Abstract
Background
Adenosine A2A receptors (A2ARs) are enriched in the striatum but are also present at lower levels in the extra-striatal forebrain (i.e., hippocampus, cortex), integrating dopamine, glutamate, and brain-derived neurotrophic factor (BDNF) signaling, and are thus essential for striatal neuroplasticity and fear and anxiety behavior.
Methods
We tested two brain region-specific A2AR knockout lines with A2ARs selectively inactivated either in the striatum only (st-A2AR KO) or the entire forebrain (striatum, hippocampus, and cortex, fb-A2AR KO) on fear and anxiety-related responses. We also examined the effect of hippocampus-specific A2AR deletion by local injection of AAV5-Cre into conditional (floxed)-A2AR knockout mice.
Results
Selective deletion of striatal A2ARs in st-A2AR KO mice increased Pavlovian fear conditioning (both context and tone), but when the A2AR deletion was extended to include extra-striatal regions in fb-A2AR KO mice, context fear conditioning was normalized and tone fear conditioning was attenuated. Moreover, focal deletion of hippocampal A2ARs by AAV5-Cre injection selectively attenuated context (but not tone) fear conditioning. Deletion of A2ARs in the entire forebrain in fb-A2AR KO mice also produced an anxiolytic phenotype in both the elevated plus maze and open field tests and increased the startle response. These extra-striatal forebrain A2AR behavioral effects were associated with reduced BDNF levels in the fb-A2AR KO hippocampus.
Conclusions
This study provides the first evidence that inactivation of striatal A2ARs facilitates Pavlovian fear conditioning while inactivation of extra-striatal A2ARs in the forebrain inhibits fear conditioning and also affects anxiety-related behavior.
Keywords: Adenosine A2A receptor, Fear conditioning, Anxiety, Startle Response, Striatum, Hippocampus, Cortex, BDNF
INTRODUCTION
Conditioned fear predicts aversive events from prior experience and its dysfunction leads to maladaptive fear responses that underlie neuropsychiatric disorders such as post-traumatic stress disorder (PTSD). Adenosine A2A receptors (A2ARs) are abundantly expressed in striatopallidal neurons where they interact with dopamine, glutamate and CB1 receptor signaling to modulate striatal neuroplasticity underlying mnemonic and mood-related behaviors (1). A2ARs are also present in the extra-striatal forebrain, namely in the hippocampus and cortex (2), where they partake in hippocampal (3, 4) and corticostriatal synaptic plasticity (5, 6). The ability of A2ARs to control brain-derived neurotrophic factor (BDNF) levels and to transactivate their TrkB receptors emerges as a candidate mechanism for A2AR-dependent modulation of emotional behavior (7). Thus, A2ARs are uniquely positioned to modulate multiple parallel pathways implicated in mnemonic and emotional processes (8, 9). However, it is not yet established if A2ARs can control fear behavior, and the limited studies on the impact of pharmacological (10–13) or genetic manipulations of A2ARs (13, 14) on avoidance responses or anxiety have produced largely inconclusive results. This is attributed to the difficulty in disentangling the: (i) different contributions of distinct brain regions to emotional responses; (ii) potentially different effects of A2ARs on different emotional processes such as conditioned fear and anxiety; and (iii) different effects of A2ARs in distinct brain regions on brain plasticity related to cognition and emotion. The latter is particularly important since A2ARs in distinct forebrain regions (such as striatum versus hippocampus and cerebral cortex) exert an opposite control over psychomotor responses (15).
To molecularly dissect the striatal and extra-striatal contributions of forebrain A2ARs to emotional processes, we analyzed conditioned and innate fear responses in two conditional A2AR knockout (KO) lines: st-A2AR KO mice with deletion of A2ARs restricted to the striatum and fb-A2AR KO mice with A2AR deletion extended beyond the striatum to the entire forebrain, including cerebral cortex and hippocampus. We found that A2AR deletion in striatal neurons enhanced context and tone fear conditioning without affecting anxiety-like behavior. Deleting the A2AR in the entire forebrain or focal deletion of hippocampal A2AR normalized or attenuated context and tone fear conditioning while also producing an anxiolytic phenotype. We conclude that striatal and extra-striatal A2ARs may exert opposite control over fear conditioning, prompting consideration of forebrain A2ARs as novel therapeutic targets to manage maladaptive fear responses.
METHODS AND MATERIALS
All procedures were approved by the IACUCs at Boston University School of Medicine, the Legacy Research Institute, the Cantonal Veterinary Office in Zurich, and the Faculty of Medicine of the University of Coimbra.
Generation of forebrain-specific and striatum-specific A2AR knockout mice
Forebrain-specific A2AR knockout mice [fb-A2AR KO, CaMKII-Cre(+)A2ARflox/flox, near congenic >97% C57BL/6J genetic background, for both fb-A2AR KO and fb-WT (CaMKII-Cre(−)A2ARflox/flox)] were generated as previously detailed (15–17). Striatum-specific A2AR knockout mice [st-A2AR KO, Dlx5/6-Cre(+)A2ARflox/flox, F5 generation of a mixed 129-Steel-C57BL/6-FVB genetic background, for both st-A2AR KO and st-WT (Dlx5/6-Cre(−)A2ARflox/flox)] mice were also generated as previously described (15, 17). Fb-A2AR KO and st-A2AR KO mice were characterized by their selective deletion of A2ARs in the forebrain or exclusively in striatal neurons, as validated by: (i) X-gal staining of a Cre-expressing Rosa26 reporter transgenic line; (ii) PCR analysis of Cre-mediated A2AR deletion; (iii) A2AR immunohistochemistry localization; and (iv) A2AR binding with 3H-ZM241385 (15, 16, 18, 19).
Intra-hippocampal injection of AAV5-Cre into conditional (floxed)-A2AR mice
Either AAV5-CMV-Cre-GFP (Vector BioLabs, 2 µl of 1×1012 genome copies/mL) or its control viral vector (AAV5-CMV-GFP) were injected stereotaxically into both hippocampi of conditional (floxed)-A2AR adult mice (AP=−2.5 mm from bregma, ML=±2.0 mm from midline, and DV=−1.5 mm from the skull surface). Mice were tested for conditioned freezing three weeks later.
Fluorescence immunohistochemistry
Mice were anesthetized with Avertin [2% 2,2,2-tribromoethanol and 1% amyl alcohol]. Brains were transcardially perfused with 4% paraformaldehyde in PBS, post-fixed, and cryopreserved. 30-µm coronal sections (Leica Microsystems) were incubated for 1 h in PBS containing 0.25% Triton X-100 and 5% donkey serum, followed by incubation with mouse anti-mouse NeuN (1:1,000; Millipore) and rabbit anti-GFP (1:1,000; Abcam) antibodies overnight at 4°C. Sections were rinsed 3×10 min in PBS and then incubated with donkey anti-mouse and donkey anti-rabbit secondary antibodies conjugated with Alexa Fluor 488 or 555 (1:200; Invitrogen) for 1 h at room temperature. Sections were rinsed, mounted with Vectashield mounting medium, and examined with a fluorescence Nikon eclipse E600 microscope.
qPCR analysis
Mice were killed by decapitation, hippocampi were homogenized in trizol, RNA was isolated (20), and cDNA was synthesized using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Real-time PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems) using the A2AR primers 5’-TAGCCCTGTGACTGAGTGCATG and 5’-GCTGCTGACCTAGAAGTGG and the GAPDH primers 5’-TGGTCCAGGGTTTCTTACTCC and 5’-AGGTTGTCTCCTGCGACTTCA as the internal control in a realplex4 thermocycler (Eppendorf). The data were analyzed as described previously (20).
Behavioral evaluation
Two cohorts of mice (the ‘forebrain’ cohort comprising 17 fb-A2AR KO mice and 13 fb-WT mice, and the ‘striatal’ cohort comprising 17 st-A2AR KO mice and 17 st-WT mice) were used. A total of eleven tests were conducted using a within-subjects approach in the following chronologic order to minimize transfer effects, as described previously (17): (1) elevated plus maze, (2) accelerating rotarod, (3) open field, (4) Y-maze, (5) acoustic startle response, (6) prepulse inhibition, (7) Pavlovian fear conditioning, (8–11) water maze (visible, working and reference memory, reversal). Here, we report the results derived from the (i) elevated plus maze test of anxiety, (ii) spontaneous acoustic startle response, and (iii) Pavlovian fear conditioning. Another cohort with 9 fb-A2AR KO and 10 fb-WT mice was used to evaluate anxiety in the open field test. Additionally, we used floxed-A2AR mice intra-hippocampally injected with either AAV5-CMV-Cre-GFP (+AAV-CRE, n=7) or AAV5-CMV-GFP (control group, +AAV-CTR, n=7) to evaluate Pavlovian fear conditioning.
Anxiety
Anxiety was assessed using the elevated plus maze (21). While the total distance provided a measure of general locomotor activity, the reluctance to venture into the open arms (i.e., percent time spent in and percent entries into the open arms relative to all arms) comprised the main measures of anxiety. The open field test was also used to evaluate anxiety, measuring the relative time spent in and entries into the central versus peripheral arenas of the activity box (22).
Associative learning: Pavlovian conditioned freezing
The apparatus consisted of two different sets of conditioning chambers to provide two distinct contexts (23). The operant chambers (context A) were from Coulbourn Instruments (Allentown, PA) and contained a grid floor to apply electric shocks (the unconditioned stimulus, US). The second set of chambers (context B) was transparent cylindrical Plexiglas enclosures resting on a white plastic floor. The conditioned stimulus (CS) was an 86 dBA tone. The experiment consisted of three phases: conditioning, context test, and CS (tone) test. Conditioning was conducted in context A by presenting three discrete paired CS-US trials. Each trial began with a 30-s tone CS followed by delivery of a 1-s foot-shock US set at 0.26 mA. Each trial was preceded and followed by a 180-s inter-trial interval. Mice were then returned to the home cage until the context freezing test on the next day when mice were again exposed to context A for 8 min in the absence of any discrete stimulus. On the third day, conditioned freezing to the tone CS was assessed in the neutral context B. Following a 120-s acclimatization period, the tone CS was turned on for 8 min. This CS test procedure was repeated over the next three days to measure extinction of the conditioned freezing response.
In the viral-mediated hippocampal A2AR knockdown experiment, we used a similar protocol with a Gemini system (San Diego Instruments, San Diego, CA). The operant chamber (context A) contained a grid floor to apply electric shocks, and the second chamber (context B) consisted of a brown plastic floor and striped walls. CS extinction was not tested.
Acoustic startle response
The acoustic startle response was examined to provide a measure of emotional reactivity. Whole-body startle response to a sudden acoustic white-noise stimulus was measured using sound-attenuated startle chambers (San Diego Instruments, San Diego, CA) (24). A test session lasted 30 min, consisting of 106 discrete single stimulus trials presented in a pseudorandom order with a variable inter-trial interval (mean=15 s). The stimulus varied among ten possible intensities (69–120 dBA) and two durations: 20 or 40 ms. All stimuli were in the form of a sudden elevation in broadband white noise with a rise time of 0.2–1 ms and were presented once in every successive block of 20 trials. There were five such test blocks. Prior to the 100 test trials, mice were presented with six consecutive 120-dBA trials to allow the startle response to stabilize.
Determination of total BDNF levels in brain regions
The striatum, cerebral cortex, and hippocampus were dissected and stored at −80°C. BDNF was extracted as described (25). Total BDNF levels were determined using an ELISA assay (BDNF Emax from Promega, WI) and are presented as ng/g wet weight tissue. The standard curve was linear between 2.5 and 250 ng of BDNF.
Statistical analysis
Data were analyzed using parametric analysis of variance (ANOVA) or Student’s t-test with genotype and sex as between-subject factors when appropriate. Within-subjects factors (bins, days, arms) were also included for repeated measures analyses when appropriate. Post-hoc pair-wise comparisons were performed to facilitate data interpretation when appropriate.
RESULTS
1. Reduced anxiety-related behaviors in fb-A2AR KO but not in st-A2AR KO mice
Striatal-selective A2AR deletion did not influence anxiety-related behavior, with both st-WT and st-A2AR KO mice showing indistinguishable behavior patterns in the elevated plus maze [F’s<1] (Figures 1A,B). However, A2AR deletion in the entire forebrain reduced anxiety-related behavior in the elevated plus maze (Figures 1D,E) and in the open field paradigm (Figures 1G,H). Thus, fb-A2AR KO mice spent a greater proportion of time in (Figure 1D) and entered more into (Figure 1E) the open arms than did fb-WT mice: ANOVA showed a genotype effect on both the percent time spent in [F(1,26)=7.58, p<0.05] and the percent frequency of entries into [F(1,26)=5.23, p<0.05] the open arms. This is likely independent of the locomotor pattern since an independent analysis of the total distance traveled failed to yield any genotypic difference in the st-A2AR KO [F(1,30)=1.87, p=0.18] (Figure 1C) or fb-A2AR KO [F(1,26)=2.42, p=0.13] (Figure 1F) cohorts. A separate cohort of fb-A2AR KO mice also displayed an anxiolytic profile in an open field test, with more time spent in [t=2.84, df=15, p<0.05] and more entries into [t=2.48, df=17, p<0.05] the center arena compared to fb-WT mice (Figures 1G,H), despite exhibiting comparable total open field locomotion [t=1.20, df=17, p=0.25] (Figure 1I). These results suggest that the suppression of extra-striatal hippocampal and/or cortical A2ARs but not striatal A2ARs may reduce anxiety.
Figure 1. Reduced anxiety-like behavior on the elevated plus maze and open field tests in fb-A2AR KO but not in st-A2AR KO mice.
To measure unlearned emotional or fear-related processing, anxiety-related behavior was evaluated in the elevated plus maze (EPM) for 5 min in naïve st-A2AR KO and fb-A2AR KO mice (A–F). Further evaluation in the open field for 8 min in a separate cohort of naïve fb-A2AR KO mice was performed (G–I). EPM (st-A2AR KO and fb-A2AR KO): (A) St-A2AR KO and st-WT mice spent a similar percentage of time in the open arms of the maze. (B) Likewise, st-A2AR KO and st-WT mice entered into the open arms of the maze at a similar frequency compared to the closed arms of the maze. (C) St-A2AR KO and st-WT mice traveled a similar distance on the maze. (D) Fb-A2AR KO mice spent a significantly greater proportion of time in the open arms relative to the closed arms, suggesting reduced anxiety compared to fb-WT mice. (E) Fb-A2AR KO mice also entered into the open arms proportionally more than the closed arms compared to fb-WT mice, further supporting reduced anxiety-like behavior in fb-A2AR KO mice. (F) Fb-A2AR KO and fb-WT mice traveled a similar distance on the maze. Open field (fb-A2AR KO): Fb-A2AR KO mice also showed reduced anxiety-related behavior in the open field. (G) Fb-A2AR KO mice spent more time in the center arena compared to fb-WT mice. (H) Fb-A2AR KO mice also entered into the center arena more frequently compared to fb-WT mice. (I) Fb-A2AR KO and fb-WT mice traveled a similar distance in the open field. Data are mean ± SEM. *p<0.05, fb-A2AR KO vs fb-WT. EPM: st-A2AR KO, n=17; st-WT, n=17; fb-A2AR KO, n=17; fb-WT, n=13. Open field: fb-A2AR KO, n=9; fb-WT, n=10.
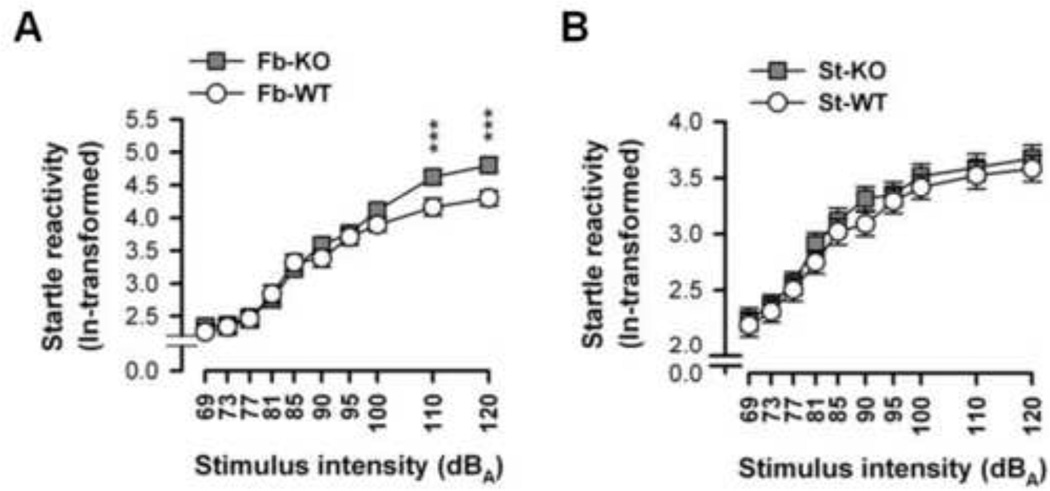
2. Increased acoustic startle response in fb-A2AR KO but not in st-A2AR KO mice
Fb-A2AR KO mice displayed higher startle responses to higher intensity stimuli compared to fb-A2AR WT mice (Figure 2A). ANOVA revealed an effect of stimulus intensity [F(9,234)=162.26, p<0.0001] and a genotype × stimulus intensity interaction [F(9,234)=2.47, p=0.01], but no genotype effect [p=0.22]. Although all mice gradually responded more to startle stimuli of increasing intensity, irrespective of stimulus duration [p=0.99], post-hoc comparisons revealed that fb-A2AR KO mice startled more to the two most intense stimuli (110 and 120 dBA) relative to fb-WT mice [p’s<0.001]. This increased startle response in fb-A2AR KO, but not in st-A2AR KO (Figure 2B), suggests that suppression of extra-striatal hippocampal and/or cortical A2ARs but not striatal A2ARs heightens startle reactivity.
Figure 2. Increased startle response in fb-A2AR KO but not in st-A2AR KO mice.
The acoustic startle response (ln-transformed) ± SEM to ten acoustic stimuli of increasing intensity is depicted (collapsed across stimuli durations of 20 and 40 ms). Startle reactivity increased monotonically as a function of stimulus intensity. (A) Compared to fb-WT mice, fb-A2AR KO mice exhibited increased startle responses to the two highest stimulus intensities (110 and 120 dBA). (B) In contrast, st-A2AR KO mice and st-WT mice displayed similar sensitivity to acoustic stimuli. ***p< 0.001, fb-A2AR KO vs fb-WT. st-A2AR KO, n=17; st-WT, n=17; fb-A2AR KO, n=17; fb-WT, n=13.
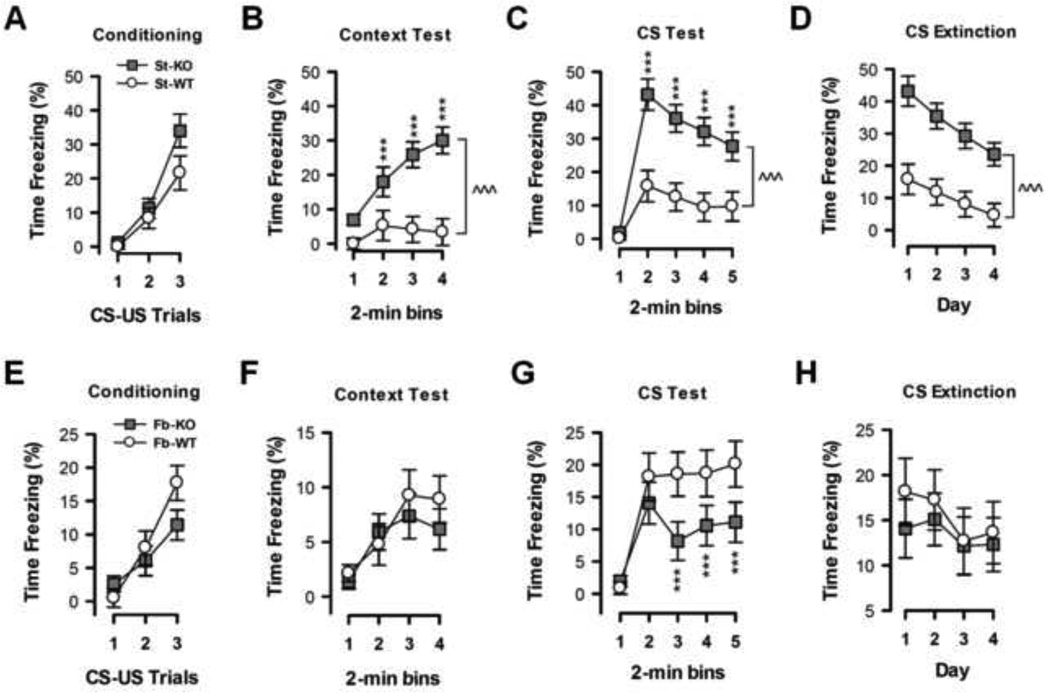
3. Effects of A2AR deletion in the striatum or in the entire forebrain on fear conditioning
(a) Preserved conditioned fear acquisition (Conditioning, Day 1)
Forebrain or striatal A2AR deletion did not affect shock responsiveness or perception and also did not alter the formation of the CS-US association or the execution of the conditioned freezing response (Figures 3A,E and Supplementary Materials).
Figure 3. Opposite effects of selective deletion of A2ARs in the striatum or in the entire forebrain on fear conditioning.
Conditioned freezing (% Time) during conditioning acquisition (day 1), context test (day 2), and tone conditioned stimulus (CS) tests (days 3–6) are shown. (A, E) Conditioning: Freezing responses to three repeated presentations of a 30-s CS paired with an unconditioned stimulus (1-s foot-shock US) are illustrated. All mice showed a comparable increase in freezing with each successive CS-US-paired trial. (B, F) Context Test: Freezing responses to the conditioning context in the absence of the CS one day later are illustrated. St-A2AR KO mice exhibited more pronounced freezing throughout the entire 8-min test relative to st-WT mice while fb-A2AR KO mice exhibited indistinguishable freezing from fb-WT mice. (C, G) CS Test: Freezing responses during four consecutive daily CS tests in a new context are illustrated; each test included a 2-min CS-free period followed by an 8-min CS presentation. St-A2AR KO mice consistently showed a marked increase in CS freezing compared to st-WT mice; by contrast, fb-A2AR KO mice consistently showed a reduced CS freezing response compared to fb-WT mice. (D, H) CS Extinction: Freezing responses (daily 2-min bin mean) to the repeated presentation of only the CS across the 4 test days are depicted. The observed reduction in freezing across days suggests extinction, which was comparable between st-A2AR KO, fb-A2AR KO mice, and their corresponding WT littermates. Data are mean ± SEM. *p<0.05, ***p<0.005, st-A2AR KO vs st-WT or fb-A2AR KO vs fb-WT. st-A2AR KO, n=17; st-WT, n=17; fb-A2AR KO, n=17; fb-WT, n=13.
(b) Enhanced context fear conditioning in st-A2AR KO mice (Context test, Day 2)
Re-exposure to the same context one day after conditioning in the absence of the tone CS or shock-US induced a freezing behavior in all mice that increased over bins (Figures 3B,F). The relatively weak (10% of freezing time) magnitude of the context fear conditioned response in WT mice offers the opportunity for detecting a potentially enhanced fear response in st-A2AR KO since inactivation of striatal A2ARs is associated with a pro-cognitive effect (17). Indeed, ANOVA of the percent time freezing revealed only a bins effect [F(3,78)=12.97 p<0.0001] without any genotype-dependent effect in fb-A2AR KO mice (Figure 3F), whereas for st-A2AR KO mice there was an effect of genotype [F(1,30)=21.44, p<0.001], bins [F(3,90)=8.89, p<0.0001], and their interaction [F(3,90)=5.02, p<0.01] (Figure 3B). This indicates that context freezing was enhanced by striatal A2AR deletion, an effect that was abrogated by the additional deletion of extra-striatal A2ARs in the forebrain. This suggests that A2ARs in striatal neurons versus A2ARs in the rest of the forebrain (including hippocampus or cortex) modified context conditioned freezing in opposite directions. Lastly, ANCOVA using the total distance traveled during the open field test as covariate (see Supplemental Materials and Supplemental Figures 1A,B) showed that the enhanced conditioned freezing phenotype of st-A2AR KO mice was not attributable to hypo-locomotion (26).
(c) Impaired tone fear conditioning in fb-A2AR KO mice but enhanced tone fear conditioning in st-A2AR KO mice (Tone CS test, Day 3)
The next day, mice were placed in a novel context and presented with the tone CS. While this elicited freezing in all groups of mice, the freezing response was significantly reduced in fb-A2AR KO mice and enhanced in st-A2AR KO mice (Figures 3C,G). ANOVA in fb-A2AR KO and fb-WT mice revealed a bins effect [F(4,104)=23.17, p<0.0001] and genotype × bins interaction [F(4,104)=3.33, p<0.05]. Post-hoc comparisons revealed that the different levels of freezing emerged in the last six minutes of testing [p’s<0.005] (Figure 3G). ANOVA in st-A2AR KO and st-WT mice found effects of genotype [F(1,30)=17.94, p<0.0001], bins [F(4,120)=34.79, p<0.0001], and their interaction [F(4,120)=7.53, p<0.0001] (Figure 3C). These data suggest that tone conditioned freezing is potentiated in st-A2AR KO mice but attenuated in fb-A2AR KO mice. ANCOVA using either the percent time spent in the open arms on the elevated plus maze or the startle response as covariate (Supplemental Materials) showed that the reduction in CS-induced freezing observed in fb-A2AR KO mice was not solely attributable to changes in general emotional dysfunction but perhaps to another, possibly mnemonic, process.
(d) Normal progression of tone fear extinction (Tone CS extinction test, Days 3–6)
Extinction of the conditioned freezing response was examined by repeated presentation of the tone CS in the same neutral context over four consecutive days. This led to a similar reduced conditioned freezing response in both cohorts (Figures 3D,H). ANOVA of the percent time freezing confirmed this impression in both the forebrain [Days: F(3,78)=2.73, p<0.05; Genotype × Days interaction: F<1] and striatal [Days: F(3,90)=15.99, p<0.0001; Genotype × Days interaction: F(3,90)=1.21, p=0.31] cohorts, thereby indicating that fear extinction was not affected in fb-A2AR KO or st-A2AR KO mice.
4. Intra-hippocampal injection of AAV-Cre into floxed-A2AR mice attenuated context (but not tone) fear conditioning
Fluorescence GFP histology confirmed that the intra-hippocampal AAV5-CMV-GFP injection was largely restricted to the hippocampus (Figure 4A), and qPCR analysis demonstrated reduced A2AR mRNA expression in the hippocampus of floxed-A2AR mice injected with AAV5-CMV-Cre-GFP (Figure 4B). During the conditioning phase, AAV-CRE and AAV-CTR groups similarly acquired fear conditioning [ANOVA: Treatment: F(1,36)=0.33, p=0.57; Trials: F(2,36)=39.66, p<0.0001] (Figure 4C). During context conditioning the next day, the AAV-CRE group displayed significantly weaker fear responses compared to the AAV-CTR group [ANOVA: Treatment: F(1,40)=29.62, p<0.0001; Bins: F(3,40)=0.24, p=0.86; Treatment × Bins interaction: F(3,40)=0.24, p=0.87] (Figure 4D). However, tone-induced fear responses indistinguishable between groups [ANOVA: Treatment: F(1,48)=0.38, p=0.54] (Figure 4E). Thus, deletion of A2ARs restricted to the hippocampus by local AAV5-CMV-Cre-GFP infusion selectively reduced context (but not tone) fear conditioning.
Figure 4. Deletion of hippocampal A2ARs by local injection of AAV5-CMV-Cre-GFP into conditional (floxed)-A2AR mice attenuates context (but not tone) fear conditioning.
AAV5-CMV-Cre-GFP (+AAV-CRE) or AAV5-CMV-GFP (+AAV-CTR) were injected into both hippocampi of floxed-A2AR mice. Fluorescence histology and qPCR analyses were conducted to confirm selective AAV5-mediated A2AR deletion in hippocampus. Fear conditioning acquisition, context and tone tests were conducted three weeks after injection. (A) Fluorescence GFP histology in the hippocampus of an AAV-CTR group mouse shows restricted infection of the hippocampus. (B) qPCR analysis of A2AR mRNA in hippocampus shows reduced A2AR expression in AAV-CRE mice. (C) Acquisition of fear responses was similar between the two treatment groups. (D) The context fear response was attenuated in the AAV-CRE group compared to the AAV-CTR group. (E) Tone fear conditioning was indistinguishable between groups. Data are mean ± SEM. *p<0.05, ^^^p<0.001, AAV-CRE vs AAV-CTR. n=7 per group.
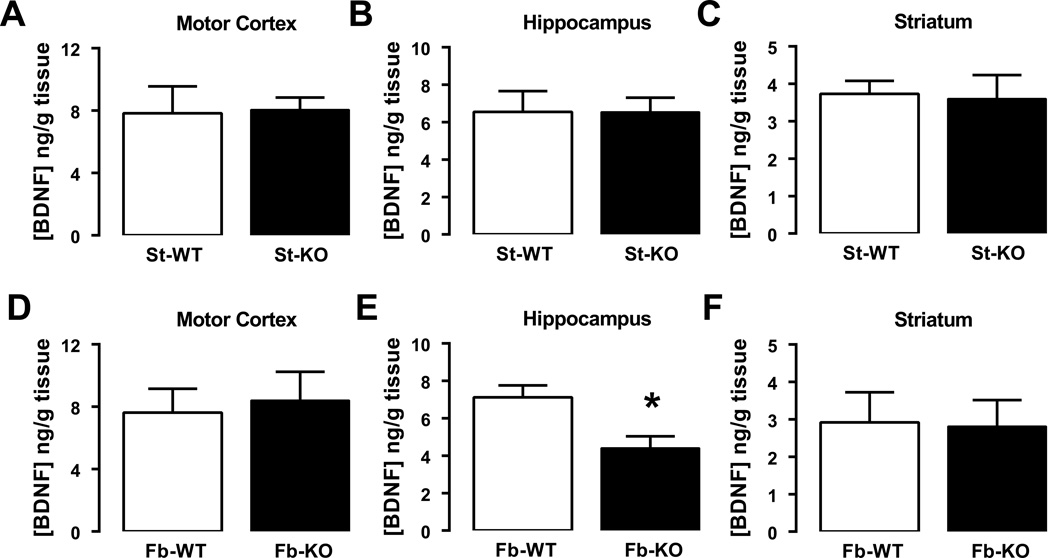
5. Altered fear and anxiety-like behaviors in fb-A2AR KO mice are associated with a selective reduction of BDNF levels in hippocampus
Since A2ARs control BDNF levels and can transactivate their TrkB receptors, and since BDNF levels in specific brain regions are coupled with aversive memory (27), we hypothesized that the A2AR-dependent modulation of fear and anxiety behaviors might be associated with altered forebrain BDNF levels. Indeed, BDNF levels were reduced in the hippocampus but not in the striatum or motor cortex in fb-A2AR KO mice compared to their WT littermates [t-test for hippocampus: t=3.03, df=10, p<0.05] (Figures 5A–F). No changes in BDNF levels were detected in st-A2AR KO mice compared to their WT littermates.
Figure 5. Brain-derived neurotrophic factor (BDNF) levels are selectively reduced in the hippocampus of fb-A2AR KO but not of st-A2AR KO mice.
The average quantification of BDNF levels in different brain regions, namely the motor cortex (A, D), hippocampus (B, E) and striatum (C, G), of fb-A2AR KO and WT mice (A–C) and str-A2AR KO and WT mice (D–F), are depicted. Total BDNF levels were quantified by ELISA. Data are mean ± SEM. *p<0.05 compared to the respective WT mice by Student’s t-test. n=5–6 per group.
DISCUSSION
Striatopallidal A2ARs inhibit Pavlovian fear conditioning
This is the first study, to the best of our knowledge, showing that striatal A2ARs regulate Pavlovian learned fear. Selective deletion of striatal A2ARs enhanced the conditioned freezing response to the tone CS and to the shocked context without appreciably altering the acquisition or extinction of the conditioned response. Importantly, these enhanced responses of st-A2AR KO mice cannot be attributed to general emotional hyper-reactivity, abnormal fear processing, or enhanced shock detection/reactivity, and the effect remained when the confounding hypolocomotor activity effect was statistically controlled. Since we excluded differences in extinction, the stronger conditioned freezing response in st-A2AR KO mice may reflect an enhanced learned fear response involving enhanced mnemonic processes, which might indicate a strong memory trace of the learned stimulus- and context-shock associations. This notion is consistent with the pro-cognitive effects seen after genetic and pharmacological inactivation of A2ARs in enhancing working memory (19, 28) and reversing age-related and pathologic memory loss (8, 9). This striatal A2AR-mediated inhibition may involve the well-documented striatal D2R-A2AR antagonism, since bolstering striatal dopamine activity, through intra-accumbal injection of amphetamine (29) or a D2R agonist (30) enhances learned fear. A similar mechanism might also underlie the inhibitory control by the activation of striatopallidal neurons over drug reward behavior (31, 32) and psychomotor sensitization (33). Our finding that striatopallidal A2ARs exert an inhibitory control over fear conditioning fits in the emerging concept that the striatopallidal pathway exerts a broad inhibitory effect on diverse types of behavior, suggesting that the striatopallidal indirect pathway may function as a “break” mechanism in the control of behavioral responses. However, this interpretation is not consistent with a recent report that selective inhibition of neurotransmission from the striatopallidal neurons attenuated fear conditioning (34). Additional studies are required to clarify this issue.
Inactivation of extra-striatal A2ARs in the forebrain counters the actions of striatal A2ARs on Pavlovian fear conditioning
When A2AR deletion was extended beyond the striatum to cortical and hippocampal regions, the impact of striatal A2AR inactivation on context and tone fear conditioning was normalized or reversed. Indeed, forebrain A2AR elimination impaired, rather than enhanced, tone fear conditioning though sparing normal context freezing. Although the genetic backgrounds differ between forebrain and striatum KO lines, which may account for the difference in freezing levels observed between fb-WT and st-WT mice, comparison of each conditional KO line against its respective littermate WT controls clearly demonstrates the opposite impacts on fear conditioning in the two KO lines.
These opposite phenotypes may be attributable to hippocampal, cortical, or amygdalar A2ARs. In particular, AAV5-Cre experiments revealed the role of hippocampal A2ARs in controlling context (but not tone) fear conditioning. Conversely, cortical A2ARs may enhance tone fear conditioning since inactivating perirhinal, parietal, or cingulate cortical activity selectively impairs fear tone conditioning (35–37). A2ARs in the amygdala also might contribute given the role of this brain region in fear responses. Additional analyses with focal deletion of prefrontal cortical and amygdalar A2ARs by AAV-Cre may identify the forebrain structure(s) where the loss of A2AR overrides the opposite impact of striatal A2AR deletion.
Extra-striatal A2ARs in the forebrain attenuate anxiety-like behavior but increase startle response
When A2AR deletion was restricted to the striatum, no change in anxiety-like behavior was observed. When the deletion was extended to the cortex and hippocampus in fb-A2AR KO mice, anxiolytic behavior in the elevated plus maze and the open field was observed. This finding is consistent with the hippocampus and cortex as relevant regions controlling anxiety (38–40). However, anxiolysis seen following forebrain A2AR constitutive inactivation contrasts with the anxiogenic effect of acute caffeine (a non-selective adenosine receptor antagonist) (41) and with genetic association studies between the A2AR gene and panic disorder (42–44). A2ARs in brain regions beyond the forebrain need to be examined to clarify this issue.
It is well known that a fearful state or heightened anxiety can potentiate startle reactions. The acoustic startle response was selectively increased only at the two highest stimulus intensities in fb-A2AR KO mice while no effect was found in st-A2AR KO mice. Such an intensity-dependent enhancement most likely reflects changes in the maximal startle response rather than an anxiogenic phenotype (45–47). The lack of a startle phenotype in st-A2AR KO mice is also consistent with the null-effect of intra-accumbal infusions of A2AR ligands on startle responses (48, 49). Notably, forebrain A2AR-dependent regulation of acoustic startle and elevated plus maze behaviors resemble the behavioral phenotype induced by septal lesions, which produced an ‘anxiolytic’ effect in the elevated plus maze and 'anxiogenic' stronger acoustic startle responses (e.g., 50). Thus, forebrain A2AR deletion may induce these seemingly paradoxical phenotypes via an effect on the septum.
A2AR-dependent control of conditioned and unconditioned fear behavior in extra-striatal forebrain is associated with selective alteration of BDNF levels
BDNF modulation of synaptic plasticity (51, 52) may underlie the BDNF-mediated control of conditioned fear and anxiety. Clearly, there are different pools of BDNF in the hippocampus, resulting from different transcripts expressed in different cellular and subcellular compartments, possibly fulfilling different functional roles. It is therefore likely and understandable that the ablation of the BDNF gene (eliminating all BDNF) should produce an impact different from the putative selective reduction of a particular pool of BDNF. Recently, several studies have reported that BDNF levels in the dorsal hippocampus are associated with increased emotional reactivity (53), context fear conditioning (54, 55), anxiety (55–57), and altered behavior in PTSD animal models (58, 59). Notably, the effect of BDNF on hippocampal synaptic plasticity requires A2AR activation (7) whereas global A2AR inactivation decreases BDNF levels (60), reduces the activation of the major BDNF receptor (TrkB), and impairs BDNF-dependent-LTP in the hippocampus (61, 62). Thus, hippocampal A2AR deletion might impair fear conditioning by interfering with the BDNF-TrkB pathway. This observation is consistent with the present finding that a selective BDNF reduction in the hippocampus is associated with reduced tone fear conditioning and anxiety behavior in fb-A2AR KO (but not in st-A2AR KO) mice. However, the differential modifications of context fear learning upon elimination of A2ARs in the forebrain (fb-A2AR KO) and selectively in the hippocampus (by viral approach) clearly indicates that the reduction of BDNF in the hippocampus of fb-A2AR KO mice is likely not causally related to increased tone freezing. Other mechanisms apart from the control of hippocampal BDNF levels are involved in extra-striatal A2AR-control of fear responses. Future studies with rescue experiments (e.g. augmenting BDNF expression) in the hippocampus and additional related brain regions like nucleus accumbens, prefrontal cortex, and lateral and central amygdala in fb-A2AR KO mice would be required to establish a causal link between the reduction in BDNF in distinct forebrain regions and the fear conditioning phenotype in fb-A2AR KO mice.
In summary, we have demonstrated distinctive roles of striatal and extra-striatal A2ARs in the control of conditioned and unconditioned fear behavior. Inactivation of striatal A2ARs facilitates Pavlovian fear conditioning while inactivation of extra-striatal A2ARs in the forebrain inhibits fear conditioning and also affects anxiety-related behavior. Our work also identifies the previously under-appreciated A2ARs in cortex and hippocampus as contributing regulators of conditioned fear, anxiety, and startle response with a parallel reduction of hippocampal BDNF. Enhanced fear conditioning and increased startle response are important behavioral elements in animal models for PTSD (61, 62). We demonstrate that selective deletion of A2ARs in the forebrain yields alterations in all three of the defining features of PTSD in rodent models (i.e., fear conditioning, startle response, and anxiety). Thus, targeting forebrain A2ARs may provide a novel therapeutic strategy for PTSD.
Supplementary Material
ACKNOWLEDGMENTS
Supported by grants from NIH (R01MH083973, RO1NS48995, RO1NS41083-07, RO1DA19362), the Department of Defense (W81XWH-071-1-0012, DARPA-BAA-09-68), and FCT (PTDC/SAU-NEU/108668/2008, PTDC/SAU-NSC/122254/2010).
Footnotes
DISCLOSURES/CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Progress in neurobiology. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Progress in neurobiology. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 3.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Costenla AR, Diogenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, et al. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. The European journal of neuroscience. 34:12–21. doi: 10.1111/j.1460-9568.2011.07719.x. [DOI] [PubMed] [Google Scholar]
- 5.d'Alcantara P, Ledent C, Swillens S, Schiffmann SN. Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience. 2001;107:455–464. doi: 10.1016/s0306-4522(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 6.Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, et al. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nature neuroscience. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastiao AM, Ribeiro JA. Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. British journal of pharmacology. 2009;158:15–22. doi: 10.1111/j.1476-5381.2009.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alzheimers Dis. 2010;20(Suppl 1):S95–S116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- 10.Dall'igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Experimental neurology. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Kopf SR, Melani A, Pedata F, Pepeu G. Adenosine and memory storage: effect of A(1) and A(2) receptor antagonists. Psychopharmacology (Berl) 1999;146:214–219. doi: 10.1007/s002130051109. [DOI] [PubMed] [Google Scholar]
- 12.Pereira GS, Rossato JI, Sarkis JJ, Cammarota M, Bonan CD, Izquierdo I. Activation of adenosine receptors in the posterior cingulate cortex impairs memory retrieval in the rat. Neurobiol Learn Mem. 2005;83:217–223. doi: 10.1016/j.nlm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology (Berl) 2000;148:153–163. doi: 10.1007/s002130050037. 150.htm. [DOI] [PubMed] [Google Scholar]
- 14.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 15.Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, et al. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knockouts. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, et al. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:891–900. doi: 10.1038/sj.npp.1300630. [DOI] [PubMed] [Google Scholar]
- 17.Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, et al. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–474. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, et al. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63:338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- 19.Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, et al. Selective inactivation of adenosine A2A receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–474. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Hagenbuch N, Feldon J, Yee BK. Use of the elevated plus-maze test with opaque or transparent walls in the detection of mouse strain differences and the anxiolytic effects of diazepam. Behavioural pharmacology. 2006;17:31–41. doi: 10.1097/01.fbp.0000189811.77049.3e. [DOI] [PubMed] [Google Scholar]
- 22.Pietropaolo S, Mintz M, Feldon J, Yee BK. The behavioral sequela following the prevention of home-cage grid-climbing activity in C57BL/6 mice. Behav Neurosci. 2007;121:345–355. doi: 10.1037/0735-7044.121.2.345. [DOI] [PubMed] [Google Scholar]
- 23.Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. The European journal of neuroscience. 2007;26:3237–3252. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 24.Yee BK, Chang DL, Feldon J. The Effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1865–1877. doi: 10.1038/sj.npp.1300480. [DOI] [PubMed] [Google Scholar]
- 25.Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, Andrews AM. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods. 2004;140:81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Singer P, Zhang CC, Boison D, Yee BK. Dysregulation of brain adenosine is detrimental to the expression of conditioned freezing but not general Pavlovian learning. Pharmacology, biochemistry, and behavior. 2013 doi: 10.1016/j.pbb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma L, Wang DD, Zhang TY, Yu H, Wang Y, Huang SH, et al. Region-specific involvement of BDNF secretion and synthesis in conditioned taste aversion memory formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2079–2090. doi: 10.1523/JNEUROSCI.5348-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, et al. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 29.White NM, Salinas JA. Mnemonic functions of dorsal striatum and hippocampus in aversive conditioning. Behav Brain Res. 2003;142:99–107. doi: 10.1016/s0166-4328(02)00402-3. [DOI] [PubMed] [Google Scholar]
- 30.White NM, Viaud M. Localized intracaudate dopamine D2 receptor activation during the post-training period improves memory for visual or olfactory conditioned emotional responses in rats. Behav Neural Biol. 1991;55:255–269. doi: 10.1016/0163-1047(91)90609-t. [DOI] [PubMed] [Google Scholar]
- 31.Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012;31:640–653. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Biedenkapp JC, Rudy JW. Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn Mem. 2009;16:38–45. doi: 10.1101/lm.1099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, et al. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biological psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C. Differential contribution of some cortical sites to the formation of memory traces supporting fear conditioning. Exp Brain Res. 2002;146:223–232. doi: 10.1007/s00221-002-1165-y. [DOI] [PubMed] [Google Scholar]
- 38.McHugh SB, Fillenz M, Lowry JP, Rawlins JN, Bannerman DM. Brain tissue oxygen amperometry in behaving rats demonstrates functional dissociation of dorsal and ventral hippocampus during spatial processing and anxiety. The European journal of neuroscience. 33:322–337. doi: 10.1111/j.1460-9568.2010.07497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE, et al. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology. 2004;29:558–565. doi: 10.1038/sj.npp.1300311. [DOI] [PubMed] [Google Scholar]
- 43.Hohoff C, Mullings EL, Heatherley SV, Freitag CM, Neumann LC, Domschke K, et al. Adenosine A(2A) receptor gene: evidence for association of risk variants with panic disorder and anxious personality. J Psychiatr Res. 2010;44:930–937. doi: 10.1016/j.jpsychires.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Deckert J, Nothen MM, Franke P, Delmo C, Fritze J, Knapp M, et al. Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Molecular psychiatry. 1998;3:81–85. doi: 10.1038/sj.mp.4000345. [DOI] [PubMed] [Google Scholar]
- 45.Hince DA, Martin-Iverson MT. Differences in prepulse inhibition (PPI) between Wistar and Sprague-Dawley rats clarified by a new method of PPI standardization. Behavioral neuroscience. 2005;119:66–77. doi: 10.1037/0735-7044.119.1.66. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Iverson MT, Stevenson KN. Apomorphine effects on emotional modulation of the startle reflex in rats. Psychopharmacology (Berl) 2005;181:60–70. doi: 10.1007/s00213-005-2217-3. [DOI] [PubMed] [Google Scholar]
- 47.Stoddart CW, Noonan J, Martin-Iverson MT. Stimulus quality affects expression of the acoustic startle response and prepulse inhibition in mice. Behavioral neuroscience. 2008;122:516–526. doi: 10.1037/0735-7044.122.3.516. [DOI] [PubMed] [Google Scholar]
- 48.Hauber W, Koch M. Adenosine A2a receptors in the nucleus accumbens modulate prepulse inhibition of the startle response. Neuroreport. 1997;8:1515–1518. doi: 10.1097/00001756-199704140-00038. [DOI] [PubMed] [Google Scholar]
- 49.Nagel J, Schladebach H, Koch M, Schwienbacher I, Muller CE, Hauber W. Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse. 2003;49:279–286. doi: 10.1002/syn.10240. [DOI] [PubMed] [Google Scholar]
- 50.Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiology of learning and memory. 1995;64:156–168. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- 51.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 52.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 53.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9857–9869. doi: 10.1523/JNEUROSCI.3145-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yee BK, Zhu SW, Mohammed AH, Feldon J. Levels of neurotrophic factors in the hippocampus and amygdala correlate with anxiety- and fear-related behaviour in C57BL6 mice. J Neural Transm. 2007;114:431–444. doi: 10.1007/s00702-006-0548-9. [DOI] [PubMed] [Google Scholar]
- 56.Texel SJ, Camandola S, Ladenheim B, Rothman SM, Mughal MR, Unger EL, et al. Ceruloplasmin deficiency results in an anxiety phenotype involving deficits in hippocampal iron, serotonin, and BDNF. Journal of neurochemistry. 120:125–134. doi: 10.1111/j.1471-4159.2011.07554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R, et al. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. Journal of neurochemistry. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- 58.Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res. 45:460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int J Neuropsychopharmacol. 2007;10:741–758. doi: 10.1017/S1461145707007560. [DOI] [PubMed] [Google Scholar]
- 60.Tebano MT, Martire A, Potenza RL, Gro C, Pepponi R, Armida M, et al. Adenosine A(2A) receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. Journal of neurochemistry. 2008;104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Chao MV, Sebastiao AM. Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 30:8468–8480. doi: 10.1523/JNEUROSCI.5695-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.