Abstract
Aims. To evaluate the effect of Punica granatum (Pg) rind extract and its spray dried biopolymeric dispersions with casein (F1) or chitosan (F2) against Diabetes mellitus (DM) and diabetic neuropathy (DN). Methods. We measured the acute (6 h) and subacute (8 days) effect of various doses of Pg, F1, and F2 and the active compounds on alloxan-induced DM mouse model. We evaluated DN utilizing latency tests for longer period of time (8 weeks). In addition, the in vivo antioxidant activity was assessed utilizing serum catalase level. Results. The results proved that the highest dose levels of Pg extract, F1, F2 exerted remarkable hypoglycemic activity with 48, 52, and 40% drop in the mice glucose levels after 6 hours, respectively. The tested compounds also improved peripheral nerve function as observed from the latency tests. Bioguided fractionation suggested that gallic acid (GA) was Pg main active ingredient responsible for its actions. Conclusion. Pg extract, F1, F2, and GA could be considered as a new therapeutic potential for the amelioration of diabetic neuropathic pain and the observed in vivo antioxidant potential may be involved in its antinociceptive effect. It is highly significant to pay attention to Pg and GA for amelioration and control of DM and its complications.
1. Introduction
Diabetes mellitus (DM) is a major endocrine disorder and the global annual cost of treating DM and its complication could reach US $ trillion [1]. Amelioration of DM is a high priority in medical research. Self-management of DM is cornerstone to achieving good glycemic control and reducing the risk of developing microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (cardiovascular and cerebrovascular) complications [2]. Natural extracts have been used in management of diabetes and its related complications because they are safe and readily available [3]. Many plant species are used in folk medicine for their hypoglycemic properties and therefore potentially used for treatment of DM [4].
Punica granatum (P. granatum) L., universally known as pomegranate, from family Punicaceae, is a distinctive fruit bearing plant with various medicinal and dietary importance, native to the Middle East. P. granatum has shown to possess free radical scavenging, anticarcinogenic, anti-inflammatory, and effectiveness in the treatment of cancer, cardiovascular disease, Alzheimer's disease, arthritis, and erectile dysfunction [5]. Folk medicine suggests some possible benefits of P. granatum to diabetes and some related complications. Some in vitro studies were done to show the antihyperglycemic effect of P. granatum [6, 7]. The available research suggests that ellagic acid is the effective antihyperglycemic compound in P. granatum [8]. None of these investigations were done to show the antinociceptive effect of P. granatum on diabetic neuropathy (DN).
Oxidative stress, mediated mainly by hyperglycemia-induced generation of free radicals, helps in the development and progression of DM and its complications [4]. Diabetic peripheral neuropathy, which is one of the most frequent long-term complications of DM, is frequently accompanied with inferior quality of life [9]. This complication occurs in about one-quarter of diabetic patients [10]. Painful diabetic neuropathy is associated with symptoms and signs such as burning, tingling, or lancing type of spontaneous pain, allodynia, and hyperalgesia [11]. Thus, novel therapeutic targets are required for the satisfactory treatment of diabetic neuropathic pain [9, 12]. Abnormal free radicals high levels cause membrane damage leading to decline of antioxidant defense mechanisms causing cell and tissue damage [13]. The recent strategy for alleviating the oxidative damage in DM is based on supplementation with certain dietary antioxidants such as vitamins E and C and flavonoids [14].
Due to its unique physicochemical properties, casein, a natural biopolymeric surfactant, has a potential use in the preparation of conventional and novel pharmaceutical drug delivery systems. A number of in vitro and in vivo studies showed that casein is a suitable material for efficient drug delivery. Researchers have noted that casein has evolved to be easily degradable by the digestive enzymes proteases. Casein microspheres, either plain or cross-linked, could be promising parenteral biodegradable carriers for sustained delivery of drugs when administered via intramuscular, intraperitoneal, or direct intratumoral or intra-arterially for embolization in solid tumor deposits [15].
Chitosan, another biodegradable polymer, is a linear polysaccharide composed of randomly distributed (1–4)-linked d-glucosamine and N-acetyl-d-glucosamine [16]. Studies on the possible biomedical applications of chitosan have been adapted due to its low cytotoxicity, biodegradability, and antimicrobial activity [17–19].
Therefore, the aim of the present work involves the study of possible hypoglycemic, in vivo antioxidant, and diabetic neuropathy management effects of ethanolic rind extract of P. granatum and its biodegradable polymeric dispersions with either casein or chitosan.
2. Materials and Methods
2.1. Materials
Dried P. granatum L. fruit rind was purchased commercially from Ibn-Al-Nafess herbalist, Beirut, Lebanon. The solvents used in extraction and standards, alloxan, casein and chitosan (150 kDa) were purchased from Sigma-Aldrich (St. Louis, USA). Glibenclamide (GB) was commercially purchased (Lansa Pharm., China). Tramadol hydrochloride was obtained commercially (Deutsche Labs, Germany). All other chemicals were analytical grade chemicals.
2.2. Preparation of Plant Extract
The dried rind was extracted using 80% ethanol. The ethanolic extract was dried in a rotary evaporator (Buchi, Germany) at temperature 40°C under vacuum. The rind was authenticated with a reference sample, and a voucher specimen (PS-13-11) was deposited in the faculty herbarium.
2.3. Preparation of the Spray Dried Polymeric Dispersions
Casein was dissolved in 0.1 N NaOH in a concentration of 1.5% w/w. The partially dried extract (soft extract) of P. granatum was then added and mixed using a magnetic stirrer to have an extract/polymer ratio of 1 : 1. The polymeric dispersion was then neutralized with a few drops of 1 N HCl till a pH of 7.4 before feeding into LabPlant Mini-Spray dryer SD-06AG (LabPlant, UK). Inlet temperature was adjusted at 165°C and the outlet at 75–80°C. The dried product (F1) was collected and kept in a desiccator till further testing.
For chitosan dispersions (F2), chitosan solution (1.5% w/w) was prepared in 1% acetic acid and the soft extract was added to the solution to have an extract/polymer ratio of 1 : 1. The dispersion was then fed into the spray dryer as described above. The dried products were collected and kept in desiccators till further testing. Placebo dispersions of the polymers were prepared for control testing.
At the used extract/polymer ratios, the produced powders should have 1 mg Pg extract/2 mg of either of the dried formula (that the given Pg extract doses of 25, 50, and 100 mg/kg are equivalent to a powder dose; of either F1 or F2; of 50, 100, and 200 mg/kg, resp.).
2.4. HPLC Analysis of P. granatum Ethanolic Extract
Chromatographic optimization was done using the HPLC conditions and the investigation of columns with different packing materials, different wavelengths from 200 nm to 400 nm, and different mobile phase systems. An ideal chromatographic separation was achieved using RP-C18 endcapped Lichrospher column (250 × 4.6 mm I. D.; 5 μM particle size) (Merck), at 40°C [20]. Isocratic elution was performed using (43 : 57) methanol/phosphate buffer 34.1 mM and pH 2.1. The flow rate was adjusted to 1 mL/min.
2.5. Determination of Total Phenolic Compounds in the Extract and Spray Dried Polymeric Dispersions
Generally, measurement of color occurred by reaction between Folin-Ciocalteu's phenol reagent [21, 22], and extract is a preferred method for the determination of the phenolic compounds, because the majority of their ingredients are polyphenols [21, 23]. Total contents of the phenolic compounds in the extracts were determined by Folin-Ciocalteu's method [22] as gallic acid equivalents (GAE) [23, 24]. In brief, 250 μL of Folin-Ciocalteu's phenol reagent was mixed with 50 μL of the samples, and 500 μL of 20% water solution of Na2CO3 was added to the mixture. Mixtures were vortexed and completed with water to 5 mL. As control, reagent without addition of extract was used. After incubation of the samples at ambient temperature for 30 min, their absorbance was measured at 765 nm. The calibration curve was assembled by using fresh prepared gallic acid solutions as a base in calculations of total phenolic compound contents in the extracts. Experiments were repeated three times for every extract and the total phenolics were given in average values as GAE (mg gallic acid/g extract) [25, 26]. For the calibration curve, 10 mg of gallic acid was dissolved in 10 mL of MeOH utilizing an ultrasonic bath (stock solution). Various dilutions of stock solution were prepared and were determined by Folin-Ciocalteu's method [22, 27]. The experiments were repeated three times for every dilution and a calibration curve was created [24].
2.6. Content and In Vitro Release Pattern of the Dispersions
An amount equivalent to 8 mg of the soft extract was digested in 100 mL PBS pH 7.4. After sonication for 15 minutes, the content was assayed for the total phenolics by Folin-Ciocalteu's method [22] after proper dilution.
The in vitro release pattern of the extract was estimated by placing an amount equivalent to 8 mg of each formulation in a dialysis tubing (Visking Dialysis Tubing) closed by tubing closures at one end, 2 mL of the release medium was then added, and the other tube end was also closed by the tubing closure. The tube assembly was clamped to the paddle of a dissolution rate apparatus immersed in 500 mL PBS (pH 7.4) placed in the dissolution apparatus jar. The temperature was adjusted at 35°C, while the paddle was rotated at 50 rpm. Samples of 5 mL each were withdrawn at fixed time intervals and replaced with prewarmed buffer solution. Each sample was assayed by Folin-Ciocalteu's method and the average reading was calculated.
2.7. Bio-Guided Chromatographic Fractionation and Identification of the Effective Compounds
The P. granatum ethanolic extract (Pg) was fractionated using column chromatography. Preparative chromatography column, 50 mm diameter and 100 cm height, was used. Elution was done using ethyl acetate, formic acid, water, and hexane at ratios of 70 : 7.5 : 7.5 : 15, respectively, as mobile phase and silica gel as stationary phase. During the entire chromatography process the eluent was collected in a series of over 200 fractions by time.
Each fraction was tested in the same way as the test solutions in this study using in vivo alloxan-diabetic mice. The most active fraction was analyzed using RP-HPLC. The injection volume was 20 μL and UV detection was performed using UV-detector at 270 nm, which has the highest absorbance for the tested active fraction, using JASCO spectrophotometer (JASCO), and confirmed utilizing TLC and standard steeping method utilizing standard calibration curves.
2.8. Animals
Male Swiss-Webster mice (Faculty of Pharmacy, Beirut Arab University) were accommodated for one week prior to the experimentation. The environment consisted of standard mouse cages with a 12 h light/dark cycle. The temperature was 22 ± 1°C; animals had open access to water and standard laboratory pellets (20% proteins, 5% fats, and 1% multivitamins) [26, 28]. The mice were kept in those conditions for a seven-day period of adaptation prior to the beginning of the experiment. Sixteen hours before the experiment, mice were fasted overnight but allowed open access to water. All animal care and experiments were done in accordance with Lebanese Ministry of Higher Education and with the approval of Beirut Arab University Institutional Review Board.
2.9. Diabetes Induction
Experimental DM was induced by intraperitoneal (IP) injection of freshly prepared alloxan dissolved in sterile saline (0.9%) every 48 h for three times at a dose of 180 mg/kg. Fasting glucose levels in the blood samples obtained from the tail of each mouse 72 h after the last alloxan injection were measured with Accu-Chek Active glucose strips in Accu-Chek Active Test Meter (Roche, USA). The glucose levels were expressed as mg/dL. Glucose (5%) was added to mice drinking water. The mice with blood glucose level more than 200 mg/dL were considered to be diabetic and were used in the experiment.
2.10. Acute Antidiabetic Effect of the P. granatum Extract in Alloxan-Induced Diabetic Mice
The diabetic mice were divided into sixteen groups of seven animals each. The animals of each group had a single IP injection as follows.
Group I received only vehicle (0.9% sterile saline) and served as control.
Group II received GB dissolved in DMSO as reference drug (5 mg/kg).
Groups III, IV, and V received the Pg extract dissolved in vehicle at doses of 25, 50, and 100 mg/kg, respectively.
Groups VI, VII, and VIII received F1, suspended in vehicle at doses equivalent to 25, 50, and 100 mg Pg extract/kg, respectively.
Group VIII received placebo F1 suspended in vehicle (200 mg/kg).
Groups IX, X, and XII received F2, suspended in vehicle at doses equivalent to 25, 50, and 100 mg Pg extract/kg, respectively.
Group XIII received placebo F2 suspended in vehicle (200 mg/kg).
Groups XIV, XV, and XVI received gallic acid (GA) dissolved in DMSO, at doses of 3, 6, and 12 mg/kg to the animals, respectively.
Blood samples were collected from the tail just prior to and at 0.5, 2, and 6 h after dosing. Blood glucose and body weight were determined.
2.11. Subacute Antidiabetic Effect of the P. granatum Extract in Alloxan-Induced Diabetic Mice
The action of Pg was also tested during a longer duration of treatment. The mice were divided into groups containing healthy and diabetic animals. Group I (healthy mice, n = 7) received only vehicle IP for 7 days and served as control [4]. The diabetic mice were divided into sixteen groups (II–XVII) of seven animals each. The animals of each group were treated for 7 days with a daily IP injection as follows.
Group II received only vehicle (0.9% sterile saline) and served as diabetic control.
Group III received GB dissolved in DMSO as reference drug (5 mg/kg, IP).
Groups IV, V, and VI received Pg extract dissolved in vehicle at doses of 25, 50, and 100 mg/kg, respectively.
Groups VII, VIII, and IX received F1 suspended in vehicle at doses equivalent to 25, 50, and 100 mg Pg extract/kg, respectively.
Group X received placebo F1 suspended in vehicle 200 mg/kg.
Groups XI, XII, and XIII received Pg F2 suspended in vehicle at doses equivalent to 25, 50, and 100 mg Pg extract/kg, respectively.
Group XIV received placebo F2 suspended in vehicle 200 mg/kg.
Groups XV, XVI, and XVII received GA, dissolved in DMSO, at doses of 3, 6, and 12 mg/kg, respectively.
The blood glucose level of each animal was monitored on the 1st, 3rd, 5th, and 8th days after 6 h of each injection given every other day.
The antioxidant enzyme (catalase) levels were measured and the body weights of the animals were recorded at the same day.
2.12. Management of Diabetic Neuropathy
After six weeks of induction of diabetes in animals, DN success rate (i.e., loss of sensory of thermal sensitivity significantly below 10 s [29]) was ca. 85%, and their neurological function was tested at one-week intervals for eight weeks, with tramadol (TRA) 10 mg/kg as a positive control, using the following tests.
2.12.1. Hot Plate Latency Test
The animals were positioned one at a time on the hot plate (hot plate analgesia meter, Ugo Basile, Italy) that is maintained at a temperature of 55 ± 0.1°C. Response latency either to jump or to a hindpaw lick was monitored by means of an electronic timer. To avoid tissue damage, a cut-off time of 30 s was selected [30].
2.12.2. Tail Flick Latency Test
In brief, a beam of light was focused on the dorsal surface of the mouse tail using tail flick apparatus with appropriate tail flick mice restrainer (Hugo Sachs Elektronik, Germany) and the time until the tail flicked was measured. The tail withdrawal latency, time from onset of the radiant heat to the withdrawal of the tail, was recorded with a timer. The light intensity in the apparatus was set so that the baseline tail withdrawal latency was about 5.6 s in all mice. A cut-off time of 10.00 ± 0.50 s was set with the purpose of preventing tissue damage [30].
2.13. Estimation of Antioxidant Activity
Catalase (CAT) activity was determined in serum using the modified method described before in the literature [31]. CAT activity was expressed as kU/L.
2.14. Statistical Analysis
All values are presented as means ± SEM. Statistical differences between the test and the control were tested by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test using the “OriginPro” statistic computer program. A difference in the mean values of P ≤ 0.05 was considered to be statistically significant.
3. Results
3.1. HPLC Analysis of Pg Extract
After the bioguided fractionation, the most active P. granatum extract fraction was injected in the HPLC instrument to study its pattern. The most active fraction major peak was GA (11.4%). Ellagic acid was not detected in the active fraction. The concentration of GA was measured using the area under peak utilizing GA calibration curve.
3.2. Total Phenolic Compounds Using Folin-Ciocalteu Assay
Total phenolic content of 0.15 ± 0.01 mg gallic acid/g Pg extract, using Folin-Ciocalteu Reagent (FCR), was found in Pg extract. All absorbance was measured at 765 nm. Results are mean ± SEM of three parallel measurements, and P < 0.01, when compared to the control.
3.3. Content and In Vitro Release Pattern
All the prepared powders contained 98 ± 2% of the claimed extract content calculated as mg gallic acid/g extract. The in vitro release pattern of the tested powders showed that F1 had a faster release pattern with 7.5% released after 30 minutes compared with 5.5% in case of F2. The difference in release was highly observed after 3 hours with F1 releasing more than 85% of the extract compared with less than 50% for F2. Retardation of drug release from such dispersions of casein is commonly achieved via surface cross-linkage (Figure 1).
Figure 1.
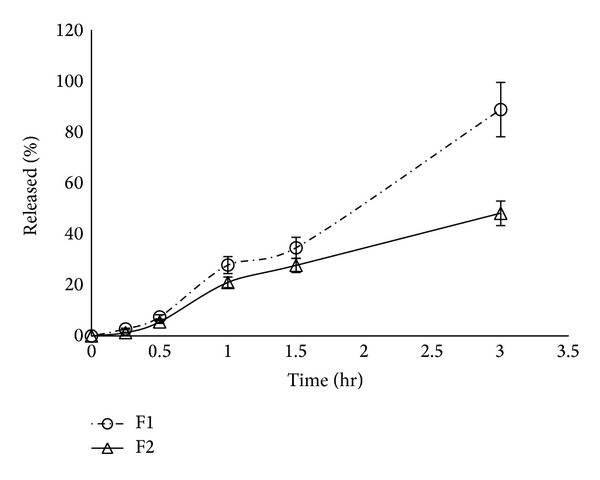
In vitro release pattern of P. granatum L. extract from different biopolymeric dispersions of casein (F1) and chitosan (F2).
3.4. Acute Antidiabetic Effect of the Pg Extract in Alloxan-Induced Diabetic Mice
The acute antidiabetic effect of various doses of the Pg extract in diabetic animals is summarized in Table 1. The Pg extract at all doses (25, 50, and 100 mg/kg) showed a significant effect compared to the control, with blood glucose levels dropping after 6 h of administration to 36.4, 34.2, and 48.4%, respectively. GB, the positive control, prevented the drastic increase of blood glucose after 1 h of glucose loading. On the other hand, GB reduced the glucose level, 2 and 6 h after the glucose loading. Dispersion F1 at all doses (equivalent to 25, 50, and 100 mg Pg extract/kg) showed significant decrease in blood glucose level after 6 h dropping to 40.6, 40.0, and 52.4%, respectively. Similar to F1, F2 showed significant decrease in blood glucose level after 6 h at all doses (equivalent to 25, 50, and 100 mg Pg extract/kg) with the glucose level dropping to 30.2, 42.6, and 40.6%, respectively. On the other hand, placebos F1 and F2 did not significantly reduce the glucose level 6 h after the glucose loading. In case of GA, standard GA at the lowest dose (3 mg/kg) did not show a significant effect from that of control after 6 h of glucose administration. At higher doses (6 and 12 mg/kg) GA showed a significant effect, with blood glucose levels dropping to 36.1 and 36.4%, respectively, compared to that of control after 6 h of glucose administration.
Table 1.
Acute effect of P. granatum ethanolic extract (Pg), F1, F2, and gallic acid (GA) on blood glucose of alloxan-induced diabetic mice (n = 7).
| Group | Dose (mg/kg) | Mean blood glucose concentration ± SEM (mg/dL) | |||
|---|---|---|---|---|---|
| 0 hr | 0.5 hr | 2 hr | 6 hr | ||
| Diabetic control | — | 202.79 ± 5.60 | 211.93 ± 4.50 | 214.21 ± 9.70 | 210.15 ± 7.30 |
| GB | 5 | 219.70 ± 3.70 | 223.64 ± 1.80 | 159.74 ± 2.10 | 130.64 ± 2.40** |
| Pg | 25 | 218.29 ± 1.50 | 142.44 ± 2.80 | 181.56 ± 2.02 | 138.89 ± 1.45* |
| Pg | 50 | 200.43 ± 2.10 | 240.93 ± 2.50 | 158.41 ± 2.60 | 131.96 ± 1.90* |
| Pg | 100 | 249.92 ± 1.80 | 292.44 ± 2.80 | 228.56 ± 2.02 | 128.98± 1.54* |
| Placebo F1 | 200 | 208.10 ± 3.10 | 227.23 ± 2.10 | 222.62 ± 2.30 | 209.98 ± 2.90 |
| F1 | 50 | 243.12 ± 1.30 | 158.44 ± 1.90 | 167.65 ± 2.10 | 144.33 ± 2.00* |
| F1 | 100 | 201.33 ± 1.70 | 130.44 ± 2.10 | 160.89 ± 2.90 | 120.77 ± 2.10* |
| F1 | 200 | 263.11 ± 2.90 | 139.56 ± 2.40 | 117.79 ± 2.80 | 125.33 ± 2.30* |
| Placebo F2 | 200 | 213.56 ± 3.10 | 202.17 ± 2.60 | 201.13 ± 2.80 | 229.56 ± 3.10 |
| F2 | 50 | 211.55 ± 2.90 | 205.12 ± 1.30 | 146.82 ± 1.40 | 147.725 ± 2.80* |
| F2 | 100 | 214.65 ± 1.30 | 213.99 ± 1.90 | 186.83 ± 1.10 | 123.27 ± 1.20* |
| F2 | 200 | 240.44 ± 2.50 | 190.825 ± 1.90 | 172.165 ± 1.80 | 142.87 ± 2.20* |
| GA | 3 | 201.29 ± 5.60 | 210.43 ± 4.50 | 213.71 ± 9.70 | 209.05 ± 7.30 |
| GA | 6 | 202.20 ± 3.70 | 222.14 ± 1.80 | 158.24 ± 2.10 | 129.14 ± 2.40** |
| GA | 12 | 218.29 ± 1.50 | 142.44 ± 2.80 | 181.56 ± 2.02 | 138.89 ± 1.45* |
SEM: standard error of the mean.
*P < 0.05 significant from the control animals.
**P < 0.01 significant from the control animals.
3.5. Subacute Effect of the Pg Extract in Alloxan-Induced Diabetic Mice
As shown in Table 2, the blood glucose levels of diabetic control (positive control) mice were significantly higher than those of the control (negative control) mice during the experiment period. The highest reduction in blood glucose using Pg extract was observed with a dose of 100 mg/kg, showing 54.2% reduction in blood glucose levels on the 8th day compared with 17.7 and 6.4% reduction in case of 25, 50 mg/kg doses, respectively.
Table 2.
Subacute effect of Pg, F1, F2, and GA on blood glucose of alloxan-induced diabetic mice (n = 7).
| Group | Dose (mg/kg) | Mean blood glucose concentration ± SEM (mg/dL) | |||
|---|---|---|---|---|---|
| 1st day | 3rd day | 5th day | 8th day | ||
| Control | — | 107.90 ± 2.50 | 109.80 ± 3.60 | 108.86 ± 3.20 | 117.50 ± 4.70 |
| Diabetic controla | — | 202.79 ± 5.60*** | 211.93 ± 4.50*** | 214.91 ± 9.70*** | 210.55 ± 7.30*** |
| GBb | 5 | 186.70 ± 3.70 | 179.53 ± 2.90 | 161.54 ± 2.40** | 171.97 ± 3.10 |
| Pg b | 25 | 138.67 ± 2.60 | 87.54 ± 1.70 | 158.63 ± 2.30 | 135.50 ± 2.10** |
| Pg b | 50 | 143.34 ± 1.90 | 119.67 ± 1.70 | 119.33 ± 1.90 | 119.10 ± 1.10** |
| Pg b | 100 | 132.12 ± 1.30 | 112.98 ± 2.50 | 124.36 ± 2.60 | 111.50 ± 2.50* |
| Placebo F1 | 200 | 193.10 ± 3.10 | 212.66 ± 2.90 | 229.54 ± 2.80 | 226.33 ± 2.10 |
| F1b | 50 | 144.11 ± 1.20 | 129.40 ± 1.60 | 130.66 ± 2.40 | 125.88 ± 3.10* |
| F1b | 100 | 120.33 ± 1.70 | 115.60 ± 2.40 | 128.65 ± 2.90 | 124.33 ± 3.20* |
| F1b | 200 | 125.21 ± 2.90 | 115.50 ± 3.10 | 108.40 ± 2.40 | 104.32 ± 1.90* |
| Placebo F2 | 200 | 229.33 ± 2.50 | 226.89 ± 2.90 | 204.98 ± 3.10 | 265.44 ± 2.90 |
| F2b | 50 | 175.50 ± 2.50 | 119.90 ± 2.10 | 119.10 ± 1.30 | 118.50 ± 1.90* |
| F2b | 100 | 118.65 ± 1.30 | 128.10 ± 1.30 | 122.49 ± 2.40 | 117.65 ± 2.10* |
| F2b | 200 | 191.33 ± 3.90 | 94.54 ± 1.40 | 108.55 ± 3.40 | 107.43 ± 2.50* |
| GAb | 3 | 198.10 ± 1.90 | 208.00 ± 2.20 | 111.36 ± 3.30 | 101.88 ± 2.22* |
| GAb | 6 | 179.65 ± 3.10 | 134.66 ± 2.60 | 130.54 ± 2.90 | 98.23 ± 2.87* |
| GAb | 12 | 168.33 ± 2.90 | 140.50 ± 3.50 | 137.25 ± 2.70 | 118.55 ± 3.50* |
SEM: standard error of the mean.
*P < 0.05 significant from the control animals.
**P < 0.01 significant from the control animals.
***P < 0.001 significant from the control animals.
aCompared to vehicle control.
bCompared to diabetic control.
The F1 at all doses (equivalent to 25, 50, and 100 mg Pg extract/kg) showed significant decrease in blood glucose level on the 8th day compared to that of the diabetic control with the mice glucose levels dropping to 40.2, 40.9, and 50.5%, respectively. Similarly, F2 at all doses (equivalent to 25, 50, and 100 mg Pg extract/kg) showed significant decrease in blood glucose level on the 8th day dropping to 43.7, 44.1, and 49.0%, respectively. Placebos F1 and F2 did not significantly reduce the glucose level on the 8th day compared to that of the diabetic control.
For GA, standard GA showed a significant effect compared to that of diabetic control on the 8th day at all doses (3, 6, and 12 mg/kg), with blood glucose levels dropping to 51.6, 53.3, and 43.7%, respectively.
During the subacute administration, mice treated with GB and various doses of the Pg extract, F1, F2, and GA were also monitored for changes in weight (Table 3). The Pg extract showed 5.9, 30.7, and 21.9% increase in body weight at doses of 25, 50, and 100 mg/kg, respectively, on the 8th day. In case of F1 at doses equivalent to 25, 50, and 100 mg Pg extract/kg showed 9.8, 10.5, and 17.8% increase in body weight on the 8th day, respectively. Similarly, F2 at doses equivalent to 25, 50, and 100 mg Pg extract/kg showed 9.8, 10.5, and 17.8% increase in body weight on the 8th day. Placebos F1 and F2 did not significantly show any increase in body weight on the 8th day compared to that of the diabetic control. Also GA at all doses (3, 6, and 12 mg/kg) showed a significant increase in body weight on the 8th day, of 23.9, 18.9, and 6.5%, respectively.
Table 3.
Subacute effect of Pg, F1, F2, and GA on body weights in alloxan-induced diabetic mice (n = 7).
| Group | Dose (mg/kg) | Mean body weight ± SEM (gm) | |||
|---|---|---|---|---|---|
| 1st day | 3rd day | 5th day | 8th day | ||
| Control | — | 25.90 ± 0.50 | 26.00 ± 0.60 | 26.01 ± 0.97 | 26.62 ± 0.70 |
| Diabetic controla | — | 28.68 ± 0.70 | 27.10 ± 0.20 | 27.15 ± 0.80 | 27.69 ± 0.50 |
| GBb | 5 | 22.90 ± 0.70 | 28.17 ± 1.70 | 28.54 ± 0.40 | 30.37 ± 1.10* |
| Pg b | 25 | 24.11 ± 2.60 | 27.70 ± 1.50 | 30.20 ± 1.60 | 30.40 ± 2.10** |
| Pg b | 50 | 29.30 ± 1.90 | 29.20 ± 1.60 | 29.30 ± 1.80 | 31.50 ± 2.20** |
| Pg b | 100 | 30.24 ± 2.60 | 30.40 ± 1.70 | 31.54 ± 3.90 | 31.98 ± 2.90* |
| Placebo F1 | 200 | 27.50 ± 2.10 | 27.50 ± 2.20 | 27.00 ± 2.40 | 26.88 ± 2.50 |
| F1b | 50 | 31.11 ± 1.20 | 31.00 ± 1.50 | 31.10 ± 1.60 | 31.50 ± 1.90 |
| F1b | 100 | 30.10 ± 1.40 | 29.50 ± 2.50 | 30.50 ± 2.40 | 31.70 ± 2.50* |
| F1b | 200 | 31.00 ± 1.90 | 29.00 ± 3.50 | 32.60 ± 2.90 | 33.80 ± 2.80* |
| Placebo F2 | 200 | 24.50 ± 2.50 | 24.45 ± 2.60 | 24.01 ± 2.50 | 23.60 ± 2.40 |
| F2b | 50 | 27.30 ± 1.90 | 27.40 ± 1.80 | 27.5 ± 1.90 | 27.8 ± 2.00 |
| F2b | 100 | 28.40 ± 1.40 | 28.5 ± 1.50 | 28.90 ± 1.60 | 30.25 ± 2.50* |
| F2b | 200 | 31.90 ± 1.90 | 32.00 ± 1.80 | 32.50 ± 1.90 | 33.11 ± 2.10* |
| GAb | 3 | 29.20 ± 1.20 | 30.30 ± 1.30 | 30.50 ± 1.10 | 32.05 ± 1.20* |
| GAb | 6 | 31.56 ± 1.10 | 32.67 ± 0.60 | 33.45 ± 1.00 | 34.10 ± 1.60* |
| GAb | 12 | 30.20 ± 1.20 | 30.30 ± 1.30 | 29.50 ± 1.50 | 30.56 ± 1.40 |
SEM: standard error of the mean.
*P < 0.05 significant from the control animals.
aCompared to vehicle control.
bCompared to diabetic control.
In order to evaluate in vivo antioxidant effect of the tested Pg extract and its polymeric dispersions, CAT level in serum of each mouse was monitored on 1st, 3rd, 5th, and 8th days after administration. As shown in Table 4, diabetic mice were monitored for changes in serum CAT level after treatment with GB and various doses of the Pg extract, F1, F2, and GA. The Pg extract at doses of 25, 50, and 100 mg/kg had a gradual rise in serum CAT activity to reach a significant difference on 5th (1.0, 9.2, and 15.1%, resp.) and 8th day (4.1, 6.1, and 15.5%, resp.) as compared with diabetic control mice. Polymeric dispersion F1 at doses equivalent to 25, 50, and 100 mg Pg extract/kg had a gradual rise in serum CAT activity to reach significant differences on 5th (2.7, 8.7, and 14.5%, resp.) and 8th day (5.2, 6.1, and 19.8%, resp.) as compared with diabetic control mice. Similarly, F2 had a gradual rise in serum CAT activity to reach a significant difference on the 8th day with CAT activities of 3.3, 4.0, and 10.9% for doses equivalent to 25, 50, and 100 mg Pg extract/kg, respectively. Placebos F1 and F2 did not significantly show any increase in serum CAT level compared to that of the diabetic control. GA at doses 6 and 12 mg/kg had also shown a gradual rise in serum CAT activity to reach a significant difference on the 8th day (3.4 and 3.1%, resp.) as compared with diabetic control mice.
Table 4.
In vivo assessment of the antioxidant activity of Pg, F1, F2, and GA using catalase levels in serum of alloxan-induced diabetic mice (n = 7).
| Group | Dose (mg/kg) | Catalase level ± SEM (kU/I) | |||
|---|---|---|---|---|---|
| 1st day | 3rd day | 5th day | 8th day | ||
| Control | — | 41.00 ± 1.50 | 41.50 ± 1.60 | 40.86 ± 1.20 | 41.62 ± 1.70 |
| Diabetic controla | — | 21.67 ± 1.60*** | 20.93 ± 1.30*** | 24.01 ± 1.90*** | 25.55 ± 1.40*** |
| GBb | 5 | 22.60 ± 1.70 | 25.00 ± 1.70 | 31.54 ± 1.40* | 32.37 ± 1.00** |
| Pg b | 25 | 22.33 ± 1.80 | 22.66 ± 1.60 | 24.26 ± 2.10* | 26.60 ± 1.90** |
| Pg b | 50 | 20.44 ± 1.30 | 22.55 ± 1.90 | 26.22 ± 1.69* | 27.10 ± 1.20** |
| Pg b | 100 | 23.33 ± 1.50 | 26.40 ± 2.10 | 27.63 ± 2.10* | 29.50 ± 2.30* |
| Placebo F1 | 200 | 21.50 ± 2.80 | 20.77 ± 2.10 | 20.84 ± 2.80 | 20.33 ± 2.20 |
| F1b | 50 | 20.11 ± 1.20 | 21.40 ± 1.60 | 24.66 ± 2.40* | 26.88 ± 3.10* |
| F1b | 100 | 22.33 ± 1.70 | 23.42 ± 2.20 | 26.10 ± 2.70* | 27.10 ± 2.90* |
| F1b | 200 | 19.44 ± 2.10 | 23.66 ± 2.70 | 27.50 ± 2.50* | 30.62 ± 1.60* |
| Placebo F2 | 200 | 22.54 ± 2.40 | 20.89 ± 2.80 | 20.89 ± 3.10 | 20.66 ± 2.80 |
| F2b | 50 | 24.44 ± 2.70 | 24.21± 2.00 | 25.40 ± 1.70* | 26.40 ± 1.70 |
| F2b | 100 | 20.14 ± 1.30 | 19.40 ± 1.40 | 24.00 ± 2.10* | 26.56 ± 2.20* |
| F2b | 200 | 21.85 ± 3.10 | 24.54 ± 2.40 | 26.66 ± 3.40* | 28.34 ± 2.40* |
| GAb | 3 | 21.44 ± 1.60 | 20.11 ± 1.10 | 22.36 ± 1.60 | 23.22 ± 1.30 |
| GAb | 6 | 20.55 ± 1.50 | 22.78 ± 1.60 | 24.85 ± 1.80* | 26.42 ± 0.80* |
| GAb | 12 | 24.00 ± 1.80 | 22.30 ± 1.40 | 24.11 ± 1.10* | 26.33 ± 1.20* |
SEM: standard error of the mean.
*P < 0.05 significant from the control animals.
**P < 0.01 significant from the control animals.
***P < 0.001 significant from the control animals.
aCompared to vehicle control.
bCompared to diabetic control.
The acute and subacute antihyperglycemic activity of the Pg extract, F1, F2, and GA were shown to be more potent and prolonged than those of GB.
3.6. Management of Diabetic Neuropathy
Deterioration of peripheral nerve conduction is a milestone indicator for diabetic patients having peripheral neuropathy [32–34]. So, we examined the effect of Pg extract, F1, F2, and GA treatment on sensory function by measuring the thermal latency with tail flick and hot plate tests on the 8th week after alloxan injection.
Treatment of the alloxan-induced diabetic mice with Pg extract markedly improved the thermal latency compared with TRA 10 mg/kg positive control (Figure 2(a)). Diabetic mice exhibited transient hyperalgesic response in thermal tests. On the 8th week after alloxan injection, treatment with Pg extract showed a marked improvement in hot plate latency compared to vehicle treated group by 33.3, 73.5, and 85.1% in doses of 25, 50, and 100 mg/kg, respectively (Figure 2(a)).
Figure 2.
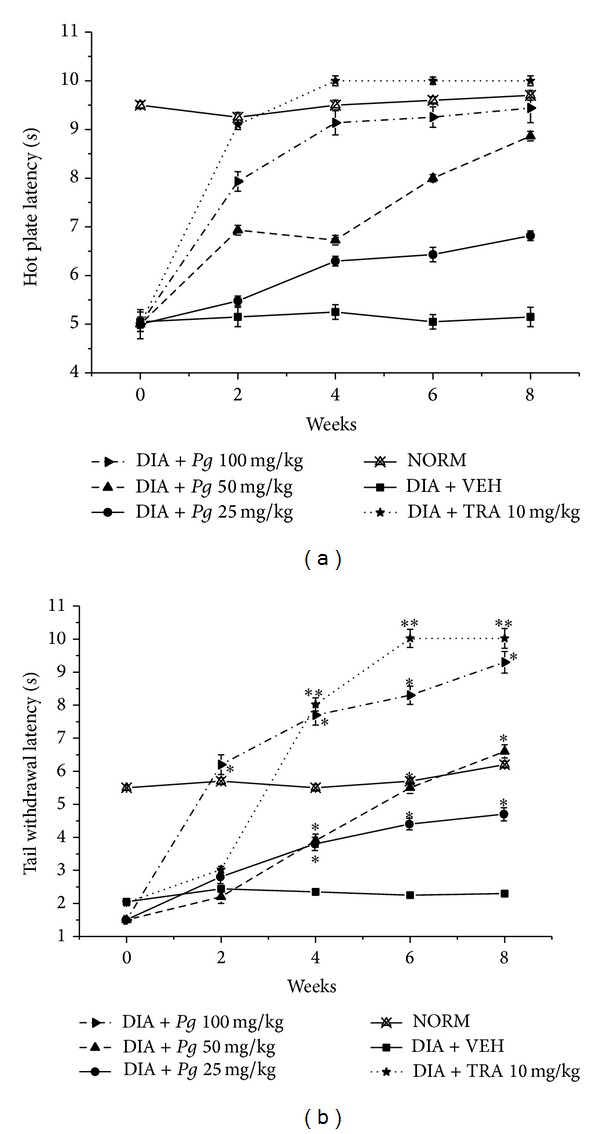
Effect of P. granatum ethanolic extract (Pg) and tramadol (TRA) 10 mg/kg, as positive control, on the hot plate and tail withdrawal latencies in alloxan-induced diabetic mice. (a) Hot plate latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + Pg 25 mg/kg: diabetic animals treated with Pg 25 mg/kg (solid-circles, straight-line); DIA + Pg 50 mg/kg: diabetic animals treated with Pg 50 mg/kg (up-triangles, dashed-line); DIA + Pg 100 mg/kg: diabetic animals treated with Pg 100 mg/kg (right-triangles, dashed-dotted-line). (b) Tail withdrawal latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + Pg 25 mg/kg: diabetic animals treated with Pg 25 mg/kg (solid-circles, straight-line); DIA + Pg 50 mg/kg: diabetic animals treated with Pg 50 mg/kg (up-triangles, dashed-line); DIA + Pg 100 mg/kg: diabetic animals treated with Pg 100 mg/kg (right-triangles, dashed-dotted-line). Data are expressed in mean ± SEM. “∗” means P < 0.05 compared with vehicle. “∗∗” means P < 0.01 compared with vehicle.
Nevertheless, treatment with all doses of Pg extract demonstrated a marked improvement in tail flick latency by ca. one-, two-, and threefold fordoses of 25, 50, and 100 mg/kg, respectively, compared to vehicle treated group (Figure 2(b)).
On the 8th week, treatment with the lowest dose of F1 did not significantly improve hot plate latency, while higher doses (equivalent to 50 and 100 mg Pg extract/kg) of F1 markedly improved hot plate latency by 15.7 and 84.3%, respectively, compared to vehicle treated group (Figure 3(a)). Nonetheless, treatment with all doses of F1 the tail flick latency have markedly improved by ca. 1.5-, 1.7-, and 3-fold in F1 doses equivalent to 25, 50, and 100 mg Pg extract/kg, respectively (Figure 3(b)).
Figure 3.
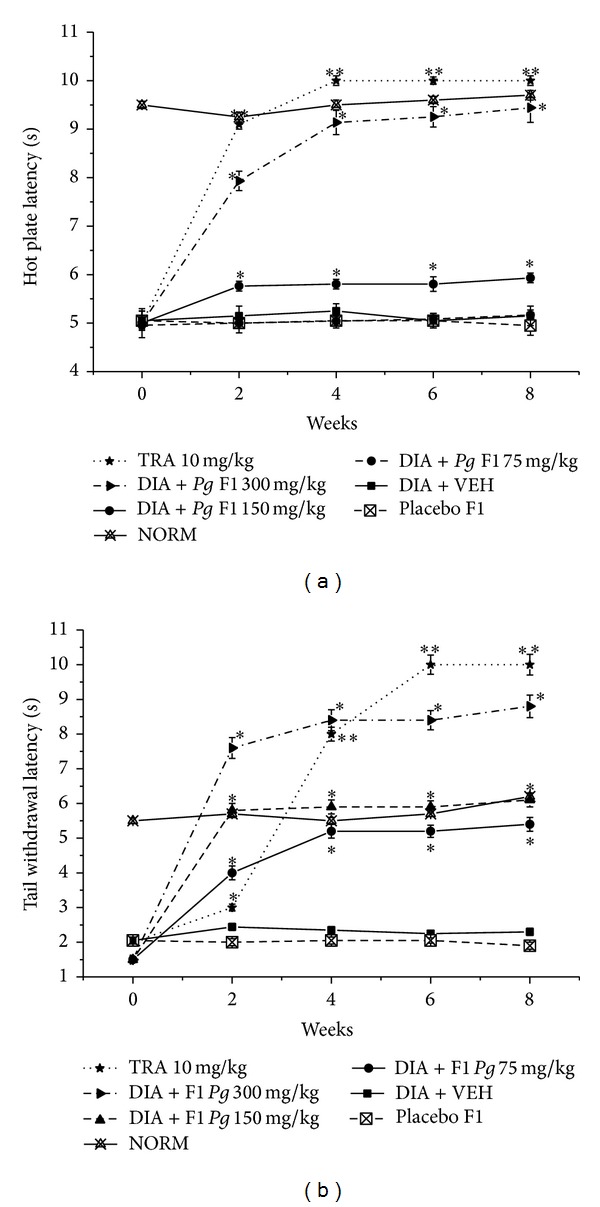
Effect of F1 and TRA 10 mg/kg, as positive control, on the hot plate and tail withdrawal latencies in alloxan-induced diabetic mice. (a) Hot plate latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); placebo F1: diabetic animals treated with placebo F1 200 mg/kg (crossed-squares, dashed-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + F1 50 mg/kg: diabetic animals treated with F1 50 mg/kg (solid-circles, straight-line); DIA + F1 100 mg/kg: diabetic animals treated with F1 100 mg/kg (up-triangles, dashed-line); DIA + F1 200 mg/kg: diabetic animals treated with Pg F1 200 mg/kg (right-triangles, dashed-dotted-line). (b) Tail withdrawal latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); placebo F1: diabetic animals treated with placebo F1 200 mg/kg (crossed-squares, dashed-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + F1 50 mg/kg: diabetic animals treated with F1 50 mg/kg (solid-circles, straight-line); DIA + F1 100 mg/kg: diabetic animals treated with F1 100 mg/kg (up-triangles, dashed-line); DIA + F1 200 mg/kg: diabetic animals treated with F1 200 mg/kg (right-triangles, dashed-dotted-line). Data are expressed in mean ± SEM. “∗” means P < 0.05 compared with vehicle. “∗∗” means P < 0.01 compared with vehicle.
Moreover, treatment with the lowest dose of F2 did not significantly improve hot plate or tail flick latencies, while higher doses (equivalent to 50 and 100 mg Pg extract/kg) of F2 markedly improved hot plate latency by 45.5 and 60.8%, respectively, and tail flick latency by ca. 0.3- and 0.9-fold compared to vehicle treated group (Figures 4(a) and 4(b)).
Figure 4.
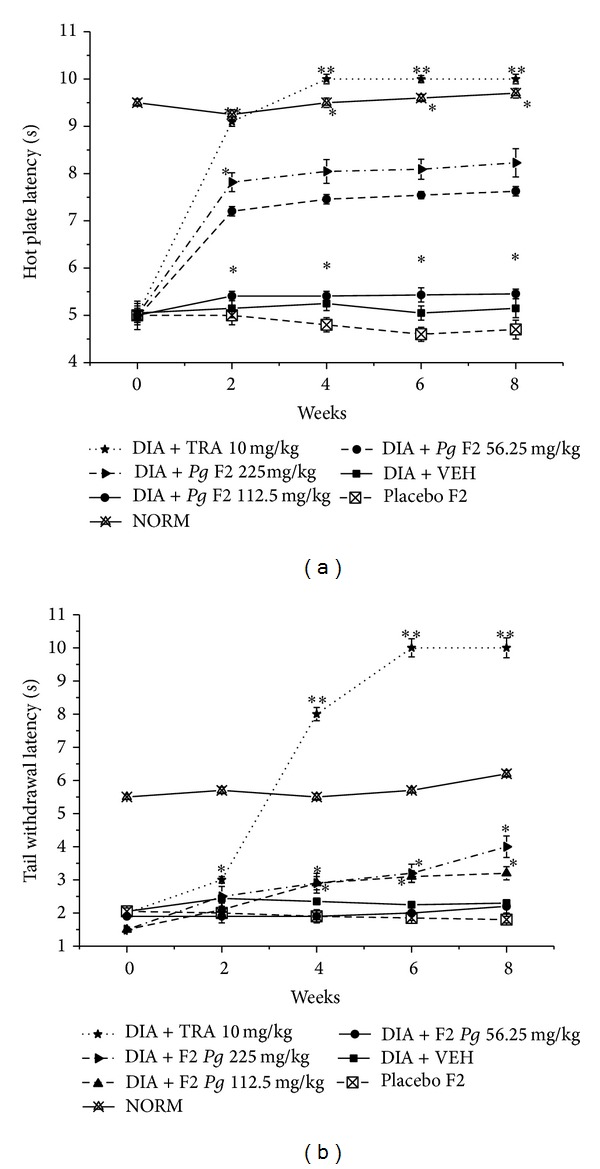
Effect of F2 and TRA 10 mg/kg, as positive control, on the hot plate and tail withdrawal latencies in alloxan-induced diabetic mice. (a) Hot plate latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); placebo F2: diabetic animals treated with placebo F2 200 mg/kg (crossed-squares, dashed-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + F2 50 mg/kg: diabetic animals treated with F2 50 mg/kg (solid-circles, straight-line); DIA + F2 100 mg/kg: diabetic animals treated with F2 100 mg/kg (up-triangles, dashed-line). DIA + Pg 200 mg/kg: diabetic animals treated with F2 200 mg/kg (right-triangles, dashed-dotted-line). (b) Tail withdrawal latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); placebo F2: diabetic animals treated with placebo F2 200 mg/kg (crossed-squares, dashed-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line). DIA + F2 50 mg/kg: diabetic animals treated with F2 50 mg/kg (solid-circles, straight-line); DIA + F2 100 mg/kg: diabetic animals treated with F2 100 mg/kg (up-triangles, dashed-line). DIA + F2 200 mg/kg: diabetic animals treated with F2 200 mg/kg (right-triangles, dashed-dotted-line). Data are expressed in mean ± SEM. “∗” means P < 0.05 compared with vehicle. “∗∗” means P < 0.01 compared with vehicle.
Both Placebos F1 and F2 did not significantly improve neither hot plate nor tail flick latencies (Figures 3 and 4).
In addition, treatment with GA (3, 6, and 12 mg/kg) showed a marked improvement in hot plate latency by 70.6, 78.6, and 86.3% and tail flick latency by ca. 1.5-, 2-, and 2.4-fold, respectively, compared to vehicle treated group (Figures 5(a) and 5(b)).
Figure 5.
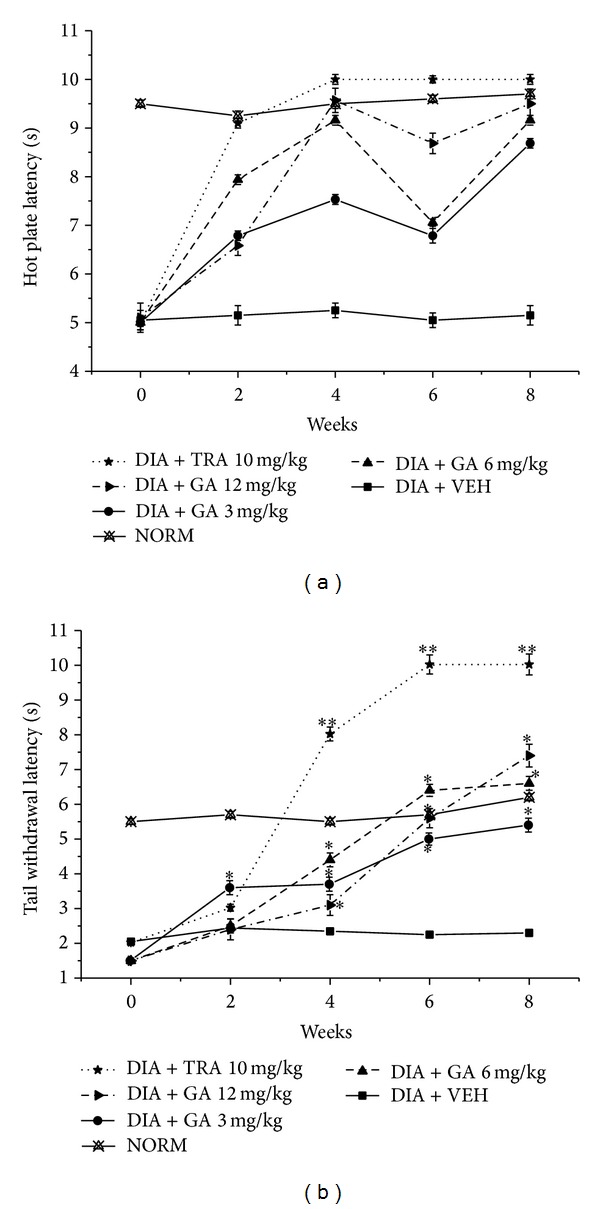
Effect of gallic acid (GA) and tramadol (TRA) 10 mg/kg, as positive control, on the hot plate and tail withdrawal latencies in alloxan-induced diabetic mice. (a) Hot plate latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + GA 3 mg/kg: diabetic animals treated with GA 3 mg/kg (solid-circles, straight-line); DIA + GA 6 mg/kg: diabetic animals treated with GA 6 mg/kg (up-triangles, dashed-line). DIA + Pg 12 mg/kg: diabetic animals treated with GA 12 mg/kg (right-triangles, dashed-dotted-line). (b) Tail withdrawal latency: NORM: normal control mice (crossed-triangles, straight line); DIA + VEH: diabetic animals treated with vehicle as control (closed-squares, straight-line); positive control TRA 10 mg/kg: alloxan treated mice with TRA 10 mg/kg (solid-stars, dotted-line); DIA + GA 3 mg/kg: diabetic animals treated with GA 3 mg/kg (solid-circles, straight-line); DIA + GA 6 mg/kg: diabetic animals treated with GA 6 mg/kg (up-triangles, dashed-line); DIA + GA 12 mg/kg: diabetic animals treated with GA 12 mg/kg (right-triangles, dashed-dotted-line). Data are expressed in mean ± SEM. “∗” means P < 0.05 compared with vehicle. “∗∗” means P < 0.01 compared with vehicle.
4. Discussion
Extended exposure to hyperglycemia promotes the development of microvascular and macrovascular complications associated with DM [35]. The high oxidative stress in diabetics considerably contributes to the complications of this disease [36] and excessive production of free radicals is a discovered phenomenon associated with diabetic complications [37]. Management of DM and diabetic complications with minimal side effect is still a major challenge to the medical system [35].
This directed scientists to broader exploration of potent natural antidiabetics with fewer side effects. In this study we chose Pg extract based on its folkloric use in treatment of DM in Lebanon. The current research was focused on assessing its possible antidiabetic and antinociceptive activities of Pg extract and its spray dried biodegradable polymeric dispersions, along with its most effective compound, GA.
There are a number of scientific reports proposing the antidiabetic potential of Pg extracts [5, 7, 8], whereas the active compound responsible for this action was assumed to be ellagic acid [8]. In the present work, bioguided fractionation utilizing column chromatography, TLC and RP-HPLC, indicated that gallic acid (GA) is the most effective compound.
The hypoglycemic action of the extract was observed to be dose dependent in hyperglycemic mice, with significant decrease of blood glucose level at the highest dose levels of 100 mg extract/kg.
It has been found that the highest dose levels of Pg extract (100 mg/kg), F1, F2, and GA (6 mg/kg) are the most effective doses in the acute and subacute groups. These doses have more significant effect on blood glucose level compared to that of the synthetic drug, GB. An initial increase in blood glucose levels after 0.5 and sometimes 2 hrs after treatment with Pg extract and GA was observed during testing the acute antidiabetic effect. This temporary initial hyperglycemia may be due to preglucose loading, while, at the same time, the test compounds did not start to give their effect directly after administration.
In the acute antidiabetic effect, F1 had shown a relatively higher reduction in glucose level compared with F2. This could be attributed to the micellar arrangement of casein that promotes solubilization within the release medium [15], thus improving the release. The same release pattern was seen in the in vitro release experiment with F1 showing faster release than F2.
In the subacute antidiabetic effect, due to F2 relatively slower release seen in its in vitro release pattern, F2 showed a relatively higher subacute effect compared to F1, an effect that could be beneficial for designing a long acting form of the extract for controlling DM.
Currently, much attention has been given on the role of oxidative stress. It has been suggested that oxidative stress may comprise the key and common events in the pathogenesis of different diabetic complications [38].
Pg extract, F1, F2, and GA showed a significant increase in body weight, as an evidence of alleviating of hyperglycemia, as demonstrated before with pharmacotherapies used in management of DM [3, 39].
It was suggested that the mechanism of action of GA appears to be through the regeneration of the damaged Langerhans β-cells and the potentiation of insulin release from the existing ones [40].
In our study, the activity of CAT decreased in diabetic mice as reported earlier [38, 41] which could be due to inactivation caused by alloxan-generated reactive oxygen species (ROS). Long-term treatment of DM with all doses, especially with the highest dose of the Pg extract, F1, F2, and GA, could have reversed the activities of this enzymatic antioxidant, which might be due to lessened oxidative stress as evidenced by the elevation in CAT activity.
Pg extract has long been known as having antioxidant molecules of gallic and ellagic acids [42, 43]. The most effective active fraction of our extract did not contain ellagic acid; on the other hand, it has shown a comparatively high percentage of GA. The effect of GA in the same concentration, relative to that present in the Pg extract, is comparatively showing equipotent antihyperglycemic and antioxidant activity to Pg extract, which may indicate that GA is the most effective bioactive component in Pg extract.
Administration of Pg extract, F1, F2, and GA also alleviated hyperalgesia in pain conditions compared to that of the positive control, tramadol (TRA).
These findings supply health care providers with a promising medication intended for symptomatic management of diabetic neuropathy, a safe antidiabetic agent, and a safe treatment of micro- and macrovascular complications of DM. Slow release dispersions (F2) may be more beneficial in long-term management (subacute antidiabetic effect) while rapid ones (F1) may be better for acute management.
In conclusion, the present study indicated that Pg extract as well as its biodegradable polymeric dispersions with casein (F1) and chitosan (F2) exerted remarkable hypoglycemic activity and improved peripheral nerve function, which might be due to GA, that prevents oxidative stress in diabetic animals beside its insulin-secretagogue action. Therefore, the observed in vivo antioxidant potential of the Pg extract might possibly be added to the mechanism of action responsible for its antinociceptive effect.
Acknowledgments
The authors would like to thank Dr. A. Yacout for partially funding this research. Thanks are also extended to Junior Research Team, Department of Pharmaceutical Sciences, Faculty of Pharmacy, BAU, for helping in methodology preparation.
Abbreviations
- DM:
Diabetes mellitus
- DN:
Diabetic neuropathy
- GA:
Gallic acid
- GB:
Glibenclamide
- IP:
Intraperitoneal
- FCR:
Folin–Ciocalteu reagent
- RP:
Reversed phase
- DMSO:
Dimethyl sulfoxide
- LD50:
Lethality towards 50% of a population
- ca.:
Approximately
- P. granatum (Pg):
Punica granatum
- ROS:
Reactive oxygen species
- CAT:
Catalase.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Stopford R, Winkley K, Ismail K. Social support and glycemic control in type 2 diabetes: a systematic review of observational studies. Patient Education and Counseling. 2013;93(3):549–558. doi: 10.1016/j.pec.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Raafat K, Boukhary R, Aboul-Ela M, El-Lakany A. Endogenous Lebanese plants treating diabetes and related complications. Natural Products Chemistry and Research. 1(3):112–120. [Google Scholar]
- 4.Bakirel T, Bakirel U, Keleş OÜ, Ülgen SG, Yardibi H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. Journal of Ethnopharmacology. 2008;116(1):64–73. doi: 10.1016/j.jep.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Baliga MS, Shivashankara AR, Shetty CB, Thilakchand KR, Periera N, Palatty PL. Antidiabetic effects of Punica granatum L, (Pomegranate): a review. In: Watson RR, Preed VR, editors. Bioactive Food as Dietary Interventions for Diabetes. Academic Press; 2013. pp. 355–369. [Google Scholar]
- 6.Huang THW, Peng G, Kota BP, et al. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-γ and identification of an active component. Toxicology and Applied Pharmacology. 2005;207(2):160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrman B, Volkova N, Aviram M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: studies in vitro and in diabetic patients. Nutrition. 2010;26(4):359–366. doi: 10.1016/j.nut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Banihani S, Swedan S, Alguraan Z. Pomegranate and type 2 diabetes. Nutrition Research. 2013;33(5):341–348. doi: 10.1016/j.nutres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes/Metabolism Research and Reviews. 2012;28(supplement 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 10.Quattrini C, Tesfaye S. Understanding the impact of painful diabetic neuropathy. Diabetes/Metabolism Research and Reviews. 2003;19(supplement 1):S2–S8. doi: 10.1002/dmrr.360. [DOI] [PubMed] [Google Scholar]
- 11.Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Current Opinion in Supportive and Palliative Care. 2009;3(2):136–143. doi: 10.1097/SPC.0b013e32832b7df5. [DOI] [PubMed] [Google Scholar]
- 12.Raafat K, Aboul-Ela M, El-Lakany A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. doi: 10.1007/s12272-014-0372-y. Archives of Pharmacal Research. In press. [DOI] [PubMed] [Google Scholar]
- 13.Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. Journal of Biochemical and Molecular Toxicology. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomedicine and Pharmacotherapy. 2005;59(7):365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Elzoghby AO, Abo El-Fotoh WS, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. Journal of Controlled Release. 2011;153(3):206–216. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Picker-Freyer KM, Brink D. Evaluation of powder and tableting properties of chitosan. AAPS PharmSciTech. 2006;7(3, article 75) doi: 10.1208/pt070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chemical Reviews. 2004;104(12):6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 18.Elgindy N, Elkhodairy K, Molokhia A, Elzoghby A. Lyophilization monophase solution technique for improvement of the physicochemical properties of an anticancer drug, flutamide. European Journal of Pharmaceutics and Biopharmaceutics. 2010;74(2):397–405. doi: 10.1016/j.ejpb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Samy W, Elgindy N, El-Gowelli HM. Biopolymeric nifedipine powder for acceleration of wound healing. International Journal of Pharmaceutics. 2012;422(1-2):323–331. doi: 10.1016/j.ijpharm.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Raafat K. Exploration of the protective effects of some natural compounds against neurodegeneration exploiting glycine receptors in vivo model. Natural Products Chemistry and Research. 2013;1(3):1–6. [Google Scholar]
- 21.Kogure K, Yamauchi I, Tokumura A, et al. Novel antioxidants isolated from plants of the genera Ferula, Inula, Prangos and Rheum collected in Uzbekistan. Phytomedicine. 2004;11(7-8):645–651. doi: 10.1016/j.phymed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Mavi A, Terzi Z, Özgen U, Yildirim A, Coşkun M. Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), Malva neglecta (Malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), Urtica dioica (Urticaceae) Biological and Pharmaceutical Bulletin. 2004;27(5):702–705. doi: 10.1248/bpb.27.702. [DOI] [PubMed] [Google Scholar]
- 23.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16(3):144–158. [Google Scholar]
- 24.Ahmed J, Güvenç A, Küçükboyaci N, Baldemir A, Coşkun M. Total phenolic contents and antioxidant activities of Prangos Lindl. (Umbelliferae) species growing in Konya province (Turkey) Turkish Journal of Biology. 2011;35(3):353–360. [Google Scholar]
- 25.Baravalia Y, Kaneria M, Vaghaslya Y, Parekh J, Chanda S. Antioxidant and antibacterial activity of Diospyros ebenum roxb. Leaf extracts. Turkish Journal of Biology. 2009;33(2):159–164. [Google Scholar]
- 26.Raafat KM, Jassar H, Aboul-Ela M, El-Lakany A. Protective effects of Origanum majorana L. against neurodegeneration: fingerprinting, isolation and in vivo glycine receptors behavioral model. Inernational Journal of Phytomedicine. 5(1):46–57. [Google Scholar]
- 27.Slinkard K, Singleton VL. Total phenols analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture. 1977;28:49–55. [Google Scholar]
- 28.Raafat K, Breitinger U, Mahran L, Ayoub N, Breitinger H. Synergistic inhibition of glycinergic transmission in vitro and in vivo by flavonoids and strychnine. Toxicological Sciences. 2010;118(1):171–182. doi: 10.1093/toxsci/kfq245. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan KA, Hayes JM, Wiggin TD, et al. Mouse models of diabetic neuropathy. Neurobiology of Disease. 2007;28(3):276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulugol A, Oltulu C, Gunduz O, et al. 5-HT7 receptor activation attenuates thermal hyperalgesia in streptozocin-induced diabetic mice. Pharmacology Biochemistry and Behavior. 2012;102(2):344–348. doi: 10.1016/j.pbb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Yasmineh WG, Kaur TP, Blazar BR, Theologides A. Serum catalase as marker of graft-vs-host disease in allogeneic bone marrow transplant recipients: pilot study. Clinical Chemistry. 1995;41(11):1574–1580. [PubMed] [Google Scholar]
- 32.Said G. Diabetic neuropathy—a review. Nature Clinical Practice Neurology. 2007;3(6):331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 33.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widenfalk J, Wu W, Hao J, Person JKE, Wiesenfeldt-Hallin Z, Risling M. Treatment of transected peripheral nerves with artemin improved motor neuron regeneration, but did not reduce nerve injury-induced pain behaviour. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 2009;43(5):245–250. doi: 10.3109/02844310903259082. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Kaur N, Kishore L, Gupta GK. Management of diabetic complications: a chemical constituents based approach. Journal of Ethnopharmacology. 2013;150(1):51–70. doi: 10.1016/j.jep.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 36.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Young IS, Tate S, Lightbody JH, McMaster D, Trimble ER. The effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic rat. Free Radical Biology and Medicine. 1995;18(5):833–840. doi: 10.1016/0891-5849(94)00202-u. [DOI] [PubMed] [Google Scholar]
- 38.Sepici-Dincel A, Açikgöz Ş, Çevik C, Sengelen M, Yeşilada E. Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. Journal of Ethnopharmacology. 2007;110(3):498–503. doi: 10.1016/j.jep.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes -causes, effects and coping strategies. Diabetes, Obesity and Metabolism. 2007;9(6):799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 40.Latha RCR, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chemico-Biological Interactions. 2011;189(1-2):112–118. doi: 10.1016/j.cbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Al-Azzawie HF, Alhamdani MS. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sciences. 2006;78(12):1371–1377. doi: 10.1016/j.lfs.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Tosun F, Kizilay ÇA. Anthraquinones and flavonoids from Rheum ribes . Ankara Universitesi Eczacilik Fakultesi Dergisi. 2003;32(1):31–35. [Google Scholar]
- 43.Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187(2):363–371. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]


