Abstract
Objectives
HIV ‘treatment as prevention’ (TasP) describes early treatment of HIV-infected patients intended to reduce viral load (VL) and transmission. Crucial assumptions for estimating TasP's effectiveness are the underlying estimates of transmission risk. We aimed to determine transmission risk during primary infection, and of the relation of HIV transmission risk to VL.
Design
Systematic review and meta-analysis.
Methods
We searched PubMed and Embase databases for studies that established a relationship between VL and transmission risk, or primary infection and transmission risk, in serodiscordant couples. We analyzed assumptions about the relationship between VL and transmission risk, and between duration of primary infection and transmission risk.
Results
We found 36 eligible articles, based on six different study populations. Studies consistently found that larger VLs lead to higher HIV transmission rates, but assumptions about the shape of this increase varied from exponential increase to saturation. The assumed duration of primary infection ranged from 1.5 to 12 months; for each additional month, the log10 transmission rate ratio between primary and asymptomatic infection decreased by 0.40.
Conclusions
Assumptions and estimates of the relationship between VL and transmission risk, and the relationship between primary infection and transmission risk, vary substantially and predictions of TasP's effectiveness should take this uncertainty into account.
Keywords: HIV, Transmission, Viral Load, Acute Disease, Primary infection, Systematic Review, Treatment as prevention
Introduction
Treatment as prevention (TasP) is a promising approach to curbing the HIV epidemic, but estimates of its effectiveness vary. A crucial assumption in models estimating the effectiveness of TasP is the high rate of HIV transmissions during primary HIV infection. How much primary infection contributes to overall transmission is a matter of debate [1], and estimates of the proportion of HIV transmission during the primary phase range from 2% to 90% [2]. Powers et al recently argued that primary infection is a major driver of the epidemic, causing up to 40% of new infections [3]; a rate that may be high enough to compromise TasP-strategies. Williams et al, however, suggested that only 2% of all HIV transmissions occur during primary infection [4], which suggests that the epidemic could be ended with TasP.
Transmission during primary infection has been estimated directly (from serodiscordant couples with recently infected index partners) and indirectly (using high viral load values during primary infection as a proxy for primary infection). Combining evidence from two studies, one using direct and one indirect estimation of transmission, Hollingsworth et al [5] concluded that the indirect approach underestimates rates of transmission during primary infection.
We conducted a systematic literature review to analyze direct and indirect estimates of transmission during primary infection and compare the results of the two approaches to determine if the measured viral load values could explain observed transmission during primary infection.
Methods
Search strategy, eligibility criteria and study selection
We systematically searched Medline and Embase databases on March 7th 2012, using free text words and Medical Subject Headings in Pubmed and Emtree-terms in Embase and variations of the following search terms: HIV, transmission rate, transmission probability, viral load and primary infection. We also examined the references of included papers. Our detailed search strategy is shown in the web appendix. Eligible studies assessed the risk of HIV transmission based on viral load values, or compared HIV transmission during the primary and the asymptomatic stage of infection. We included studies of serodiscordant couples and systematic reviews of such studies. We excluded studies of non-human populations and studies on non-sexual transmission (e.g., mother-to-child transmission, blood transfusion). Two reviewers independently screened abstracts and selected full text articles in accord with the above criteria. Disagreements were resolved by consensus.
Data collection
Two reviewers used a standardized sheet to extract the following: 1) characteristics of the study (study locations, study period, follow-up time, monitoring frequency, inclusion/exclusion criteria, number of participants); 2) characteristics of couples (sex and age of both partners, viral load, CD4 cell count, and ART status of index partner); 3) factors that determine the risk of HIV transmission (sexually transmitted diseases of both partners, circumcision status of male partner, reported number of unprotected sex acts); and, 4) the relation between HIV transmission and viral load values and stage of infection. We resolved disagreements by consensus.
Standardization of study results
We standardized the unit of transmission risk to compare the results from different studies: we report transmission rates per year or transmission rate ratios. Transmission probabilities per sex act were transformed into transmission rates. We assumed an average of four sex acts per month, which is consistent with previous studies [6]. Sexual activity was assumed to be independent of viral load and phase of infection. The transmission rate per year r is therefore related to the per act transmission probability p by the formula
wheren = 48 is the average number of sex acts per year. A detailed description of these calculations is shown in the web appendix. All analyses were done in R version 2.14.2 [7].
Meta-analysis of two types of studies
We separately analyzed the relationship between viral load and transmission risk, and the relationship between primary infection and transmission risk. We compared results of these separate analyses to determine if high viral load values could explain the high transmission risk during primary infection.
1) Relationship between viral load and transmission risk
For each study, we described the relationship between HIV transmission rates per year and log10 viral load. We graphically compared different patterns of this relationship (e.g. proportional hazards models, step functions, saturating curve). We conducted a random-effects meta-analysis of the transmission hazard ratio per log10 increase in viral load. If multiple studies were conducted on the same study population, we included the largest study in the meta-analysis. As a sensitivity analysis, we re-analyzed data from Fideli et al [8] to separate the heterogeneity caused by different assumptions and the heterogeneity caused by different data.
2) Relationship between primary infection and transmission risk
We extracted the assumed duration of primary infection, and calculated corresponding transmission rate ratios between primary and asymptomatic infection. The result was presented graphically. For one study population (Rakai [9]), which had been analyzed with different assumed durations of the primary infection, we used a regression model to relate the rate ratio to the assumed duration of primary infection.
3) Viral load and primary infection compared
We compared estimated transmission rates based on viral load values (approach 1) with observed transmission rates during primary infection (approach 2), as follows: In approach 1, we assumed the viral load was 3 log10 higher during the primary infection than during the asymptomatic infection, an assumption consistent with a previous study by Pilcher et al. [10] in which it was assumed that viral load values remained elevated for two months. Based on our meta-analysis of the relationship between viral load and transmission risk, we calculated the estimated increase in transmission during primary infection based on viral load values. We used the regression model of the relationship between primary infection and transmission risk to calculate the number of expected transmissions during primary infection (again two months), and then compared that number to the number of expected transmissions during the asymptomatic phase. We also used other association patterns (i.e. step functions and saturating curves) to describe the relation between viral loads and HIV transmission.
Results
Identification of relevant studies
We found 788 potentially eligible articles (765 from search and 23 from references) and included 34 in our analysis. Of 788 potentially eligible articles, we excluded 173 duplicates and 515 that were conducted in non-human populations, did not focus on sexual HIV transmission, studied other diseases than HIV, were case reports, or did not report on individual viral load values. We excluded 64 more studies that were not based on serodiscordant couples (n=39), did not calculate a transmission rate or probability based on viral load or primary infection (n=20), or reported results from previous studies (n=5). Twenty articles described the relationship between viral load level and risk of HIV transmission during the asymptomatic phase of HIV infection; 12 articles compared the risk of HIV transmission during primary infection to the risk during the asymptomatic phase, but did not consider viral load levels; four studies reported on both (Figure 1). The 20 articles that used primary data were based on only six different primary study populations of serodiscordant couples: the European Study Group on Heterosexual Transmission of HIV, the Medical Research Council Programme on AIDS in Uganda, a cohort study in Tanzania, the Partners in Prevention HSV/HIV Transmission Study Team in seven countries in Eastern and Southern Africa, the Zambian HIV Research Project, and the Rakai Project Study Group in Uganda (Table 1). Thirteen of the included articles were reviews and meta-analyses, and another three articles estimated the effect of primary infection on HIV transmission from a mathematical model fitted to HIV prevalence data during the early epidemic.
Figure 1. Identification and selection of studies.
N: number of studies, VL: viral load, PI: primary infection
Table 1.
Characteristics of included studies
| Author (publication year) | Time period | Serodsicordant couples | Person-years of follow-up | Number of transmissions | Transmission risk depends on VL/PI | Transmission rate r(x) as a function of x=log10(VL) | Duration of primary infection (days) | Transmission rate ratio between primary and asymptomatic infection |
|---|---|---|---|---|---|---|---|---|
| European Study Group on Heterosexual Transmission of HIV | ||||||||
| Leynaert (1998) | 1987-1992 | 499 | NA | 94 | PI | 90 | 3.15 | |
| Medical Research Council Programme on AIDS in Uganda | ||||||||
| Carpenter (1999) | 1989-1999 | 29 | 85 | 6 | PI | 365 | 2.4 | |
| Tanzania cohort study | ||||||||
| Hugonnet (2002) | 1991-1995 | 61 | 122 | 9 | PI | 365 | 2.4 | |
| Partners in Prevention HSV/HIV Transmission Study Team | ||||||||
| Baeten (2010) | 2004-2008 | 1096 | 1685 | 64 | VL | step function | ||
| Baeten (2011) | 2004-2008 | 2521 | 3509 | 113 | VL | step function, r(x) = c*bx | ||
| Celum (2010) | 2004-2007 | 3360 | 4868 | 132 | VL | step function | ||
| Donnell (2010) | 2004-2008 | 3381 | 4467 | 142 | VL | step function | ||
| Hughes (2012) | 2004-2008 | 3297 | NA | 86 | VL | r(x) = c* bx | ||
| Lingappa (2010) | 2004-2008 | 3381 | 4756 | 108 | VL | step function, r(x) = c* bx | ||
| Rakai Project Study Group | ||||||||
| Gray (2000) | 1994-1998 | 411 | 752 | 89 | VL | step function | ||
| Gray (2001) | 1994-1998 | 174 | 328 | 38 | VL | step function | ||
| Kiwanuka (2009) | 1997-2002 | 268 | 812 | 92 | VL/PI | r(x) = c* bx | 365 | 0.8 |
| Quinn (2000) | 1994-1998 | 415 | 760 | 90 | VL | step function, r(x) = c* bx | ||
| Wawer (2005) | 1994-1999 | 235 | 558 | 68 | VL/PI | step function | 150 | 11.71 |
| Zambia HIV Research Project | ||||||||
| Fideli (2001) | 1994-2000 | 317 | NA | 109 | VL | step function, r(x) = c* bx | ||
| Malhotra (2011) | 1995-2006 | 567 | NA | 240 | VL | r(x) = c* bx | ||
| Merino (2011) | 1995-2006 | 566 | 1697 | 240 | VL | r(x) = c* bx | ||
| Song (2011) | 1995-2006 | 568 | NA | 240 | VL | r(x) = c*b^x | ||
| Tang (2004) | 1995-2002 | 292 | NA | 141 | VL | step function | ||
| Tang (2008) | 1995-2006 | 429 | 1336 | 205 | VL | step function | ||
| No primary data | ||||||||
| Attia (2009) | step function | |||||||
| Boily (2009) | 90-150 | 9.17 | ||||||
| Chakraborty (2001) | r(x) = −n * log(1-c*b^x) | |||||||
| Corey (2004) | step function | |||||||
| Fox (2011) | step function | 150 | 4.98 | |||||
| Fraser (2007) | Hill function | 180 | NA | |||||
| Gisselquist (2005) | not reported | NA | ||||||
| Hollingsworth (2008) | 87 | 26.04 | ||||||
| Jacquez (1994) | 60 | NA | ||||||
| Modjarrad (2008) | r(x) = c*b^x | |||||||
| Pilcher (2004) | 54 | NA | ||||||
| Pinkerton (2008) | 49 | 43.68 | ||||||
| Powers (2008) | 90-150 | 6.59 | ||||||
| Shiboski (1992) | not reported | NA | ||||||
| Shiboski (1998) | 690 - 1530 | NA | ||||||
| Wilson (2008) | r(x) = −n * log(1-c*b^x) | |||||||
VL: viral load, PI: primary infection; Step function: , Hill function: ; z = 10x.
Study populations
The six study populations consisted of heterosexual HIV-serodiscordant couples. Studies included between 29 and 3381 couples and observed between 6 and 426 transmissions (Table 1) and were conducted after 1987, in different samples of the general population. In the Partners in Prevention study group, index partners were Herpes Simplex virus (HSV-)-2 seropositive and had a CD4 count of at least 250 cells/μl. Other inclusion criteria were married or cohabiting couples, age ranges, known circumcision status, minimum follow-up duration, reported monogamy, reported unprotected sex, or a high risk of HIV-infection.
Relationship between viral load and transmission risk
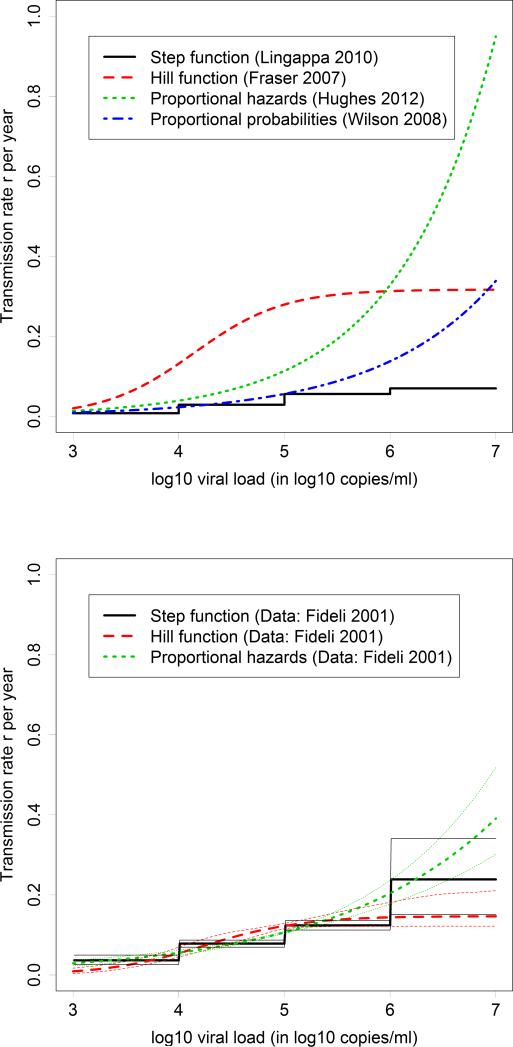
Most studies made one of three different assumptions about the relationship between HIV viral load values and the risk of HIV transmission (Table 1): 1) step functions where viral load values are grouped into categories [6, 8, 9, 11-22]; 2) Cox proportional hazards models where the transmission rate r depends on the log10 viral load x according to the formula (x)= c*b^x, with constants b and c [6, 8, 19, 20, 23-28]; and, 3) a variation of the Cox proportional hazards model in which the per-act transmission probability was assumed to be proportional instead of the rate, which results in the function r(x)=-n*log(1-c*b^x) for the transmission rate, with constants b and c and annual number of sex acts n [29, 30]. For high viral load values, Cox models assume that HIV transmission rates will continue to increase. Step functions assumed a constant transmission rate for high viral loads or reported transmission rates only for log10 viral load values below 7. Fraser et al [31] assumed a saturating curve (Hill function). Figure 2 (top panel) illustrates the different patterns, using the most recent study in each category. While transmission rates all increase with higher viral load values, their shapes are quite different, and differences become more pronounced for high viral load values. Figure 2 (bottom panel) shows the results of a re-analysis of data by Fideli et al, which illustrates that the heterogeneity between models persists for high viral load values when analyzing the same data.
Figure 2. Examples of the four assumed relationships between viral load and transmission rates.
Top panel: The most recent study of each example is presented. Bottom panel: Re-analysis of the data by Fideli et al, which shows that the heterogeneity in transmission risk for lower viral load values is mostly due to different data sources, but for high viral load values the heterogeneity is also due to different assumptions.
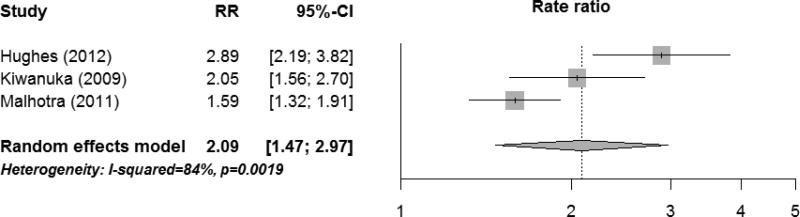
Cox proportional hazard models assume a constant increase in risk of HIV infection per log10 increase in viral load. Across the nine studies, the HIV transmission rate ratio per log10 increase in viral load ranged from 1.5 to 2.9 (Figure S2). Our meta-analysis of the largest studies of each study population (n=3) resulted in a combined rate ratio of 2.09 (95%CI: 1.47-2.97) per log10 increase in viral load (Figure 3).
Figure 3. Meta-analysis of transmission rate ratio per log10 increase in viral load.
For each study population, we used the largest study reporting rate ratio per log10 increase in viral load. Figure S2 summarizes all studies on rate ratio per log10 increase.
Relationship between primary infection and transmission risk
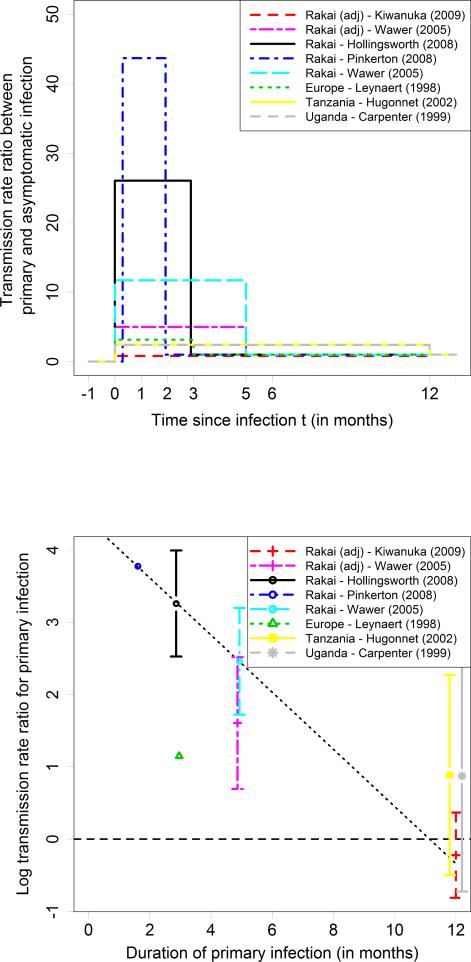
The assumed duration of primary infection varied from 1.5 to 12 months; corresponding HIV transmission rate ratios that compared primary with asymptomatic infection varied from 43.7 to 0.8 (Table 3; Figure 4, top panel). Two studies [5, 32] estimated both duration and rate ratio. Based on the Rakai data, Hollingsworth et al found that primary infection lasted 87 days, and Pinkerton et al found that it lasted 49 days. Studies that assumed primary infection lasted for a year consistently found that it had little effect on transmission rates. For the Rakai study we found a linear relationship between duration of primary infection and log rate ratio: each month increase in duration of the primary infection decreased the log rate ratio between primary and asymptomatic infection by 0.40 (Figure 4, bottom panel).
Figure 4. Duration of primary infection and transmission rate ratio between primary and asymptomatic infection.
Top panel: Transmission rates in the first year after infection. Bottom panel: Transmission rate ratio between primary and asymptomatic infection and linear regression for Rakai studies that did not adjust for viral load values.
Viral load and primary infection compared
Viral load values during primary infection do not entirely explain the higher risk of HIV transmission during this period. Our meta-analysis (Figure 3) shows that a 3 log10 increase in viral load values (consistent with Pilcher et al [10] who assumed that the primary infection lasts for 2 months) corresponds to a 9.1-fold higher risk of HIV transmission during primary infection than during the asymptomatic period (=2.09^3). If we use the highest reported rate ratio of 2.89 [23], the transmission rate ratio between primary and asymptomatic infection reaches 24 (=2.89^3). When we assumed other relationships between viral load and transmission rates (e.g. saturating curves) the estimate was lower.
In contrast, observed HIV transmissions during primary infection were higher than we expected based on viral load values. Based on the meta-regression (Figure 4, bottom panel), the rate ratio for a primary infection of two months was 37 (=e^3.61).
Discussion
The relationship between viral load values and HIV transmission rate was described in four different ways: step functions, Cox proportional hazard models, proportional probability models and Hill functions. Risk of HIV transmission increased with viral load values, but for high viral load values the pattern of the relationship is unclear. For high viral load values, transmission rates across the studies ranged from saturating to exponentially increasing. Between studies, assumed duration of primary infection varied from 1.5 to 12 months. Studies that assumed longer primary infections estimated lower rates of HIV transmission during primary infection. Predictions based on high viral load values alone underestimated the risk of HIV transmission during primary infection.
The discrepancy in transmission risk between studies that focused on viral load and studies that focused on primary infection could be because viral load values were measured during the asymptomatic phase, when the virus may be less infectious [33]. We could not determine if viral load was measured during primary or asymptomatic infection. High estimated transmission risk during primary infection might also be caused by co-infections [5, 9, 32], greater susceptibility in newly exposed uninfected partners [9], or biological differences between strains [32]. None of these hypotheses has been tested.
The high risk of HIV transmission during primary infection (beyond what may be explained by high viral load values during primary infection) is supported by Wawer et al [9]. After adjusting for viral load values, Wawer et al found that HIV transmission rates during primary infection was 4.98 (95% CI: 2.00-12.39) times higher than during asymptomatic infection. In contrast, Kiwanuka et al [24] assumed that primary infection lasted a year and estimated that the transmission rate ratio adjusted for viral load was 0.8 (0.4-1.4). Other authors [34, 35] used different methods to determine the influence of primary infection on the HIV epidemic. For instance, Brenner et al [34] used phylogenetic clustering analysis and found that early infection accounted for approximately half of onward transmissions.
Earlier meta-analyses compared HIV transmission during primary and asymptomatic infection without accounting for different durations of primary infection. Boily et al and Powers et al [36, 37] meta-analyzed the estimated rates during primary infection from the Rakai study and the European study group (with different durations of primary infection), and found that transmission rates were 6.6 and 9.2 times higher during primary infection than in the asymptomatic period. The Rakai study and the European study are difficult to compare because they are designed differently. The Rakai study included couples that started out sero-negative, and in which one partner seroconverted during follow-up. Leynaert et al [38], estimated time of infection based on decline in CD4 cell counts, and thus was less reliable than the Rakai study. Though the Rakai study consisted of only 23 couples and transmissions were not genetically confirmed, it is still the best data to assess transmission rates during primary infection. Whether this study provides solid evidence about the transmission rate during primary infection is a matter of debate [1]. Studies by Hugonnet et al [39] and by Carpenter et al [40] estimated transmission rates during primary infection under the assumption that primary infection lasted a year. These two studies might be interesting to reanalyze with different durations.
Several models assessed the effect of a test and treat strategy on HIV transmission, with conflicting results. The model used by Granich et al [41] showed that, in a high HIV prevalence setting, annual HIV testing and immediate treatment could reduce HIV incidence to below one per 1000 person-years. Powers et al [3] showed that the proposed intervention (annual HIV testing and immediate treatment) did not reduce HIV transmission as much as Granich et al had estimated. The validity of these findings and the differences between them were discussed at length [1, 4, 42]. Granich et al assumed a rate ratio of ten for the first two months, while Powers et al assumed a risk ratio of 30.3 over 4.8 months. Our review suggests that these assumptions are opposite extremes: the rate ratio of ten would be appropriate if primary infection lasted for six months instead of two, while a rate ratio of 30 would be reasonable if the primary infection lasted for two months instead of 4.8. Although we are not sure how long primary infection lasts, our review shows that duration and transmission rate during this period are negatively associated and bivariate estimates are necessary.
We standardized transmission risk before we analyzed the data to compare the results of different studies. We thus made assumptions about sexual behavior, which are probably inaccurate. We could not account for changes in sexual behavior patterns over time [43] or inaccurate self-reporting of sexual contacts [44]. Because we used transmission rates per year instead of transmission probabilities per sex act, our assumptions about sexual behavior did not substantially affect the results. Moreover, we only used these assumptions in five of 24 viral load studies, which reported probabilities instead of rates. For primary infection studies we report rate ratios, and these are less sensitive to sexual behavior than rates. We acknowledge these additional limitations: Most of the studies that used a step function stratified risk of HIV transmission according to variables (e.g. circumcision status, female to male versus male to female transmission, baseline CD4 value, baseline age), which made comparisons difficult. The number of steps and change points also varied between studies. Studies used different inclusion criteria. There was some heterogeneity in HIV transmission between studies. Transmission rates in the Partners in Prevention study were lower than in the Rakai study. It is unlikely that the functional dependence between transmission rate and viral load values depends on different transmission rates between studies. In a sensitivity analysis, where we analyzed data from Fideli et al [8] under different assumptions about the relationship between viral load and transmission rates, we found that heterogeneity was smaller, but remained substantial.
Ours is the first review to consider assumed durations of primary infection. The strength of our study is its unique design: we systematically reviewed the literature and analyzed rates of HIV transmission based on different relationships between viral load and transmission rate. Our viral load models were not restricted to one parametric form, and we could fully compare estimates of transmission rates for high viral loads. We analyzed rates of HIV transmission for different durations of primary infection. This is an improvement over previous reviews that meta-analyzed transmission rates without adjusting for the underlying assumptions about the duration of primary infection.
Reliable estimates of transmission rates for high viral loads are urgently needed. All studies were based on six primary data sources. Collecting more primary data on HIV-negative couples that seroconvert, and on serodiscordant couples will improve estimates. Current studies capture additional transmission risk for an assumed duration of primary infection, but accurate estimation of this duration requires more complete data on initially seronegative couples. Available primary data on viral load should be reanalyzed to estimate the shape of the increase in transmission rates when viral load increases.
Conclusion
The relation between viral load values and HIV transmission, and the duration and amount of transmission during primary infection are poorly understood. HIV transmission risk increases as viral load values increase, but the pattern of this relationship is unclear. If transmission rates increase exponentially with viral load values, then finding and treating patients with high viral load values should be a public health priority [45]. But if the function is saturating, then TasP may not reduce HIV incidence. Estimates of the relationship between HIV transmission risk and viral load, and the contribution of primary infection diverge because researchers make different assumptions about the shape of the relationship between transmission rate and viral load, and the duration of primary infection. TasP is widely advocated as a public health approach for HIV prevention, but we cannot assess its effectiveness until HIV transmission rates in the primary phase, and effect of viral load on transmission, are accurately described.
Supplementary Material
Acknowledgments
We would like to thank K. Tal for her editorial assistance.
This work was supported by the National Institute of Allergy and Infectious Diseases [5U01-AI069924–05] and the Swiss National Science Foundation [Prosper grant 32333B_131629 to O.K. and ProDoc PhD grant PDFMP3_137106 to N.B. and O.K.].
Footnotes
Conflicts of Interest and Sources of Funding:
The authors have no conflicts of interest to declare.
Author Contributions: Conceived and designed the systematic review: NB, CW, ME, OK. Performed data extraction: NB, CW, JE, LSV. Analyzed the data: NB. Wrote the manuscript: NB, OK. Revised the manuscript: GW, ME. All authors contributed to the interpretation of the results and to the final version of the manuscript.
References
- 1.Cohen MS, Dye C, Fraser C, Miller WC, Powers KA, Williams BG. HIV treatment as prevention: debate and commentary--will early infection compromise treatment-as-prevention strategies? PLoS Med. 2012;9:e1001232. doi: 10.1371/journal.pmed.1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams BG, Granich R, Dye C. Role of acute infection in HIV transmission. Lancet. 2011;378:1913. doi: 10.1016/S0140-6736(11)61832-1. author reply 1914-1915. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. Journal of Infectious Diseases. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 6.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Science Translational Medicine. 2011;3::77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: 2012. [Google Scholar]
- 8.Fideli US, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. Journal of Infectious Diseases. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 10.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. Aids. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: Systematic review and meta-analysis. Aids. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 12.Baeten JM, Donnell D, Kapiga SH, Ronald A, John-Stewart G, Inambao M, et al. Male circumcision and risk of male-to-female HIV-1 transmission: A multinational prospective study in African HIV-1-serodiscordant couples. Aids. 2010;24:737–744. doi: 10.1097/QAD.0b013e32833616e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. The Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox J, White PJ, Weber J, Garnett GP, Ward H, Fidler S. Quantifying sexual exposure to HIV within an HIV-serodiscordant relationship: Development of an algorithm. Aids. 2011;25:1065–1082. doi: 10.1097/QAD.0b013e328344fe4a. [DOI] [PubMed] [Google Scholar]
- 17.Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, Mangen FW, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. Aids. 2000;14:2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 18.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 19.Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS ONE. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England Journal of Medicine. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Penman-Aguilar A, Lobashevsky E, Allen S, Kaslow RA. HLA-DRB1 and -DQB1 alleles and haplotypes in Zambian couples and their associations with heterosexual transmission of HIV type 1. J Infect Dis. 2004;189:1696–1704. doi: 10.1086/383280. [DOI] [PubMed] [Google Scholar]
- 22.Tang J, Shao W, Yoo YJ, Brill I, Mulenga J, Allen S, et al. Human leukocyte antigen class I genotypes in relation to heterosexual HIV type 1 transmission within discordant couples. J Immunol. 2008;181:2626–2635. doi: 10.4049/jimmunol.181.4.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiwanuka N, Laeyendecker O, Quinn TC, Wawer MJ, Shepherd J, Robb M, et al. HIV-1 subtypes and differences in heterosexual HIV transmission among HIV-discordant couples in Rakai, Uganda. Aids. 2009;23:2479–2484. doi: 10.1097/QAD.0b013e328330cc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra R, Hu L, Song W, Brill I, Mulenga J, Allen S, et al. Association of chemokine receptor gene (CCR2-CCR5) haplotypes with acquisition and control of HIV-1 infection in Zambians. Retrovirology. 2011;8:22. doi: 10.1186/1742-4690-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merino A, Malhotra R, Morton M, Mulenga J, Allen S, Hunter E, et al. Impact of a functional KIR2DS4 allele on heterosexual HIV-1 transmission among discordant Zambian couples. J Infect Dis. 2011;203:487–495. doi: 10.1093/infdis/jiq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. Aids. 2008;22:2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song W, He D, Brill I, Malhotra R, Mulenga J, Allen S, et al. Disparate associations of HLA class I markers with HIV-1 acquisition and control of viremia in an African population. PLoS ONE. 2011;6:e23469. doi: 10.1371/journal.pone.0023469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: A probabilistic empiric model. Aids. 2001;15:621–627. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 30.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. The Lancet. 2008;372:314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 31.Fraser C, Hollingsworth TD, Chapman R, De Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: Epidemiological analysis and an evolutionary hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinkerton SD. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS and Behavior. 2008;12:677–684. doi: 10.1007/s10461-007-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma ZM, Stone M, Piatak M, Jr., Schweighardt B, Haigwood NL, Montefiori D, et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83:3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 35.Pao D, Fisher M, Hue S, Dean G, Murphy G, Cane PA, et al. Transmission of HIV-1 during primary infection: relationship to sexual risk and sexually transmitted infections. Aids. 2005;19:85–90. doi: 10.1097/00002030-200501030-00010. [DOI] [PubMed] [Google Scholar]
- 36.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leynaert B, Downs AM, De Vincenzi I. Heterosexual transmission of human immunodeficiency virus: Variability of infectivity throughout the course of infection. American Journal of Epidemiology. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 39.Hugonnet S, Mosha F, Todd J, Mugeye K, Klokke A, Ndeki L, et al. Incidence of HIV infection in stable sexual partnerships: a retrospective cohort study of 1802 couples in Mwanza Region, Tanzania. J Acquir Immune Defic Syndr 2002. 30:73–80. doi: 10.1097/00042560-200205010-00010. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter LM, Kamali A, Ruberantwari A, Malamba SS, Whitworth JA. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. Aids. 1999;13:1083–1089. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- 41.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 42.Hayes RJ, White RG. Role of acute infection in HIV transmission. Lancet. 2011;378:1913–1914. doi: 10.1016/S0140-6736(11)61833-3. author reply 1914-1915. [DOI] [PubMed] [Google Scholar]
- 43.Hargreaves JR, Slaymaker E, Fearon E, Howe LD. Changes over time in sexual behaviour among young people with different levels of educational attainment in Tanzania. J Int AIDS Soc. 2012;15(Suppl 1):1–7. doi: 10.7448/IAS.15.3.17363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JL, Sales JM, DiClemente RJ, Salazar LF, Vanable PA, Carey MP, et al. Predicting discordance between self-reports of sexual behavior and incident sexually transmitted infections with African American female adolescents: results from a 4-city study. AIDS Behav. 2012;16:1491–1500. doi: 10.1007/s10461-012-0163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novitsky V, Essex M. Using HIV viral load to guide treatment-for-prevention interventions. Curr Opin HIV AIDS. 2012;7:117–124. doi: 10.1097/COH.0b013e32834fe8ff. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.