Summary
Chimeric antigen receptor–modified T cells with specificity for CD19 have shown promise in the treatment of chronic lymphocytic leukemia (CLL). It remains to be established whether chimeric antigen receptor T cells have clinical activity in acute lymphoblastic leukemia (ALL). Two children with relapsed and refractory pre–B-cell ALL received infusions of T cells transduced with anti-CD19 antibody and a T-cell signaling molecule (CTL019 chimeric antigen receptor T cells), at a dose of 1.4×106 to 1.2×107 CTL019 cells per kilogram of body weight. In both patients, CTL019 T cells expanded to a level that was more than 1000 times as high as the initial engraftment level, and the cells were identified in bone marrow. In addition, the chimeric antigen receptor T cells were observed in the cerebrospinal fluid (CSF), where they persisted at high levels for at least 6 months. Eight grade 3 or 4 adverse events were noted. The cytokine-release syndrome and B-cell aplasia developed in both patients. In one child, the cytokine-release syndrome was severe; cytokine blockade with etanercept and tocilizumab was effective in reversing the syndrome and did not prevent expansion of chimeric antigen receptor T cells or reduce anti-leukemic efficacy. Complete remission was observed in both patients and is ongoing in one patient at 11 months after treatment. The other patient had a relapse, with blast cells that no longer expressed CD19, approximately 2 months after treatment. Chimeric antigen receptor–modified T cells are capable of killing even aggressive, treatment-refractory acute leukemia cells in vivo. The emergence of tumor cells that no longer express the target indicates a need to target other molecules in addition to CD19 in some patients with ALL.
Patients with relapsed and chemotherapy-refractory pre–B-cell ALL have a poor prognosis despite the use of aggressive therapies such as allogeneic hematopoietic stem-cell transplantation1,2 and bispecific CD19 antibody fragments.3 Chimeric antigen receptor–modified T cells that target the lineage-specific antigens CD19 and CD20 have been reported to be effective in adults with CLL and B-cell lymphomas.4-9 However, the effects of chimeric antigen receptor T cells on ALL blasts, a more immature leukemia that progresses more rapidly, have not been fully investigated. In particular, there has been uncertainty about whether chimeric antigen receptor T cells would expand in vivo in patients with ALL and whether they would have antileukemic efficacy in patients with relapsed disease, high tumor burdens, or both.
We previously reported the in vivo expansion and robust antileukemic effects of CTL019 (formerly CART19) cells in three patients with CLL.7,8 CTL019 is a chimeric antigen receptor that includes a CD137 (4-1BB) signaling domain and is expressed with the use of lentiviral-vector technology.10 Here we report the use of CTL019 in two children with refractory and relapsed ALL. Both children had remission of leukemia, accompanied by the robust expansion of CTL019 in vivo, with CTL019 detected in bone marrow and the CSF. The antileukemic effects were potent, given that one child had chemotherapy-refractory disease that precluded allogeneic donor stem-cell transplantation, and the other child had had a relapse after allogeneic cord-blood transplantation and had resistance to blinatumomab, a chimeric bispecific anti-CD3 and anti-CD19 monoclonal antibody.
Case Reports
Patient 1 was a 7-year-old girl with a second recurrence of ALL. She had received a diagnosis 2 years earlier. A remission with a negative test for minimal residual disease had been achieved, then she had a relapse 17 months after the original diagnosis. She had a second remission after reinduction chemo-therapy, but the cancer recurred 4 months later, and she did not have a response to further intensive chemotherapy, including clofarabine, etoposide, and cyclophosphamide. Her karyotype at baseline was 48,XX,del(9)(p21.3),+11,del(14)(q2?q24),+16/46, XX[4]. Peripheral-blood mononuclear cells (PBMCs) were collected by means of apheresis before administration of the intensive chemotherapy, with the anticipation that there might be an insufficient number of circulating T cells available for cell manufacturing after such intensive treatment. The patient received an infusion of CTL019 cells that had been expanded with anti-CD3 and anti-CD28 antibodies and lentivirally transduced to express the anti-CD19 chimeric antigen receptor; the total dose was 108 CD3+ cells per kilogram (1.2×107 CTL019 cells per kilogram), given over a period of 3 consecutive days, as previously described.7,8 She did not receive lymphocyte-depleting chemotherapy before treatment with the CTL019 infusions, with the most recent cytotoxic therapy having been given 6 weeks before CTL019 infusion. No immediate infusion-related toxic effects were noted, but she was hospitalized for low-grade fevers that progressed to high fevers by day 4, and on day 5 (Fig. 1A), she was transferred to the pediatric intensive care unit. This was followed by rapid progression to respiratory and cardiovascular compromise requiring mechanical ventilation and blood-pressure support.
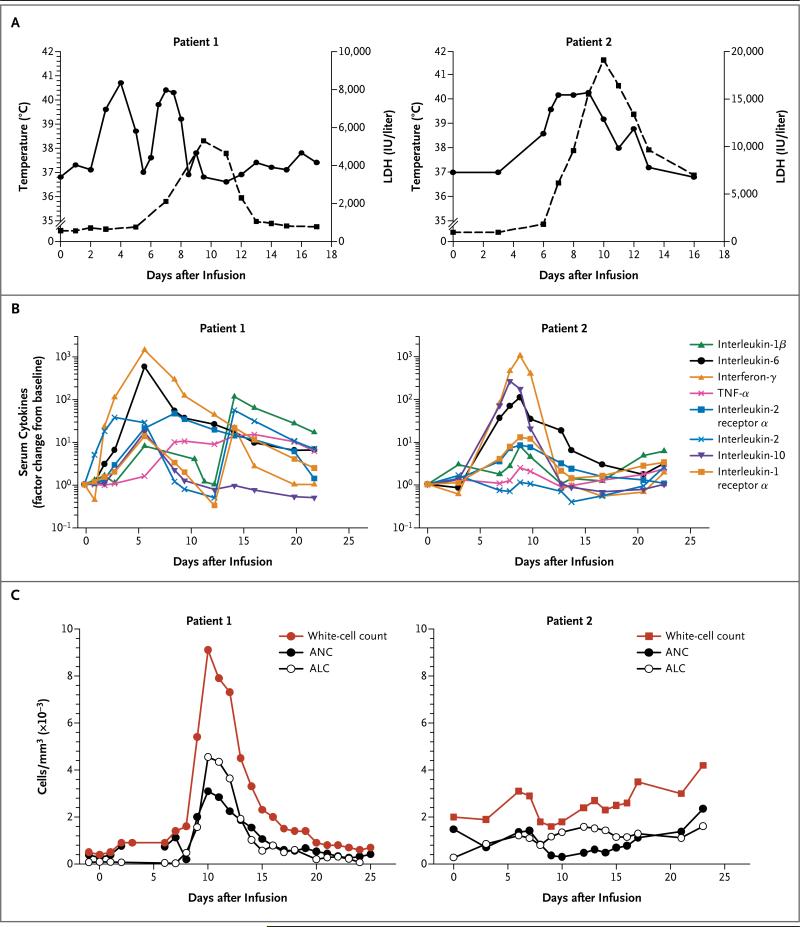
Figure 1. Clinical Responses to CTL019 Infusion in Two Children with Relapsed, Chemotherapy-Refractory Acute Lymphoblastic Leukemia (ALL).
The two children, both of whom had CD19+ B-cell– precursor ALL, received infusions of CTL019 cells on day 0. Panel A shows changes in serum lactate dehydrogenase (LDH) levels and body temperature after CTL019 infusion, with the maximum temperature per 24-hour period indicated by the circles. Patient 1 was given methylprednisolone starting on day 5 at a dose of 2 mg per kilogram of body weight per day, tapered to 0 by day 12. On the morning of day 7, etanercept was given at a dose of 0.8 mg per kilogram. At 6 p.m. on day 7, tocilizumab was given at a dose of 8 mg per kilogram. A transient improvement in pyrexia occurred after the administration of glucocorticoids on day 5, with complete resolution of fevers occurring after the administration of cytokine-directed therapy. Panel B shows serum levels of cytokines and inflammatory markers measured at the indicated time points after CTL019 infusion. Cytokine values are shown with the use of a semilogarithmic plot indicating change from baseline. Baseline values (on day 0 before infusion) in Patient 1 and Patient 2, respectively, were as follows: interleukin-1β, 0.9 and 0.2 pg per milliliter; interleukin-6, 4.3 and 1.9 pg per milliliter; interferon-γ, 0.08 and 0.23 pg per milliliter; tumor necrosis factor α (TNF-α), 1.5 and 0.4 pg per milliliter; interleukin-2 receptor α, 418.8 and 205.7 pg per milliliter; interleukin-2, 0.7 and 0.4 pg per milliliter; interleukin-10, 9.9 and 2.3 pg per milliliter; and interleukin-1 receptor α, 43.9 and 27.9 pg per milliliter. Pronounced elevations in a number of cytokines and cytokine receptors developed in both patients, including soluble interleukin-1 receptor α; interleukin-2 receptor; interleukin-2, 6, and 10; TNF-α; and inter fer on-γ. Panel C shows changes in the circulating absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and white-cell count. The increase in the ALC was primarily from activated CTL019 T lymphocytes.
Patient 2 was a 10-year-old girl with ALL who had had a second relapse after undergoing transplantation of umbilical-cord blood from an unrelated donor (HLA-4/6) 28 months after diagnosis and 10 months before CTL019 infusion. She had had graft-versus-host disease (GVHD) after the transplantation, which resolved with treatment; she was not receiving immunosuppressive therapy at the time of her relapse. She did not have another remission, in spite of multiple cytotoxic and biologic therapies. Her baseline karyotype was 46,XX,del(1)(p13),t(2;9)(q?21;q?21), t(3;17)(p24;q23),del(6)(q16q21),del(9)(q13q22), der(16)t(1;?;16)(p13;?p13.3)[9]//46,XY[1]. Before PBMC collection, she received two cycles of blinatumo mab,3 a CD19-targeted bispecific antibody treatment for ALL, with no response. Sixty-eight percent of her peripheral-blood cells were of donor origin at the time of PBMC collection. CTL019 T cells were manufactured and infused at a total dose of 107 CD3+ cells per kilogram (1.4×106 CTL019 cells per kilogram) in a single dose; etoposide–cyclophosphamide chemotherapy had been administered for lymphocyte depletion the week before. Her bone marrow on the day before CTL019 infusion was replaced by a population of CD19+CD34+ ALL cells, with variable expression of CD19 on flow cytometry (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The patient had no immediate infusion-related toxic effects, but a fever developed on day 6 and she was admitted to the hospital. She had no cardio-pulmonary toxic effects and did not receive glucocorticoids or anticytokine therapy. Her fever was of unknown origin but was suspected to be due to the cytokine-release syndrome (Fig. 1B); she also had myalgias and 2 days of confusion (grade 3), all of which spontaneously resolved. She had no evidence of GVHD after the infusion of the CTL019 cells. Although these cells had been collected from the patient, the infused cells were all of donor (cord blood) origin.
Methods
The patients were the first two enrolled in an institutional review board–approved phase 1 clinical trial (ClinicalTrials.gov number, NCT01626495) that was designed to assess the safety and feasibility of infusing autologous CTL019 T cells in children with relapsed or refractory B-cell cancers. All authors discussed and interpreted the results and vouch for the data and analyses. No commercial sponsor was involved in the study.
The materials and methods used in CTL019 production have been reported previously,7,8 with the exception of the lentiviral vector for this protocol, which was produced at Children's Hospital of Philadelphia. CTL019 was detected and quantified in clinical specimens as previously reported.7,8 Additional experimental details are included in the Supplementary Appendix.
Results
Induction of Remission in Both Patients
Both children had an increase in circulating lymphocytes and neutrophils in the 2 weeks after CTL019 infusion, as shown by plots depicting the total white-cell count, absolute lymphocyte count, and absolute neutrophil count relative to the timing of CTL019 infusion (Fig. 1C). Most of the lymphocytes were T cells that expressed the chimeric antigen receptor (Fig. 2, and Fig. S2 in the Supplementary Appendix). In both children, high-grade, most likely noninfectious fevers were documented, followed by elevations in lactate dehydrogenase (LDH) levels (Fig. 1A). The elevations in LDH levels and the high-grade fevers were similar to those previously described in patients with CLL after CTL019 infusion.7,8 Approximately 1 month after infusion, morphologic remission of leukemia (minimal residual disease, <0.01%) was achieved in both children. Results of flow-cytometric assays of minimal residual disease were confirmed by molecular detection of clonal IgH transcripts by means of deep sequencing (Table 1).
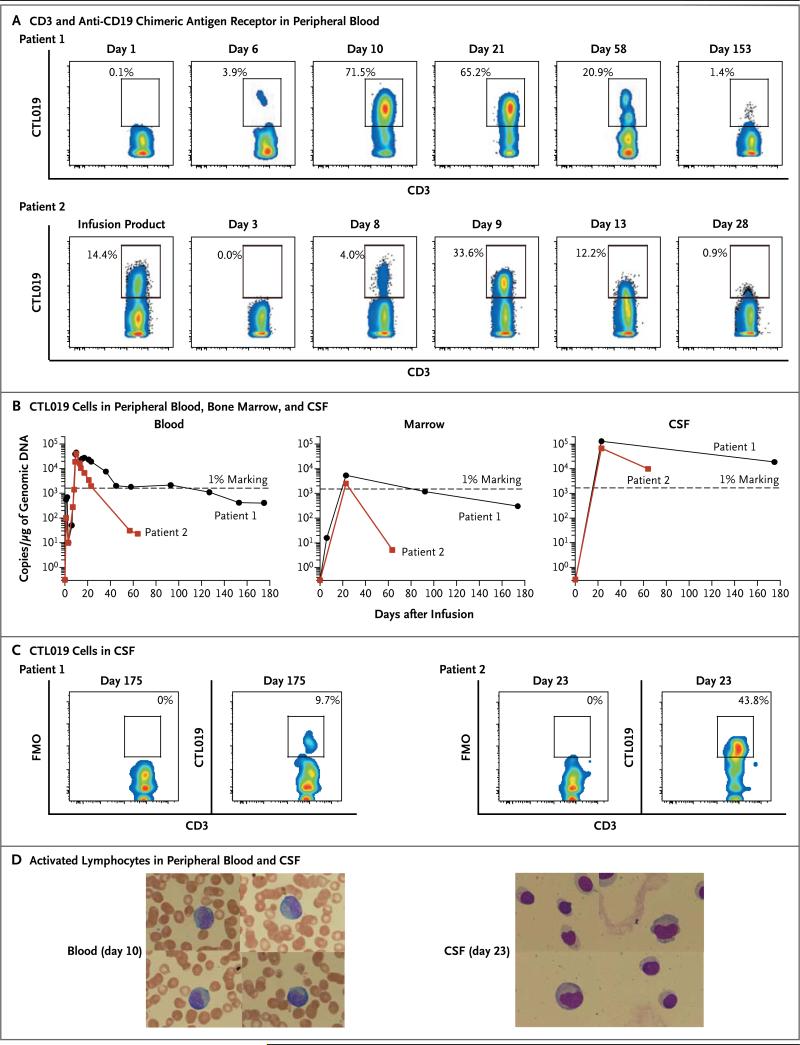
Figure 2. Expansion and Visualization of CTL019 Cells in Peripheral Blood, Bone Marrow, and Cerebrospinal Fluid (CSF).
Panel A shows the results of flow-cytometric analysis of peripheral blood stained with antibodies to detect CD3 and the anti-CD19 chimeric antigen receptor. Both the x and y axes are log10 scales. Depicted is the percentage of CD3 cells expressing the chimeric antigen receptor in Patients 1 and 2. Panel B shows the presence of CTL019 T cells in peripheral blood, bone marrow, and CSF as assessed by means of a quantitative real-time polymerase-chain-reaction (PCR) assay. Genomic DNA was isolated from samples of whole blood, bone marrow aspirate, and CSF collected at serial time points before and after CTL019 infusion. The 1% marking line represents the number of detected transgene copies that would be expected if 1% of the total cells in the sample contained a single integration of the chimeric antigen receptor transgene. Panel C shows flow-cyto-metric detection of CTL019 cells in CSF from Patients 1 and 2. FMO denotes fluorescence minus one. Both the x and y axes are log10 scales. Panel D shows activated large granular lymphocytes in Wright-stained smears of the peripheral blood and cytospin preparations of CSF from Patient 2.
Table 1.
Induction of Molecular Remission in the Blood and Bone Marrow of the Patients.*
| Patient and Tissue | No. of Cell Equivalents Analyzed | Total Reads of T-Cell Receptor β | Total Reads of IGH | Total Unique Reads of IGH | Dominant Clone Reads | Tumor Clone Frequency % |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| Blood | ||||||
| Day –1 | 111,340 | 525,717 | 189 | 6 | 185 | 97.88 |
| Day 23 | 218,210 | 1,651,129 | 0 | 0 | 0 | 0 |
| Day 87 | 288,152 | 1,416,378 | 0 | 0 | 0 | 0 |
| Day 180 | 420,571 | 1,276,098 | 6 | 2 | 0 | 0 |
| Marrow | ||||||
| Day –1 | 317,460 | 348,687 | 59,791 | 318 | 59,774 | 99.97 |
| Day 23 | 362,819 | 1,712,507 | 37 | 2 | 33 | 89.19 |
| Day 87 | 645,333 | 425,128 | 10 | 1 | 10 | 100.00 |
| Day 180 | 952,381 | 800,670 | 45 | 7 | 0 | 0 |
| Patient 2 | ||||||
| Blood | ||||||
| Day –1 | 152,584 | 1,873,116 | 38,170 | 52 | 30,425 | 79.71 |
| Day 23 | 417,371 | 1,462,911 | 92 | 5 | 18 | 19.60 |
| Marrow | ||||||
| Day –1 | 158,730 | 2,417,992 | 68,368 | 65 | 50,887 | 74.43 |
| Day 23 | 305,067 | 1,978,600 | 1,414 | 11 | 946 | 66.90 |
| Day 60 | 916,571 | NA | 530,833 | 206 | 363,736 | 68.90 |
Molecular analysis of minimal residual disease was performed as described in the Supplementary Appendix on DNA isolated from whole blood or bone marrow. Day –1 indicates the day before infusion of CTL019 cells. NA denotes not available.
The clinical remission in Patient 1 was associated with a molecular remission that had persisted for 9 months as of January 2013. High-throughput DNA sequencing of the IGH locus revealed a pronounced decrease in total rearranged IGH sequence reads on day 23 in blood and marrow specimens. The malignant clone was not detected in the blood or marrow in more than 1 million cell equivalents that were sequenced on day 180 (Table 1). In contrast, rearranged T-cell– receptor sequences were readily detected in blood and marrow, findings that indicate the integrity of the DNA tested at all time points.
Toxicity of CTL019
Grade 3 and 4 adverse events are summarized in Table 2. Both patients had acute toxic effects, which consisted of fever and a cytokine-release syndrome that evolved into the macrophage activation syndrome. Both patients were monitored and given prophylaxis for the tumor lysis syndrome. Both had substantial elevations in LDH levels, the causes of which were probably multi-factorial but could have included the tumor lysis syndrome. Each uric acid value in Patient 1 was either below or within the normal range, and she received allopurinol on days 5 and 6 only. Patient 2 received prophylactic allopurinol on days 0 through 14 and had abnormal uric acid values of 4.8 to 5.7 mg per deciliter (286 to 339 μmol per liter) on days 8 through 10, which were consistent with mild tumor lysis syndrome.
Table 2.
Grade 3 or 4 Adverse Events.*
| Event | Grade | Description | Duration |
|---|---|---|---|
| Patient 1 | |||
| Febrile neutropenia | 3 | Peak temperature of 40.7°C; event resolved on day 7 (within hours after administration of tocilizumab) | 7 days |
| Hypotension | 4 | Shock requiring pressor support; by day 7, only pressor support was tapered dose of dobutamine; by day 12, no pressors required | 4 days at grade 4 |
| Acute vascular leak syndrome | 4 | Life-threatening; pressor support or ventilatory support required | 4 days at grade 4 |
| Acute respiratory distress syndrome | 4 | Intubation required; chest radiograph clear on day 8 | 12 days |
| Patient 2 | |||
| Febrile neutropenia | 3 | Peak temperature of 40.3°C; event resolved on day 6 | 6 days |
| Encephalopathy | 3 | Parents reported confusion; MRI scan was normal | 3 days |
| Elevated AST | 4 | Peak AST value: 1060 U/liter (grade 4) | 1 day at grade 4 |
| Elevated ALT | 4 | Peak ALT value: 748 U/liter (grade 4) | 1 day at grade 4 |
Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 3.0. ALT denotes alanine aminotransferase, AST aspartate aminotransferase, and MRI magnetic resonance imaging.
Severe cytokine-release syndrome developed in Patient 1. Glucocorticoids were administered to this patient on day 5, with a brief response in the fever curve but without remission of hypotension. A single course of anticytokine therapy, consisting of etanercept and tocilizumab, was given on day 7, with rapid clinical effects: within hours, defervescence occurred, and the patient was weaned from vasoactive medications and ventila-tory support as the clinical and radiologic manifestations of the acute respiratory distress syndrome resolved. She did not have laboratory evidence of the tumor lysis syndrome; however, biochemical evidence of the macrophage activation syndrome was noted, with elevation of the ferritin level to 45,529 ng per deciliter on day 11, coagulopathy with an elevated d-dimer level and hypofibrinogenemia, hepatosplenomegaly, and elevated levels of aminotransferases, LDH (Fig. 1A), and triglycerides, as well as a cytokine profile that was consistent with the macrophage activation syndrome. Her ferritin level decreased to 2368 ng per deciliter by day 26, and the clinical and laboratory abnormalities of the macrophage activation syndrome resolved.
In Patient 2, although there was no direct evidence of the tumor lysis syndrome other than fever and changes in the LDH level (Fig. 1A), features of the macrophage activation syndrome also developed, with elevations in the ferritin level to 33,360 ng per deciliter on day 7, peaking at 74,899 ng per deciliter on day 11; aminotransferase levels that reached grade 4 for 1 day; and an elevated serum d-dimer level. These biochemical changes were reversible: on day 21, the amino-transferase elevations were grade 1, and the ferritin level 3894 ng per deciliter. The patient was discharged from the hospital on day 16.
Both children had prominent elevations in a number of cytokines and cytokine receptors in the serum (Fig. 1B). In both, elevations in interferon-γ and interleukin-6 were most prominent. These observations are similar to the pattern observed previously in patients with CLL who also had a remission of leukemia after CTL019 infusion.8 The peak cytokine elevations were temporally correlated with systemic inflammation as determined by changes in core body temperature (Fig. 1A and 1B).
In Vivo Expansion of CTL019
The fraction of CTL019 T cells in circulation progressively increased in vivo to 72% of T cells in Patient 1 and 34% of T cells in Patient 2 (Fig. 2A). The initial transduction efficiency was 11.6% and 14.4% for the T cells infused in Patient 1 and Patient 2, respectively. In both children, the absolute lymphocyte count increased substantially (Fig. 1C) and the number of CTL019 cells progressively increased from baseline in vivo (Fig. 2A, and Fig. S2 in the Supplementary Appendix), reflecting a robust and selective expansion of CTL019 cells. The selective increase in T cells expressing CTL019 in both children is consistent with an antileukemic mechanism involving CD19-driven expansion and with the subsequent elimination of cells expressing CD19 (Fig. 3, and Fig. S3A and S3B in the Supplementary Appendix).
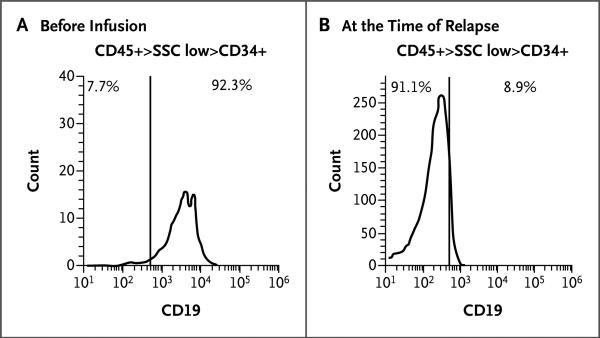
Figure 3. CD19 Expression at Baseline and at the Time of Relapse in Patient 2.
Bone marrow samples were obtained from Patient 2 before CTL019 infusion and at the time of relapse, 2 months later. Mononuclear cells isolated from marrow samples were stained for CD45, CD34, and CD19 and analyzed on an Accuri C6 flow cytometer. After a gating on live cells, the blast gate (CD45+ side scatter [SSC] low) was subgated on CD34+ cells, and histograms were generated for CD19 expression. The vertical line in each graph represents the threshold for the same gating on isotype controls. Pretherapy blasts (Panel A) have a range of distribution of CD19, with a small population of very dim-staining cells seen as the tail at the left of the histogram at 102 on the x axis. The numbers on the x axis are arbitrary fluorescence intensity units. The sample obtained at the time of relapse (Panel B) does not have any CD19+ blasts. Analysis of CD19 expression on the pretreatment blast population revealed a small population of CD19+dim or CD19– cells. The mean fluorescence intensity of this small population of cells was 187 units, which is similar to that of the anti-CD19–stained blast cells at relapse, 201 units. The pretherapy marrow sample was hypocellular, with 10% blasts, and the marrow sample at relapse was normocellular, with 68% blasts, accounting for differences in the number of events (cells) available for acquisition.
Molecular deep-sequence analysis of T-cell receptors (TCRs) in the peripheral-blood and marrow samples obtained from Patient 1 on day 23, when more than 65% of CD3+ cells in peripheral blood and marrow were shown to be CTL019+ on flow cytometry, revealed the absence of a dominant T-cell TCR clonotype in both compartments, with the 10 most abundant T cells present at frequencies of 0.2 to 0.7% in bone marrow and 0.2 to 0.8% in peripheral blood. Six of the 10 dominant clones were shared between the two compartments. In addition, both CD4 and CD8 chimeric antigen receptor T cells were present. Thus, the chimeric antigen receptor T cells appeared to proliferate after CD19-stimulated expansion and not by means of TCR signals or clone-specific events such as activation by integration of the lentivirus.
Expansion and Morphologic Features of CTL019 Chimeric Antigen Receptor T Cells
In both children, CTL019 cells expanded in the peripheral blood and bone marrow to levels that were more than 1000 times as high as the original engraftment levels (Fig. 2A and 2B). The frequency of CTL019 cells increased to more than 10% of circulating T cells by day 20 in both children (Fig. S2 in the Supplementary Appendix), with the absolute magnitude of CTL019 expansion similar to that observed in patients with CLL.8 Unexpectedly, cells in the CSF also showed a high degree of CTL019 gene marking and persisted at a high frequency at 6 months (Fig. 2B). The presence of CTL019 cells in the CSF was surprising, given that neither child had detectable central nervous system (CNS) leukemia according to analysis of cytospin preparations at the time of infusion or at the evaluation 1 month after treatment. Furthermore, prior studies of chimeric antigen receptor therapy for B-cell cancers have not shown the presence of chimeric antigen receptor T cells in the CNS.4,6,9,11-13 The morphologic features of the lymphocytes in the blood and CSF are shown for both children in Figure 2D. Because more than 70% of lymphocytes in circulation on day 10 were CTL019 cells (Fig. 2A and 2B), most of the large granular lymphocytes in the peripheral blood, as shown in Figure 2D, are probably CTL019 cells. Similarly, because many lymphocytes in the CSF obtained from Patient 2 on day 23 were CTL019 cells (Fig. 2B and 2C), the CSF lymphocytes shown in Figure 2D most likely represent the morphologic features of CTL019 cells in vivo that have migrated to the CSF.
Induction of B-Cell Aplasia
In both children, CD19+ cells in bone marrow and blood were eliminated within 1 month after CTL019 infusion (Fig. 3, and Fig. S3A and S3B in the Supplementary Appendix). In Patient 1, a large proportion of cells remaining in the marrow at day 6 after infusion were CD19+CD20+ leukemic blast cells. This population of cells was not detectable by day 23, an effect that has been maintained (Fig. S3A in the Supplementary Appendix). Marrow in this patient remained in remission for 9 months, and peripheral-blood counts remained normal for more than 11 months. Patient 1 did not receive chemotherapy in the 6 weeks before CTL019 infusion, which indicates that the CTL019 cells were sufficient to ablate normal and leukemic B cells in this patient.
Emergence of CD19 Escape Variant in Patient 2
Patient 2 had a clinical relapse that was apparent in the peripheral blood 2 months after CTL019 infusion, as evidenced by the reappearance of blast cells in the circulation. These cells were CD45+dim, CD34+ and did not express CD19 (Fig. 3). The absence of the original dominant CD34dim+CD34+CD19dim+ cells is consistent with a potent antileukemic selective pressure of the CTL019 chimeric antigen receptor T cells directed to CD19 (Fig. S3B in the Supplementary Appendix). Deep sequencing of IGH revealed the presence of the malignant clone in peripheral blood and marrow as early as day 23 (Table 1), despite a clinical assessment of no residual disease by means of flow cytometry at this time point (Fig. S1 in the Supplementary Appendix). In addition, deep sequencing of DNA isolated from bone marrow cells obtained at the time of clinical relapse revealed that the CD45+dimCD34+CD19– cells are clonally related to the initial dominant CD45dim+CD34+CD19dim+ cells, since they share the same IGH sequence.
Discussion
We report the induction of remission of relapsed and refractory leukemia in the first two patients treated on this protocol. Remission has been sustained in one patient and was accompanied by relapse due to the emergence of CD19– blasts in the other patient. High levels of genetically modified CTL019 cells were detected in the CNS in both patients. Systemic elevations of proinflammatory cytokines, which were reversible, were concomitant with peak T-cell expansion and tumor-cell elimination and therefore consistent with on-target activity of CTL019 cells against CD19+ target cells. The induction of complete remission in refractory CD19+ ALL after infusion of chimeric antigen receptor T cells is encouraging, particularly given the low frequency of remissions after the infusion of allogeneic donor lymphocytes that do not express chimeric antigen receptors.14-16 Deep sequencing indicated that the CTL019 chimeric antigen receptor infusion was associated with a sustained 5-log10 reduction in the frequency of malignant B cells in Patient 1, providing further evidence of potent antitumor effects in chemotherapy-refractory leukemia.
The unfortunate emergence of CD19– blast cells in one patient is consistent with previous reports that document the presence of CD19– precursor cells in some patients with ALL.17,18 It is possible that the coinfusion of chimeric antigen receptor T cells redirected to specificities, such as CD22, in addition to CD19 might decrease the likelihood of this event. Thus far, we have not observed a relapse with CD19– escape cells in adults with CLL after treatment with CTL019 cells,8 a finding that suggests this issue may be specific to a subset of acute leukemias. Finally, the induction of remission in Patient 1 did not require concomitant chemotherapy and is consistent with our previous observation that remissions in CLL could be delayed for several weeks after chemotherapy.7 Thus, concomitant administration of cytotoxic chemotherapy may not be necessary for chimeric antigen receptor– mediated antitumor effects.
Both children with ALL had substantial toxic effects after CTL019 infusion. The induction of B-cell aplasia was expected and indicates that the chimeric antigen receptor T cells can function in patients with relapsed acute leukemia. Both children also had clinical and laboratory evidence of the cytokine-release syndrome and the macrophage activation syndrome within a week after infusion. The cytokine profile observed in these children is similar to the cytokine patterns previously observed in children with hemophagocytosis and the macrophage activation syndrome or hemophagocytic lymphohistiocytosis.19,20 The macrophage activation syndrome is characterized by hyperinflammation with prolonged fever, hepatosplenomegaly, and cytopenias. Laboratory findings that are characteristic of this syndrome are elevated levels of ferritin, triglycerides, aminotransferases, bilirubin (primarily conjugated), and soluble interleukin-2 receptor α chain and decreased levels of fibrinogen.21 Recent studies indicate that tocilizumab (anti–interleukin-6 receptor monoclonal antibody) holds promise for glucocorticoid-resistant GVHD,22-24 and our results are consistent with these data.
The vigorous in vivo expansion of CTL019, persistent B-cell aplasia, and prominent antileukemic activity suggest that CTL019 cells have substantial and sustained effector functions in children with advanced ALL. The highly efficient migration of chimeric antigen receptor T cells to the CSF is encouraging as a mechanism for surveillance to prevent relapse in the CNS25 and supports the testing of chimeric antigen receptor T-cell–directed therapies for CNS lymphomas and primary CNS cancers. With the exception of B-cell aplasia, the duration of which is currently undefined, immune-based therapies such as CTL019 may have a favorable profile of long-term adverse effects, as compared with the high-dose regimens of chemotherapy and radiation therapy that are the current standard of care for most cases of relapsed pediatric leukemia.26
Supplementary Material
Acknowledgments
Supported in part by grants from the National Institutes of Health (1R01CA165206, to Dr. June; and R01CA102646 and R01CA116660, to Dr. Grupp) and the Leukemia and Lymphoma Society.
We thank Timothy Macatee for sample processing and flow-cytometric analysis; Irina Kulikovskaya and Minnal Gupta for the quantitative polymerase-chain-reaction assay; Erica Suppa for the Luminex assay; Saar Gill, Marybeth Helfrich, and Jessica Hulitt for assistance with images and flow-cytometric data analysis; Zhaohui Zheng, Andrea Brennan, Julio Cotte, and members of the Clinical Cell and Vaccine Production Facility for development of methods for clinical-scale ex vivo lentiviral transduction and for cell manufacturing; the Human Immunology Core for provision of reagents; Christine Strait, Margaret Tartaglione, Elizabeth Veloso, Lester Lledo, and Joan Gilmore for assistance in clinical research support; Edward Behrens and Donald Siegel for advice; Children's Hospital of Philadelphia Vector Core (Katherine High) for clinical-grade vector production; Kenneth Cornetta and the National Gene Vector Bioreposi-tory at the Indiana University School of Medicine for postinfusion testing of the product for replication-competent lentivirus; Cindy Desmarais, Harlan Robins, and Nishanth Marthandan at Adaptive Biotechnologies for assistance with molecular analysis of minimal residual disease; and Bipulendu Jena and Laurence Cooper for provision of the chimeric antigen receptor anti-idio-type detection reagent.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Barrett AJ, Horowitz MM, Pollock BH, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med. 1994;331:1253–8. doi: 10.1056/NEJM199411103311902. [DOI] [PubMed] [Google Scholar]
- 2.Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–41. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- 3.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 4.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, et al. T cells expressing chimeric receptors establish memory and potent antitumor effects in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen re ceptor–modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MC, Popplewell L, Cooper LJ, et al. Anti-transgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–50. [PubMed] [Google Scholar]
- 15.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 16.Collins RH, Jr, Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–6. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 17.Hotfilder M, Röttgers S, Rosemann A, et al. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19− cells. Cancer Res. 2005;65:1442–9. doi: 10.1158/0008-5472.CAN-04-1356. [DOI] [PubMed] [Google Scholar]
- 18.le Viseur C, Hotfilder M, Bomken S, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Xu X, Song H, et al. Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol. 2008;143:84–91. doi: 10.1111/j.1365-2141.2008.07298.x. [DOI] [PubMed] [Google Scholar]
- 20.Behrens EM, Canna SW, Slade K, et al. Repeated TLR9 stimulation results in macrophage activation syndrome–like disease in mice. J Clin Invest. 2011;121:2264–77. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–46. doi: 10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]
- 22.Drobyski WR, Pasquini M, Kovatovic K, et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1862–8. doi: 10.1016/j.bbmt.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17:77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Huu D, Matsushita T, Jin G, et al. IL-6 blockade attenuates the development of murine sclerodermatous chronic graft-versus-host disease. J Invest Dermatol. 2012;132:2752–61. doi: 10.1038/jid.2012.226. [DOI] [PubMed] [Google Scholar]
- 25.Pullen J, Boyett J, Shuster J, et al. Extended triple intrathecal chemotherapy trial for prevention of CNS relapse in good-risk and poor-risk patients with B-progenitor acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol. 1993;11:839–49. doi: 10.1200/JCO.1993.11.5.839. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Manero G, Thomas DA. Salvage therapy for refractory or relapsed acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15:163–205. doi: 10.1016/s0889-8588(05)70204-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.