those in either intervention condition had significant reductions in alcohol use compared to controls. However, exercising was based entirely on self-report, and the study had significant attrition.
Adherence and attrition to an exercise regimen are common methodological weaknesses of intervention studies involving exercise (Banks-Wallace & Conn, 2002; Oman & King, 2000). Data suggest that short-term dropout (i.e., within the first three to six months) from exercise programs ranges from 35% to 70%, while long-term adherence to exercise also remains very poor (Dishman, 1988; Marcus et al., 1998). Therefore, interventions that address motivation to adhere to exercise are needed.
One intervention that is widely used to increase treatment retention and adherence, as well as to improve outcomes across multiple populations and health behaviors, including with sedentary individuals is MET (Burke et al., 2004). Meta-analyses find that motivational interviewing interventions for physical activity may be efficacious, with small to large effect sizes (d = 0.20 to 0.78; Conn, Hafdahl, & Mehr, 2011; Hettema, Steele, & Miller, 2005). In this study MET was used as a platform therapy to increase intrinsic motivation to maintain an exercise routine (Buckworth, Lee, Regan, Schneider, & DiClemente, 2007).
Contingency management (CM) is a behavioral intervention that provides external motivation to change behaviors (Stitzer & Petry, 2006). This intervention has been successfully applied to increase drug abstinence, medication adherence, and treatment retention (e.g., Petry, et al., 2005; Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002). The principles of CM can also be applied toward increasing exercise participation. Several studies have shown that providing reinforcers (e.g., television viewing, money, or tangible items) contingent upon exercise improves exercise frequency, intensity, and health outcomes (Epstein, Plauch, Kilanowski, & Raynor, 2004; Epstein & Roemmich, 2001; Harland et al., 1999; Jeffery, Wing, Thorson, & Burton, 1998; Petry, Andrade, Barry & Byrne, 2013). Therefore, we developed and evaluated a novel intervention combining MET with CM for exercise in sedentary hazardous drinking college students. We hypothesize that MET paired with a CM intervention that provided the chance to win tangible prizes for exercise will increase adherence to exercise over the short-term over MET alone. Concerns may exist about combining these interventions, as CM may thwart the development of intrinsic motivation for the target behavior; however, prior studies show that incentives for health behavior change do not negatively impact motivation (Ledgerwood & Petry, 2006; Promberger & Marteau, 2013).
Conducting an exercise intervention with college students is particularly relevant as the majority are sedentary with only about one fifth currently meeting the current exercise recommendations (American College Health Association, 2011). Further, a substantial subset of sedentary college students, approximately 40 to 50%, desire to change their sedentary behavior (Keating, Guan, Piñero, & Bridges, 2005).
The primary goals of this pilot study were to (1) explore the feasibility of recruiting and retaining sedentary hazardous heavy drinkers into an exercise intervention; (2) ascertain the acceptability of the intervention as evidenced by intervention attendance; (3) conduct a preliminary analysis regarding the effect of adding CM to MET in relation to changes in exercise behavior in sedentary college students who engage in hazardous drinking; and (4) examine whether the combined MET+CM intervention decreases drinking in comparison to the MET alone intervention, including its potential effect size.
Methods
Participants
Participants were 31 sedentary hazardous drinking college students. Individuals were recruited via screening efforts, flyers posted on campus, and email list-serve announcements. As few students voluntarily seek alcohol-reducing interventions, the ads focused on recruitment of individuals who desired to become more physically active. A screening questionnaire was administered in university common areas, study information sessions, and over the telephone. The screen consisted of demographics, the Physical Activity Readiness Questionnaire (PAR-Q; American College of Sports Medicine [ACSM], 2013), Alcohol Use Disorders Identification Test (AUDIT; Flemming, Barry, & MacDonald, 1991; O’Hare & Sherrer, 1999; Saunders, Aasland, Amundsen, & Grant, 1993), questions about exercise and heavy drinking patterns over the past two months, and whether the individual was currently receiving or desired treatment for problems related to alcohol.
Students who were ≥ 18 and ≤ 27 years old, scored ≥ 8 on the AUDIT, did not endorse any contraindications for exercise on the PAR-Q, reported ≥ 4 heavy drinking episodes (4/5 criterion) in the past two months, exercising less than ≤ 12 times in the past two months, and were not receiving or did not desire treatment for alcohol-related problems were invited to participate in the study. The heavy drinking episode criterion was enacted to ensure that those identified as past year hazardous drinkers had recently engaged in binge drinking. As an aim of this pilot study was assessing feasibility of recruitment methods (Leon, Davis, & Kraemer, 2011), the recruitment goal was at least 30 participants (15 per group) during the one academic year allotted for study data collection.
As approved by the university’s Institutional Review Board, students initially provided assent for screening. Those who appeared to meet inclusion criteria were invited to an in-person evaluation where informed consent was obtained and study eligibility was verified. Data were collected in the 2008–2009 academic year.
Measures
Demographic Questionnaire assessed age, gender, ethnicity, marital status, grade point average (GPA), monthly income, affiliation in fraternities/sororities, and year in school.
Body Mass Index (BMI) and Waist Circumference (WC)
BMI was calculated from height (assessed in stocking feet and measured to the nearest 0.10cm) and weight (with excessive clothing and materials such as keys and wallet removed) and measured to the nearest 0.10 kg with a Health-o-meter® Professional scale 597 KL (Pelstar, Bridgeville, IL). BMI is weight in kg/height in m2. WC was measured twice at the height of the iliac crest with a tape measure to the nearest cm and averaged.
YMCA Submaximal Ergometer Test (YSET) is a measure of cardiorespiratory endurance (ACSM, 2013; Poldermans et al., 1993). The YSET is a reliable and valid means of predicting V02 peak with correlations ≥ .77 to V02 peak tests (Beekley et al., 2004; Garatachea, García-López, González-Gallego, & de Paz, 2007). The YSET is a multistage cardiorespiratory measure that began with an initial 2 min warm-up of 0 kgm resistance on a cycle ergometer (Monark Ergometric 818, Stockholm, Sweden). Following the initial warm-up period, a 150 kgm workload was applied for 3 minutes, representing stage one. All subsequent stages were progressed incrementally in 3 minute intervals based upon the heart rate (HR) response observed in the prior stage. Participant’s HR was measured in bpm and recorded at rest and during the second and third minute of each stage using a Polar Heart Rate monitor, model 190027142 (Polar Electro Oy, Kempele, Finland). Participant’s BP was also taken at rest and after the second minute of each stage using a Baumanometer Kompak model sphygmomanometer (W.A. Baum Co. Inc., Copiague, NY) and a Cardiology Stethoscope (Adscope model 602, Stuart Drug and Surgical Supply, La Mirada, CA). The rating of perceived exertion was monitored near the end of the third minute of each stage using the Borg 6–20 scale (Borg, 1998). YSET termination occurred when subjects reached 70% of their age-predicted peak HR, failed to conform to the exercise test protocol, or experienced signs or symptoms of excessive discomfort. The YSET concluded with a 5 min recovery period of seated rest. HR readings were used to estimate V02 peak, an accepted criterion of cardiorespiratory fitness (ACSM, 2013).
Timeline Followback (TLFB; Sobell & Sobell, 1992) is a retrospective method for assessing a trained research assistants. The TLFB assessed alcohol use (i.e., standard drinks) in the 60-days prior to baseline and throughout the intervention period and was used to calculate drinking outcomes.
The TLFB also assessed the frequency, duration, and intensity of exercise over the same time periods and was used to calculate self-report exercise outcomes. Intensity was assessed via a rating of perceived exertion using the Borg scale with a range of 6 to 20. The Borg scale is positively correlated with V02 and HR during exercise (Borg, 1998). The Borg scale was used to calculate estimated HR during each exercise episode (Scherr, Wolfarth, Christle, Pressler, Wagenpfeil, & Halle, 2013), and calories expended during each exercise session was calculated using the formula developed by Keytel and colleagues (2005). The TLFB demonstrates good test-retest reliability and validity for assessing alcohol use in numerous drinking populations (Sobell & Sobell, 1992) and is reliable and valid for assessing numerous other health behaviors, including exercise (Panza, Weinstock, Ash, & Pescatello, 2012).
Actical® Physical Activity Monitor is an omnidirectional accelerometer (Mini Mitter Company, Bend, OR). Rothney and colleagues (2007) found the Actical “was generally good at estimating the time spent in moderate and vigorous PA” (p. 1951) and has acceptable reliability (Chen et al., 2003). Omnidirectional accelerometers, such as the Actical, are considered the standard by which other measurement methods used to monitor ambulatory physical activity are compared (e.g., Panza et al., 2012). Participants securely fastened the Actical to their hip on the side of the dominant hand. The device was set to record physical activity in 60 second epochs and was initialized to begin recording data immediately. In order to be included in the subsequent analysis, participants were required to wear the Actical for at least four consecutive days (96 hr; two days during the week and two days over the weekend) and were asked to only remove the Actical when they were swimming, bathing, showering, or sleeping. Participants were asked to inform the research assistant if they removed the Actical to go swimming at any point during the four days they were wearing the device. A valid day was defined as at least 8 consecutive hours of wear time.
Exercise variables extracted from the accelerometers were (1) total kilocalories (Kcals) expended and (2) percent of time spent in moderate to vigorous intensity physical activity, as these intensities correspond to engaging in exercise. Cut points (in units of activity energy expenditure) used to define moderate and vigorous intensity physical activity were as follows: 0.0310 kcal·kg−1·min−1 ≤ moderate intensity < 0.0832 kcal·kg−1·min−1; and vigorous ≥ 0.0832 kcal·kg−1·min−1 (Heil, 2006).
Procedures
After providing informed consent, participants were fitted for the accelerometer and provided with instructions to wear the monitor continuously for four days. Upon returning the research laboratory four days later, the participant was asked about compliance with wearing the accelerometer and the device was checked to ensure compliance. Participants then completed a baseline assessment battery. The battery consisted of self-report questionnaires assessing alcohol use, exercise participation, and psychosocial functioning. Participants also completed a physical fitness battery. A post-treatment evaluation occurred at 2-months that was identical to the baseline assessment with four days of continuous accelerometer monitoring and then completion of the study assessment battery. In total, participants were compensated $70 if they completed both study evaluations, $20 for the baseline evaluation and $50 for the post-treatment.
Randomization to Interventions
Immediately after completing the baseline assessment, participants selected a slip of paper from an envelope with slips numbered 1 through 40. Those selecting a slip of paper with an odd number were randomized to the MET alone condition. Participants selecting a slip of paper with an even number were randomized to the MET + CM intervention. Subsequent participants choose from the remaining slips. Thus, sample sizes are not equal across groups.
MET
The MET session lasted approximately 50 minutes and was framed as a “wellness intervention” for increasing exercise. Interventionists provided personalized feedback about the student’s exercise habits in comparison to population norms (Keating et al., 2005) and exercise guidelines at the time (ACSM, 2007). Next, a discussion about how exercise fit within the participant’s lifestyle goals and values was conducted. Last, the student in conjunction with the interventionist developed a change plan to begin exercising. Drinking was not discussed as part of the MET intervention, unless the participant brought up alcohol use as an impediment to exercising.
MET+CM
Participants randomly assigned to this condition received the same MET intervention as outlined above, plus 8 weeks of CM for exercise. A CM intervention offers tangible reinforcement when target behaviors are completed and verified. In this study exercise was the target behavior being reinforced.
The eight CM sessions were structured such that the interventionist and participant met briefly each week to review the previous week’s exercise activity contract, award any reinforcement earned for completion and verification of exercise activities, and collaboratively develop a new exercise activity contract for the upcoming week. The exercise activity contracts identified three specific exercise activities to be completed by the participant within the upcoming week. Options included walking with a pedometer, walking or jogging on a treadmill for 15 minutes, or attending an exercise class at the gym. Each exercise bout was explicitly defined in terms of duration and length, as well as objective verification needed for proving completion. Pedometers were used for objective verification of aerobic exercise such as running and aerobic classes. Brief video clips (via a cellular phone or digital camera) of resistance exercises or an aerobics instructor’s note confirming an individual’s attendance also served as verification of exercise bout completion. Participants were sedentary at the start of the study. Thus, initial exercise activities were in the light (<40% V02 peak) to moderate (40% – <60% V02 peak) intensity range as tolerated by the participant. Over time, the goal was to have participants exercise in a manner consistent with recommended guidelines (ACSM, 2007). Towards the end of the intervention period, the exercise activities contracts could contain more than one bout of exercise per “activity” as some individuals were exercising five days per week and there were spots for only three activities per contract.
Participants earned a draw from a prize bowl for each verified exercise activity completed. If all three activities were completed and verified within one week, s/he got bonus draws that started at three draws and escalated over time with successful completion of the all three exercise activities. For example, if participants completed and provided verification for three activities two weeks in a row, they got four bonus draws, and so on up to a maximum of 10 bonus draws/week. In total, participants could earn up to 79 draws from the prize bowl if s/he completed and verified 24 exercise activities (three per week for 8 weeks). If participants did not complete three exercise activities in a week, or failed to provide verification, they earned a draw for each activity completed and verified (if any) but forfeited the bonus draws. In addition, the bonus draws were reset back to three on the next week’s contract. Participants were informed at the outset of the intervention that if they failed to provide verification for completed activities at the weekly CM appointments they would not receive the associated draws from the prize bowl. The prize bowl for drawings contained 80 slips of paper, and all slips were returned to the prize bowl after participant drawings to maintain probabilities. Half of the slips stated “Good job!”, but were not associated with a prize. The other half were winning slips: 34 stated “small prize”, five stated “large prize”, and one stated “jumbo prize”. When participants drew a winning slip, they chose amongst the available prizes in that category: small, large, or jumbo. Examples of small prizes were $1 coffee gift certificates, granola bars, etc. Examples of large prizes were $20 gift certificates to book/music stores, sporting equipment stores, etc. Examples of a jumbo prize were a choice of a gift certificate to shoe or clothing stores, Best Buy, iTunes, etc. Maximal costs of respective types of prizes were $1, $20, and $100. In total, participants were expected to earn on average $230 if they completed all the exercise activities.
Interventionists
Two interventionists (one M.S. kinesiologist and one Ph.D. clinical psychologist) delivered the MET and CM interventions. Each provided the intervention to approximately half of the participants.
Data Analysis
Prior to analysis all variables were examined for outliers and fit between their distributions and the assumptions of multivariate analysis. One outlier (i.e., greater than three SDs from the mean) was detected and winsorized to within three standard deviations for each of the following variables: baseline weekly exercise duration, baseline estimated weekly calories expended, post-treatment weekly exercise duration, and post-treatment estimated weekly calories expended. No other outliers were detected. The intervention groups were compared on baseline variables using ANOVA for continuous measures and chi-square tests for categorical measures.
Validity of accelerometer data was assessed by participant self-report of wearing the monitor and screening for prolonged periods of inactivity (i.e., > 14 consecutive hours with energy expenditures equal to zero). During the follow-up evaluation, one participant was identified as not having worn the accelerometer as requested by the researchers and subsequently was not included in the accelerometer analyses. Accelerometer data was obtained on all participants at baseline yielding an average of 96.82 hours (SD = 2.43) of available data. At post-treatment accelerometer data was obtained on 28 of the 31 participants (90.3%) yielding an average of 96.01 hours (SD = 0.05) of available data.
Primary outcomes for exercise included self-reported exercise: (1) frequency, (2) weekly duration (minutes), (3) weekly estimated calories expended as recorded on the TLFB for exercise, and (4) estimated V02 peak from the YSET. Accelerometer outcomes included: (1) total Kcals expended, and (2) percent of time spent in moderate to vigorous physical activity. Primary drinking self-reported outcomes were (1) number of days drank alcohol, (2) number of heavy drinking episodes (5/4 criterion), and (3) total number of drinks per week as assessed by the TLFB. Three separate repeated measures MANOVA were used to examine each class of outcomes: (1) self reported exercise outcomes, (2) accelerometer outcomes, and (3) the drinking outcomes. In these analyses, the dependent variables included the relevant indices associated with that class of outcomes, and time and intervention condition were included as a between subjects variable in each analysis. No covariates were included in the analyses due to small sample size. Effect size estimates (d) of the MET+CM intervention in comparison to the MET intervention using baseline to post-treatment change score means and standard deviations were also calculated as this was a pilot study with a small sample size and concerns about the possibility of a type 2 error exist. IBM SPSS version 21 was used.
Results
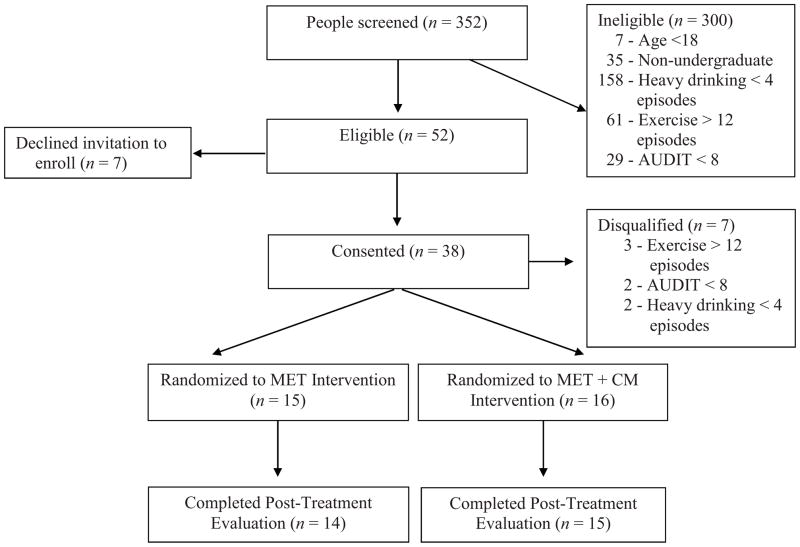
As shown in Figure 1, 352 individuals were screened of whom 52 (14.8%) were deemed potentially eligible participants. Of those 52 individuals, 31 (59.6%) were eligible, signed informed consent, and randomized to an intervention condition, which represented 8.8% of all individuals screened. Less than 15% of potentially eligible individuals were not interested in participating in the study.
Figure 1.
Flowchart of participants in the study.
Table 1 shows baseline characteristics for participants randomized to each treatment condition. No significant group differences were detected for any of the demographic characteristics or baseline health fitness variables, p > .05. Tables 2 and 3 display past two-month exercise and drinking behavior at baseline and post-treatment. At baseline, participants in the MET+CM intervention reported drinking significantly more days than participants in the MET intervention, F(1,29) = 5.81, p < .05. No significant differences were detected among the remaining drinking variables or exercise variables, p > .05.
Table 1.
Participant demographic characteristics.
| Variable | MET (n = 15) | MET + CM (n = 16) | Statistic (df) | p-value |
|---|---|---|---|---|
| Gender | χ2(1) = 0.99 | .458 | ||
| Male | 4 (26.7%) | 7 (43.8%) | ||
| Female | 11 (73.7%) | 9 (56.3%) | ||
| Ethnicity | χ2(1) = 0.44 | .600 | ||
| Caucasian | 13 (86.7%) | 15 (93.8%) | ||
| Non-Caucasian | 2 (13.3%) | 1 (6.3%) | ||
| Year in School | χ2(4) = 4.35 | .361 | ||
| ≤1Year | 3 (20.0%) | 3 (18.8%) | ||
| 2 Years | 2 (13.3%) | 2 (12.5%) | ||
| 3 Years | 7 (46.7%) | 3 (18.8%) | ||
| 4 Years | 2 (13.3%) | 7 (43.8%) | ||
| ≥5 Years | 1 (6.7%) | 1 (6.3%) | ||
| Fraternity/sorority Affiliation | χ2(1) = 0.44 | .600 | ||
| Yes | 2 (13.3%) | 1 (6.3%) | ||
| No | 13 (86.7%) | 15 (93.7%) | ||
| Mean (SD) | Mean (SD) | |||
| Age (years) | 20.1 (1.2) | 21.0 (2.3) | F(1,29) = 1.69 | .204 |
| Grade Point Average | 2.9 (0.4) | 3.1 (0.3) | F(1,29) = 0.66 | .422 |
| Body Mass Index | 23.9 (3.8) | 26.2 (5.0) | F(1,29) = 2.01 | .167 |
| Resting Systolic BP | 110.7 (8.5) | 114.9 (8.2) | F(1,29) = 1.97 | .172 |
| Resting Diastolic BP | 74.4 (5.4) | 75.7 (7.8) | F(1,29) = 0.27 | .605 |
| Waist Circumference | 76.0 (9.8) | 81.4 (13.8) | F(1,28) = 1.58 | .219 |
Note. BP = Blood pressure; RPE = Rating of Perceived Exertion; Brown-Forsythe F-statistic reported for weekly exercise duration.
Table 2.
Exercise behavior at baseline and post-treatment.
| Outcome Measures mean (SD) | Univariate F Test, p-value | |||
|---|---|---|---|---|
|
| ||||
| Variable | Baseline | Post-Treatment | Time | Group × Time |
| Exercise Frequency | 48.26, p = .001 | 17.35, p =.001 | ||
| MET | 5.31 (3.75) | 8.62 (5.42) | ||
| MET+CM | 7.00 (4.11) | 20.21 (6.81) | ||
| Weekly Duration (min) | 1.45, p = .240 | 0.78, p = .387 | ||
| MET | 46.56 (26.46) | 44.38 (14.18) | ||
| MET+CM | 63.78 (38.23) | 49.63 (20.09) | ||
| Weekly Calories Expended | 6.61, p = .016 | 3.62, p = .069 | ||
| MET | 352.29 (408.38) | 456.01 (367.61) | ||
| MET+CM | 763.69 (825.19) | 1458.33 (1169.37) | ||
| Estimated V02 Peak | 7.24, p = .013 | 1.60, p = .218 | ||
| MET | 33.88 (4.97) | 35.51 (6.51) | ||
| MET+CM | 31.63 (4.99) | 36.14 (6.89) | ||
| Total Kcals | 2.52, p = .124 | 0.12, p = .733 | ||
| MET | 2422.89 (1094.07) | 1953.48 (1151.26) | ||
| MET+CM | 2629.92 (1282.51) | 2328.03 (1167.37) | ||
| Percent Time (%) M-V PA | 3.11, p = .089 | 0.33, p = .573 | ||
| MET | 9.06 (2.92) | 9.95 (3.52) | ||
| MET+CM | 9.01 (3.80) | 10.75 (4.15) | ||
Note. MET = Motivational Enhancement Therapy; MET+CM = Motivational Enhancement Therapy plus Contingency Management; Kcals = Kilocalories; M-V PA = moderate to vigorous physical activity.
Table 3.
Drinking behavior at baseline and post-treatment.
| Outcome Measures means (SD) | Univariate F Test, p-value | |||
|---|---|---|---|---|
|
| ||||
| Variable | Baseline | Post-Treatment | Time | Group × Time |
| Drinking Days | 3.66, p = .067 | 1.64, p = .211 | ||
| MET | 15.07 (7.62) | 14.21 (5.85) | ||
| MET+CM | 22.60 (9.23) | 18.27 (8.76) | ||
| Heavy Drinking Days | 3.44, p = .075 | 0.48, p = .494 | ||
| MET | 13.29 (7.85) | 10.21 (7.87) | ||
| MET+CM | 12.67 (8.38) | 11.27 (7.97) | ||
| Total Drinks per Week | 0.27, p = .609 | 0.17, p = .681 | ||
| MET | 11.83 (7.26) | 11.71 (9.28) | ||
| MET+CM | 14.72 (7.90) | 13.66 (8.62) | ||
Note. MET = Motivational Enhancement Therapy; MET+CM = Motivational Enhancement Therapy plus Contingency Management.
All participants received the MET session as it was held immediately after the baseline assessment. For MET+CM participants the MET session ended with an introduction to the CM procedures. On average MET+CM participants attended 6.94 CM sessions (SD = 2.24) out a total possible of 8, with a range of 1–8. MET+CM participants completed and verified 17.9 (SD = 8.8) exercise activities, earned an average of 49.9 draws (SD = 26.8) resulting in mean winnings of $182.0 (SD = $102.3). No study-related adverse events were detected during the study.
The repeated measures MANOVA found significant multivariate effects on the self-reported exercise outcome variables for time, F(4, 22) = 14.29, p < .001, intervention condition, F(4, 22) = 4.47, p < .01, and intervention condition by time interaction, F(4, 22) = 5.04, p < .01. As shown in Table 2, post-hoc tests found significant increases for time from baseline to post-treatment in exercise frequency, estimated weekly calories expended, and improved estimated V02 peak, ps < .05. Weekly duration did not differ significantly over time, p = .055. Only exercise frequency differed significantly over time by condition, p < .001. Weekly duration, estimated weekly calories expended, and estimated V02 peak did not differ significantly over time by condition, ps > .05, but estimated weekly calories expended approached significance, p = .069. While both groups increased exercise frequency during the intervention period, the MET+CM participants exercised significantly more often than the MET participants. During the intervention period the MET+CM participants’ exercised 20.21 times (SE = 1.65); meanwhile, MET participants exercised 8.50 times (SE = 1.76). These exercise frequencies translated to weekly averages of 2.5 and 1.0 times, respectively.
Repeated measures MANOVA of accelerometer data found a significant multivariate effect for time, F(2, 26) = 7.80, p < .01, but not for intervention condition, F (2,26) = 0.16, p = .852, or for the time by intervention condition interaction, F(2,26) = 0.16, p = .855. The post-hoc tests for time were not significant for total Kcals expended, F(1, 26) = 2.52, p =.124, or percent of time spent in moderate to vigorous physical activity, F(1, 26) = 3.11, p =.089.
The estimated effect size of the change from baseline to post-treatment of MET+CM intervention in comparison to the MET intervention was large for exercise frequency (d = 1.60) and estimated weekly calories expended (d = 0.76), moderate on estimated V02 peak (d = 0.49), and small to no effect for percent of time spent in moderate to vigorous physical activity (d = 0.22) and total Kcal expended (d = 0.14). A moderate effect size was found in favor of the MET intervention over the MET+CM intervention for weekly exercise duration (d = −0.34).
No significant multivariate effects were found with the repeated measures MANOVA on the drinking outcome variables for time, F(3, 25) = 1.71, p = .189, intervention condition, F(3, 25) = 2.08, p = .128, or the time by intervention condition interaction, F(3, 25) = 1.70, p = .193 (Table 3). The estimated effect size of the change from baseline to post-treatment of the MET+CM intervention in comparison to the MET intervention was moderate for days drinking (d = 0.48) and small for total number of drinks per week (d = 0.15). The MET alone condition resulted in a numerically greater reduction in heavy drinking episodes than the MET+CM condition, with an estimated effect size of d = −0.26.
Discussion
Results from this pilot study suggest that sedentary hazardous drinking college students are interested in participating in an exercise intervention and will engage in interventions that seek to facilitate initiation and maintenance of exercise. Moreover, adding CM to MET was successful in increasing the self-reported frequency of exercising, but the addition of CM did not improve any other self-report or objective indices of exercise relative to MET alone. On average, participants exercised more often during the intervention period in comparison to baseline, and participants in the MET+CM intervention exercised more frequently than MET only participants, with weekly averages of 2.5 and 1.0 times, respectively. This result is consistent with prior research that finds providing reinforcers contingent upon exercise improves engagement (Epstein et al., 2001; 2004; Jeffery et al., 1998; Harland et al., 1999; Petry et al., 2013). Additionally, the results of this pilot study suggest the combination of MET and CM warrant further examination as an exercise intervention strategy for sedentary college students.
While it is possible that MET+CM participants may have overrepresented their exercise engagement to obtain the incentives, we believe it is unlikely as all exercise activities were verified during the CM sessions and incentives were not provided for the exercise reported at the post-treatment assessment. The discrepancy between self-report and the accelerometers is possibly due to the different timeframes of the assessments: four days for the accelerometer and past two months for the TLFB. MET+CM participants may have completed much of their weekly exercise prior to the wearing of the accelerometer for the post-treatment evaluation.
Although exercise appeared to increase across participants as a whole, there were no significant changes or differences in drinking behavior over time or by treatment condition over time. Participants’ number of drinking days, number of binge drinking episodes, and number of drinks per week remained relatively static. We found that while participants in both interventions increased exercise frequency no corresponding changes in heavy drinking occurred. There are many possible explanations for the lack of change in drinking behavior in this pilot study. Firstly, the interventions did not directly address drinking. Secondly, other studies of college students have found either no relationship or a positive association between alcohol use and exercise, with greater exercise engagement justifying higher alcohol use (Downs, & Ashton, 2011; Musselman & Rutledge, 2010). Additionally, exercise may not effectively compete with drinking in this population because many college environments support and facilitate heavy drinking as an important aspect of the college experience. These interventions, while potentially not efficacious in this population, may yield benefits on reducing drinking in other populations or clinical samples.
Some limitations of the present study include the small sample size, the lack of a “no intervention” control group, and the short duration of the intervention. These limitations hampered data analysis, increased the likelihood of type 2 error, and allowed detection of only very large effect sizes. Because all participants received an intervention that focused on increasing exercise behavior, the effects of the MET may have muted those of CM. Further, other research suggests that in order to gain the many of the benefits of exercise, at least 12–16 weeks of regular exercise participation are needed (ACSM, 2013). With a longer intervention time period, significant reductions in drinking may have manifested as the level of physical fitness increases and the benefits of exercise become noticeable. Moreover, effects of the intervention may have been different with a heavier drinking population. Despite random assignment, a significant difference was found on baseline drinking days between the two treatment groups, with the MET+CM participants drinking significantly more days in the pre-treatment period than the MET participants.
This pilot study found that the intervention was well received by sedentary hazardous drinking college students, the addition of CM to MET significantly increased frequency of self-reported exercise, but not any other indices of exercising, and no effects of these interventions were noted with respect to alcohol use. Efficacy of this combined intervention remains to be tested on a large scale for a longer duration including long-term follow-up, and in these circumstances may yield beneficial effects. Evaluating motivations to drink and drinking consequences may highlight specific groups for whom the intervention may be most beneficial. Hazardous drinking in college students is a major public health concern; alternate interventions are needed to address this problem in a manner that is not stigmatizing and efficacious in decreasing drinking.
Highlights.
Exercise is proposed to reduce drinking in sedentary hazardous drinking students
Self-reported exercise frequency increased over time for all participants
Participants receiving the MET+CM intervention reported exercising more often
No changes in drinking were reported
Acknowledgments
This research and preparation of this report was funded by National Institutes of Health Grants P60-AA-003510and R21-AA-017717.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College Health Association. National College Health Assessment II: Reference Group Data Report Spring 2011. Hanover, MD: Author; 2011. [Google Scholar]
- American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. 7. Baltimore, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 9. Baltimore, MD: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Fetzner MG, DeBoer LB, Powers MB, Otto MW, Smits JAJ. Let’s get physical: A contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depression and Anxiety. 2013;30:362–373. doi: 10.1002/da.22043. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, et al. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Banks-Wallace J, Conn V. Interventions to promote physical activity among African American women. Public Health Nursing. 2002;19:321–335. doi: 10.1046/j.1525-1446.2002.19502.x. [DOI] [PubMed] [Google Scholar]
- Beekley MD, Brechue WF, Dehoyos DV, Garzarella L, Werber-Zion G, Pollock ML. Cross-validation of the YMCA submaximal cycle ergometer test to predict VO2 max. Research Quarterly for Exercise and Sport. 2004;75:337–342. doi: 10.1080/02701367.2004.10609165. [DOI] [PubMed] [Google Scholar]
- Blanco C, Okuda M, Wright C, Hasin DS, Grant BF, Lui S, Olfson M. Mental health of college students and their non-college-attending peers: Results from the National Epidemiologic Study on Alcohol and Related Conditions. Archives of General Psychiatry. 2008;65:1429–1437. doi: 10.1001/archpsyc.65.12.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24:399–410. doi: 10.1016/S0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ. Does adolescent self-esteem predict later life outcomes? A test of the causal role of self-esteem. Development and Psychopathology. 2008;20:319–339. doi: 10.1017/S0954579408000151. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- Buckworth J, Lee RE, Regan G, Schneider LK, DiClemente LC. Decomposing intrinsic and extrinsic motivation for exercise: Application to stages of motivational readiness. Psychology of Sport and Exercise. 2007;8:399–410. doi: 10.1016/j.psychsport.2006.06.007. [DOI] [Google Scholar]
- Burke BL, Dunn CW, Atkins DC, Phelps JS. The emerging evidence base for motivational interviewing: A meta-analytic and qualitative inquiry. Journal of Cognitive Psychotherapy. 2004;18:309–322. doi: 10.1891/jcop.18.4.309.64002. [DOI] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10:54–63. doi: 10.1037/1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Chen KY, Acra SA, Majchrzak K, Donahue CL, Baker L, Clemens L, Buchowski MS. Predicting energy expenditure of physical activity using hip- and wrist-worn accelerometers. Diabetes Technology & Therapuetics. 2003;5:1023–1033. doi: 10.1089/152091503322641088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. American Journal of Public Health. 2011;101(4):751–758. doi: 10.2105/AJPH.2010.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia CJ, Carey KB, Simons J, Borsari BE. Relationship between binge drinking and substance-free reinforcement in a sample of college students: A preliminary investigation. Addictive Behaviors. 2003;28:361–368. doi: 10.1016/S0306-4603(01)00229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia CJ, Benson TA, Carey KB. Decreased substance use following increases in alternative behaviors: A preliminary investigation. Addictive Behaviors. 2005;30:19–27. doi: 10.1016/j.addbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Cronce JM, Larimer ME. Individual-focused approaches to the prevention of college student drinking. Alcohol Research & Health. 2011;34(2):210. SPS-AR&H-33. [PMC free article] [PubMed] [Google Scholar]
- Dishman RK. Exercise Adherence: Its Impact on Public Health. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- Downs A, Ashton J. Vigorous physical activity, sports participation, and athletic identity: Implications for mental and physical health in college students. Journal of Sport Behavior. 2011;34:228–249. [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Hunt J, Speer N, Zivin K. Mental health service utilization among college students in the United States. Journal of Nervous and Mental Disease. 2011;199:301–308. doi: 10.1097/NMD.0b013e3182175123. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Plauch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in treatment of pediatric obesity. Health Psychology. 2004;23:135–145. doi: 10.1037/0278-6133.23.4.371. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN. Reducing sedentary behavior: Role in modifying physical activity. Exercise and Sports Medicine Reviews. 2001;29:103–108. doi: 10.1097/00003677-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Barry KL, MacDonald R. The Alcohol Use Disorders Identification Test (AUDIT) in a college sample. International Journal of the Addictions. 1991;26:1173–1185. doi: 10.3109/10826089109062153. [DOI] [PubMed] [Google Scholar]
- Eyre H, Baune BT. Neuroimmunological effects of physical exercise in depression. Brain, Behavior, and Immunity. 2012;26:251–266. doi: 10.1016/j.bbi.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Frankel A, Murphy J. Physical fitness and personality in alcoholism: Canonical analysis of measure before and after treatment. Quarterly Journal on Studies on Alcohol. 1974;35:1272–1278. [PubMed] [Google Scholar]
- Garatachea EC, García-López D, González-Gallego J, de Paz JA. Estimation of energy expenditure in healthy adults from the YMCA submaximal cycle ergometer test. Evaluation & the Health Professions. 2007;30:138–149. doi: 10.1177/0163278707300628. [DOI] [PubMed] [Google Scholar]
- Ham LS, Hope DA. College students and problematic drinking: A review of the literature. Clinical Psychology Review. 2003;23(5):719–759. doi: 10.1016/S0272-7358(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: A randomized controlled trial of methods to promote physical activity in primary care. British Medical Journal. 1999;319:828–832. doi: 10.1136/bmj.319.7213.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil D. Predicting activity energy expenditure using the Actical activity monitor. Research Quarterly for Exercise and Sport. 2006;77:64–80. doi: 10.1080/02701367.2006.10599333. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Wing RR, Thorson C, Burton L. Use of personal trainers and financial incentives to increase exercise in a behavioral weight loss program. Journal of Consulting and Clinical Psychology. 1998;66:777–783. doi: 10.1037/0022-006X.66.5.777. [DOI] [PubMed] [Google Scholar]
- Johnston JJE, McGovern SJ. Alcohol related falls: An interesting pattern of injuries. Emergency Medicine Journal. 2004;21(2):185–188. doi: 10.1136/emj.2003.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating XD, Guan J, Piñero JC, Bridges DM. A meta-analysis of college students’ physical activity behaviors. Journal of American College Health. 2005;54:116–125. doi: 10.3200/JACH.54.2.116-126. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg JS, Anthony JC. Lifetime co-occurrence of DSM-IIIR alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Keytel LR, Goedecke JH, Noakes TD, Hiiloskorpi H, Laukkanen R, van der Merwe L, Lambert EV. Prediction of energy expenditure from hear rate monitoring during submaximal exercise. Journal of Sports Sciences. 2005;23(3):289–297. doi: 10.1080/02640410470001730089. doi: 10/1080.02610440470001730089. [DOI] [PubMed] [Google Scholar]
- Knight JR, Wechsler H, Kuo M, Seibring M, Weitzman ER, Schuckit MA. Alcohol abuse and dependence among U.S. college students. Journal of Studies on Alcohol. 2002;63:263–270. doi: 10.15288/jsa.2002.63.263. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug and Alcohol Dependence. 2006;83:65–72. doi: 10.1016/j.drugalcdep.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research. 2011;45:626–629. doi: 10.1016/j.psychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisha NE, Sussman S, Leventhal AM. Physical activity and alcohol use disorders. American Journal of Drug and Alcohol Abuse. 2013;39:115–120. doi: 10.3109/00952990.2012.713060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neuroscience & Biobehavioral Reviews. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Bock BC, Pinto BM, Forsyth LAH, Roberts MB, Traficante RM. Efficacy of an individualized, motivationally-tailored physical activity intervention. Annals of Behavioral Medicine. 1998;20(3):174–180. doi: 10.1007/BF02884958. [DOI] [PubMed] [Google Scholar]
- Maher JP, Doerksen SE, Elavsky S, Hyder AL, Pincus AL, Ram N, Conroy DE. A daily analysis of physical activity and satisfaction with life in emerging adults. Health Psychology. 2013;32:647–656. doi: 10.1037/a0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Dennhardt AA, Skidmore JR, Borsari B, Barnett NP, Colby SM, Martens MP. A randomized controlled trial of a behavioral economic supplement to brief motivational interventions for college drinking. Journal of Consulting and Clinical Psychology. 2012;80(5):876–886. doi: 10.1037/a0028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Pagano RR, Marlatt GA. Lifestyle modification with heavy alcohol drinkers: Effects of aerobic exercise and meditation. Addictive Behaviors. 1986;11:175–186. doi: 10.1016/0306-4603(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Musselman JR, Rutledge PC. The incongruous alcohol-activity association: Physical activity and alcohol consumption in college students. Psychology of Sport and Exercise. 2010;11(6):609–618. doi: 10.1016/j.psychsport.2010.07.005. [DOI] [Google Scholar]
- O’Hare T, Sherrer MV. Validating the alcohol use disorders identification test with college first-offenders. Journal of Substance Abuse Treatment. 1999;17:113–119. doi: 10.1016/S0740-5472(98)00063-4. [DOI] [PubMed] [Google Scholar]
- Oleski J, Mota N, Cox BJ, Sareen J. Perceived need for care, help seeking, and perceived barriers to care for alcohol use disorders in a national sample. Psychiatric Services. 2010;61:1223–1231. doi: 10.1176/appi.ps.61.12.1223. [DOI] [PubMed] [Google Scholar]
- Oman RF, King AC. The effect of life events and exercise program format on the adoption and maintenance of exercise behavior. Health Psychology. 2000;19:605–612. doi: 10.1016/S0740-5472(98)00063-4. [DOI] [PubMed] [Google Scholar]
- Palmer JA, Palmer LK, Michiels K, Thigpen B. Effects of type of exercise on depression in recovering substance abusers. Perceptual and Motor Skills. 1995;80:523–530. doi: 10.2466/pms.1995.80.2.523. [DOI] [PubMed] [Google Scholar]
- Panza G, Weinstock J, Ash GI, Pescatello LS. Psychometric evaluation of the Timeline Followback for exercise among college students. Psychology of Sport & Exercise. 2012;13:779–788. doi: 10.1016/j.psychsport.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Andrade LF, Barry D, Byrne SA. Randomized study of reinforcing ambulatory exercise in older adults. Psychology and Aging. 2013;28:1164–1173. doi: 10.1037/a0032563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldermans D, Fioretti PM, Forster T, Thompson IR, Boersma E, el-Said EM, et al. Dobutamine stress echocardiography for assessment of perioperative cardiac risk in patients undergoing major vascular surgery. Circulation. 1993;87:1506–1512. doi: 10.1161/01.CIR.87.5.1506. [DOI] [PubMed] [Google Scholar]
- Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? Comparing behaviors studied in psychological and economic literatures. Health Psychology. 2013;32:950–957. doi: 10.1037/a0032727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Brown RA, Marcus BH, Kahler CW, Ramsey SE, Dubreuil ME, Francione C. Exercise attitudes and behaviors among persons in treatment for alcohol use disorders. Journal of Substance Abuse Treatment. 2001;21:199–206. doi: 10.1016/S0740-5472(01)00203-3. [DOI] [PubMed] [Google Scholar]
- Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of physical activity intensity predictions by ActiGraph, Actical and RT3. Obesity. 2008;16:1946–1952. doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clinical Psychology Review. 2001;21:33–61. doi: 10.1016/S0272-7358(99)00032-X. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of person with harmful alcohol consumption, I. Addiction. 1993;88:349–362. doi: 10.1111/j.1360-0443.1993.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. European Journal of Applied Physiology. 2013;113(1):147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback User’s Guide: A Calendar Method for Assessing Alcohol and Drug Use. Toronto, Ontario, Canada: Addiction Research Foundation; 1992. [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Oh H, Cullin S. Acute effect of exercise on alcohol urges and attentional bias towards alcohol-related images in high alcohol consumers. Mental Health & Physical Activity. 2013;6:220–226. doi: 10.1016/j.mhpa.2013.09.004. [DOI] [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99:1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in the 1990s: A continuing problem: Results of the Harvard School of Public Health 1999 College Alcohol Study. Journal of American College Health. 2000;48:199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Nelson TF. Binge drinking and the American college student: What’s five drinks? Psychology of Addictive Behaviors. 2001;15:287–291. doi: 10.1037/0893-164X.15.4.287. [DOI] [PubMed] [Google Scholar]
- Weinstock J. A review of exercise as intervention for sedentary hazardous drinking college students: Rationale and issues. Journal of American College Health. 2010;58(6):539–544. doi: 10.1080/07448481003686034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: Effects of age and housing condition. Drug and Alcohol Dependence. 2013;129:240–246. doi: 10.1016/j.drugalcdep.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]