Abstract
The accuracy and noninvasive nature of the doubly labeled water (DLW) method makes it ideal for the study of human energy metabolism in free-living conditions. However, the DLW method is not always practical in many developing and Asian countries because of the high costs of isotopes and equipment for isotope analysis as well as the expertise required for analysis. This review provides information about the theoretical background and practical aspects of the DLW method, including optimal dose, basic protocols of two- and multiple-point approaches, experimental procedures, and isotopic analysis. We also introduce applications of DLW data, such as determining the equations of estimated energy requirement and validation studies of energy intake.
Keywords: Doubly labeled water, estimated energy requirement, energy intake
INTRODUCTION
Changing body composition or reducing body weight requires modifications to energy balance, i.e., the balance between energy intake (EI) and energy expenditure (EE): body weight can be reduced when EI is lower than EE over a given period. Thus, measuring EE precisely is important for determining daily food intake targets.
Direct calorimetry measures and records the heat released from a subject in a thermally-isolated chamber. However, direct calorimetry is often impractical. Therefore, indirect calorimetry, which uses CO2 production and/or oxygen consumption measured from subjects, is generally used [1]. However, it is difficult to assess EE in a daily free-living state using indirect calorimetry methods without any interference. Instead, non-calorimetric techniques such as questionnaires, heart rate monitors, motion sensors, and the doubly labeled water (DLW) method are used to obtain various indices of daily physical activities in free-living populations [2]. However, the greatest problems with the use of these non-calorimetric techniques in humans are the errors associated with determining total EE (TEE) and physical activity level (PAL,TEE/basal metabolic rate) in the free-living state. Among the non-calorimetric techniques, the DLW method using two stable isotopes, 2H and 18O, has become the gold standard for measuring TEE under free-living conditions because of its precision and accuracy [3,4,5].
Accurately assessing TEE is critical for understanding the estimated energy requirement (EER) of populations [6]. This could help prevent and/or reduce the high prevalence of obesity, which is associated with diseases such as cardiovascular disease, diabetes, and hypertension [7,8]. To derive a proper EER equation, the TEE of individuals who maintain an energy balance must be measured accurately. EER is usually determined by assessing sex, age, weight, height, and physical activity status. PAL has been calculated from data taken from a pooled analysis of DLW studies to determine physical activity coefficients used in EER equations [9]. On the other hand, assessing TEE by the DLW method is challenging because of the high costs of isotopes and equipment for isotope analysis as well as the expertise required for analysis. The EER equations for Western populations have already been derived using the DLW method [9]. However, it remains unclear whether these equations are appropriate for Asian populations or populations in developing countries, who have different lifestyles from those of Western populations. Furthermore, information about the practical aspects of performing the DLW method is limited.
This review provides information about the theoretical background and practical aspects of the DLW method. We also introduce applications of DLW data, including the determination of EER equations and validation studies of EI.
Principles of the DLW method
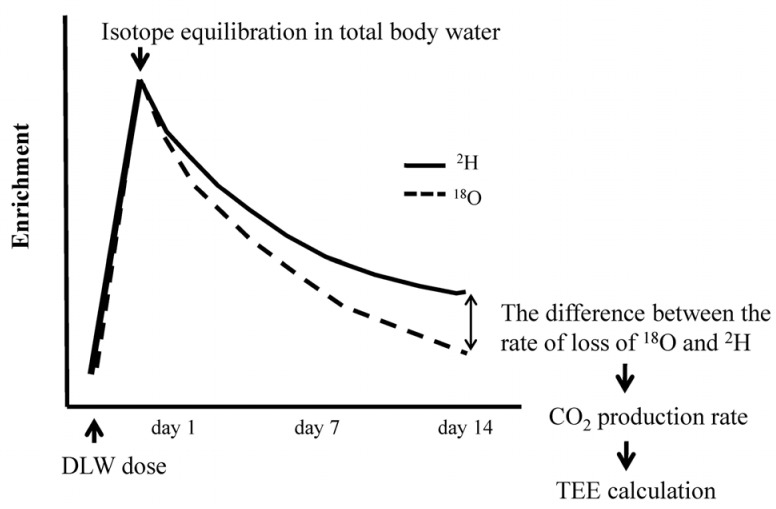
The DLW method was introduced for human use approximately 30 years ago [10]. This method provides information on TEE in free-living individuals over a period of 4-20 days. The principle of the method is as follows. Subjects receive a loading dose of water labeled with the stable 2H and 18O isotopes, and these isotopes mix with the hydrogen and oxygen in body water within a few hours. As energy is expended, CO2 and water are excreted. The CO2 is lost from the body only via the breath, while the water (including both 2H and 18O) is lost not only via the breath but also in urine, sweat, and through other means such as evaporation. As 18O is contained in both CO2 and water, it is lost from the body more rapidly than 2H, which is contained in water but not in CO2 (Fig. 1). Lifson [11] observed that the isotope ratio of O2 in water is rapid and complete isotopic equilibrium with the O2 in CO2. Therefore, a water molecule labeled with 18O will not only mix with body water and exit the body the same way as 2H-labeled water, but will mix with CO2 and exit the body as CO2. During a period of 4-20 days, the difference between the rate of loss of 18O and 2H from the body reflects the rate at which CO2 is produced, which in turn can be used to estimate EE by using a modified Weir's formula [12] based on the CO2 production rate (rCO2) and respiratory quotient (RQ).
Fig. 1.
Decline of 2H and 18O levels in total body water during a doubly labeled water experiment. TEE, total energy expenditure; FQ, food quotient.
Dose of DLW
The dose of DLW is based on body size to match the required body water enrichment. In practice, given that the dose should be prescribed per unit of total body water (TBW), investigators must estimate TBW for each subject. However, because measuring TBW is inconvenient, most researchers use the assumption that TBW represents 60% of body weight in non-obese adults. In our previous studies, we used a single dose of approximately 0.06 g 2H2O∙kg-1 body weight (99.8 atom %, Cambridge Isotope Laboratories, MA, USA) and 1.4 g H218O∙kg-1 body weight (10.0 atom %, Taiyo Nippon Sanso, Tokyo, Japan) [13,14]. The 99 atom % 2H and 10 atom % 18O levels are the most commonly used enrichment levels of labeled water available in the market. Although DLW doses are not always the same among studies, most studies follow the recommendation of the International Atomic Energy Agency (IAEA), which suggests optimal doses for a typical DLW study in adults with 0.12 g∙kg-1 body water of 99 atom % 2H-labeled water and 1.8 g∙kg-1 body water of 10 atom % 18O water [15].
During DLW preparation, the dose must be sterilized by performing distillation or filtration through a 0.22-µm filtering system. The weight of the water should be recorded to four significant digits to allow the determination of the amount of labeled water each subject is administered. The administration container, which should be easy to drink from, should be washed with 50-100 mL tap water after the dose is consumed, and that water should also be consumed; this ensures that the entire dose is received (most containers will not deliver the total dose without washing). For children and the elderly, a straw can be used in order to prevent water loss during administration.
Two basic DLW protocols: two-point and multiple-point approaches
The original DLW method evaluated isotope elimination curves after taking only two samples (from blood, urine or saliva), an initial and a final measurement. This approach allows subjects to continue their normal lives without any restriction between the two time points, providing a valuable technique for quantifying the energy demands of free-living animals and humans [16,17,18]. The first DLW study in human subjects employed the two-point sampling methodology that had previously been used in animal studies [10]. After Schoeller and Van Santen's study was published [10], Klein et al. [19] tested the approach of taking samples at much more frequent intervals and reconstructed a time course for isotope elimination from the body by using a fitted regression curve. They suggested that their multiple-sample method could better determine the production rate of CO2 from multiple samples rather than from measurements at only two points of time. On the other hand, some studies found that there was little bias when simultaneously applying the two-point and multiple-point methods [20]. Below, we summarize the general methods for performing two-point and multiple-point DLW protocols and try to compare the relative precision of the two-point and multiple-point methods.
Experimental procedure for the two-point method
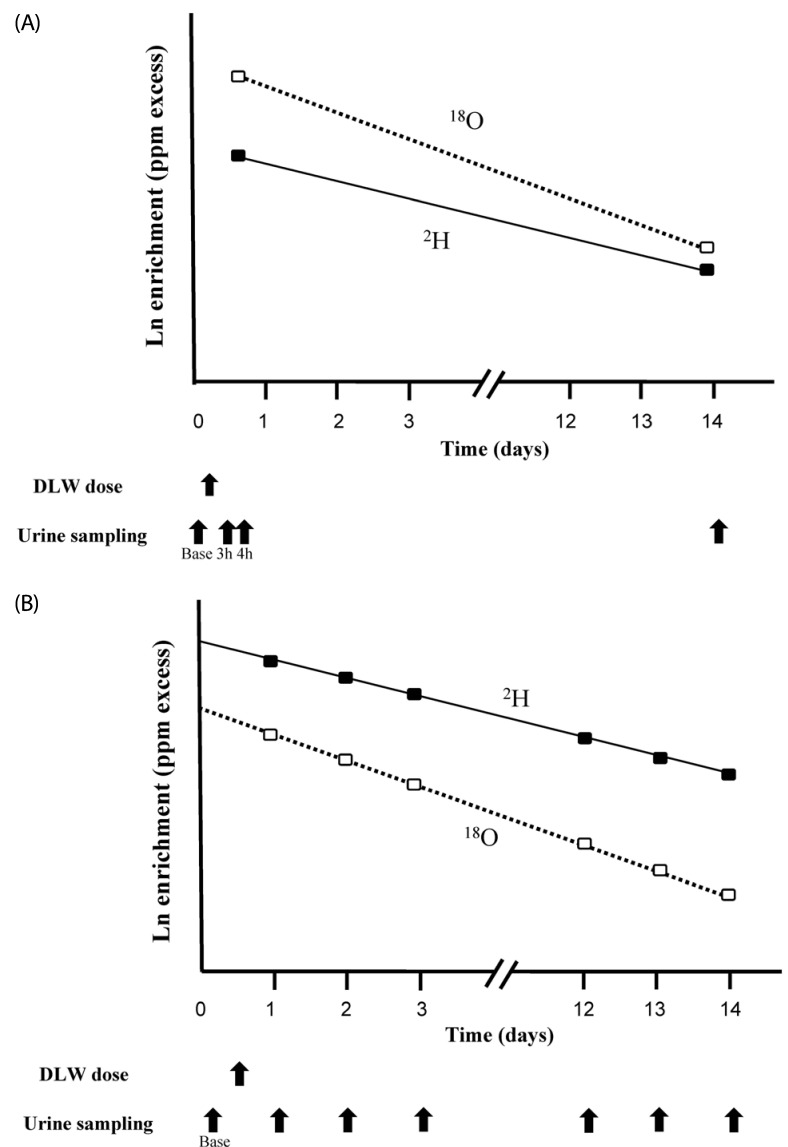
Fig. 2-A shows the time points of urine sampling and log-linear plot of isotope elimination in a two-point DLW protocol [15]. The Experimental procedure for the two-point method is described in detail in the IAEA Human Health [15]. A brief description of the experimental procedure for two-point method follows. The most common protocol for the two-point approach employs morning dosing and sampling. The participant's body weight and height are measured before the start of the experimental period. After an overnight fast and collection of the baseline specimen, the participant drinks the DLW preparation. Importantly, the participant should void and empty their bladder 1 h after the dose. This voided urine need not be collected for analysis, but the time of the void should be recorded. The participant may consume a small meal and should consume fluid to maintain a hydrated state for TBW determination. Additional urine samples should be collected at 3 and 4 h after the dose. The participant should not drink or eat between the 3- and 4-h urine specimens to minimize any short-term effects of water intake on urine enrichment. On the final day of the experimental period, the participant should provide urine samples. Elimination rates of 2H and 18O are determined by using urine samples collected at the two time-points. The typical interval between dosing and final urine collection is 7 or 14 days because a week includes both weekdays and weekends. Because the elimination rate of 18O is dependent of both metabolic rates and water elimination, the final urine collection should be performed within day 7 in case of children and athletes.
Fig. 2.
The time points of urine sampling and log-linear plot of isotope elimination in a two-point (A) and multiple-point (B) doubly labeled water (DLW) protocol. (A) On day 0, the 2H218O (DLW) dose was given orally to each subject after collecting a baseline urine sample. Additional urine samples should be collected at 3 and 4 h after the dose on day 0. On the final day of experimental period, the participant should provide the one more urine sample. The isotope elimination rates (kx) are calculated from the gradient of the isotope elimination curve. (B) On day 0, the 2H2 18O (DLW) dose is given orally to each subject after collecting baseline urine sample. After administration of this dose, the participants are requested to collect urine samples on the following day and at two additional sampling points at the same time of the day during the study period. In the two-point protocol (A), kx = ln(E2 / E1) / (t2 - t1) where E is the enrichment calculated as abundancex - abundancebaseline and t is the time interval after the dose administration. The subscripts 1 and 2 refer to the specimen, where 1 is the post-dose specimen and 2 is the final specimen. In the multi-point protocol (B), kx is the gradient of the linear regression line through the isotope elimination data.
TEE calculation by using the two-point method
The principle of the TEE calculation is described in detail in the IAEA Human Health Series [15]. To help understating how TEE is calculated by using the two-point method, this section is described based on reports from Saitoh's group in Japan [21]. Saitoh and colleagues have calculated TEE by using the two-point protocol and have published approximately 12 articles on this method pertaining to Japanese populations. Notably, there is only one DLW study that has been published for the Korean population, which was conducted in soccer players [22]; this study used the method of Saitoh's group. Ebine et al. [21] used saliva samples (3 and 4 hours after the DLW dose) for the measurement of TBW, whereas the elimination rates of 2H and 18O were determined by using urine samples collected according to the two-point method (Fig. 2-A). The dilution space was determined from saliva 2H enrichment by using the following equation:
N = [WA (δa - δt)] / [18.02a (δs - δp)]
where N (moles) is the dilution space of 2H, W (g) is the amount of tap water in which the dose is diluted for analysis, A (g) is the given dose, a (g) is the amount of the dose diluted with tap water for analysis, and δ (%) is the enrichment of the diluted dose (a), tap water (t), saliva sample after dosing (s), and saliva sample at baseline (p). TBW (moles) was calculated as N/1.041 [23]. Note that the Saitoh's group used only 2H dilution space when calculating N space, however, N space could be calculated by the average dilution space of 2H and 18O to reduce the measurement error of 2H [15].
TBW (mol) =N(mol) / 1.041
rCO2 was calculated as follows:
rCO2 (mol/day) = 0.4554 × TBW (1.007 kO - 1.041 kH)
where rCO2 (mol/day) is the rate of CO2 production, TBW is the total body water in moles, and kO and kH are the rates of 18O and 2H elimination, respectively. TEE is determined from the rate of CO2 production and the food quotient (FQ), which is derived from food consumption data [24], using the following modified Weir's formula [12]:
TEE (kcal/day) = 22.4 {3.9 (rCO2/FQ) + 1.1 (rCO2)} × 4.184 / 1000
Black et al. [24] suggested that the RQ is quite similar to the FQ and that the former can be predicted from the latter. They also demonstrated that the error in calculating energy expenditure from the FQ is less than 3% in most situations.
Experimental procedure for the multiple-point method
Fig. 2-B shows the time points of urine sampling and log-linear plot of isotope elimination in multiple-point DLW protocol [15]. The Experimental procedure for the multiple-point method is described in detail in the IAEA Human Health Series [15]. A brief description of the experimental procedure for multiple-point method differed from the two-point method follows. After the administration of this dose, the participants are asked to collect urine samples on the following day and at multiple time-points. Samples should be collected at the same time of the day during the study period. The 2H and 18O zero-time intercepts and elimination rates (kH and kO) are calculated by using a least-squares linear regression method based on the isotope concentrations in multiple specimens.
TEE calculation by using the multiple-point protocol
The following section describes how TEE is calculated by using the multiple-point method based on reports from the National Institute of Health and Nutrition (NIHN) in Japan [25]. The 2H and 18O zero-time intercepts and elimination rates (kH and kO) are calculated by using least-squares linear regression of the natural logarithm of the isotope concentration as a function of the elapsed time from dose administration (Fig. 2-B). The zero-time intercepts are used to determine the isotope pool sizes. TBW is calculated from the mean value of the isotope pool size of 2H divided by 1.041 and that of 18O divided by 1.007. The equation for calculating rCO2 is that used for the two-point method. The TEE calculation is also performed by using a modified Weir's formula [12] based on the rCO2 and the FQ, but a modified equation is used, as follows:
TEE (kcal/day) = 1.1 rCO2 + 3.9 rCO2 / FQ
Relative precision of the two-point and multiple-point methods
Two previous studies have compared in detail the data generated by the simultaneous application of the two-point and multiple-point methodologies [26,27]. The studies found that there was little bias when using the two-point method, probably because systematic temporal variation in metabolism in the subjects offset any benefit of the multiple-point approach. However, theoretically, if such systematic temporal variation in energy demands were absent, the multiple-sampling approach would provide a more precise result [28,29,30]. Djafarian et al. [20] also compared data generated by using the two-point and multiple-point methodologies in children and suggested that the using the two-point method may be most appropriate for population-based comparisons while using the multi-point method may be best suited for exploring individual variation in TEE. Thus, using the multiple-sample approach may be more suited to situations where maximum precision is required. In the studies to compare the two-point and multiple-point methodologies [20,26], timed samples for multi-point methods were collected on a daily basis for the 10 or 14 days. However, it should be noted that human DLW studies using multiple-point methodology does not always analyze everyday samples, but analyzes some samples at the points of the first, middle, and end during the study period [13,14]. In addition, with urine samples, there is concern about whether water stored in the bladder is incomplete equilibrium with the body water. Especially in the elderly, voiding may be incomplete because of urine retention. Blanc et al. [31] suggested that 24 hours would be necessary for the urine to precisely calculate dilution spaces and the intercept method in multiple-point method should reduce the problem of delay in isotope equilibration. Because urine samples are generally collected by each subject personally at their residence, it could be that sample collection occurs at the wrong time or that subjects do not discard the first urine samples voided after waking up (the urine in such samples may be concentrated). Furthermore, urine samples may have leaked from containers during transit. Thus, the greatest advantage of the multiple-point method is that it minimizes the impact of imprecise analyses. It must be remembered that the multiple-point sampling method and curve-fitting approach involves substantially more isotope analysis, and consequently greater cost. However, the extra cost associated with the multiple-point method could be offset by the benefits of the resultant estimates of energy demands being more accurate than estimates based on the two-point method [29,32].
Isotopic analysis
Here, we summarize isotopic analysis procedures by using a general isotopes ratio mass spectrometer (IRMS) based on the method introduced by the IAEA [15]. Before analyzing urine samples with the IRMS, the urine samples must be equilibrated to gas samples, because the IRMS system can analyze only gas materials (sample preparation before the analysis). The gas for equilibration of 18O is CO2 [33], and that for 2H is H2 [34]. A platinum, chrome or zinc catalyst is used for equilibration of 2H. An IRMS system has a magnetic sector mass spectrometer with multiple internal detectors. The sample must be introduced into the mass spectrometer as an ion source in the form of pure gas molecules (CO2 or H2). Sample gas is ionized by the impact of electrons emitted from a hot filament within a high vacuum. The ions are separated in a magnetic field according to molecular weight. Each sample is compared with a reference gas of known composition. In the case of the NIHN in Japan, isotopic analyses are conducted using the DELTA Plus device (Thermo Electron Corporation, Bremen, Germany) calibrated using Vienna Standard Mean Ocean Water, 302B and the Greenland Ice Sheet Precipitation standard provided by the IAEA [25]. There are two kinds of IRMS systems categorized according to their inlet systems: dual inlet gas IRMS and continuous flow IRMS systems. Dual inlet gas IRMS is the classical technique. Briefly, dual inlet gas IRMS has superior precision, while continuous flow IRMS systems have the advantages of better automation, higher throughput, and reduced sample sizes. Each sample and the corresponding reference are analyzed in duplicate or triplicate. Ishikawa-Takata et al. [25] reported the average standard deviations through the analyses performed by using the dual inlet gas IRMS system are 0.5% for 2H and 0.03% for 18O.
Applications of the DLW data Determining EER
For many decades, energy requirements were based on estimates of EI. However, in 1985, the World Health Organization (WHO) proposed using TEE for more accurate estimates of energy requirements. Around this time, reports on the DLW method for measuring TEE in free-living humans began to be published in Western countries [10,35]. Therefore, the DLW data were based on subsequent estimates of energy requirements [9].
As an example of daily recommended intake (DRI) determination for energy using DLW data in the United States, Black et al. [36] compiled the DLW data of 574 subjects and found the recommended EI levels for American infants and children are high, whereas those for adolescents and adults are low. Approximately 10 years later, the DRI for energy in the United States was determined on the basis of pooled DLW data [9] and energy requirements were determined on the basis of TEE calculated by the following formula:
EER for men (kcal) = 662 - 9.53 × age + PA [15.91 × body wt. (kg) + 539.6 × height (m)]
PA (physical activity coefficient) = 1.0 (sedentary), 1.11 (low active), 1.25 (active), 1.48 (very active)
EER for women (kcal) = 354 - 6.91 × age + PA [9.36 × body wt. (kg) + 726 × height (m)]
PA = 1.0 (sedentary), 1.12 (low active), 1.27 (active), 1.45 (very active)
When the PAL is 1.00 to 1.39, 1.40 to 1.59, 1.60 to 1.89, and 1.90 to 2.50, PA is categorized as "sedentary," "low active," "active," and "very active," respectively. After the EER equations were determined using the DLW data, further DLW studies were performed to assess the accuracy of these equations in various subject groups [37,38].
In the study determining the PAL of healthy Japanese adults (n = 150, age 20-59 years), the mean PAL values of men and women were 1.72 ± 0.22 and 1.72 ± 0.27, respectively. The study also compared PAL values obtained using the DLW method according to categories used for DRI for Japanese people (Table 1). The distribution of four categories across sex and age groups was uniform. Categories III (light heavy) and IV (heavy) had relatively higher PAL compared with categories I (light) and II (moderate). When they combined categories III and IV together (n = 10, PAL = 1.88 ± 0.29) because of their small number, this category had significantly higher PAL than category I (P = 0.036). The DLW data of Ishikawa-Takata et al. [25] have been used for the determination of the EER for Japanese people. In determining the EER for other age groups including infants, children, and the elderly, they used PAL data from DLW studies performed in other countries [39]. In China, researchers have measured TEE values for Chinese people using the DLW method, in order to determine if the current levels of nutrient intake are appropriate. Yao et al. [40] measured the TEE of 73 Chinese adults aged 35-49 years from a wide variety of occupations. Their results suggested that current energy requirement recommendations slightly underestimate the energy needs of women in occupations requiring light physical activity, but were accurate for men and women engaged in occupations requiring moderate and heavy physical activity. Zhuo et al. [41] recently reported that the TEE of young Chinese men measured by using the DLW method is approximately 10% lower than the current diet energy-based recommended nutrient intake, suggesting that the EER equation for Chinese men may be overestimated. In Korea, the Korean Nutrition Society determined energy requirements on the basis of the WHO equation, which predicts TEE as resting metabolic rate multiplied by PAL with the DRI for Koreans in 1995 and 2000 [42,43]. However, the Korean Nutrition Society determined energy requirements from the DRI for Koreans in 2005 using the same method used to determine the DRI for Americans using the DLW method [9,44].
Table 1.
PAL among categories according to Dietary Reference Intake in Japan
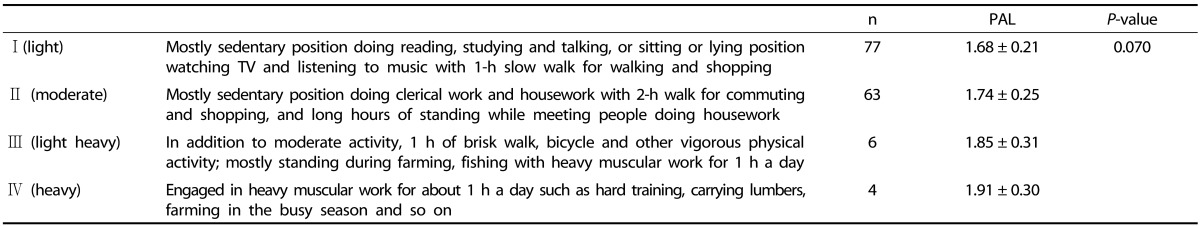
Abbreviations: PAL, physical activity level. PAL was calculated from total energy expenditure obtained from DLW method divided by basal metabolic rate. P-values were calculated by one-way analysis of variance for PAL. Data was modified from Ishikawa-takata et al.'study (2008).
Application in validating EI
Before the development of the DLW method, self-reported habitual EI was often used as a proxy measure of TEE. It has become clear that TEE obtained by the DLW method is being used as a biomarker of EI [5]. Energy balance is the principle used to validate EI measures determined using EE measures obtained by the DLW method. Energy balance occurs when EI equals EE under the conditions of stable body weight during an experimental period. Thus, this principle of energy metabolism enables the determination of the energy requirements of certain populations from either EI or EE.
The many available methods for obtaining information regarding dietary intake can be divided into three general categories: (1) recall of foods eaten, (2) diet histories or retrospective questionnaires, and (3) diet records [45]. Studies published from the late 1980s using the DLW method indicate underreporting is a common problem associated with the self-assessment of dietary EI. Numerous subsequent studies comparing self-reported EI and EE values assessed by the DLW method have been published [45,46], and the problem of misreporting, particularly underreporting, by participants has been noted [45].
Okubo et al. [47] found 11% underreporting in 158 Japanese adults aged 20-59 years on a dietary assessment questionnaire compared with TEE measured by the DLW method. Similarly, Redman et al. [48] studied 217 healthy American adults aged 21-50 years and found 15% underreporting of EI assessed by self-report in the DLW period. Furthermore, Scagliusi et al. [49] found underreporting in 65 Brazilian adult women aged 18-57 years in a cross-sectional study; the authors assessed EI by 24-h recalls, a 3-day food record, and a food frequency questionnaire (FFQ) and assessed TEE by DLW. Using these 3 methods, they found a bias of > 20% towards the underreporting of food intake; these values are similar to those reported in other DLW studies of adults using various methods to assess EI [50,51].
Misreporting is also prominent in children because of their lower literacy levels and limited cognitive abilities as well as difficulties estimating portion sizes for children [52,53]. Recently, Burrows et al. [46] systematically reviewed DLW studies to determine which dietary assessment method(s) provides valid and accurate estimates of EI. The critical appraisal process resulted in the inclusion of 15 articles. They classified "misreporting" as follows: adequate reporting, EI/TEE within the 95% confidence limits (0.84-1.16); underreporting, EI/TEE < 0.84; or overreporting, EI/TEE > 1.16. All 15 studies were associated with a degree of misreporting; in particular, significant underreporting of EI was found for estimated food records (19-41% of estimated EI, three out of five studies) and weighed food records (11-27%, one out of two studies), and overreporting for 24-h multiple pass recalls (7-11%, two out of four studies) and FFQs (up to 59%, one out of two studies). The authors note the degree of misreporting may depend on sample size, the dietary assessment method used, and whether parents helped their children complete the survey [46].
It should be noted that "undereating" may be misinterpreted as underreporting. Shahar et al. [54] found undereating and not underreporting in elderly subjects aged 70-79 years (n = 296); in that study, TEE and EI were assessed over 2 weeks by the DLW method and a self-reported FFQ, respectively. The authors categorized misreporting as follows: participants with an EI/TEE ratio < 0.77 and > 1.28 were categorized as low and high energy reporters, respectively. The results showed 43% of participants were low energy reporters. Among them, almost 30% were losing weight, probably because they were eating less than their EE level; therefore, these subjects were categorized as undereaters.
There is a vast body of literature related to the underreporting problem in obese individuals. Several studies found a bias of > 35% towards the underreporting of food intake in obese adults, children, and adolescents populations [55,56,57,58]. Westerterp [59] demonstrated that TEE linearly increases as body weight increases, although reported EI does not. Studies of the reasons for the underestimation of dietary intake among obese subjects have indicated that body image issues and weight consciousness may play significant roles [60,61,62].
Needless to say, an underestimation of EI will lead to false conclusions when establishing the nutritional requirements of a certain group; therefore, it is important that populations at risk of underreporting be identified. Because EI derived from any method of food recording can often be imprecise in a given subject group, results from studies using such methods should be interpreted with caution.
CONCLUSIONS
Although the number of applications of the DLW method has significantly increased in many areas of study including nutrition and clinical medicine, the DLW method is not always practical in many developing or Asian countries. To prevent and/or reduce the high prevalence of obesity and various diseases due to excess energy intake, it is essential to determine proper EER equations for general populations or provide an accurate and convenient tool for assessing EI. The DLW method is currently considered the best solution for these problems.
ACKNOWLEDGEMENT
Jonghoon Park was supported by the SMART Research Professor Program of Konkuk University.
References
- 1.Ainslie P, Reilly T, Westerterp K. Estimating human energy expenditure: a review of techniques with particular reference to doubly labelled water. Sports Med. 2003;33:683–698. doi: 10.2165/00007256-200333090-00004. [DOI] [PubMed] [Google Scholar]
- 2.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol. 2009;105:823–828. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- 3.Schoeller DA. Energy expenditure from doubly labeled water: some fundamental considerations in humans. Am J Clin Nutr. 1983;38:999–1005. doi: 10.1093/ajcn/38.6.999. [DOI] [PubMed] [Google Scholar]
- 4.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jéquier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 5.Schoeller DA. Recent advances from application of doubly labeled water to measurement of human energy expenditure. J Nutr. 1999;129:1765–1768. doi: 10.1093/jn/129.10.1765. [DOI] [PubMed] [Google Scholar]
- 6.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E CALERIE Study Group. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99:71–78. doi: 10.3945/ajcn.113.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 8.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–338. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine (US) Panel on Macronutrients; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C.: National Academies Press; 2005. 5. Energy. [Google Scholar]
- 10.Schoeller DA, van Santen E. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:955–959. doi: 10.1152/jappl.1982.53.4.955. [DOI] [PubMed] [Google Scholar]
- 11.Lifson N. Theory of use of the turnover rates of body water for measuring energy and material balance. J Theor Biol. 1966;12:46–74. doi: 10.1016/0022-5193(66)90185-8. [DOI] [PubMed] [Google Scholar]
- 12.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Ishikawa-Takata K, Tanaka S, Hikihara Y, Ohkawara K, Watanabe S, Miyachi M, Morita A, Aiba N, Tabata I. Relation of body composition to daily physical activity in free-living Japanese adult women. Br J Nutr. 2011;106:1117–1127. doi: 10.1017/S0007114511001358. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Ishikawa-Takata K, Tanaka S, Hikihara Y, Ohkawara K, Watanabe S, Miyachi M, Morita A, Aiba N, Tabata I. The relationship of body composition to daily physical activity in free-living Japanese adult men. Br J Nutr. 2014;111:182–188. doi: 10.1017/S0007114513001918. [DOI] [PubMed] [Google Scholar]
- 15.International Atomic Energy Agency (AT) IAEA Human Health Series No. 3. Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques. Vienna: International Atomic Energy Agency; 2009. [Google Scholar]
- 16.Butler PJ, Green JA, Boyd IL, Speakman JR. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct Ecol. 2004;18:168–183. [Google Scholar]
- 17.Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ, Bennett NC. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature. 2006;440:795–797. doi: 10.1038/nature04578. [DOI] [PubMed] [Google Scholar]
- 18.Speakman JR. The role of technology in the past and future development of the doubly labelled water method. Isotopes Environ Health Stud. 2005;41:335–343. doi: 10.1080/10256010500384283. [DOI] [PubMed] [Google Scholar]
- 19.Klein PD, James WP, Wong WW, Irving CS, Murgatroyd PR, Cabrera M, Dallosso HM, Klein ER, Nichols BL. Calorimetric validation of the doubly-labelled water method for determination of energy expenditure in man. Hum Nutr Clin Nutr. 1984;38:95–106. [PubMed] [Google Scholar]
- 20.Djafarian K, Jackson DM, Milne E, Roger P, Speakman JR. Doubly labelled water: multi-point and two-point methods in pre-school children. Int J Pediatr Obes. 2010;5:102–110. doi: 10.3109/17477160903039915. [DOI] [PubMed] [Google Scholar]
- 21.Ebine N, Feng JY, Homma M, Saitoh S, Jones PJ. Total energy expenditure of elite synchronized swimmers measured by the doubly labeled water method. Eur J Appl Physiol. 2000;83:1–6. doi: 10.1007/s004210000253. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Kim HR. Total energy expenditure of professional soccer players measured by the doubly labeled water method. Korean J Exerc Nutr. 2003;7:241–246. [Google Scholar]
- 23.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267:E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 24.Black AE, Prentice AM, Coward WA. Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure. Hum Nutr Clin Nutr. 1986;40:381–391. [PubMed] [Google Scholar]
- 25.Ishikawa-Takata K, Tabata I, Sasaki S, Rafamantanantsoa HH, Okazaki H, Okubo H, Tanaka S, Yamamoto S, Shirota T, Uchida K, Murata M. Physical activity level in healthy free-living Japanese estimated by doubly labelled water method and International Physical Activity Questionnaire. Eur J Clin Nutr. 2008;62:885–891. doi: 10.1038/sj.ejcn.1602805. [DOI] [PubMed] [Google Scholar]
- 26.Welle S. Two-point vs multipoint sample collection for the analysis of energy expenditure by use of the doubly labeled water method. Am J Clin Nutr. 1990;52:1134–1138. doi: 10.1093/ajcn/52.6.1134. [DOI] [PubMed] [Google Scholar]
- 27.Speakman JR, Racey PA. Measurement of CO2 production by the doubly labeled water technique. J Appl Physiol (1985) 1986;61:1200–1202. doi: 10.1152/jappl.1986.61.3.1200. [DOI] [PubMed] [Google Scholar]
- 28.Speakman JR. Doubly Labelled Water: Theory and Practice. London: Chapman & Hall; 1997. [Google Scholar]
- 29.Prentice AM. Stable isotopic methods for measuring energy expenditure. Applications of the doubly-labelled-water (2H2(18)O) method in free-living adults. Proc Nutr Soc. 1988;47:259–268. doi: 10.1079/pns19880043. [DOI] [PubMed] [Google Scholar]
- 30.Speakman JR. Principles, problems and a paradox with the measurement of energy expenditure of free-living subjects using doubly-labelled water. Stat Med. 1990;9:1365–1380. doi: 10.1002/sim.4780091113. [DOI] [PubMed] [Google Scholar]
- 31.Blanc S, Colligan AS, Trabulsi J, Harris T, Everhart JE, Bauer D, Schoeller DA. Influence of delayed isotopic equilibration in urine on the accuracy of the (2)H(2)(18)O method in the elderly. J Appl Physiol (1985) 2002;92:1036–1044. doi: 10.1152/japplphysiol.00743.2001. [DOI] [PubMed] [Google Scholar]
- 32.Speakman JR. Estimation of precision in DLW studies using the two-point methodology. Obes Res. 1995;3(Suppl 1):31–39. doi: 10.1002/j.1550-8528.1995.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 33.Schoeller DA, Luke AH. Rapid 18O analysis of CO2 samples by continuous-flow isotope ratio mass spectrometry. J Mass Spectrom. 1997;32:1332–1336. doi: 10.1002/(SICI)1096-9888(199712)32:12<1332::AID-JMS598>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Prosser SJ, Scrimgeour CM. High-precision determination of 2H/1H in H2 and H2O by continuous-flow isotope ratio mass spectrometry. Anal Chem. 1995;67:1992–1997. [Google Scholar]
- 35.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118:1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 36.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 37.Tooze JA, Schoeller DA, Subar AF, Kipnis V, Schatzkin A, Troiano RP. Total daily energy expenditure among middle-aged men and women: the OPEN Study. Am J Clin Nutr. 2007;86:382–387. doi: 10.1093/ajcn/86.2.382. [DOI] [PubMed] [Google Scholar]
- 38.Bandini LG, Lividini K, Phillips SM, Must A. Accuracy of Dietary Reference Intakes for determining energy requirements in girls. Am J Clin Nutr. 2013;98:700–704. doi: 10.3945/ajcn.112.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabata I, Ebine N, Kawashima Y, Ishikawa-Takata K, Tanaka S, Higuchi M, Yoshitake Y. Dietary reference intakes for Japanese 2010: energy. J Nutr Sci Vitaminol (Tokyo) 2013;59:S26–S35. [Google Scholar]
- 40.Yao M, McCrory MA, Ma G, Li Y, Dolnikowski GG, Roberts SB. Energy requirements of urban Chinese adults with manual or sedentary occupations, determined using the doubly labeled water method. Eur J Clin Nutr. 2002;56:575–584. doi: 10.1038/sj.ejcn.1601361. [DOI] [PubMed] [Google Scholar]
- 41.Zhuo Q, Sun R, Gou LY, Piao JH, Liu JM, Tian Y, Zhang YH, Yang XG. Total energy expenditure of 16 Chinese young men measured by the doubly labeled water method. Biomed Environ Sci. 2013;26:413–420. doi: 10.3967/0895-3988.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 42.The Korean Nutrition Society. Dietary Reference Intakes for Koreans. Seoul: The Korean Nutrition Society; 1995. [Google Scholar]
- 43.The Korean Nutrition Society. Dietary Reference Intakes for Koreans. Seoul: The Korean Nutrition Society; 2000. [Google Scholar]
- 44.The Korean Nutrition Society. Dietary Reference Intakes for Koreans. Seoul: The Korean Nutrition Society; 2010. [Google Scholar]
- 45.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85:415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 46.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110:1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Okubo H, Sasaki S, Rafamantanantsoa HH, Ishikawa-Takata K, Okazaki H, Tabata I. Validation of self-reported energy intake by a self-administered diet history questionnaire using the doubly labeled water method in 140 Japanese adults. Eur J Clin Nutr. 2008;62:1343–1350. doi: 10.1038/sj.ejcn.1602858. [DOI] [PubMed] [Google Scholar]
- 48.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E CALERIE Study Group. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99:71–78. doi: 10.3945/ajcn.113.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scagliusi FB, Ferriolli E, Pfrimer K, Laureano C, Cunha CS, Gualano B, Lourenço B, Lancha AH. Under-reporting of energy intake is more prevalent in a healthy dietary pattern cluster. Br J Nutr. 2008;100:1060–1068. doi: 10.1017/S0007114508971300. [DOI] [PubMed] [Google Scholar]
- 50.Seale JL, Rumpler WV. Comparison of energy expenditure measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. Eur J Clin Nutr. 1997;51:856–863. doi: 10.1038/sj.ejcn.1600498. [DOI] [PubMed] [Google Scholar]
- 51.Kroke A, Klipstein-Grobusch K, Voss S, Möseneder J, Thielecke F, Noack R, Boeing H. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70:439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 52.Livingstone MB, Robson PJ. Measurement of dietary intake in children. Proc Nutr Soc. 2000;59:279–293. doi: 10.1017/s0029665100000318. [DOI] [PubMed] [Google Scholar]
- 53.Livingstone MB, Robson PJ, Wallace JM. Issues in dietary intake assessment of children and adolescents. Br J Nutr. 2004;92(Suppl 2):S213–S222. doi: 10.1079/bjn20041169. [DOI] [PubMed] [Google Scholar]
- 54.Shahar DR, Yu B, Houston DK, Kritchevsky SB, Newman AB, Sellmeyer DE, Tylavsky FA, Lee JS, Harris TB Health, Aging, and Body Composition Study. Misreporting of energy intake in the elderly using doubly labeled water to measure total energy expenditure and weight change. J Am Coll Nutr. 2010;29:14–24. doi: 10.1080/07315724.2010.10719812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Weisel H, Heshka S, Matthews DE, Heymsfield SB. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327:1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- 56.Buhl KM, Gallagher D, Hoy K, Matthews DE, Heymsfield SB. Unexplained disturbance in body weight regulation: diagnostic outcome assessed by doubly labeled water and body composition analyses in obese patients reporting low energy intakes. J Am Diet Assoc. 1995;95:1393–1400. doi: 10.1016/S0002-8223(95)00367-3. [DOI] [PubMed] [Google Scholar]
- 57.Bandini LG, Schoeller DA, Cyr HN, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. Am J Clin Nutr. 1990;52:421–425. doi: 10.1093/ajcn/52.3.421. [DOI] [PubMed] [Google Scholar]
- 58.Singh R, Martin BR, Hickey Y, Teegarden D, Campbell WW, Craig BA, Schoeller DA, Kerr DA, Weaver CM. Comparison of self-reported, measured, metabolizable energy intake with total energy expenditure in overweight teens. Am J Clin Nutr. 2009;89:1744–1750. doi: 10.3945/ajcn.2008.26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev. 2010;68:148–154. doi: 10.1111/j.1753-4887.2010.00270.x. [DOI] [PubMed] [Google Scholar]
- 60.Heitmann BL. The influence of fatness, weight change, slimming history and other lifestyle variables on diet reporting in Danish men and women aged 35-65 years. Int J Obes Relat Metab Disord. 1993;17:329–336. [PubMed] [Google Scholar]
- 61.Crawley H, Summerbell C. Feeding frequency and BMI among teenagers aged 16-17 years. Int J Obes Relat Metab Disord. 1997;21:159–161. doi: 10.1038/sj.ijo.0800380. [DOI] [PubMed] [Google Scholar]
- 62.Lafay L, Basdevant A, Charles MA, Vray M, Balkau B, Borys JM, Eschwège E, Romon M. Determinants and nature of dietary underreporting in a free-living population: the Fleurbaix Laventie Ville Santé (FLVS) Study. Int J Obes Relat Metab Disord. 1997;21:567–573. doi: 10.1038/sj.ijo.0800443. [DOI] [PubMed] [Google Scholar]