Abstract
BACKGROUND/OBJECTIVES
The aim of this study was to investigate the effects of exercise (EX) and Korean red ginseng (KRG) on inflammation mechanism in aging model rats with diet-induced atherosclerosis.
MATERIALS/METHODS
Forty-eight male Sprague-Dawley rats were divided into 6 groups: Young control (Y-C), Aging control (A-C), A-C with HFD (AHF), AHF with EX (AHF-EX), AHF-EX with KRG (AHF-EX+RG), and AHF with KRG (AHF-RG). Aging was induced by D-gal (100mg/kg) and atherosclerosis was induced by HFD (60% fat) for 9 weeks. The experimental rats were performed swimming (60 min/day, 5 days/week) and supplied KRG orally (dose of 200 mg/kg) for 8 weeks. All rat aorta samples were harvested for biochemical and immunohistochemical analyses.
REULTS
The EX and KRG supplementation significantly inhibited body weight and levels of TC, TG, LDL-C, and enhance of HDL-C compared with untreated AHF groups. AHF-EX, AHF-EX+RG, and AHF-RG group showed a decreased plasma CRP and increase plasma NO activities compared to AHF group. In addition, these groups revealed reduced 4-HNE, NF-kB, TNF-α, IL-6, COX-2, ICAM-1, VCAM-1 and enhanced eNOS expression in the aorta.
CONCLUSION
These results suggest that EX alone, KRG alone, and combined treatment of EX and KRG may be an effective anti-inflammatory therapeutic for the atherosclerosis, possibly acting via the decreased of CRP and pro-inflammation proteins and the increased NO and eNOS.
Keywords: Korea red ginseng, exercise, lipid profiles, inflammation, atherosclerosis
INTRODUCTION
Aging is a biological process characterized by time-dependent, progressive decrease in physiological capacity and the increased age related diseases [1] due to overproduce of cellular reactive oxidative stress (ROS) [2]. Atherosclerosis development during aging is increased the morbidity and mortality the age vascular diseases such as cardiovascular disease (CVD) and cerebrovascular disease (CBVD) in elderly [3, 4]. It is caused by lipid accumulation including hypercholesteroles, dyslipidemia, activation inflammatory cytokines and oxidative stress [5,6].
Several studies demonstrated that high-fat diet (HFD) feeding in rats produces obesity and atherosclerosis and involved in endothelial dysfunction, vascular oxidative stress, and activated inflammatory molecular factors [5,6,7].
Aging related atherosclerosis play a pivotal role in the vascular inflammation via the release of potent inflammatory factors including Nuclear factor-κB (NF-κB) and cytokines which are activated in all the phases of the atherosclerotic process [4].
Nuclear factor-κB (NF-κB), pro-inflammatory transcription factor, is known as the mater regulator of the inflammatory process and induced by oxidative stress stimuli [8]. NF-κB is up-regulated the transcription of pro-inflammatory molecules, such as tumor necrosis factor-α (TNF-α), and interleukins (IL-1β, and IL-6), cyclo-oxygenase-2 (COX-2), nitric oxide synthase (iNOS). Other pro-inflammatory mediators, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) are increased through NF-κB activation in aorta during the aging process and induced atherosclerosis [9,10]. Inflammatory molecules are all enhanced during the aging process and leads to a chronic pro-inflammatory state [11] and induced age related vascular diseases [12].
Thus atherosclerosis couple with aging therapy is a useful means of preventing in CVD and CBVD and require comprehensive management to control their lipid.
Gingeng, the root Panax ginseng CA Meyer, has been used for more than 2000 years in Asia as a traditional medicine and dietary supplement for health [13,14]. Red ginseng (RG) is produced by steaming and drying fresh and raw ginseng. During the steaming process, ginsenosides produce chemical changes that confer the potential to induce special physical activities [15].
Korean RG (KRG), which is a famous as the red ginseng with Korean origin, and have beneficial effects against obesity [16], hyperglycemia [17], thrombotic and platelet [13]. Although several studies have reported that KRG has broad efficacious [13,16,17], the lipid profile control, and anti-inflammation activities of KRG in aged atherosclerosis still remains unclear.
Physical exercise has been associated with a decreasing in CVD morbidity and mortality [18,19]. It reduces body weight and serum TC and TG levels, and inflammatory markers in older adults [14,20,21]. It can also prevents cell damage by antiapoptoic proteins, antioxidative enzymes, nitric oxide (NO), and endothelial NO synthase (eNOS) [14,19].
Nitric oxide (NO) is synthesized by endothelial NO synthases (eNOS) which is present in the vascular wall [22] and plays an important role in the control of vascular tone [23] and inhibits platelet aggregation [24]. Indeed, eNOS activation prevented damage of L-NAME induced hypertensive heart and coronary artery in rats [14]. It has previously demonstrated that basal and stimulated bioactivity of eNOS is inhibited in aging rats [25]. Thus, one of the treatment options to prevent atherosclerosis is to elevate NO, eNOS and inhibit inflammation molecules.
Many studies have shown that long-term production of oxidative stress by administering D-galactose (D-gal) can induce aging and shows age related diseases in experimental animals [26]. The administration of D-gal and HFD feeding has various adverse health effects including aging related disease, oxidative stress, hyperlipidemia, atherosclerosis, endothelial impairment and obesity [5,7,27,28]. Therefore, inflammatory inhibitors by exercise (EX) and KRG might be a promising approach for the treatment of elderly peoples with atherosclerosis.
In the present study, we used D-gal and high fat diet (HFD) to induce stage of aging with atherosclerosis in SD rats to investigate the anti-inflammatory effects and anti-atherosclerosis effects of EX and KRG supplementation in process of the initiation of atherosclerosis at age and its possible mechanisms in vivo.
MATERIAL AND METHODS
Animals and diet
48 male Sprague-Dawley (SD) rats (body weight 200-250g, 2 month old) were provided by Orient Bio laboratory animal center (Seoul, Korea) and all procedures relating to animals and their care conformed to the international guidelines 'Principles of Laboratory Animals Care' (NIH publication no. 85-23, revised 1985). All experimental rats were randomly assigned into six groups after 1 week adaptation as following groups: Young control (Y-C), Aging control (A-C), A-C with HFD (AHF), AHF with exercise training (AHF-EX), AHF-EX with Korean red ginseng (AHF-EX+RG), AHF with RG (AHF-RG). The animal diets were prepared using a modified America Institute of Nutrition (AIN)-76 and shows in Table 1. Control groups were fed a normal diet with 10 kcal % fat (100000, Dyets Inc, Bethlehem, PA, USA) and AHF rats were fed a HFD with 60% kcal fat (100496, Dyets Inc) for 9 weeks. All experimental animals were given free access to food and water. Treatment (exercise training and/or RG supplementation) started on 1 week after D-gal injection and high fat diet. All rats were handled in an accredited Korea FDA animal facility in accordance with the AAALAC International Animal Care Policies (Accredited Unit-Korea food and Drug Administration: Unit Number-HY-IACUC-11-050).
Table 1.
Composition of normal and high fat diets (g/kg diet)
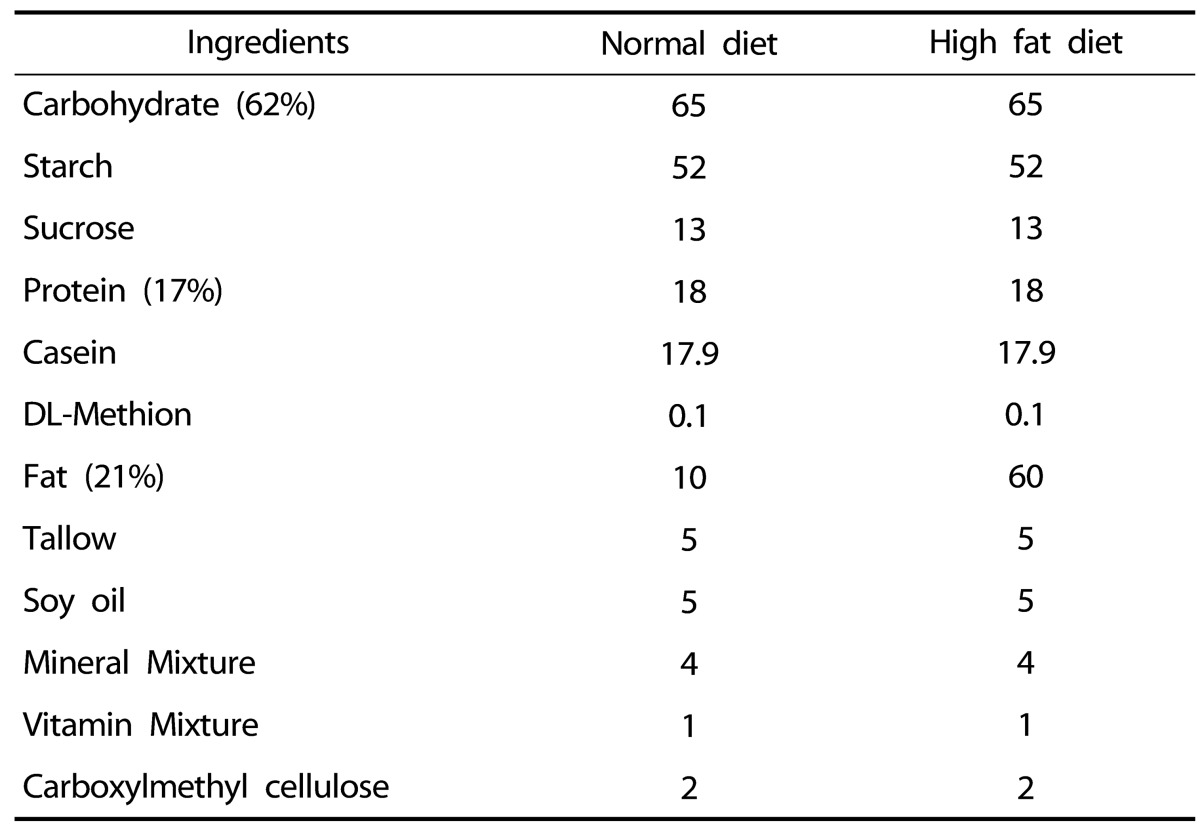
Normal diet : AIN-76 # 100000 (10% kcal fat)
High fat diet : AIN-76A # 100496 (60% kcal fat)
Aging induced D-gal
Aging was induced by D-gal (Sigma ST. Louis, MO) melted in normal saline. In Aging group, D-gal injection (100mg/kg) was given to intraperitoneally (IP) in the morning (10:00 am) everyday for 9 weeks. Y-C group was only injected normal saline in IP without D-gal [28].
Exercise training
The total 8-week exercise training was performed in swimming chamber (diameter: 150 cm, height: 70 cm) at 30-32℃ water temperature. The swim exercise was performed for 60 min per day, 5days per week for 8 weeks. To allow adaptation for the swimming exercise, the exercise time was gradually increased for a week (for 5min initially and then lengthened by 10 min daily for total 60 min) [14].
KRG administration
The experimental rats were orally administered with KRG (manufactured by Korean Ginseng Corporation, Seoul, Korea) dally and does of 200 mg/kg in distil water for 8 weeks [16]. The total content of ginsenosides in the KRG was 19.64mg/g, and this compositions were 0.71 (Rg1), 0.93 (Re), 1.21 (Rf), 0.78 (Rf1), 1.92 (Rg2(s)), 1.29 (Rg2(r)), 4.62 (Rb1), 2.41 (Rc), 1.83 (Rb2), 0.89 (Rd), 2.14 (Rg3(s)), 0.91 (Rg3(r)), respectively.
Blood and tissue preparation
After the experimental period, all rats were fasted for 12h, and anesthetized with ketamine/xylazine mixture, after which whole blood sample was collected from their abdominal aorta. Plasma was obtained by centrifuging the blood (3000 rpm, 4℃, 10 min), followed by incubation for 15 min at room temperature, and then stored at -80℃ until analysis. The aorta was rapidly removed and washed in phosphate-buffered solution (PBS). The thoracic aorta tissue was snap-frozen on dry ice and stored at -80℃ for western blot analysis, and was fixed with 4% paraformaldehyde-0.1% glutaraldehyde in 0.1M PBS for morphological analysis. After fixation, samples were dehydrated in ethanol and embedded in paraffin and cross sectioned into 5-6 µm thickness.
Plasma lipid and C-reactive protein (CRP) analysis
Total cholesterol (TC), Triglycerides (TG), HDL cholesterol (HDL-C), and LDL cholesterol (LDL-C) levels were measured with the commercial kit (Sigma, St. Louis, MO, USA), respectively.
CRP was evaluated by CRP assay kit (Immundiagnostik, Bensheim, Germany) in according to the manufacturer's protocol. The absorbance was read at 540nm via ELISA (Molecular Device E Max, Sunnyvale, CA, USA).
Atherogenic index calculation
Atherogenic index (AI) calculated according to the Friedewald formula [29] : AI = (TC - HDL-C) / HDL-C
Plasma nitric oxide (NO) level
Serum NO levels were detected using the Griess assay in accordance with the methods established by Dawson et al. [30].
Griess reagent mixed with NED solution (0.1% naphthlenediamine · dihydrochloride) and sulf-anilamide solution (1% sulfanilamide in 5% phosphoric acid) at 1:1 ratio, was combined with the samples at 1:1 ratio. This mixture was maintained for 15 minutes, while the light was interrupted at a room temperature. The mixture was then assessed at a wavelength of 450 nm via ELISA (Molecular Device E Max, Sunnyvale, CA, USA).
Immunohistochemistry
For immunohistochemistry, tissue sections were deparaffinzed, cleared, and hydrated to PBS using a descending series of ethanol. The sections were blocked for 40 min at 37℃ with 3% goat serum in PBS followed by quenching endogenous peroxidase activity by exposing slides to 0.3% H2O2 and 10% methanol for 5 min. Primary antibodies for NF-κβ (1:50) (Santa Cruz Biotechnology, CA, USA) and 4-HNE (1:250) (abcam, UK) were added and incubated overnight at 4℃. The next day, the slides were washed in PBS three times for 5 min and secondary antibodies (1:200, Santa Cruz Biotechnology) were incubated for 40 min in room temperature, and then washed in PBS several times for 3min before DAB visualization. Finally, the slides were counterstained with 0.5% methyl green and observed on a light microscope (Olympus U-LH 100HG, Tokyo, Japan).
Western blot analysis
The aorta was homogenized in lysis buffer (Cell Signaling technology, Danvers, MA, USA) with PMSF at 4℃, and centrifuged (10,000 × g). After this, the supernatants were centrifuged at 13,000 × g. The protein content of each of the samples was determined using Bradford's method, with bovine serum albumin as a standard. The protein samples (35 µg) were boiled in 5× sample buffer followed by polyacrylamide resolving gel and stacking gel, and then transferred overnight to a nitrocellulose membrane at 15 volts. The membranes were washed, blocked, and incubated with Western blotting to detect NF-κβ, TNF-α, IL-6, Cox-2 (Cell Signaling, Danvers, MA, State, 1:1000), and eNOS (1:2000) (BD Biosciences, Bedford, MA) for overnight at -4℃. Secondary antibodies were incubated with horseradish peroxidase-conjugated secondary antibody for 1hr (1:3000, Santa Cruz Biotechnology) at room temperature. The membranes were washed and visualized by autoradiography after development with ECL (Amersham Life Sciences, Arlington Heights, IL, USA). Densitometry was performed with gel documentation (Gel Doc 2000, Quantity One Bio Red, Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS software (version 18.0). All data were presented as means ± standard error of mean (SEM) and analyzed by one-way ANOVA with a following Tukey's. P < 0.05 was considered as statistically significant.
RESULTS
BW
The results of BW were shown in Table 2. Although initial BW of 6 groups were similar and rats fed on the normal and HFD continued to show elevated BW until the experimental end. After 8 weeks of EX and RG, the BW was significantly lowered (P < 0.05, P < 0.001) in AHF-EX, AHF-EX+RG, and AHF-RG groups than in the AHF group.
Table 2.
Body Weight (BW, unit g) and Food intake (g/day)
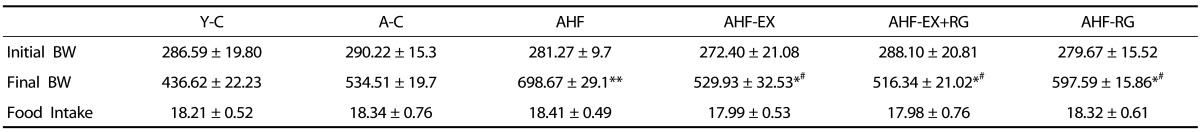
Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. Values are means ± SEM (n=8). *P < 0.05, **P < 0.01 vs Y-C, A-C, #P < 0.05 vs AHF
Plasma lipid profiles
The plasma lipid profiles were evaluated and shown in Table. 3. TC, TG, LDL-C levels and AI were significantly increased in AHF groups. However, AHF-EX, AHF-EX+RG, and AHF-RG groups were markedly decreased compared to AHF group.
Table 3.
Serum Lipid status (unit mg/L)
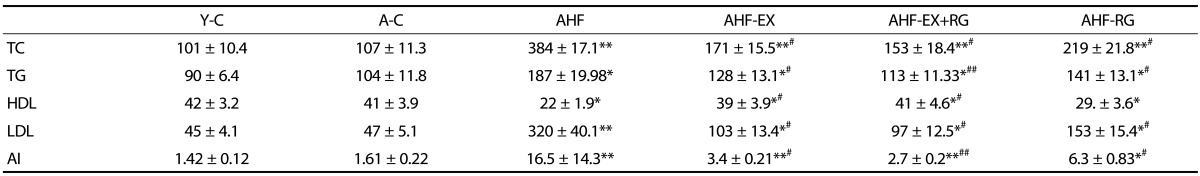
Plasma levels of total cholesterol (TC), triglyceride (TG), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C) levels and atherogenic index (AI) in D-gal-induced aging rats with high fat diet. Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. Values are means±SEM (n=8). *P < 0.05, **P < 0.01 vs Y-C, A-C, #P < 0.05, ##P < 0.01 vs AHF
The HDL-C was markedly increased in AHF-EX and AHF-EX+RG groups compared to AHF group.
Plasma CRP level
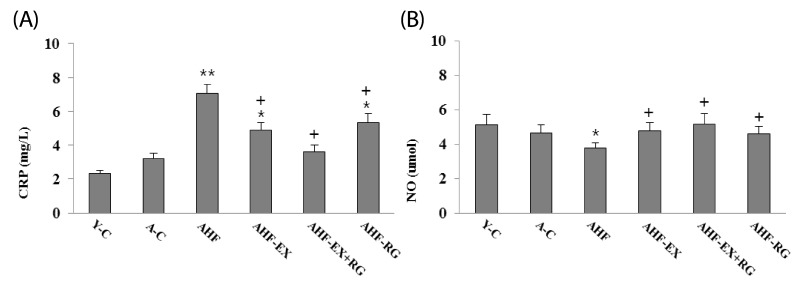
As shown in Fig 1, CRP, inflammatory marker, was significantly elevated in AHF, AHF-EX and AHF-RG groups when compared to control. However, the CRP level was reduced remarkably after combined treatment with EX or RG compared to AHF group.
Fig. 1.
Plasma levels of CRP (A), NO levels (B) in blood of D-gal-induced aging rats with high fat diet. Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. Values are means ± SEM (n = 8). *P < 0.05, **P < 0.01 vs Y-C, A-C, †P < 0.05 vs AHF
Plasma nitric oxide (NO) level
The plasma NO activities were evaluated and shown in Fig. 1. AHF group showed significantly lower than control groups. However, the NO activities was elevated remarkably after combined treatment with EX and RG groups compared to AHF group.
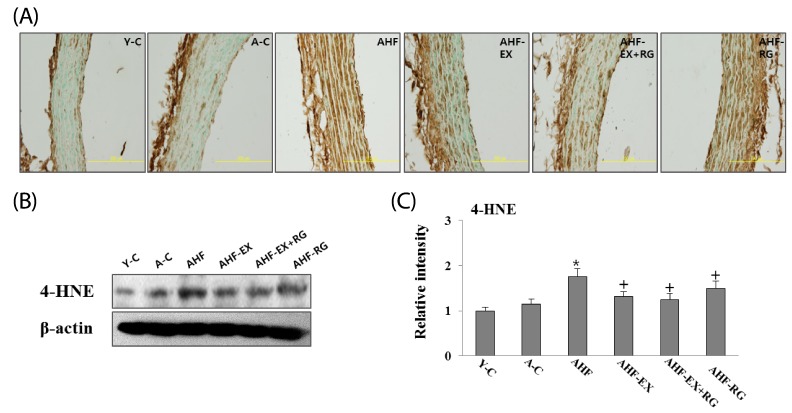
4-HNE in the aorta
As shown in Fig 2, 4-HNE, oxidative stress marker in aorta, was performed using immunohistochemistry (A) and western blotting (B). 4-HNE protein was significantly decreased in aortas of AHF-EX, AHF-EX+RG, and AHF-RG compared to AHF groups. In histological preparations of aortas, 4-HNE was observed by brown staining in the vessel walls. EX and KRG appeared to inhibit 4-HNE expression in all arterial compartment.
Fig. 2.
Immunohistochemical (A) and Western blotting (B) analysis of 4-HNE expression. Densitometric analysis of Western blots (C) in aorta of D-gal-induced aging rats with high fat diet are shown. Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. *P < 0.05, **P < 0.01 vs Y-C, A-C, †P < 0.05 vs AHF. Magnification = ×400, Bar = 200 µm. Values are means ± SEM (n = 8).
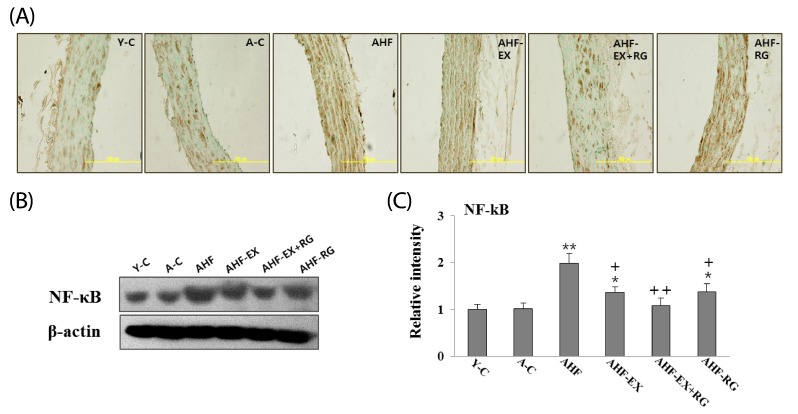
NF-kB in the aorta
As shown in Fig 3, NF-kB in aorta was performed using immunohistochemistry (A) and western blotting (B). Aortic concentration of NF-kB was increased in AHF group compared to control group, whereas EX and KRG were remarkably inhibited compared to AHF group. Immunohistochemical analysis yielded patterns similar to western blotting. NF-kB immunostaining was stronger in the AHF group compared to other groups. In contrast, EX and KRG were reduced in the aorta walls.
Fig. 3.
Immunohistochemical (A) and Western blotting (B) analysis of NF-kB expression. Densitometric analysis of Western blots (C) in aorta of D-gal-induced aging rats with high fat diet are shown. Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. *P < 0.05, **P < 0.01 vs Y-C, A-C, †P < 0.05, ††P < 0.01 vs AHF. Magnification = ×400, Bar = 200 µm. Values are means ± SEM (n = 8).
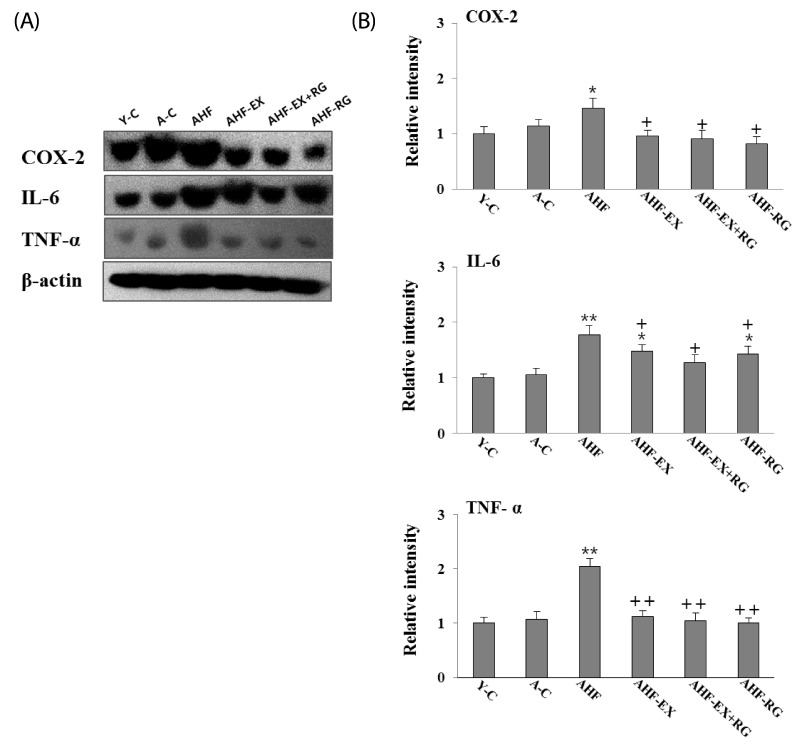
TNF-α, IL-6 and COX-2 in the aorta
Pro-inflammatory proteins NF-κβ, TNF-α, IL-6 and COX-2 in aorta are measured by western blotting. The TNF-α, IL-6 and COX-2 were significantly lowered (P < 0.05, P < 0.01) in AHF-EX, AHF-EX+RG and AHF-RG groups than in the AHF group (Fig. 4 A,B).
Fig. 4.
Western blotting analysis of TNF-α, IL-6 and COX-2 (A). Densitometric analyses of western blotting (B) in aorta of D-gal-induced aging rats with high fat diet are shown. Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. Values are means ± SEM (n = 8). *P < 0.05, **P < 0.01 vs Y-C, A-C, †P < 0.05, ††P < 0.01 vs AHF.
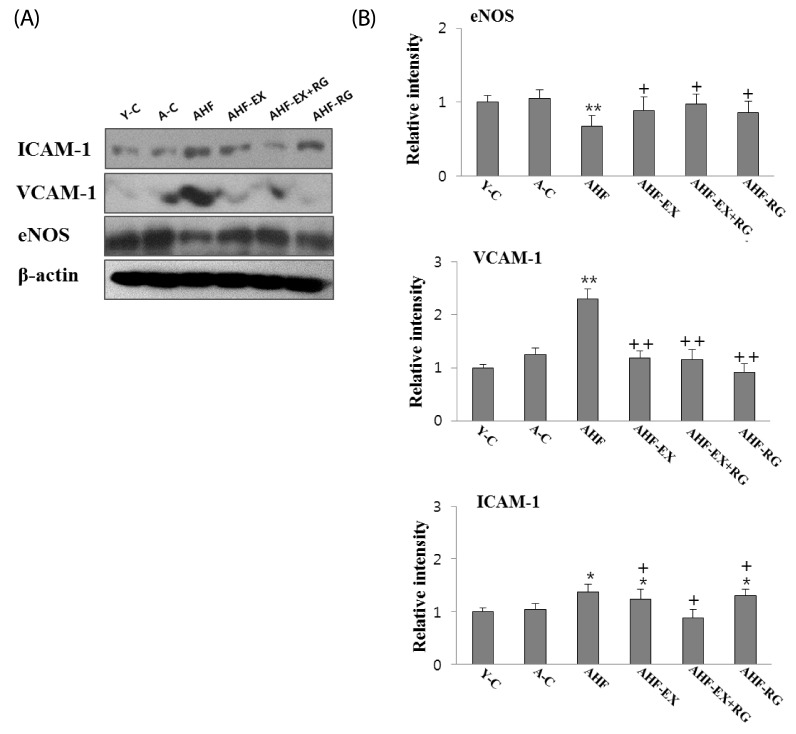
ICAM-1, VCAM-1 and eNOS in the aorta
Adhesion molecule protein ICAM-1 and VCAM-1 expressions in aorta are shown in Fig 5. The ICAM-1 and VCAM-1 were significantly lowered (P < 0.05, P < 0.01) in AHF-EX, AHF-EX+RG and AHF-RG groups than in the AHF group. In contrast, eNOS expression was significantly higher in the AHF-EX, AHF-EX+RG and AHF-RG groups than in the AHF group (Fig. 5A, B).
Fig. 5.
Western blotting analysis of ICAM-1, VCAM-1 and eNOS (A). The Densitometric analyses of western blotting (B) in aorta of D-gal-induced aging rats with high fat diet are shown. Y-C: Young control group, A-C: Aging control group, AHF: Aging with high fat diet group, AHF-EX: AHF with exercise training group, AHF-EX+RG: AHF-EX with Korean red ginseng, AHF-RG: AHF with Korean red ginseng. Values are means ± SEM (n = 8). *P < 0.05, **P < 0.01 vs Y-C, A-C, †P < 0.05, ††P < 0.01 vs AHF.
DISCUSSION
The major risk factors of atherosclerosis are associated with hyperlipidemia, inflammation and ROS with increasing age [4,31]. Many risk factors for the atherosclerotic vascular diseases can be treated physically or by life-style changes. The therapies for atherosclerosis are effective and protective against risk of myocardial infarction, stroke and CVD death [32]. The present study established a rat model of atherosclerosis by feeding with HFD and aging with D-gal. Chronic administration of D-gal significantly elevates ROS including oxidative stress and induces natural age related diseases such as diabetes mellitus, atherosclerosis, renal failure, and Alzheimer's in animals [26, 28]. Several studies have demonstrated that aging inducement by D-gal led to oxidative stress overproduction in tissue, Ca++ homeostasis damage, and mitochondria dysfunction [28, 33], glycation end products (AGE) [26]. These changes by long-term D-gal administration can be damaged the tissues and induced similarly to aging state. Aging not only promotes the development of atherosclerosis vascular disease through endothelial dysfunction, but also associated with significant metabolic changes, resulting in age-dependent increase of BW as well as changes in lipid metabolism [34].
Altered lipid metabolism both in cell and in plasma are constantly show in atherosclerosis patients [31]. In animal models, HFD is clearly associated with increased BW, levels of cholesterol, triglycerides, LDL, AI and reduce in HDL-C levels in aged HFD fed rats when compared with normal diet and an induced atherosclerosis [6,35]. This suggested that lipids are required for vascular disease onset and advancement of atherosclerosis already early during life [25] and AI is closely related to the occurrence of CVD including atherosclerosis [36]. Thus, in this study we used D-gal induced aging rat models with HFD induced natural atherosclerosis.
Although several studies have been tried to prevent atherosclerosis in rodent [6,37], physical EX and KRG administration are still unclear in aged atherosclerotic aorta. Therefore, we investigated the preventive effects of EX and KRG on lipid profile associated with age related atherosclerosis.
In the present study, treatment of EX and KRG reduced BW, plasma TC, TG, and LDL-C levels. Although there was no significant change in HDL-C of AHF-RG group, AHF-EX and AHF-EX+RG groups showed higher HDL-C level than AHF group. Interestingly, we found that combination of EX and KRG has more effective in controlling BW and lipid profiles than HFD groups. Particular HDL-C level was the highest in AHF-EX+RG group and AI was the lowest.
Regular exercise has prevented weight gain and fat weight as well as decrease fat deposition in animals and human [38,39,40] and also markedly improve the lipid profiles [39]. Recently, it was reported that the regular aerobic EX reduces blood serum TC, TG, and LDL-C and increase HDL-C in ovariectomized rats [41].
In addition, KRG is known to exert multiple efficacies on the cholesterol, hypertension and CVD [13,42] and obesity [16]. One of KRG major constituents is ginsenoside, which harbors wide range of biological activity has significant protective effect on and induced a decrease in plasma TG, LDL-C and liver levels in rat [43]. It has been reported that chronic KRG administration is increased energy expenditure by increasing adaptive thermogenesis and decreasing weight gain including lipid metabolism [16,14]. Lee et al. [14] also showed that KRG markedly reduced BW, serum TC, TG, and LDL-C in fatty rats [16]. Therefore, these results indicate that combined EX and KRG for 8 weeks may play a potential role in increasing HDL-C and have a synergy effect on serum lipid parameters.
Atherosclerosis is pivotal to vascular inflammatory processes, and monocytes/macrophages are critical participants. Among the inflammatory molecules, NF-κB is an important mediator in the vascular inflammatory process during aging and its associated with oxidative stress production or with age related disease [12] and suggest that activated mitochondrial oxidative stress leads to endothelial NF-κB activation, which changes the pro-inflammatory phenotype in aged blood vessels [44]. Studies also have shown NF-κB activation in advanced human atherosclerotic lesions [45] and early atherosclerotic plaques in mice [46]. In addition, studies suggest that activation of NF-κB accelerate the expression of pro-inflammatory cytokines like TNF-α, IL-6, and COX-2 [9], atherosclerosis progressive lipid accumulation lead to enhance in expression of pro-inflammatory cytokines and infiltration of inflammatory cells [47].
Adhesion molecules (VCAM-1 and ICAM-1) play important role in cellular interactions during inflammatory responses and these molecules were increased by a high-cholesterol diet in experimental animals [48]. Expression of VCAM-1 and ICAM-1 also may influence the organization of cells that promote the production of cytokines in inflammatory cells and is influenced by NF-kB, and expression of these in endothelium may represent a pathogenic mechanism or a phenotypic marker of predisposition to atherogenesis [48]. TNF-α, IL-6, and ICAM increases with aging, as do C-reactive protein (CRP) and inflammatory cell such as neutrophils and monocytes, and contributes to atherosclerosis [11,12,49]. These inflammatory cytokines are associated with activation of age related vascular dysfunction like atherosclerosis while NF-κB inhibition associated with amelioration of age-related inflammation.
Exercise contributes to the anti-inflammation effects associated with aging in mice [50] and human [20,21]. It also increases anti-oxidants enzyme to scavenge ROS [19] and reduces circulating concentrations of inflammatory proteins in elderly men [50]. These results reported that aerobics exercise in apo E-deficient mice can reverse macrophage accumulation and CD4+ cell accumulation within fatty streak lesions in aortic lumens [51], and suggested that macrophages may be the more important physiological change affecting vascular function in settings of aging and exercise [50].
Furthermore, KRG suppresses inflammatory responses in diabetes by remarkably reducing ICAM, VCAM, and oxidized LDL [52] and suggest that KRG has antioxidant therapeutic effects on superoxide dismutase inhibitor induced in pancreatitis by significantly inhibiting NF-kB, TNF-α and oxidant stress markers, 4-HNE [53], anti-thrombotic and anti-platelet activities on high risks of thrombotic and CVD in rat [13].
We have shown that alone or combination of EX and KRG supplements decreased serum CRP, 4-HNE, NF-kB, TNF-α, COX-2, IL-6, ICAM-1 and VCAM-1 in aorta of D-gal with HFD induced aging atherosclerosis rats. This suggests that inhibits CRP level, TNF-α, COX-2, IL-6, ICAM-1 and VCAM-1 are associated with decreased 4-HNE and NF-kB, in aorta, as previously indicated, implying that EX alone or KRG alone or combination of EX and KRG may play a potential role in inhibitive pro-inflammatory activations via the NF-kB signaling system by decreasing oxidative stress in the aorta of D-gal with HFD induced aging atherosclerosis rats.
NO is released in the endothelium, from which it diffuses both into the lumen and out into the wall of the vessel by eNOS [23], playing a key role in vasodilator and basal vascular tone and regulates blood flow [14]. eNOS is not only a vasodilator but also inhibits cell growth and inflammation [48]. Although NO and eNOS expression down-regulated with aging atherosclerosis [25], increasing NO and eNOS bioactivity can attenuated inflammation and subsequent disease progression and will have anti-inflammatory effects on aged atherosclerosis. The present finding of decreased serum NO revel and eNOS protein in D-gal with HFD induced aging atherosclerosis rats are in agreement with previous observations in rat [54] and suggesting a promontory role endothelial dysfunction and inflammation in aged obesity and atherosclerosis rat [25, 54].
Regular aerobics exercise is preservation of arterial function with aging in humans [55] and it also activate serum NO revel and eNOS expression in rat aorta [41]. There are several lines of evidence suggesting that the EX is closely related to the increased in the acetylcholine synthesis which induces shear stress and eNOS expression, increasing blood NO levels [41, 56]. Okabe et al. [51] reported that swimming training reduced atherosclerosis by antioxidant effects via the vascular NO system in apolipoprotein E deficient mice and it lode did not affect energy metabolism efficacies in the heart.
Furthmore, KRG administration improves NO and eNOS, has a anti-hypertensive effect due to induce an expansion of the blood vessels [57]. The data of present study further showed that regular Ex and KRG for 8 weeks remarkably increased serum NO levels and eNOS protein expression in aorta of D-gal with HFD-induced aging atherosclerosis rats, interestingly combination of EX and KRG supplementation produced highest NO levels and eNOS. It is a possible that regular EX and KRG may be synergistic effect in age associated atherosclerosis rats.
In summary, we have demonstrated that the improvement in plasma lipid profiles and inhibition of aortic inflammation and prolonged protective effects against the atherosclerosis development of D-gal induced aging rat models with HFD after EX and KRG supplementation may be due to (1) increased energy expenditure associated with loss of BW, (2) inhibited serum TC, TG, LDL-C, and AI by increased serum HDL-C levels, (3) inhibition of the inflammation pathway by serum CRP, NF-kB, TNF-α, IL-6, COX-2, ICAM-1, and VCAM-1 (4) decreased of the oxidative stress by reducing 4-HNE (5) enhanced aorta vasodilatation by increased NO and eNOS expression in aorta.
In conclusion, EX and KRG may be mediated in part via inhibition of pro-inflammation pathway and reversed age associated reduction vascular NO system. These anti-inflammatory actions may be a potentially useful role for the treatment of atherosclerosis and prevention of CVD.
Footnotes
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-358-2011-1-G00036).
References
- 1.Dice JF. Cellular and molecular mechanisms of aging. Physiol Rev. 1993;73:149–159. doi: 10.1152/physrev.1993.73.1.149. [DOI] [PubMed] [Google Scholar]
- 2.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Ma Z, Zhang J, Du R, Ji E, Chu L. Rho kinase inhibition by fasudil has anti-inflammatory effects in hypercholesterolemic rats. Biol Pharm Bull. 2011;34:1684–1689. doi: 10.1248/bpb.34.1684. [DOI] [PubMed] [Google Scholar]
- 6.Quan Y, Qian MZ. Effect and mechanism of gypenoside on the inflammatory molecular expression in high-fat induced atherosclerosis rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:403–406. [PubMed] [Google Scholar]
- 7.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung HY, Kim HJ, Kim KW, Choi JS, Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech. 2002;59:264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- 11.Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006;127:436–443. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, Yu BP. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 13.Jin YR, Yu JY, Lee JJ, You SH, Chung JH, Noh JY, Im JH, Han XH, Kim TJ, Shin KS, Wee JJ, Yun YP. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Cho HS, Park S, Kim WK. Regular exercise produced cardioprotective effects on rat's heart with hypertension induced by L-NAME administration. Clin Exp Hypertens. 2009;31:364–375. doi: 10.1080/10641960902977924. [DOI] [PubMed] [Google Scholar]
- 15.Kim ND. Pharmacologic effect of red ginseng. J Ginseng Res. 2001;25:2–10. [Google Scholar]
- 16.Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124–131. doi: 10.1254/jphs.fp0040184. [DOI] [PubMed] [Google Scholar]
- 17.Yun SN, Moon SJ, Ko SK, Im BO, Chung SH. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch Pharm Res. 2004;27:790–796. doi: 10.1007/BF02980150. [DOI] [PubMed] [Google Scholar]
- 18.Haddock BL, Marshak HP, Mason JJ, Blix G. The effect of hormone replacement therapy and exercise on cardiovascular disease risk factors in postmenopausal women. Sports Med. 2000;29:39–49. doi: 10.2165/00007256-200029010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Husain K. Physical conditioning modulates rat cardiac vascular endothelial growth factor gene expression in nitric oxide-deficient hypertension. Biochem Biophys Res Commun. 2004;320:1169–1174. doi: 10.1016/j.bbrc.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 20.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 21.Leaf DA. The effect of physical exercise on reverse cholesterol transport. Metabolism. 2003;52:950–957. doi: 10.1016/s0026-0495(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 22.Förstermann U, Boissel JP, Kleinert H. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 23.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 24.Tanner FC, Meier P, Greutert H, Champion C, Nabel EG, Lüscher TF. Nitric oxide modulates expression of cell cycle regulatory proteins: a cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation. 2000;101:1982–1989. doi: 10.1161/01.cir.101.16.1982. [DOI] [PubMed] [Google Scholar]
- 25.Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflugers Arch. 2010;460:825–837. doi: 10.1007/s00424-010-0860-y. [DOI] [PubMed] [Google Scholar]
- 26.Song X, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999;108:239–251. doi: 10.1016/s0047-6374(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 27.Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei M, Hua X, Xiao M, Ding J, Han Q, Hu G. Impairments of astrocytes are involved in the d-galactose-induced brain aging. Biochem Biophys Res Commun. 2008;369:1082–1087. doi: 10.1016/j.bbrc.2008.02.151. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Dawson TM, Dawson VL. Nitric oxide: actions and pathological roles. Neuroscientist. 1995;1:7–18. [Google Scholar]
- 31.Batetta B, Mulas MF, Petruzzo P, Putzolu M, Bonatesta RR, Sanna F, Cappai A, Brotzu G, Dessì S. Opposite pattern of MDR1 and caveolin-1 gene expression in human atherosclerotic lesions and proliferating human smooth muscle cells. Cell Mol Life Sci. 2001;58:1113–1120. doi: 10.1007/PL00000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Runge MS, Molnar K, Madamanchi NR. "Old" hearts and arteries: the role of oxidative stress. Trans Am Clin Climatol Assoc. 2010;121:52–58. [PMC free article] [PubMed] [Google Scholar]
- 33.Long J, Wang X, Gao H, Liu Z, Liu C, Miao M, Cui X, Packer L, Liu J. D-galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology. 2007;8:373–381. doi: 10.1007/s10522-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 34.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Lüscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- 35.Stranahan AM, Cutler RG, Button C, Telljohann R, Mattson MP. Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. J Neurochem. 2011;118:611–615. doi: 10.1111/j.1471-4159.2011.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez ML, Webb D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J Am Coll Nutr. 2008;27:1–5. doi: 10.1080/07315724.2008.10719668. [DOI] [PubMed] [Google Scholar]
- 37.Pang J, Xu Q, Xu X, Yin H, Xu R, Guo S, Hao W, Wang L, Chen C, Cao JM. Hexarelin suppresses high lipid diet and vitamin D3-induced atherosclerosis in the rat. Peptides. 2010;31:630–638. doi: 10.1016/j.peptides.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Mazzeo RS, Horvath SM. Effects of training on weight, food intake, and body composition in aging rats. Am J Clin Nutr. 1986;44:732–738. doi: 10.1093/ajcn/44.6.732. [DOI] [PubMed] [Google Scholar]
- 39.Motoyama M, Sunami Y, Kinoshita F, Irie T, Sasaki J, Arakawa K, Kiyonaga A, Tanaka H, Shindo M. The effects of long-term low intensity aerobic training and detraining on serum lipid and lipoprotein concentrations in elderly men and women. Eur J Appl Physiol Occup Physiol. 1995;70:126–131. doi: 10.1007/BF00361539. [DOI] [PubMed] [Google Scholar]
- 40.Shinoda M, Latour MG, Lavoie JM. Effects of physical training on body composition and organ weights in ovariectomized and hyperestrogenic rats. Int J Obes Relat Metab Disord. 2002;26:335–343. doi: 10.1038/sj.ijo.0801900. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Cho HS, Kim DY, Cho JY, Chung JS, Lee HK, Seong NH, Kim WK. Combined effects of exercise and soy isoflavone diet on paraoxonase, nitric oxide and aortic apoptosis in ovariectomized rats. Appetite. 2012;58:462–469. doi: 10.1016/j.appet.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Chen X. Experimental study on the cardiovascular effects of ginsenosides. Zhonghua Xin Xue Guan Bing Za Zhi. 1982;10:147–150. [PubMed] [Google Scholar]
- 43.Yamamoto M, Kumagai A, Yamamura Y. Plasma lipid-lowering action of ginseng saponins and mechanism of the action. Am J Chin Med. 1983;11:84–87. doi: 10.1142/S0192415X83000148. [DOI] [PubMed] [Google Scholar]
- 44.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol (1985) 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowalski J, Okopien B, Madej A, Makowiecka K, Zielinski M, Kalina Z, Herman ZS. Levels of sICAM-1, sVCAM-1 and MCP-1 in patients with hyperlipoproteinemia IIa and -IIb. Int J Clin Pharmacol Ther. 2001;39:48–52. doi: 10.5414/cpp39048. [DOI] [PubMed] [Google Scholar]
- 46.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 47.Ning Y, Bai Q, Lu H, Li X, Pandak WM, Zhao F, Chen S, Ren S, Yin L. Overexpression of mitochondrial cholesterol delivery protein, StAR, decreases intracellular lipids and inflammatory factors secretion in macrophages. Atherosclerosis. 2009;204:114–120. doi: 10.1016/j.atherosclerosis.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 49.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 50.Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 51.Okabe TA, Shimada K, Hattori M, Murayama T, Yokode M, Kita T, Kishimoto C. Swimming reduces the severity of atherosclerosis in apolipoprotein E deficient mice by antioxidant effects. Cardiovasc Res. 2007;74:537–545. doi: 10.1016/j.cardiores.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Kim HJ, Yoon KH, Kang MJ, Yim HW, Lee KS, Vuksan V, Sung MK. A six-month supplementation of mulberry, korean red ginseng, and banaba decreases biomarkers of systemic low-grade inflammation in subjects with impaired glucose tolerance and type 2 diabetes. Evid Based Complement Alternat Med. 2012;2012:735191. doi: 10.1155/2012/735191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joo KR, Shin HP, Cha JM, Nam S, Huh Y. Effect of Korean red ginseng on superoxide dismutase inhibitor-induced pancreatitis in rats: a histopathologic and immunohistochemical study. Pancreas. 2009;38:661–666. doi: 10.1097/MPA.0b013e3181a9eb85. [DOI] [PubMed] [Google Scholar]
- 54.Sena CM, Nunes E, Louro T, Proença T, Fernandes R, Boarder MR, Seiça RM. Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol. 2008;153:894–906. doi: 10.1038/sj.bjp.0707474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 56.Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 57.Jeon BH, Kim CS, Kim HS, Park JB, Nam KY, Chang SJ. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]