Abstract
Antiretroviral therapy has been a spectacular success. People are now asking if the end of AIDS is possible. For those who are motivated to take therapy and who have access to lifelong treatment, AIDS-related illnesses are no longer the primary threat, but a new set of HIV-associated complications have emerged, resulting in a novel chronic disease that for many will span several decades of life. Treatment does not fully restore immune health; as a consequence, a number of inflammation-associated and/or immunodeficiency complications such as cardiovascular disease and cancer are increasing in importance. Cumulative toxicities from exposure to antiretroviral drugs for decades cause clinically-relevant metabolic disturbances and end-organ damage. There are growing concerns that the multi-morbidity associated with HIV disease may impact healthy aging and could overwhelm some health care systems, particularly those in resource-limited regions that have yet to fully develop a chronic care model. Given the problems inherent in treating and caring for a chronic disease that might persist for several decades, a global effort to identify a cure is now underway.
INTRODUCTION
The face of HIV as a chronic disease has changed as a result of advances in HIV treatment in the last three decades (Table 1). Combination ART (ART) improves health, prolongs life and substantially reduces the risk of HIV transmission. In both high and low income countries, the life expectancy of HIV-infected patients who have access to ART is now measured in decades, and may approach that observed in uninfected population among those who are optimally treated(1, 2).
Table 1.
HIV as a Chronic Disease
| Past | Present | Future | |
|---|---|---|---|
| Epidemiology |
|
|
|
| Immune Profile |
|
|
|
| Disease Burden |
|
|
|
| Health System |
|
|
|
Men having sex with men (MSM), transgender, sex workers, injection drug users
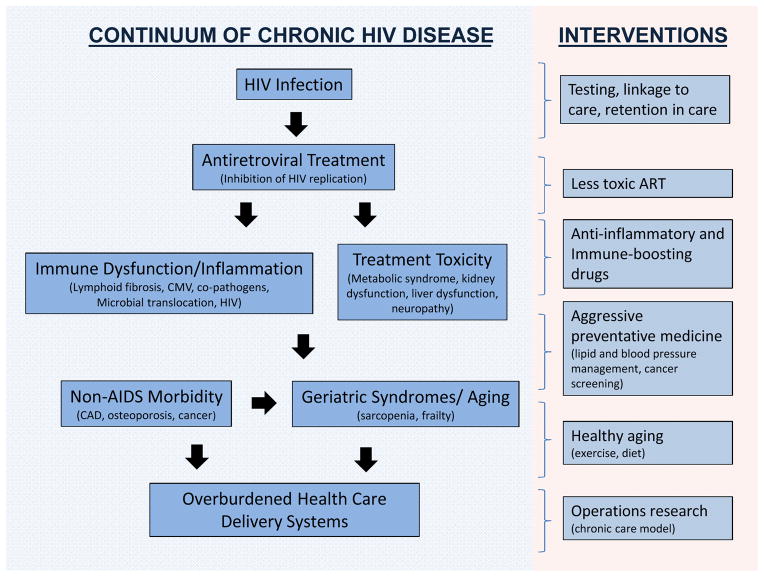
Advances in treatment and prevention have led some to now ask if the “end of AIDS” is possible(3). Making the bold assumption that challenges of HIV testing and linkage to care can be overcome, we are of the opinion that although AIDS is now preventable, substantial limitations of current therapeutic approaches persist (Figure 1). First, ART does not fully restore health. For reasons that remain to be elucidated, antiretroviral-treated HIV disease is associated with a new constellation of problems, generally referred to as “non-AIDS morbidity”, and, in the popular press, “premature aging”. Second, health care systems in those regions where most people with HIV reside (e.g., sub-Saharan Africa) were designed to provide acute care only and are ill-equipped to provide the chronic care which is now required to manage this disease. Finally, ART is not curative, meaning that a young adult who acquires HIV will need to take expensive and potentially toxic drugs for several decades, a daunting task for both the individual and the health care system. In this review, we argue that while AIDS as a syndrome will diminish in frequency among persons identified early and properly treated, solutions to the seemingly three disparate issues—HIV-associated inflammation, an overburdened health care system and HIV persistence—are needed to further transform HIV disease.
Figure 1. HIV Infection as a Chronic Disease.
Antiretroviral therapy has transformed HIV infection from a progressive, typically fatal infection to a chronic disease that persists for many decades. A typical young adult who acquires HIV is expected to be on therapy for up to 50 years. Cumulative exposure to antiretroviral drugs and/or chronic inflammation is expected to have profound effects on health and aging. Novel health care delivery systems are needed to provide optimal management of treatment and the many co-morbidities associated with HIV disease.
THE CASCADE OF CARE
People have to access and adhere to antiretroviral therapy if HIV infection is to become a truly chronic disease. Unfortunately, even within the most sophisticated health care systems, effective delivery of HIV-related care is far from ideal. The “treatment cascade” is now a commonly used conceptual model that quantifies the delivery of services to persons living with HIV across the entire continuum of care(4). In order to maximize the benefits of therapy on an individual and community levels, at risk individuals need first to get tested, and those who are infected have to access care, start treatment, stay in care and remain adherent to HIV therapy. Currently in the US, for every 100 patients with HIV infection, it is estimated by the Centers for Disease Control (CDC) that only 28 patients have successfully managed each of these steps (REF). The success rate is much lower in resource poor regions of the world, particularly sub-Saharan Africa, where identification of HIV status remains a huge challenge. (5).
DISEASE PERSISTS DURING EFFECTIVE ART
When used correctly, ART results in rapid control of HIV and partial restoration of immune function, leading to the prevention of the various complications that define AIDS. This does not mean that health is fully restored, however. Studies conducted in high income countries tell us that HIV-infected adults experiencing durable treatment-mediated suppression of HIV replication are at risk for developing a number of non-AIDS conditions, including cardiovascular disease, cancer, kidney disease, liver disease, osteopenia/osteoporosis and neurocognitive disease (collectively referred to as “serious non-AIDS events”). Consider, for example, cardiovascular disease. In the large US-based VA medical system, after adjusting for traditional risk factors, HIV-infected adults had about a 1.5 fold increased risk of having a myocardial infarction(6). This effect was seen in the subset with durable control of HIV replication, and had an overall effect comparable to other well-accepted risk factors, such as hypertension, hyperlipidemia and presence of diabetes mellitus. The level of risk attributed to HIV infection was higher in younger persons in this and other studies(7). Malignancies associated with infections such as human papilloma virus (including uro-genital and head and neck cancers), Epstein Barr Virus (including Hodgkins disease), and hepatitis B and C (hepatocellular carcinoma), are also relatively common in HIV-infected adults.
The impact of antiretroviral-treated HIV disease on risk of these non-AIDS events is expected to be similar in high and low income regions, although the nature of this risk in Africa and other low income countries has yet to be well-defined(8). One small study which compared cohorts from Botswana to the US found that crude rates of non-AIDS defining events were similar, but that age and gender adjusted rates were actually higher in Botswana(9). HIV infected patients are not spared the risk for diseases such as hypertension and diabetes, both of which are increasingly recognized as major health problems across Africa(10). Cancer prevention and treatment capabilities in much of Africa are not accessible, regardless of HIV status. Obesity among those living with HIV is well documented in high income countries but is also already is a major challenge to African health(11). Increased smoking in countries such as South Africa is likely to influence the epidemiology of co-morbidities seen in chronic HIV infection including lung, renal and liver disease, but data are lacking. There is no reason to expect the overall burden of these co-morbid conditions to be lower in Africa and elsewhere than in high income countries. Indeed, given the lack of primary prevention, the high burden of inflammatory co-infections, and the fact that therapy is often started late (which is a consistent predictor of developing non-AIDS morbidity(6, 12-14)), it must be assumed that these age-associated complications will emerge as a major problem as the current generation of relatively young adults begin to age.
Why do antiretroviral-treated adults have an excess risk of these seemingly unrelated non-AIDS events? An excess burden of the traditional risk factors such as smoking, alcohol and other substance abuse is almost certainly part of the issue(15). Direct toxicity of antiretroviral drugs also contributes to these complications, although each successive generation of antiretroviral therapy has been associated with less toxicity. For example, tenofovir—which is now included in most first-line regimens—and some commonly used protease inhibitors have subtle but measurable effects on kidney function(16, 17). Metabolic changes, incuding body fat redistribution (peripheral lipoatrophy, central lipoaccumulation), insulin resistance, diabetes mellitus, and hyperlipidemia are associated with cumulative exposure to antiretroviral therapy. Since even subtle toxicities might result in large burden of disease when the drugs are used for decades, treatment guidelines now recommend regimens based as much on their long-term toxicity as on their antiviral potency.
Traditional risk factors and antiretroviral drug toxicity, however, do not fully explain all of the excess risk for non-AIDS morbidity. A rapidly growing and remarkably consistent evidence base indicates that many markers of inflammation are higher in antiretroviral-treated adults than in age-matched uninfected individuals(18, 19). Subtle elevations in many of these biomarkers are associated with dramatic increases in the risk of subsequent disease, including all-cause mortality. Key among these biomarkers is a series of immune mediators that reflect chronic activation of the innate immune system. For example, as compared to a well-matched uninfected population, treated HIV-infected adults have approximately 50 to 100% higher levels of the inflammatory cytokine interleukin-6 (IL-6)(19). In large international multi-site studies (INSIGHT), elevations in IL-6 levels were strongly associated with all-cause mortality, with odds ratios that were much higher than that observed in the general population(20). A single determination of IL-6 predicted excess risk of mortality through several years of observation. Other well-validated biomarkers include soluble CD14 (sCD14) and sCD163, both of which are released by monocytes/macrophages into plasma upon activation. Elevated levels of sCD14 was associated with increased risk of death in one large study(21) while sCD163 has been associated with increased risk of coronary artery inflammation and atherosclerosis(22). The frequency of “inflammatory” CD16+ monocytes is also associated with risk of coronary artery progression(23). Other non-specific markers of inflammation such as C-reactive protein and cystatin C are more variably increased during HIV disease.
Measures more directly related to the adaptive immune system also have prognostic significance during treated disease. The rate at which CD4+ T-cells increase during ART is highly variable. A minority of well-treated individuals fail to achieve normal levels, traditionally defined as anything above 500 cells/ul (although truly normal levels are probably much higher). Risk factors for impaired CD4 T-cell recovery include low pre-treatment CD4+ T cell count nadir, co-infection with other viruses such as hepatitis C, older age, and perhaps viral factors(24). Suboptimal treatment-mediated CD4+ T cell outcomes likely have clinical consequences given the consistent association between CD4+ T cell counts during ART and elevated risk of many co-morbidities (e.g., heart disease, cancer) and all-cause mortality(6, 12, 13). Chronic signalling through the interferon-alpha pathway may contribute to this inflammatory disease(25), as can the impact of virus production/entry (without productive infection) on pyroptosis, which is a highly inflammatory process that can cause death of affected and neighbouring cells(26). The frequency of activated T cells remains elevated during chronic treatment(27) and appears related to size of the HIV reservoir and pace of immune reconstitution(28, 29), although the effect of this marker in predicting overall morbidity and mortality is not as strong as some of the innate immune system inflammatory markers (30).
Markers of hypercoagulation are also elevated in HIV-infected patients on ART and associated with risk of disease progression. D-dimers and to a lesser extent fibrinogen levels are elevated and associated with increased risk of disease(20, 31, 32). It has been postulated that lipopolysaccharide (LPS), a marker of microbial translocation and increased in HIV-infected patients, activates the coagulation process (perhaps via expression of tissue factor activated monocytes)(33) and that this leads to systemic clotting, tissue damage and disease(34). Liver dysfunction leading to altered production of coagulant factors and clearance of LPS may also contribute to this process(35).
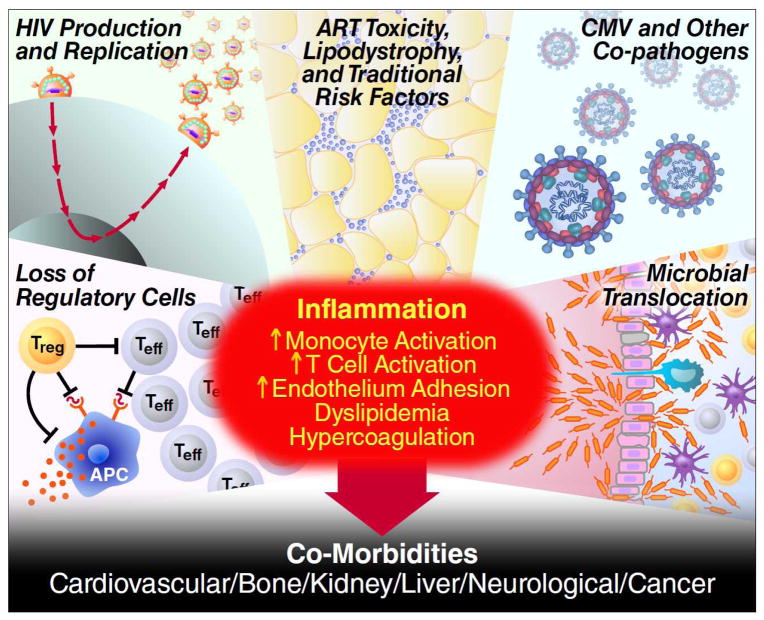
There is intense interest in defining the cause and consequence of chronic inflammation during ART (Figure 2). A number of small biomarker-driven clinical trials have provided insights into why inflammation is elevated and how it might be controlled(36). Intensification of apparently fully effective ART with additional antiretroviral drugs reduces T-cell activation(37) and measures of coagulation(38), suggesting that low-level HIV replication contributes to the inflammatory process in some patients. Treating specific co-infections such as CMV(39) and hepatitis C virus(40) reduces T-cell activation, indicating that these common chronic viral infections also contribute to inflammatory environment during ART. As HIV-mediated breakdown in the integrity of the gut mucosa and chronic translocation of gut microbial products in the systemic circulation is widely assumed to be a major cause of inflammation(41), a series of clinical trials reversing this process have been performed, with variable success(42-44). HIV-mediated deposition of collagen in lymphoid tissues is also another well-established cause of persistent immune dysfunction and inflammation(45) that is actively being addressed in prospective clinical trials.
Figure 2. Causes and consequences of chronic inflammation during antiretroviral treatment.
Causes and Consequences of HIV-associated Inflammation. Despite effective antiretroviral therapy, many if not most HIV-infected adults have evidence of persistent inflammation and immune dysfunction. Root causes of inflammation include ongoing HIV production, high levels of other co-pathogens, irreversible damage to immunoregulatory system, and translocation of microbial products across damaged mucosal surfaces. This inflammatory environment causes end-organ damage through several potential pathways.
There is also intense interest in the use of more broad non-specific immunomodulators aimed at reducing inflammation. The statins have well-established anti-inflammatory effects in the general population, and may have a mortality benefit in HIV disease(46). Promising anti-inflammatory effects have been observed in prospective interventional studies(47). Choloroquine, hydroxychloroquine, COX-2 inhibitors, aspirin, methotrexate and a number of other anti-inflammatory drugs are being developed as possible adjuncts to standard antiretroviral drugs (Table 2). Interleukin-7 is being developed as means to enhance CD4 T-cell recovery(48), although interest in this approach is affected by the failure of interleukin-2 to provide clinical benefit in two very large and expensive clinical end-point studies(49). There are dozens of promising interventions which might reduce inflammation and inflammation-associated disease burden in development. These phase I/II-type studies rely almost entirely on biomarkers that have unclear clinical significance. A key question for the field is to decide which if any of these promising drugs to move into clinical endpoint testing, which will be an expensive and logistically challenging study to complete.
Table 2.
Novel therapeutic drugs in development for management of HIV disease
| Anti-inflammatory Drugs | HIV Cure Interventions | |
|---|---|---|
| Phase I |
|
|
| Phase II |
|
|
| Phase III |
|
|
DOES HIV INFECTION ACCELERATE AGING?
Because many of these non-AIDS events are typically associated with aging in the general population(50), the popular but vague terms “accelerated aging” or “premature aging” is often used to characterize the new spectrum of HIV-associated diseases, but there are mixed opinions on what defines these terms. There is ongoing debate as to whether HIV-associated diseases which have been associated with aging are simply more common at any given age, or are occurring earlier than expected. In either case, it is well accepted that HIV-infected adults have high burden of co-morbid conditions, including cardiovascular disease, neuropathy, anemia, osteoporosis, liver disease and kidney disease. An index designed to characterize the impact of multi-morbidity in HIV disease on prognosis has been developed and validated (the VACS Index)(51). Multi-morbidity, polypharmacy, chronic inflammation, hypercoagulation, and traditional risk factors such as substance abuse are all relatively common in the HIV infected population, and all are linked to greater risk of developing the clinical manifestations of aging in later life in the general population and presumably in the HIV-infected population (50, 52-54).
The controversies and research opportunities provided by the study of aging and HIV disease is best illustrated by a discussion of frailty. The frailty phenotype is classically defined based on the presence of weight loss, exhaustion, low physical activity, muscle weakness and slow walking speed (55). The syndrome is marked by the inability to compensate and maintain normal function (e.g., mobility) when confronted with some form of stress (e.g., minor surgery, acute infection or loss of a partner)(52). Frailty emerges when multiple physiologic systems begin to decline or fail, leading to a loss of physiologic redundancy and an inability to compensate to stress. The greater the number of abnormal physiologic systems or diseases such as anemia, inflammation, cardiovascular disease, or metabolic abnormalities, the more likely one is to be frail(56, 57). Other risk factors for frailty include polypharmacy and social isolation. Since treated HIV disease is now a chronic condition with many of these risk factors, it is reasonable to assume that HIV-infected adults have higher than normal risk for developing frailty as they age(54). Indeed, despite the relatively young age of most HIV-infected adults, a number of studies have found that frailty (or a frailty-like syndrome) is more common in treated HIV disease than in the general population(58-61), and that among those with HIV, frailty is associated higher levels of inflammation(59).
The biology of fraily is the focus of intense research. Many biologic factors are known or assumed to contribute to the development of frailty, including chronic inflammation (as measured by IL-6 and other biomarkers), hypercoagulability, mitochondria dysfunction, DNA mutagenesis, alterations in telomerase activity/telomere length phenotype and endocrinopathies(62). HIV infection and its treatment impacts in a potential detrimental manner all of these pathways(20, 35, 63-65). A highly contentious issue ripe for future mechanistic studies is whether there are distinct aspects of HIV disease that alters the biology of aging in some fundamental manner (64, 66).
THE HEALTH SYSTEMS GAP
HIV disease as a chronic illness requiring life-long therapy and characterized by multiple co-morbidities represents unique problems for health care delivery. Identifying people with HIV, linking them to care, providing them with access to therapy and addressing the multiple potential complications requires a well-resourced health care system(67). Barriers to success exist at each step and have been well documented(68).
The lack of a well-resourced chronic care model is particularly urgent in resource-limited areas such as sub-Saharan Africa (69-72). Some of the critical elements needed for a sustainable HIV chronic care model in Africa include (1) efficient, effective and safe antiretroviral management, (2) services for reproductive health, non-AIDS morbidity such as cardiovascular disease, and aging and (3) fail safe TB prevention and treatment services. The recent global discourse on “health systems strengthening” that singled out HIV resource allocation as an impediment to progress has been eclipsed by thoughtful analysis of the architecture of successful systems supported by relatively few resources(73, 74). New models for health care delivery must build upon lessons learned during antiretroviral scale up while incorporating new concepts such as quality improvement “dashboards” for clinical care. Separating acute from chronic care is an essential step in the transition to a chronic disease model. Specialized largely urban clinics with staff and a structure catering to the complex medical management of advanced AIDS made sense at the launch of antiretroviral programs. As patient populations evolve from those with active AIDS illnesses and low CD4+ T cells requiring physician expertise and long visits to one enriched with stable, antiretroviral treated patient populations, new care models are necessary. These models need to absorb millions more patients into chronic disease care in a sustainable, efficient and affordable manner. Decentralization of services, task shifting and streamlined monitoring has already successfully begun, reducing transport barriers, increasing retention and reducing costs(75-77). Shifting towards a more community-based model of chronic care will require continued investments in supply chain management and the development of point-of-care diagnostics. The measurement of HIV RNA levels in real time using affordable and sensitive assays that work in a variety of settings—including those without phlebotomy and electricity—is particularly important as such measurements can be used to monitor treatment adherence and determine when therapy needs to be modified(78).
Despite an aging population, HIV disease in Africa still predominantly affects youth and adults of reproductive age. Reproductive health services encompassing both ART and family planning are essential components of chronic HIV disease care. Characterization of drug interactions between treatments required for HIV and those used to prevent pregnancy and other chronic diseases are urgently needed. In an era where HIV transmission to children can be eliminated with aggressive antiretroviral drug management at birth, future research will need to address whether exposure to these drugs impact health even as they prevent infection(79).
Care of an aging HIV epidemic in Africa has already been raised as an area of concern and unmet need(80). The increased life expectancy of HIV infected persons receiving ART will result in a progressively older population, with changes in life expectancy already detectable at a population level in South Africa(81). The number of persons over 50 years of age living with HIV is expected to triple by 2040 to 9 million persons, based on estimates of ART coverage that many would consider conservative(82). Neurocognitive impairment and frailty is more common in the elderly; tuberculosis and other non-communicable diseases will occur with a higher frequency, demanding care systems that address these needs and a research agenda focused on key issues related to this population.
Although many AIDS related illnesses can be nearly eliminated with successful ART, tuberculosis is an exception. Tuberculosis rates remain several-fold higher for persons with chronic treated HIV infection compared to HIV uninfected persons living in the same region(83). The underlying deficit to explain this vulnerability is unknown but likely includes factors related to incomplete immune restoration and ongoing inflammation. On a practical level, isoniazid prophylaxis, TB screening, and treatment should be incorporated into the chronic care model through systems that prioritize convenience to the patient(84).
What are the next steps with regard to HIV as a chronic disease in Africa? A single solution to the current challenge in health delivery for the millions with chronic HIV and other non-communicable diseases is a fallacy. The needs and face of HIV as a chronic disease will be shaped by many factors including background infections (e.g. tuberculosis, hepatitis); lifestyles (e.g., smoking, obesity): reproductive trends and socioeconomic structures. The pace of access to the diagnosis and treatment of non-AIDS disease is not predictable and will vary by region. However a unified approach between the HIV and non-communicable disease communities has a greater chance to accelerate access for both(85).There is a growing literature describing the advantages and pitfalls of integrating HIV with other chronic care and a need for rigorous implementation science that measures efficiencies from perspective of the patients, providers and health systems(86, 87). There needs to be an openness to a variety of models that could work and consideration of public as well as private sector solutions(88). It is indeed a reflection of success in HIV medicine that the creation of new systems has emerged as a critical issue in the next chapter of the AIDS response.
HIV PERSISTENCE AND NEED FOR A CURE
It is hard to overstate the clinical effectiveness of ART. Still, as outlined above, disease persists during effective ART and delivering ART on a global level for decades to all in need of therapy will be a daunting and resource-expensive endeavour. Recognition of these limitations has led to growing recognition that a safe, affordable and effective cure for HIV disease may be needed to address the limitions of current therapeutic strategies (89). Although a cure for HIV remains an aspirational goal, a number of recent observations in the clinic suggest it might be possible.
Several mechanisms account for the inability of antiretroviral drugs to eliminate HIV. Despite complete or near complete inhibition of HIV replication with ART, virus persists in long lived infected resting T-cells that contained integrated, transcriptionally silent (or “latent”) HIV DNA(90). These memory T-cells are designed to persist indefinitely. Other cells that likely harbour HIV during long-term therapy include naïve CD4+ T cells and CD4-expressing cells of the monocyte/macrophage lineage.
That a cure is possible was demonstrated by the “Berlin Patient”, who several years ago received an allogeneic hematopoietic stem cell transplant for the management of his leukaemia. After extensive conditioning with a myeloablative regimen (which likely eliminated much of the HIV reservoir), donor stem cells that were naturally resistant to HIV were successfully transplanted(91). He is now more than six years out from the transplant and meets any definition of a clinical cure(92). More recently, two cases of potential cure after myeloablation and allogenic stem cell transplant were identified in Boston(93), while several groups are attempting to repeat the Berlin Patient experiment using similar donors or by gene therapy to make autologous cells resistant to HIV infection. Curing HIV infection with such interventions is clearly possible, but these intensive, expensive and potentially life-threatening interventions are unlikely to be ever widely used.
A more promising approach may be to use ART to prevent seeding of the reservoir. The initiation of a potent regimen approximately 30 hours after birth to an infant subsequently shown to be HIV infected appeared to be curative (REF). As a single case, it is unknown what led to a cure of this infant, but there is intense interest in studies aimed repeating this observation, and determining the mechanism for the apparent cure.
Can such an outcome be achieved by aggressively treating recently infected adults? Provocative data in this regard were provided in a study of adults in Thailand who received therapy within 10 to 14 days of their exposure. Within a few months of starting therapy, HIV DNA could not be detected in longest lived cells, raising the possibility that with time, the relatively shorter-lived T-cells harbouring the virus might die off, recapitulating the cured baby story. In another unrelated study, 14 adults living in France were identified who (1) started therapy during acute/early infection, (2) remained on therapy for several years, (3) stopped therapy for a variety of uncontrolled reasons, and (4) failed to exhibit any virologic rebound, even after a few years of observations(94). Replication-competent virus was detected in these individuals, suggesting that some host mechanism was controlling the virus. It remains to be determined as to whether early treatment altered their natural history, or whether these 14 individuals were destined to be “elite” controllers and would have done well even without treatment.
Stem cell transplants and very early therapy have shown promise, but none of these studies are relevant to the vast majority of HIV infected individuals who started therapy during chronic infection and who lack any clinical condition that might necessitate a risky stem cell transplant. For these individuals, interventions that safely reverse latency while enabling the death of the virus-producing cells might be the only viable way to a cure (Table 2). Two groups have recently shown that the chromatin-modifying drug vorinostat—which alters gene regulation and can therefore activate transcription—increases HIV RNA production in resting T-cells in long-term treated adults(95). An increase in detectable virus in plasma was rare, making it unlikely that there were sufficient levels of protein made to cause cell death or stimulate a host immune response. However, in these studies, vorinostat was only given for a short period of time. Further studies using longer duration of vorinostat, more potent activating agents or combining latency activation with immune stimulation are underway. Other promising approaches to clearing the reservoir are slowly being moved into the clinic (see Table 3).
CONCLUSIONS
By virtue of the success of ART, HIV has evolved into a chronic disease in which the typical complications AIDS are no longer the dominant problem in many parts of the world. Rather than dealing with acute potentially life-threatening complications, clinicians now are confronted with managing a chronic disease that in the absence of a cure will persist for many decades. HIV care requires new skills on the part of the clinical workforce and a reshaping of those health care systems initially designed for acute care. The clinician of the future will still require knowledge of antiretroviral management but will need more expertise in preventing and managing cardiovascular disease and other co-morbidities, including many of the complications typically associated with aging. Biomedical research will need to evolve accordingly. Understanding why inflammation persists during ART, how it causes morbidity, and how to reverse the process is a high-priority for HIV disease, as it is for many other chronic conditions. The research community will also need to identify optimal, cost-effective ways that integrate non-communicable disease and TB services to deliver chronic care to an aging population who largely reside in areas that lack solid primary care health care systems. Since curing HIV infection might prove to the best solution for all of these problems, basic discovery, early clinical investigation, and the establishment of large collaborations aimed at tackling HIV persistence during ART are needed.
Acknowledgments
We acknowledge the support of John Carroll and Warner Greene of the Gladstone Institute of Virology and Immunology of assistance on the figures. SGD was supported by NIAID (K24 AI069994), the DARE: Delaney AIDS Research Enterprise (U19AI096109), and the UCSF/Gladstone CFAR (P30 AI027763). SRL is a National Health and Medical Research Council (NHMRC) of Australia Practitioner Fellow and is supported by DARE: Delaney AIDS Research Enterprise (U19AI096109) and NIAID 1R56AI095073-01A1. SRL gratefully acknowledges the contribution to this work of the Victorian Operational Infrastructure Support Program received by the Burnet Institute. DVH was supported by NIAID (A151982) and the UCSF/Gladstone CFAR (A1027763).
References
- 1.Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, Fox MP, Wood R, Prozesky H, Giddy J, Garone DB, Cornell M, Egger M, Boulle A. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2013;26:17–25. doi: 10.1097/QCO.0b013e32835ba6b1. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA : the journal of the American Medical Association. 2012;308:343–4. doi: 10.1001/jama.2012.8142. [DOI] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piot P, Quinn TC. Response to the AIDS pandemic--a global health model. The New England journal of medicine. 2013;368:2210–8. doi: 10.1056/NEJMra1201533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA internal medicine. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 8.Hirschhorn LR, Kaaya SF, Garrity PS, Chopyak E, Fawzi MC. Cancer and the ‘other’ noncommunicable chronic diseases in older people living with HIV/AIDS in resource-limited settings: a challenge to success. Aids. 2012;26(Suppl 1):S65–75. doi: 10.1097/QAD.0b013e328355ab72. [DOI] [PubMed] [Google Scholar]
- 9.Wester CW, Koethe JR, Shepherd BE, Stinnette SE, Rebeiro PF, Kipp AM, Hong H, Bussmann H, Gaolathe T, McGowan CC, Sterling TR, Marlink RG. Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS. 2011;25:1471–9. doi: 10.1097/QAD.0b013e328347f9d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 11.Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, Kimaiyo S. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One. 2011;6:e22288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, Cavert WP, Henry WK, Neaton JD. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, Wood K, Holmberg SD, Brooks JT. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51:435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 15.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, Goetz M, Tracy R, Oursler KA, Rimland D, Crothers K, Rodriguez-Barradas M, Crystal S, Gordon A, Kraemer K, Brown S, Gerschenson M, Leaf DA, Deeks SG, Rinaldo C, Kuller LH, Justice A, Freiberg M. HIV Status, Burden of Comorbid Disease and Biomarkers of Inflammation, Altered Coagulation and Monocyte Activation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, Ross M, Fux CA, Morlat P, Moranne O, Smith C, Lundgren JD. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. The Journal of infectious diseases. 2013;207:1359–69. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–75. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T Cell Activation Is Associated with Lower CD4+ T Cell Gains in Human Immunodeficiency Virus-Infected Patients with Sustained Viral Suppression during Antiretroviral Therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdo TH, Lo J, Abbara S, Wei J, Delelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a Novel Marker of Activated Macrophages, Is Elevated and Associated With Noncalcified Coronary Plaque in HIV-Infected Patients. The Journal of infectious diseases. 2011;204:1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker JV, Huppler Hullsiek K, Singh A, Wilson E, Henry WK, Lichtenstein KA, Onen O, Kojic E, Patel P, Brooks JT, Hodis HN, Budoff M, Sereti I Investigators. ftCS. . Monocyte Activation, but Not T Cell Activation, Predicts Progression of Coronary Artery Calcium in a Contemporary HIV Cohort. 20th Conference on Retroviruses and Opportunistic Infections (CROI 2013); Atlanta, GA. March 2013; (Abstract #66LB) [Google Scholar]
- 24.Battegay M, Nuesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280–7. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 25.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG. Relationship between T Cell Activation and CD4(+) T Cell Count in HIV-Seropositive Individuals with Undetectable Plasma HIV RNA Levels in the Absence of Therapy. J Infect Dis. 2008;197:126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, Hoh R, Martin JN, McCune JM, Hecht F, Busch MP, Deeks SG. Cell-Based Measures of Viral Persistence Are Associated With Immune Activation and Programmed Cell Death Protein 1 (PD-1)-Expressing CD4+ T cells. The Journal of infectious diseases. 2012 doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasula S, Lempicki RA, Adelsberger JW, Huang CY, Roark J, Lee PI, Rupert A, Stevens R, Sereti I, Lane HC, Di Mascio M, Kovacs JA. Differential effects of HIV viral load and CD4 count on proliferation of naive and memory CD4 and CD8 T lymphocytes. Blood. 2011;118:262–70. doi: 10.1182/blood-2011-02-335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, Kembabazi A, Neilands TB, Bangsberg DR, Deeks SG, Martin JN. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–31. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, Natarajan V, Rehm C, Hadigan C, Sereti I. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–17. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, Bryant K. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:984–94. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, Douek DC, Lederman MM. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2009 doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J, Xu C, Haret-Richter GS, Trichel A, Apetrei C, Landay A, Tracy R. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012;120:1357–66. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker JV, Brummel-Ziedins K, Neuhaus J, Duprez D, Cummins N, Dalmau D, Dehovitz J, Lehmann C, Sullivan A, Woolley I, Kuller L, Neaton JD, Tracy RP. HIV Replication Alters the Composition of Extrinsic Pathway Coagulation Factors and Increases Thrombin Generation. Journal of the American Heart Association. 2013;2:e000264. doi: 10.1161/JAHA.113.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work? Aids. 2013 doi: 10.1097/QAD.0b013e32835ecb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, Palmer S, Stevenson M, Clotet B, Blanco J, Martinez-Picado J. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 38.Hatano H, Strain MC, Scherzer R, Bacchetti P, Wentworth D, Hoh R, Martin JN, McCune JM, Neaton JD, Tracy R, Hsue PY, Richman DD, Deeks SG. Increase in 2-LTR Circles and Decrease in D-dimer After Raltegravir Intensification in Treated HIV-Infected Patients: A Randomized, Placebo-Controlled Trial. Journal of Infectious Dis. doi: 10.1093/infdis/jit453. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, Deeks SG. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez VD, Falconer K, Blom KG, Reichard O, Morn B, Laursen AL, Weis N, Alaeus A, Sandberg JK. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol. 2009;83:11407–11. doi: 10.1128/JVI.01211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 42.Byakwaga H, Kelly M, Purcell DF, French MA, Amin J, Lewin SR, Haskelberg H, Kelleher AD, Garsia R, Boyd MA, Cooper DA, Emery S. Intensification of antiretroviral therapy with raltegravir or addition of hyperimmune bovine colostrum in HIV-infected patients with suboptimal CD4+ T-cell response: a randomized controlled trial. The Journal of infectious diseases. 2011;204:1532–40. doi: 10.1093/infdis/jir559. [DOI] [PubMed] [Google Scholar]
- 43.Gori A, Rizzardini G, Van’t Land B, Amor KB, van Schaik J, Torti C, Quirino T, Tincati C, Bandera A, Knol J, Benlhassan-Chahour K, Trabattoni D, Bray D, Vriesema A, Welling G, Garssen J, Clerici M. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal immunology. 2011;4:554–63. doi: 10.1038/mi.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cahn P, Ruxrungtham K, Gazzard B, Diaz RS, Gori A, Kotler DP, Vriesema A, Georgiou NA, Garssen J, Clerici M, Lange JM. The Immunomodulatory Nutritional Intervention NR100157 Reduced CD4+ T-Cell Decline and Immune Activation: A 1-Year Multicenter Randomized Controlled Double-Blind Trial in HIV-Infected Persons Not Receiving Antiretroviral Therapy (The BITE Study) Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:139–46. doi: 10.1093/cid/cit171. [DOI] [PubMed] [Google Scholar]
- 45.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. The Journal of clinical investigation. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PloS one. 2011;6:e21843. doi: 10.1371/journal.pone.0021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, Brandt C, Vita J, Decker CF, Sklar P, Bavaro M, Tasker S, Follmann D, Maldarelli F. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. The Journal of infectious diseases. 2011;203:756–64. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Avettand-Fenoel V, Croughs T, Beq S, Thiebaut R, Chene G, Morre M, Delfraissy JF. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–59. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 51.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Nattermann J, Lampe FC, Bucher HC, Sterling TR, Crane HM, Kitahata MM, May M, Sterne JA. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–72. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tinetti ME, McAvay GJ, Chang SS, Newman AB, Fitzpatrick AL, Fried TR, Peduzzi PN. Contribution of multiple chronic conditions to universal health outcomes. Journal of the American Geriatrics Society. 2011;59:1686–91. doi: 10.1111/j.1532-5415.2011.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 55.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 56.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:1049–57. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–41. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 58.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–86. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 59.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, Wilson CC, Mawhinney S, Kohrt WM, Campbell TB. Association of Functional Impairment with Inflammation and Immune Activation in HIV Type 1-Infected Adults Receiving Effective Antiretroviral Therapy. The Journal of infectious diseases. 2013 doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, Kirk GD. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PloS one. 2013;8:e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. Journal of the American Geriatrics Society. 2012;60:545–9. doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 63.Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nature reviews. Endocrinology. 2012;8:11–21. doi: 10.1038/nrendo.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leeansyah E, Cameron PU, Solomon A, Tennakoon S, Velayudham P, Gouillou M, Spelman T, Hearps A, Fairley C, Smit de V, Pierce AB, Armishaw J, Crowe SM, Cooper DA, Koelsch KK, Liu JP, Chuah J, Lewin SR. Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: a potential factor contributing to HIV-associated accelerated aging. The Journal of infectious diseases. 2013;207:1157–65. doi: 10.1093/infdis/jit006. [DOI] [PubMed] [Google Scholar]
- 65.Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, Samuels DC, Price DA, Chinnery PF. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nature genetics. 2011;43:806–10. doi: 10.1038/ng.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, Keller M, Grubeck-Loebenstein B, Simon A, Lambotte O, Hunt PW, Deeks SG, Costagliola D, Autran B, Sauce D. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–22. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 67.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, Weinstein MC, Seage GR, 3rd, Moore RD, Freedberg KA. The lifetime cost of current human immunodeficiency virus care in the United States. Medical care. 2006;44:990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 68.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, Gray KM, Cohen SM, Mermin J, Skarbinski J. Differences in Human Immunodeficiency Virus Care and Treatment Among Subpopulations in the United States. JAMA internal medicine. 2013:1–7. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 69.Levitt NS, Steyn K, Dave J, Bradshaw D. Chronic noncommunicable diseases and HIV-AIDS on a collision course: relevance for health care delivery, particularly in low-resource settings--insights from South Africa. Am J Clin Nutr. 2011;94:1690S–6S. doi: 10.3945/ajcn.111.019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maher D, Smeeth L, Sekajugo J. Health transition in Africa: practical policy proposals for primary care. Bull World Health Organ. 2010;88:943–8. doi: 10.2471/BLT.10.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mills EJ, Ford N. Political lessons from the global HIV/AIDS response to inform a rapid noncommunicable disease response. Aids. 2012;26:1171–3. doi: 10.1097/QAD.0b013e32834f3319. [DOI] [PubMed] [Google Scholar]
- 72.Rabkin M, Nishtar S. Scaling up chronic care systems: leveraging HIV programs to support noncommunicable disease services. J Acquir Immune Defic Syndr. 2011;57(Suppl 2):S87–90. doi: 10.1097/QAI.0b013e31821db92a. [DOI] [PubMed] [Google Scholar]
- 73.Balabanova D, Mills A, Conteh L, Akkazieva B, Banteyerga H, Dash U, Gilson L, Harmer A, Ibraimova A, Islam Z, Kidanu A, Koehlmoos TP, Limwattananon S, Muraleedharan V, Murzalieva G, Palafox B, Panichkriangkrai W, Patcharanarumol W, Penn-Kekana L, Powell-Jackson T, Tangcharoensathien V, McKee M. Good Health at Low Cost 25 years on: lessons for the future of health systems strengthening. Lancet. 2013;381:2118–33. doi: 10.1016/S0140-6736(12)62000-5. [DOI] [PubMed] [Google Scholar]
- 74.Samb B, Desai N, Nishtar S, Mendis S, Bekedam H, Wright A, Hsu J, Martiniuk A, Celletti F, Patel K, Adshead F, McKee M, Evans T, Alwan A, Etienne C. Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet. 2010;376:1785–97. doi: 10.1016/S0140-6736(10)61353-0. [DOI] [PubMed] [Google Scholar]
- 75.Barnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell ML. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis. 2011;11:942–51. doi: 10.1016/S1473-3099(11)70181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Callaghan M, Ford N, Schneider H. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. doi: 10.1186/1478-4491-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ford N, Mills EJ. Simplified ART delivery models are needed for the next phase of scale up. PLoS Med. 2011;8:e1001060. doi: 10.1371/journal.pmed.1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts T, Bygrave H, Fajardo E, Ford N. Challenges and opportunities for the implementation of virological testing in resource-limited settings. J Int AIDS Soc. 2012;15:17324. doi: 10.7448/IAS.15.2.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heidari S, Mofenson L, Cotton MF, Marlink R, Cahn P, Katabira E. Antiretroviral drugs for preventing mother-to-child transmission of HIV: a review of potential effects on HIV-exposed but uninfected children. Journal of acquired immune deficiency syndromes. 2011;57:290–6. doi: 10.1097/QAI.0b013e318221c56a. [DOI] [PubMed] [Google Scholar]
- 80.Bendavid E, Ford N, Mills EJ. HIV and Africa’s elderly: the problems and possibilities. Aids. 2012;26(Suppl 1):S85–91. doi: 10.1097/QAD.0b013e3283558513. [DOI] [PubMed] [Google Scholar]
- 81.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–5. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hontelez JA, Lurie MN, Newell ML, Bakker R, Tanser F, Barnighausen T, Baltussen R, de Vlas SJ. Ageing with HIV in South Africa. Aids. 2011;25:1665–7. doi: 10.1097/QAD.0b013e32834982ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLoS One. 2013;8:e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.WHO Policy on collaborative TB/HIV activities: Guidelines for national programmes and other stakeholders. World Health Organization; 2012. [PubMed] [Google Scholar]
- 85.Geneau R, Hallen G. Toward a systemic research agenda for addressing the joint epidemics of HIV/AIDS and noncommunicable diseases. Aids. 2012;26(Suppl 1):S7–10. doi: 10.1097/QAD.0b013e328355cf60. [DOI] [PubMed] [Google Scholar]
- 86.Rabkin M, Melaku Z, Bruce K, Reja A, Koler A, Tadesse Y, Kamiru HN, Sibanyoni LT, El-Sadr W. Strengthening Health Systems for Chronic Care: Leveraging HIV Programs to Support Diabetes Services in Ethiopia and Swaziland. J Trop Med. 2012;2012:137460. doi: 10.1155/2012/137460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Olmen J, Schellevis F, Van Damme W, Kegels G, Rasschaert F. Management of Chronic Diseases in Sub-Saharan Africa: Cross-Fertilisation between HIV/AIDS and Diabetes Care. J Trop Med. 2012;2012:349312. doi: 10.1155/2012/349312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sambo LG, Kirigia JM. Africa’s health: could the private sector accelerate the progress towards health MDGs? Int Arch Med. 2011;4:39. doi: 10.1186/1755-7682-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Mascio MD, Katlama C, Lafeuillade A, Landay A, Lederman M, Lewin SR, Maldarelli F, Margolis D, Markowitz M, Martinez-Picado J, Mullins JI, Mellors J, Moreno S, O’Doherty U, Palmer S, Penicaud MC, Peterlin M, Poli G, Routy JP, Rouzioux C, Silvestri G, Stevenson M, Telenti A, Lint CV, Verdin E, Woolfrey A, Zaia J, Barre-Sinoussi F. Towards an HIV cure: a global scientific strategy. Nature reviews. Immunology. 2012 doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 91.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 92.Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, Eisele E, Haase A, Ho YC, Hutter G, Justement JS, Keating S, Lee TH, Li P, Murray D, Palmer S, Pilcher C, Pillai S, Price RW, Rothenberger M, Schacker T, Siliciano J, Siliciano R, Sinclair E, Strain M, Wong J, Richman D, Deeks SG. Challenges in Detecting HIV Persistence during Potentially Curative Interventions: A Study of the Berlin Patient. PLoS pathogens. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, Gallien S, Lin NH, Giguel FF, Lavoie L, Ho VT, Armand P, Soiffer RJ, Sagar M, Lacasce AS, Kuritzkes DR. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. The Journal of infectious diseases. 2013;207:1694–702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS pathogens. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]