Abstract
Purpose
To investigate pulmonary function test (PFT) and arterial blood gas changes (complete PFT) following stereotactic body radiation therapy (SBRT) and to see whether baseline PFT correlates with lung toxicity and overall survival in medically inoperable patients receiving SBRT for early stage, peripheral, non-small cell lung cancer (NSCLC).
Methods and materials
During the 2-year follow-up, PFT data was collected for patients with T1-T2N0M0 peripheral NSCLC who received effectively 18 Gy × 3 on Phase II North American multicenter study (RTOG 0236). Pulmonary toxicity was graded utilizing the RTOG SBRT pulmonary toxicity scale. Paired Wilcoxon signed rank test, Logistic Regression model, and Kaplan-Meier method were used for the statistical analysis.
Results
At 2 years, mean % predicted FEV1 and DLCO declines were 5.8% and 6.3%, respectively, with minimal changes of arterial blood gases, and no significant decline of oxygen saturation. Baseline PFT was not predictive of any pulmonary toxicity following SBRT. Whole lung V5, V10, V20 and mean dose to the whole lung were almost identical between patients who developed pneumonitis and patients who were pneumonitis-free. Poor baseline PFT did not predict decreased overall survival. Patients with poor baseline PFT as a reason for medical inoperability had higher median and overall survivals than patients with normal baseline PFT but with cardiac morbidity.
Conclusions
Poor baseline PFT did not appear to predict toxicity, or decreased overall survival after SBRT in this medically inoperable population. Poor baseline PFT alone should not be used to exclude patients with early stage lung cancer from treatment with SBRT.
Keywords: stereotactic body radiation therapy, non-small cell lung cancer, pulmonary function test, radiation pneumonitis, overall survival
Introduction
Stereotactic body radiation therapy (SBRT) has been shown to be an effective treatment in medically inoperable patients with early stage non-small cell lung cancer (NSCLC) (1-3). However, relatively little has been published about the influence of pre-treatment pulmonary function test (PFT) and dosimetry as it relates to toxicity and post-treatment PFT. Several clinical studies (4-8) analyzed changes in PFT following SBRT. However, these studies were generally limited to spirometry parameters and diffusing capacity for carbon monoxide (DLCO) with variable follow-up periods.
The Radiation Therapy Oncology Group (RTOG) 0236 protocol used SBRT in treating patients with medically inoperable early stage lung cancer (3). By design, this phase II protocol collected pre- and post-treatment PFT data as well as comprehensive dose deposition information centrally. The goal of this analysis was to use this data to conduct a comprehensive analysis of PFT and arterial blood gas changes (complete PFT) following SBRT, and to see whether baseline PFT correlates with any pulmonary toxicity and overall survival in medically inoperable patients receiving SBRT for early stage, peripheral, non-small cell lung cancer (NSCLC). An additional goal was to analyze the normal lung tissue dose deposition and see whether there is a relationship predicting the development of radiation pneumonitis or any high grade pulmonary toxicity.
Materials and Methods
Patient Eligibility
Patient eligibility criteria were described by Timmerman and colleagues in the primary analysis of RTOG 0236 (3). Briefly, patients above the age of 18 with a Zubrod performance status score 0-2 and cytological or histological proof of NSCLC were required for the entry. Eligible patients had AJCC stages T1-T3 (≤ 5 cm) and peripherally located NSCLC at least 2 cm from the proximal bronchial tree. The protocol specifically treated patients unable to tolerate a definitive surgical extirpation of the primary tumor due to medical problems (i.e., medically inoperable patients). Indicators defining a patient to be medically inoperable included baseline forced expiratory volume in one second (FEV1) of less than 40% predicted, predicted postoperative FEV1 of less than 30% predicted, DLCO of less than 40% predicted, baseline hypoxemia or hypercapnia, severe pulmonary hypertension; severe cerebral, cardiovascular, or peripheral vascular disease, severe chronic heart disease, or diabetes mellitus with end-organ damage.
Radiation Therapy Specifications
The gross tumor volume was contoured on lung CT windows and no additional margin was added for possible microscopic tumor extension. The planning target volume margin for set-up error and error related to tumor motion was limited to ≤ 5mm in the axial dimension and ≤ 10 mm in the craniocaudal dimension. Patients received a dose of 60 Gy in 3 fractions without heterogeneity correction, which approximates 54 Gy in 3 fractions when heterogeneity correction is applied (9). Each fraction was separated by at least 40 hours and the entire 3-fraction regimen was required to be completed within 14 days. Adequate target coverage was achieved when 95% of the planning target volume was covered by 60 Gy. High and intermediate dose spillages were measured by calculating the conformality index (ratio of the volume receiving 60 Gy to the planning target volume: ≤1.2), ratio of 50% prescription isodose volume to the planning target volume (R50), and by measuring the maximum dose 2 cm from the planning target volume (PTV) in any direction (D2cm). The criteria for deviation from the protocol are given in RTOG 0236 primary analysis report (3). Readers are also directed to the original publication for more information on dose gradient requirements and normal tissue dose constraints (10).
Follow-up Specifications
Patients were seen every 3 months during years 1 and 2 following SBRT and then every 6 months until year 4. PFT with spirometry parameters, lung volumes and diffusing capacity, and arterial blood gas analysis (PaCO2, PaO2 and SaO2) were to be performed every 3 months for year 1 posttreatment and every 6 months for year 2 posttreatment. The National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used for grading adverse events (11) except for spirometry and diffusing capacity where a modified version scaled to the pre-treatment baseline was used called the RTOG SBRT pulmonary toxicity scale. The CTCAE version 3 specified grading criteria for PFT assumed that all patients have normal baseline PFT. This assumption is not appropriate for medically inoperable patients. As a remedy to monitor treatment effects on PFT, a protocol specific toxicity classification for PFT that adjusts for baseline abnormalities was defined. Changes that occur after SBRT were referenced to the baseline for a given patient, which was abnormal for most patients. A proportional decline from the baseline was defined. Therefore, In the RTOG SBRT pulmonary toxicity scale, events are scaled to the baseline with a FEV1, FVC or DCLO decline to 0.90-0.75 times the patient's baseline value representing grade 1 toxicity, <0.75-0.50 times baseline representing grade 2 toxicity, <0.50-0.25 times baseline representing grade 3 toxicity, and <0.25 times baseline representing grade 4 toxicity. Grade 5 toxicity is death.
Statistical Methods
Changes in PFT were calculated by subtracting the baseline value from the follow-up value. For tests where a higher score indicates better functioning, this difference was negative for patients with a decline in functioning; for tests where a higher score indicates poorer functioning, the difference was positive for patients with a decline in functioning. In addition, categorized changes in pulmonary function (decline versus no decline) following SBRT were reported. The decline was defined as a decrease in functioning since baseline of at least 15% (7). PFT done within 28 – 56 days following completion of SBRT were used for the 6-week follow-up. PFT done within 60 – 122 days following completion of SBRT were used for the 3-month follow-up, while PFT done within 18 and 24 months following completion of SBRT were used for the 2-year follow-up. Changes in PFT were evaluated using the paired Wilcoxon signed rank test. Logistic regression models were used to investigate the relationship between PFT and pulmonary toxicity. Since the timing of required PFT was subject to assessment bias, time-to-event analysis was less appropriate than logistic regression. Each model consisted of a single PFT as the predictor and an outcome variable where an event was either any grade of pulmonary toxicity or a grade 3 or 4 pulmonary toxicity. Overall survival rates were calculated using the Kaplan-Meier method and comparisons tested using the Log-rank test. The relationship between PFT and overall survival was also evaluated using the Cox proportional hazards model. All analyses were performed with SAS software, version 9.2.
Results
Baseline Patient Characteristics
Ultimately, 55 patients of the 59 accrued patients were evaluable. The patient demographic characteristics, performance status, tumor stage, and reasons for medical inoperability are given in Table 1. The majority of patients were elderly, female, and with stage IA disease. Notably, the majority of patients (69.1%) had more than one reason for medical inoperability. Severely reduced DLCO and FEV1 < 40% predicted at baseline were among the most common abnormalities seen in patients with poor pulmonary function. Almost a half of the patients had severe cardiovascular disease as one of the reasons for medical inoperability.
Table 1. Patient Characteristics (n=55).
| Age (years) | |
| Median | 72 |
| Min - Max | 48 - 89 |
| Gender | |
| Male | 21 (38.2%) |
| Female | 34 (61.8%) |
| Race | |
| Asian | 2 (3.6%) |
| Black or African American | 2 (3.6%) |
| White | 51 (92.7%) |
| Zubrod Score | |
| 0 | 12 (21.8%) |
| 1 | 35 (63.6%) |
| 2 | 8 (14.5%) |
| Non-Small Cell Lung Cancer Stage (AJCC, 6th edition) | |
| IA | 44 (80.0%) |
| IB | 11 (20.0%) |
| Reason Medically Inoperable (Patient may have more than one) | |
| 1. Baseline FEV1 < 40% Predicted | 19 (34.6%) |
| 2. Predicted post-op FEV1 < 30% of predicted | 7 (12.7%) |
| 3. Severely reduced diffusion capacity | 22 (40.0%) |
| 4. Baseline hypoxemia and/or hypercapnia | 10 (18.5%) |
| 5. Exercise oxygen consumption < 50% of predicted | 0 (0.0%) |
| 6. Severe pulmonary hypertension | 6 (10.9%) |
| 7. Diabetes mellitus with severe end organ damage | 3 (5.5%) |
| 8. Severe cerebral, cardiac, or peripheral vascular disease | 24 (43.6%) |
| 9. Severe chronic cardiac disease | 22 (40.0%) |
| Total with more than one reason | 38 (69.1%) |
Baseline Pulmonary Function
Table 2 shows PFT distributions. At baseline, the mean FEV1 for the entire cohort was 60.8% predicted and mean FEV1/FVC was 70% predicted consistent with an obstructive pattern of chronic lung disease. The residual volume (RV) and total lung capacity (TLC) were increased: mean 154.6% predicted, and 113.1% predicted, respectively. The mean forced vital capacity (FVC) was 80% predicted. The mean DLCO was 10.6 ml/min/mmHg and mean % predicted DLCO was 60.7%. Collectively, these data indicate that the majority of patients had an obstructive or mixed (obstructive and restrictive) pattern of chronic lung disease.
Table 2. Pulmonary Function Test Distributions.
| Baseline (n=55) |
Week 6 Post-SBRT (n=41) | Month 3 Post-SBRT (n=27) | 2 Year Post-SBRT (n=24) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulmonary Function Test |
n | Raw | n | Raw | n† | Change since Baseline* |
n | Raw | n† | Change since Baseline* |
n | Raw | n† | Change since Baseline* |
| FVC (L) | 51 | 2.3 ± 0.7 | 40 | 2.4 ± 0.8 | 37 | 0.0 ± 0.5 | 26 | 2.3 ± 1.0 | 23 | -0.02 ± 0.4 | 23 | 2.5 ± 0.9 | 20 | 0.0 ± 0.5 |
| FVC (% Predicted) | 51 | 80.0 ± 23.0 | 40 | 81.4 ± 26.0 | 37 | -0.32 ± 18.6 | 26 | 78.7 ± 29.8 | 23 | 0.8 ± 10.5 | 23 | 81.4 ± 26.6 | 20 | 0.9 ± 16.6 |
| FEV1 (L) | 51 | 1.3 ± 0.6 | 40 | 1.2 ± 0.6 | 37 | -0.08 ± 0.3 | 26 | 1.1 ± 0.7 | 23 | -0.13 ± 0.3 | 23 | 1.1 ± 0.6 | 20 | -0.12 ± 0.3 |
| FEV1 (% Predicted) | 51 | 60.8 ± 33.2 | 40 | 57.1 ± 27.0 | 37 | -6.09 ± 25.2 | 26 | 53.3 ± 29.6 | 23 | -4.8 ± 11.0 | 23 | 51.0 ± 25.3 | 20 | -5.79 ± 13.5 |
| FEV1/FVC | 51 | 0.5 ± 0.2 | 40 | 0.5 ± 0.2 | 37 | -0.02 ± 0.1 | 26 | 0.5 ± 0.2 | 23 | -0.05 ± 0.1 | 23 | 0.5 ± 0.1 | 20 | -0.05 ± 0.1 |
| FEV1/FVC (% Predicted) | 51 | 70.0 ± 30.0 | 40 | 70.0 ± 20.0 | 37 | -5.0 ± 20.0 | 26 | 70.0 ± 20.0 | 23 | -7.0 ± 20.0 | 23 | 60 ± 20.0 | 20 | -7.0 ± 10.0 |
| RV (L) | 39 | 3.2 ± 1.0 | 34 | 3.3 ± 1.4 | 25 | 0.1 ± 1.2 | 18 | 3.1 ± 0.8 | 12 | -0.31 ± 0.4 | 12 | 3.5 ± 1.3 | 8 | -0.34 ± 1.1 |
| RV (% Predicted) | 39 | 154.6 ± 56.9 | 34 | 157.2 ± 59.4 | 25 | 5.0 ± 50.7 | 18 | 154.1 ± 45.7 | 12 | -17.8 ± 20.8 | 12 | 148.1 ± 49.8 | 8 | -20.8 ± 46.6 |
| TLC (L) | 39 | 5.7 ± 1.4 | 34 | 5.9 ± 1.6 | 25 | 0.2 ± 0.8 | 18 | 5.7 ± 0.9 | 12 | -0.23 ± 0.4 | 12 | 6.5 ± 1.6 | 8 | -0.38 ± 0.8 |
| TLC (% Predicted) | 39 | 113.1 ± 19.4 | 34 | 112.7 ± 20.8 | 25 | 2.6 ± 13.0 | 18 | 115.4 ± 16.7 | 12 | -1.24 ± 22.7 | 12 | 113.2 ± 18.5 | 8 | -9.17 ± 18.6 |
| DLCO (ml/min/mmHg) | 42 | 10.6 ± 3.6 | 38 | 9.5 ± 4.0 | 29 | -0.23 ± 1.7 | 25 | 9.5 ± 4.8 | 17 | -0.46 ± 2.3 | 17 | 10.7 ± 4.8 | 13 | -0.74 ± 2.4 |
| DLCO (% Predicted) | 42 | 60.7 ± 30.4 | 38 | 48.3 ± 23.0 | 29 | -3.4 ± 6.7 | 25 | 44.6 ± 18.1 | 17 | -5.39 ± 7.6 | 17 | 46.6 ± 18.1 | 13 | -6.29 ± 10.1 |
| PaCO2 (mmHg) | 37 | 40.5 ± 6.4 | 33 | 39.2 ± 7.4 | 25 | 0.0 ± 3.1 | 15 | 41.9 ± 10.8 | 14 | 0.3 ± 4.6 | 9 | 38.0 ± 5.5 | 7 | -0.9 ± 6.4 |
| PaO2 (mmHg) | 37 | 72.6 ± 13.9 | 33 | 72.0 ± 14.1 | 25 | 1.2 ± 15.2 | 15 | 68.4 ± 12.4 | 14 | -1.3 ± 16.0 | 9 | 74.0 ± 16.8 | 7 | 1.7 ± 5.4 |
| SaO2 (%) | 33 | 93.3 ± 4.4 | 31 | 93.2 ± 4.0 | 24 | 0.9 ± 4.7 | 15 | 92.5 ± 4.0 | 14 | -0.1 ± 4.9 | 10 | 94.0 ± 2.8 | 8 | 0.4 ± 3.9 |
L = Liter; n = number of patients; values are expressed as mean ± standard deviation;
Actual raw changes were calculated by subtracting the baseline value from the follow-up value;
Has both baseline and follow-up PFT. With the exception of DLCO (% predicted), all PFT changes were not statistically significant (p > 0.05). The change in DLCO (% predicted) was significant at 6 weeks (p = 0.008) and at 3 months (p = 0.007).
Changes in Pulmonary Function Following SBRT
PFT compliance rates at 6 weeks, 3 months, and 2 years were 74.5%, 49.1% and 60.0%, respectively. At 2 years, mean % predicted FEV1 and DLCO declines, shown as percent changes, were 5.8% and 6.3%, respectively, with minimal changes of arterial blood gases and no significant decline of oxygen saturation. A similar pattern of PFT test changes was seen at the 6-week and 3-month follow-ups. With the exception of DLCO (% predicted), all PFT changes were not statistically significant (p > 0.05). The change in DLCO (% predicted) was significant at 6 weeks (p = 0.008) and at 3 months (p = 0.007). Overall, non-significant PFT changes were seen following SBRT during the 2-year follow-up.
Table 3 shows changes in PFT following SBRT utilizing the RTOG SBRT pulmonary toxicity scale. As shown, the majority of patients had grade 0-1 PFT changes following SBRT. Comparison of follow-up PFT scored using CTCAE version 3.0 and the RTOG SBRT pulmonary toxicity scale is given in Table e1 (refer to supplementary materials and appendices). Categorized changes (15% decline versus no 15% decline) in PFT and arterial blood gas are shown in Table e2 (refer to supplementary materials and appendices). At 2-year follow-up ≥ 70% of the patients had no decline in PFT.
Table 3. Changes in Pulmonary Function (PF) Following SBRT: The RTOG SBRT Pulmonary Toxicity Scale Percent (%) of Patients Experiencing Each Grade of PF Toxicity is Shown.
| PF Toxicity at Any Time During the 2-year Post-SBRT Follow-up | PF Toxicity at 6 Weeks Post-SBRT | PF Toxicity at 2 Years Post-SBRT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulmonary Function Test | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
| (n=49) | (n=37) | (n=21) | ||||||||||
| FVC (L) | 42.9% | 40.8% | 16.3% | 0.0% | 73.0% | 24.3% | 2.7% | 0.0% | 71.4% | 14.3% | 14.3% | 0.0% |
| FVC (% Predicted) | 40.8% | 40.8% | 18.4% | 0.0% | 75.7% | 16.2% | 8.1% | 0.0% | 71.4% | 19.0% | 9.5% | 0.0% |
| (n=49) | (n=37) | (n=21) | ||||||||||
| FEV1 (L) | 38.8% | 36.7% | 18.4% | 6.1% | 81.1% | 13.5% | 2.7% | 2.7% | 47.6% | 38.1% | 14.3% | 0.0% |
| FEV1 (% Predicted) | 32.7% | 42.9% | 22.4% | 2.0% | 75.7% | 16.2% | 5.4% | 2.7% | 52.4% | 38.1% | 9.5% | 0.0% |
| (n=40) | (n=29) | (n=15) | ||||||||||
| DLCO (ml/min/mmHg)* | 35.1% | 18.9% | 45.9% | 0.0% | 75.9% | 13.8% | 10.3% | 0.0% | 46.7% | 26.7% | 26.7% | 0.0% |
| DLCO (% Predicted)* | 21.6% | 29.7% | 48.6% | 0.0% | 65.5% | 27.6% | 6.9% | 0.0% | 33.3% | 26.7% | 40.0% | 0.0% |
L = Liter; n = number of patients.
Correlation between Pulmonary Function Tests and Lung Toxicity
Pulmonary and upper respiratory toxicity is shown in Table e3 (refer to supplementary materials and appendices). Nine patients developed grade 1-3 pneumonitis. No patient experienced grade 4 or grade 5 pneumonitis. Logistic regression model analysis of baseline PFT and any pulmonary toxicity, including high-grade pulmonary toxicity is shown in Table 4. None of the baseline PFT parameters correlated with any pulmonary toxicity including pleural effusion and pneumonitis (Table e4, refer to supplementary materials and appendices). Furthermore, none of the baseline PFT parameters correlated with cough, dyspnea, hypoxia and PFT decrease, p > 0.05 (data not shown). When the 6-week PFT parameters were analyzed, no correlation was found between the 6-week PFT and any subsequent pulmonary toxicity including pneumonitis (Tables e5, refer to supplementary materials and appendices). These results indicate that PFTs do not appear to be predictive of any pulmonary toxicity, including high-grade toxicity following SBRT.
Table 4. Logistic Regression Models Any Pulmonary/Upper Respiratory Toxicity Baseline Pulmonary Function Tests.
| Any Pulmonary Toxicity | Grade 3+ Pulmonary Toxicity | |||||
|---|---|---|---|---|---|---|
| Pulmonary Function Test | Events/Total | OR (95% CI) | p-value | Events/Total | OR (95% CI) | p-value |
| FVC (Actual) | 31 / 51 | 1.74 (0.71, 4.24) | 0.2037 | 8 / 51 | 1.21 (0.42, 3.47) | 0.7317 |
| FVC (% Predicted) | 31 / 51 | 1.01 (0.99, 1.04) | 0.2867 | 8 / 51 | 0.99 (0.96, 1.03) | 0.7450 |
| FEV1 (Actual) | 31 / 51 | 2.52 (0.87, 7.33) | 0.0665 | 8 / 51 | 0.85 (0.25, 2.94) | 0.7958 |
| FEV1 (% Predicted) | 31 / 51 | 1.01 (0.99, 1.03) | 0.1589 | 8 / 51 | 0.99 (0.97, 1.02) | 0.4973 |
| FEV1/FVC (Actual) | 31 / 51 | NA - unstable | -- | 8 / 51 | NA - unstable | -- |
| FEV1/FVC (% Pred.) | 31 / 51 | NA - unstable | -- | 8 / 51 | 0.34 (0.01, 8.21) | 0.5004 |
| RV (Actual) | 26 / 39 | 0.74 (0.38, 1.45) | 0.3760 | 7 / 39 | 1.09 (0.49, 2.45) | 0.8284 |
| RV (% Predicted) | 26 / 39 | 1.00 (0.99, 1.01) | 0.7236 | 7 / 39 | 0.99 (0.98, 1.01) | 0.4692 |
| TLC (Actual) | 26 / 39 | 0.84 (0.51, 1.37) | 0.4791 | 7 / 39 | 1.10 (0.61, 1.99) | 0.7488 |
| TLC (% Predicted) | 26 / 39 | 0.98 (0.94, 1.01) | 0.1944 | 7 / 39 | 0.97 (0.93, 1.02) | 0.2359 |
| DLCO (Actual) | 26 / 42 | 1.03 (0.86, 1.23) | 0.7468 | 8 / 42 | 0.90 (0.71, 1.13) | 0.3504 |
| DLCO (% Predicted) | 26 / 42 | 1.00 (0.98, 1.02) | 0.8829 | 8 / 42 | 0.97 (0.92, 1.01) | 0.0617 |
| PaCO2 | 22 / 37 | 1.03 (0.92, 1.14) | 0.6364 | 9 / 37 | 1.01 (0.90, 1.13) | 0.9028 |
| PaO2 | 22 / 37 | 1.00 (0.95, 1.05) | 0.9705 | 9 / 37 | 1.00 (0.95, 1.06) | 0.8601 |
| SaO2 | 20 / 33 | 1.01 (0.86, 1.19) | 0.8765 | 9 / 33 | 1.02 (0.85, 1.22) | 0.8230 |
OR = odds ratio
Normal Lung Tissue Dose and Radiation Pneumonitis
Normal lung tissue dose volume distributions are summarized in Table 5. Although the RTOG 0236 clinical trial required the percent of normal lung tissue receiving 20 Gy (V20) to be below 10% (3), the patients treated in this study had a relatively low normal lung tissue dose; mean V20 was only 4.8% and mean dose to the whole lung was only 4.0 Gy. Normal lung tissue dose volume distributions by the occurrence of pneumonitis are also shown in Table 5. While the range of these values was low compared to conventionally fractionated radiotherapy, the occurrence of pneumonitis did not correlate with normal lung tissue dose: mean whole lung receiving V5, V10, V20, and mean whole lung dose were similar in patients who developed radiation pneumonitis and patients who were radiation pneumonitis-free. Logistic regression models showed no existing correlation (p > 0.6) between any normal lung tissue dose (V20, V10, V5, and mean dose to the whole lung) and the occurrence of pneumonitis (Table 5).
Table 5. Normal Lung Dose Volume Distributions by Development of Pneumonitis.
| Parameter | All Patients (n = 45) | Patients without Pneumonitis (n = 36) | * Patients with Pneumonitis (n = 9) | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Whole Lung V20 (%) | 4.8 ± 2.3 | 4.7 ± 2.3 | 5.2 ± 2.4 | 1.08 (0.80, 1.47) | p = 0.62 |
| Whole Lung V10 (%) | 11.9 ± 5.1 | 12.0 ± 5.6 | 11.8 ± 3.3 | 0.99 (0.86, 1.15) | p = 0.94 |
| Whole Lung V5 (%) | 19.0 ± 6.9 | 18.8 ± 7.2 | 19.8 ± 5.7 | 1.02 (0.92, 1.13) | p = 0.71 |
| Mean Dose to Whole Lung (Gy) | 4.0 ± 1.5 | 3.9 ± 1.6 | 4.1 ± 1.2 | 1.10 (0.69, 1.76) | p = 0.69 |
Values are expressed as mean ± SD, n = number of patients
OR = odds ratio, CI = confidence interval
V20 = volume receiving 20 Gy, V10 = volume receiving 10 Gy, V5 = volume receiving 5 Gy
Toxicity was graded using CTCAE v3.0. Nine patients had grade 1-3 pneumonitis. No patient had grade 4, or grade 5 pneumonitis
Intermediate Dose Spillage and Pulmonary Toxicity
By the trial design, dose at 2 cm (D2cm) from the PTV and R50 ratio were strictly limited to result in very compact dose distributions, even for the category defined as a major deviation of D2cm and R50 per the protocol guidance. Within this context of strictly limited intermediate dose spillage by the protocol, no correlation was seen between these intermediate dose spillage parameters (D2cm and R50) and any pneumonitis toxicity, including high grade pulmonary toxicity.
Overall Survival and Pulmonary Function
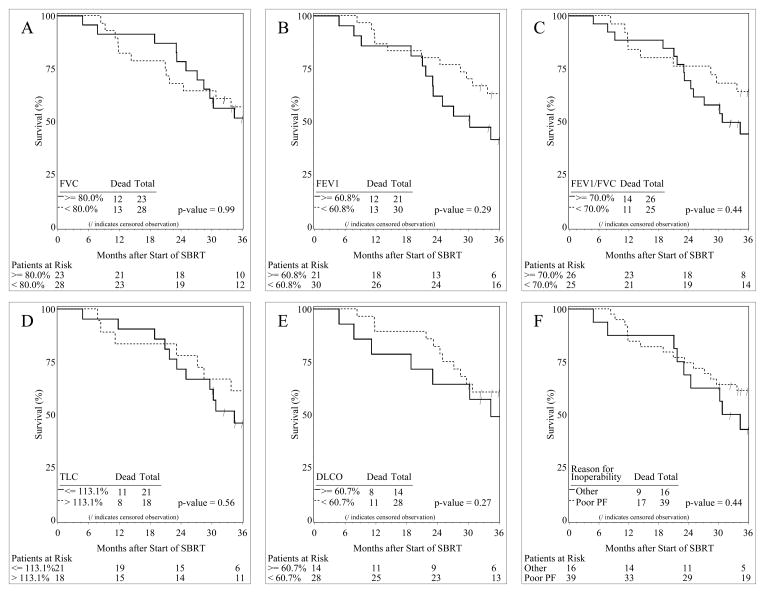
Overall survival analysis was performed in order to find out whether baseline PFT parameters have a potential predictive value. Figure 1 shows the overall survival curves for patients with % predicted PFT parameters above and below their mean values.
Figure 1.
Kaplan-Meier overall survival (OS) curves of patients with baseline pulmonary function test values greater and less than the mean % predicted values. A. forced vital capacity (FVC) greater (solid line) and less than (dashed line) its mean value; B. forced expiratory volume in the first second of expiration (FEV1) greater (solid line) and less than (dashed line) its mean value; C. FEV1/FVC greater (solid line) and less than (dashed line) its mean value; D. total lung capacity (TLC) greater (solid line) and less than (dashed line) its mean value; E. diffusing capacity for carbon monoxide (DLCO) greater (solid line) and less than (dashed line) its mean value; F. poor baseline pulmonary function (PF) and normal baseline PF but with other reasons for medical inoperability.
Median survival (MS) in patients with FVC greater than or equal to the mean value (80%) was 48 months, and less than the mean value was 42 months (p = 0.99). MS in patients with FEV1 greater than or equal to and less than its mean value (60.8% predicted) was 30 and 48 months respectively (p = 0.29). A similar pattern of MS was seen in patients with FEV1/FVC greater than or equal to and less than its mean value (70% predicted) and was found to be 31 and 48 months, respectively (p = 0.44). Difference in MS was also seen in patients with TLC greater than and less than or equal to its mean value (113.1% predicted) and was 42 and 34 months, respectively (p = 0.56). MS in patients with DLCO greater than or equal to its mean value (60.7% predicted) was 34 months, while MS in patients with DLCO less than its mean value was not reached (p = 0.27). None of the baseline PFT parameters statistically significantly correlated with overall survival. The patients with poor and normal baseline PFT values did not have statistically significant difference in 3-year overall survivals.
When overall survival was analyzed in patients with poor PFT as one of the reasons for medical inoperability, medically inoperable patients with poor baseline PFT had higher median and overall survivals than patients with normal baseline PFT but with other reasons for medical inoperability (e.g., cardiac morbidity); MS was 48 and 33 months, respectively. However, this difference in survival was not statistically significant, p = 0.44 (Figure 1). When overall survival was specifically analyzed in patients with cardiac morbidity as one of the reasons for medical inoperability, medically inoperable patients with cardiac morbidity had a trend for a worse overall survival than patients without cardiac morbidity. MS was 30 and 48 months, respectively; however, no statistically significant difference in overall survival between these two groups was seen, p = 0.10 (data not shown).
Discussion
This study unveiled several key observations after treating and following the study cohort. First, following SBRT in this population of medically inoperable patients, non-significant declines in PFT were seen with no significant change of oxygen saturation at the 2-year follow-up. Second, baseline PF was not predictive of radiation pneumonitis or any pulmonary toxicity following SBRT. Third, there was no relationship between the occurrence of radiation pneumonitis and the common normal lung tissue dose parameters used for predicting radiation pneumonitis after conventional radiation. Fourth, poor baseline PF did not predict decreased survival following SBRT, and higher median and overall survivals were seen in medically inoperable patients without cardiovascular morbidity compared to the patients with cardiovascular morbidity.
Henderson and colleagues (7) at Indiana University analyzed pulmonary function changes in medically inoperable patients with stage I NSCLC treated with SBRT. Baseline FEV1 significantly predicted for post-treatment survival and patients with poor PFT had significantly longer overall survival. Another study by Stephans and associates (8) at Cleveland Clinic showed the same. A closer look at overall survival by cause of death in both studies demonstrated that patients who were inoperable because of cardiac morbidity had inferior survival to patients without cardiac morbidity. In our study, medically inoperable patients with poor baseline PFT had higher median and overall survivals than patients with normal baseline PFT but with other reasons for medical inoperability (e.g., cardiac morbidity). As shown in Figure 1, 3-year overall survival in patients with poor baseline PFT and patients with normal PFT but with other reasons for medical inoperability were 61% and 43%, respectively. This however, did not reach statistical significance (p = 0.44), possibly due to the small sample size. The majority of inoperable patients with normal PFT have cardiac morbidity. One-year mortality after onset of heart failure in people aged 75 years is 21% in men and 17% in women, while 5-year mortality is 50% in men and 46% in women (12). Therefore, inoperable patients with lung cancer and severe cardiac disease appear to have less chance of long term survival than inoperable patients with poor PFT but without severe cardiac morbidity.
In this study, the occurrence of radiation pneumonitis did not correlate with the normal lung tissue dose parameters commonly used to predict toxicity for conventionally fractionated radiotherapy. Whole lung V5, V10, V20, and mean dose to the lung had no predictive value for the occurrence of radiation pneumonitis after SBRT. Admittedly, the absolute range of these parameters was small because of a mandated compact dose distribution. This appears to be different from conventionally fractionated radiotherapy in which whole lung V20 and mean lung dose are considered to be independent predictors of pneumonitis (13), and even V5, V10 and V13 for the whole and ipsilateral lung are reported by single centers to correlate with the incidence of pneumonitis (14-16).
This study clearly has limitations. Stage IA NSCLC was present in 80% of patients and generalizations about treating larger tumors based on this data should not necessarily be drawn. Treatment of larger tumors includes more normal lung tissue and can potentially give more lung toxicity. It has been shown that the volume of irradiated normal lung correlates with the decrease of TLC and DLCO (17). On the other hand, although more PFT decline can be seen when larger tumors are treated, the phase I dose escalation trial of SBRT at Indiana University showed no obvious relationship between tumor volume and toxicity (18). The study population in this report was limited to patients with peripheral tumors away from the central airways. Therefore, the results would not necessarily apply to patients being treated for more central tumors. Most of the study population was women while the majority of patients diagnosed with lung cancer are men. This unintended selection may have implications to health status unique from an unselected population of lung cancer patients. Overall survival analyses included patients with any cardiac morbidity, and the small sample size precluded additional overall survival analyses for each type of cardiac morbidity (e.g., coronary artery disease, congestive heart failure, etc.). Yet, another limitation of this study is the PFT compliance rate during the follow-up period. As previously mentioned, the PFT compliance rate at 6 weeks was 74.5%. The compliance steadily declined to 60% with ongoing follow-up. Higher PFT compliance rate would result in a more robust estimate of PFT change, and PFT changes might be worse in non-complaint patients. Reasons for non-compliance with protocol-specified PFT were not collected. Though the observed rate of PFT change could be higher with a better compliance, a very small number of patients experienced grade 3 pulmonary toxicity in the study. This is different from SBRT for central tumors where even grade 5 toxicity was reported (19).
In summary, following SBRT for patients treated in the RTOG 0236 trial, overall, non-significant changes in PFT were seen with no significant change of oxygen saturation. Baseline PFT was not predictive of radiation pneumonitis or any pulmonary toxicity following SBRT. There did not appear to be a relationship between the occurrence of radiation pneumonitis and normal lung tissue dose; mean whole lung V5, V10, V20 and mean dose to the whole lung were almost identical in patients who developed radiation pneumonitis and patients who were radiation pneumonitis-free. Poor baseline PFT did not appear to predict decreased overall survival, and patients with cardiac morbidity appear to have poorer overall survival than those with poor baseline PFT but without cardiac morbidity. Based on this analysis, poor baseline PFT alone should not be used to exclude patients with early stage NSCLC from treatment with SBRT.
Supplementary Material
Acknowledgments
(1) This trial was conducted by the Radiation Therapy Oncology Group (RTOG), and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. (2) Dr. Sinisa Stanic proposed this secondary analysis of RTOG 0236 as a part of his ACR/RTOG Radiation Oncology Research Fellowship in 2011, while he was a resident in Radiation Oncology at the University of California Davis, Sacramento, CA. (3) The authors would like to thank Mr. William Straube of Image-guided Therapy QA Center at Washington University in St Louis for his assistance in providing dosimetric data for the analyses in this study.
R. Paulus reported a grant support from the National Cancer Institute (NCI), Grant U10 CA032115. This grant supports statistical efforts relating to RTOG study development, monitoring and analysis. R. Timmerman reported receiving research grants for technology development from Varian Medical Systems (Palo Alto, California) and Accuray, Inc. (Sunnyvale, California). Both of these companies manufacture equipment for stereotactic body radiation therapy. In addition, he reported receiving grants from the US National Institutes of Health and Department of Defense to carry out separate protocols for stereotactic body radiation therapy in patients with lung and prostate cancer. J. Michalski reported receiving grants and support for travel to meetings from NCI (RTOG and ATC grants). R. Barriger reported his employment relationship with D3 Oncology Solutions. He is a member of the Via Oncology Pathways Physicians Advisory Committee and Co-chair of Radiation Oncology Committee for Esophageal and Lung cancer.
Footnotes
Conflict of interests: None of the other authors reported any financial disclosures.
Previous presentations: The manuscript was presented in the abstract form at the American Society for Radiation Oncology (ASTRO) 54th Annual Meeting in Boston, MA, October 28-31, 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–31. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–55. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 5.Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126–35. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 6.Baumann P, Nyman J, Hoyer M, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer – a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88:359–67. doi: 10.1016/j.radonc.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:404–9. doi: 10.1016/j.ijrobp.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4:838–44. doi: 10.1097/JTO.0b013e3181a99ff6. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:1235–42. doi: 10.1016/j.ijrobp.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmerman R, Galvin J, Michalski J, et al. Accreditation and quality assurance for Radiation Therapy Oncology Group: Multicenter clinical trials using Stereotactic Body Radiation Therapy in lung cancer. Acta Oncol. 2006;45:779–86. doi: 10.1080/02841860600902213. [DOI] [PubMed] [Google Scholar]
- 11.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 13.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–9. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 14.Seppenwoolde Y, Lebesque JV, de Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability. Int J Radiat Oncol Biol Phys. 2003;55:724–35. doi: 10.1016/s0360-3016(02)03986-x. [DOI] [PubMed] [Google Scholar]
- 15.Yorke ED, Jackson A, Rosenzweig KE, et al. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672–82. doi: 10.1016/j.ijrobp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Gopal R, Starkschall G, Tucker SL, et al. Effects of radiotherapy and chemotherapy on lung function in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:114–20. doi: 10.1016/s0360-3016(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 18.McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010–5. doi: 10.1016/j.ijrobp.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.