Abstract
Background
Epilepsy is a common chronic neurological disease with an estimated prevalence of 1% in the UK. Approximately one third of these people continue to have seizures despite drug treatment. In order to try to improve outcomes a number of new antiepileptic drugs have been developed and pregabalin is one of these. This review is an update of a previous Cochrane review (Lozsadi 2008).
Objectives
To summarise evidence from randomised controlled trials regarding the efficacy and tolerability of pregabalin when used as an add-on antiepileptic drug in treatment-resistant partial epilepsy.
Search methods
We searched the Cochrane Epilepsy Group Specialized Register (May 2012), CENTRAL (the Cochrane Central Register of Controlled Trials issue 5 of 12, The Cochrane Library 2012), MEDLINE (Ovid, 1946 to May week 4 2012) and contacted Pfizer Ltd. (the manufacturers of pregabalin) to identify published, unpublished and ongoing trials.
Selection criteria
We included randomised controlled double-blind trials comparing pregabalin with placebo for people with drug-refractory partial epilepsy. Outcomes included 50% or greater reduction in seizure frequency, seizure freedom, treatment withdrawal for any reason, treatment withdrawal for adverse events and nature of adverse events.
Data collection and analysis
Two review authors (JP and AGM) independently selected and assessed suitable trials and extracted data. Primary analyses were by intention-to-treat (ITT). Results are presented as risk ratios (RR) with 95% confidence intervals (CI).
Main results
Six suitable industry-sponsored trials (2009 participants) were identified and included in the analysis. Trials tested doses of pregabalin ranging from 50 mg/day to 600 mg/day. For the primary outcome, 50% or higher seizure reduction was significantly more likely in patients randomised to pregabalin than to placebo (RR 2.61; 95% CI 1.70 to 4.01). A dose-response analysis suggested increasing effect with increasing dose. Pregabalin was significantly associated with seizure freedom (RR 2.59; 95% CI 1.05 to 6.36). Patients were significantly more likely to have withdrawn from pregabalin treatment than placebo treatment for any reason (RR 1.39; 95% CI 1.13 to 1.72) or for adverse effects (RR 2.69; 95% CI 1.88 to 3.86). Ataxia, dizziness, somnolence and weight gain were significantly associated with pregabalin. The odds of response doubled with an increase in dose from 300 mg/day to 600 mg/day (OR 2.12; 95% CI 1.76 to 2.54).
Authors’ conclusions
Pregabalin, when used as an add-on drug for treatment-resistant partial epilepsy, is significantly more effective than placebo at achieving a 50% or greater seizure reduction and significantly increasing seizure freedom. Results demonstrate efficacy for doses from 150 mg/day to 600 mg/day, with increasing effectiveness at 600 mg doses. The trials included in this review were of short duration and longer-term trials are needed to inform clinical decision making better.
Medical Subject Headings (MesH): Anticonvulsants [adverse effects; *therapeutic use]; Drug Resistance; Epilepsies, Partial [*drug therapy]; Randomized Controlled Trials as Topic; gamma-Aminobutyric Acid [adverse effects; *analogs & derivatives; therapeutic use]
MeSH check words: Humans
BACKGROUND
Epilepsy is a common neurological chronic condition affecting up to 1% of the UK population (Hauser 1990). A single antiepileptic drug (AED) (monotherapy) will induce remission for the majority of those diagnosed. However, up to 30% of patients will fail to respond to monotherapy (Cockerell 1995). Their continuing attacks will result in reduced quality of life, and may also lead to injuries, social isolation and depression (Villeneuve 2004). This group poses a significant therapeutic problem, leading to the development of new AEDs as well as exploration of non-pharmacological treatment options, such as vagal nerve stimulation and epilepsy surgery.
Since the 1990s numerous new AEDs have become available, raising hope for more potent and better tolerated treatment of epilepsy. Pregabalin is one of these new compounds with antiepileptic, analgesic and anxiolytic (anxiety reducing) properties. It acts through binding to an auxiliary protein (alpha 2 delta) of the voltagegated calcium channels. It has been shown to reduce calcium influx into nerve terminals resulting in reduced presynaptic release of glutamate. In addition, by acting on AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors it indirectly reduces synaptic noradrenaline release (Fink 2002). These actions are thought to mediate its antiepileptic, anxiolytic and analgesic properties. Pregabalin has favourable pharmacokinetics; it is not protein bound, is 90% bioavailable and reaches peak plasma concentrations within 1.5 hours of administration of an oral dose. With repeated doses, a steady state is achieved within 24 to 48 hours. Furthermore, 90% of the drug is eliminated, unmetabolised, by the kidneys and it has no drug interactions (Brodie 2005). Pregabalin was launched onto the UK market in 2004 as an add-on AED for partial-onset seizures, as well as a treatment for neuropathic pain, and more recently (2006) as an anxiolytic.
Clinical trials published on the antiepileptic properties of pregabalin so far have focused on patients with refractory partialonset epilepsy. In these randomised placebo-controlled trials, patients are randomised to have either pregabalin or placebo added to their existing AED treatment. This is in keeping with international guidelines on the development of AEDs (ILAE Commission 1989). Once a drug has confirmed efficacy and safety as an add-on therapy, it can be tested as monotherapy. The use of pregabalin as monotherapy will be the subject of a future Cochrane review.
This review is an update of a previous Cochrane review and aims to summarise existing data regarding the effects of add-on pregabalin for patients with refractory partial-onset seizures. This review is one in a series in which the efficacy and tolerability of seven new AEDs (gabapentin, lamotrigine, tiagabine, topiramate, vigabatrin, zonisamide and pregabalin) are investigated in patients with drug-resistant partial epilepsy. Findings from the previous review showed significant seizure reduction in users of pregabalin compared to controls. Seizure freedom was not significantly associated with pregabalin. Pregabalin users were more likely to withdraw from the study than controls and significant adverse events associated with pregabalin were ataxia, dizziness, somnolence and weight gain.
OBJECTIVES
Our review aims to identify and evaluate data on the anticonvulsant effects and tolerability of add-on pregabalin in patients with drug-resistant partial epilepsy. The definitions of drug resistance used will be those employed by the authors of the included trials.
METHODS
Criteria for considering studies for this review
Types of studies
To be included in our review, studies had to meet all of the following criteria:
randomised controlled trials, in which an adequate method of randomisation and allocation concealment was used;
double-blind trials, in which both participant and clinician treating or assessing outcome were blinded to the treatment allocated;
placebo controlled; or
active controlled;
parallel group or cross-over studies.
Types of participants
People of any age with drug-resistant partial epilepsy (i.e. experiencing simple partial, complex partial or secondary generalised tonic-clonic seizures).
Types of interventions
The active treatment group received pregabalin in addition to an existing AED regimen taken at time of randomisation.
The control group received a matched placebo in addition to an existing AED regimen taken at time of randomisation.
The control group received an active control in addition to an existing AED regimen taken at time of randomisation.
Types of outcome measures
Primary outcomes
50% or greater reduction in seizure frequency
The proportion of people with a 50% or greater reduction in seizure frequency in the treatment period compared to the pre-randomisation baseline period was chosen as the primary outcome. It was chosen because it is a commonly reported outcome, and can be calculated for studies that do not report this outcome provided that baseline seizure data were recorded.
Secondary outcomes
Seizure freedom
The proportion of patients with a complete cessation of seizures during the treatment period.
Treatment withdrawal
The proportion of people having treatment withdrawn for any reason during the course of the treatment period was used as a measure of global effectiveness. Treatment is likely to be withdrawn due to adverse effects, lack of efficacy or a combination of both, and this is an outcome to which the individual makes a direct contribution. In trials of short duration, it is likely that adverse effects will be the most common reason for withdrawal. We have also assessed the proportion of people having treatment withdrawn for adverse effects.
Adverse effects
- The proportion of individuals experiencing the following five adverse effects:
- ataxia (co-ordination problems),
- dizziness,
- fatigue,
- nausea,
- somnolence (unusual drowsiness).
The proportion of individuals experiencing the five most common adverse effects mentioned in the included trials if these differed from those listed in (1) above.
Search methods for identification of studies
Electronic searches
We searched:
The Cochrane Epilepsy Group’s Specialized Register (31 May 2012) using the search term ‘pregabalin OR Lyrica’
the Cochrane Central Register of Controlled Trials (CENTRAL issue 5 of 12, The Cochrane Library, 2012) using the search strategy outlined in Appendix 1;
MEDLINE (Ovid, 1946 to May week 4, 2012) using the search strategy outlined in Appendix 2.
We did not impose any language restrictions.
Searching other resources
We reviewed the reference lists of retrieved studies to check for additional reports of relevant studies. We also contacted Pfizer Ltd. (manufacturers of pregabalin), and colleagues in the field.
Data collection and analysis
Two review authors (JP and AGM) independently assessed trials for inclusion. Any disagreements were resolved by mutual discussion.
The same two review authors extracted the following information from included trials. Again disagreements were resolved by mutual discussion.
Methodological/trial design
Method of randomisation and concealment.
Method of double blinding.
Whether any participants had been excluded from reported analyses.
Duration of baseline period.
Duration of treatment period.
Dose(s) of pregabalin tested.
Participant/demographic information
Total number of participants allocated to each treatment group.
Age/sex.
Number with partial/generalised epilepsy.
Seizure types.
Seizure frequency during the baseline period.
Number of background drugs.
For all trials sponsored by Pfizer Ltd. the following information was confirmed
The method of randomisation.
The total number randomised to each group.
The number of participants in each group achieving a 50% or greater reduction in seizure frequency per treatment group.
The number of participants having treatment withdrawn post randomisation per treatment group.
- For those excluded:
- the reason for exclusion,
- whether any of those excluded completed the treatment phase,
- whether any of those excluded had a 50% or greater reduction in seizure frequency during the treatment phase.
Outcomes
The number of participants experiencing each outcome (see Types of outcome measures) was recorded per randomised group.
Analysis
Initial analyses were undertaken using RevMan, the Cochrane Collaboration software package (RevMan 2011). Statistical heterogeneity was assessed using the I2 statistic with an I2 greater than 70% indicating heterogeneity. A Chi2 test for heterogeneity was also used with a P value < 0.10 providing evidence of heterogeneity. Provided no significant statistical heterogeneity was present, the analyses used a fixed-effect model using the Mantel-Haenszel method. Where statistical heterogeneity was present then randomeffects models are presented using Mantel-Haenszel methods. Results are presented as risk ratios (RR) with 95% confidence intervals (95% CI). For individual adverse effects 99% CI is quoted to make allowance for multiple testing.
All analyses included all participants in the treatment groups to which they had been allocated. For the efficacy outcome (50% or greater reduction in seizure frequency) three analyses were planned.
Primary (intention-to-treat) analysis - where participants not completing follow-up or with inadequate seizure data were assumed to be non-responders.
Worst case - where participants not completing follow-up or with inadequate seizure data were assumed to be non-responders in the pregabalin group and responders in the placebo group.
Best case - where participants not completing follow-up or with inadequate seizure data were assumed to be responders in the pregabalin group and non-responders in the placebo group.
Dose regression analysis
Dose-response analysis was evaluated using a generalised linear mixed model with the logit link function xtmelogit in STATA SE version 11. Study and dose were included as fixed effects and a random effect was included for the treatment (no random effect for the constant term) as described in Turner 2000. Dose was standardised by its standard deviation (277 mg). This method estimates an odds ratio (OR) as opposed to RR.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of studies awaiting classification.
Six randomised, controlled, parallel trials were identified; these had been sponsored by the company Pfizer Ltd. A total of 2009 participants were randomised into these studies. All six of the included studies recruited participants with treatment-resistant partial seizures. Participants were taking between one and four AEDs and had at least three partial seizures per month in the pre-randomisation baseline period. Further details are given below and in the table of included studies.
Arroyo et al (Arroyo 2004) published a multicentre (45 sites in Europe, Australia and Africa) parallel trial in 2004 on 288 patients. Inclusion criteria were defined as patients 18 years or older with partial seizures. As an electroencephalogram (EEG) was not required to confirm the above, some of the 18 patients included who were stated to have ‘generalised seizures’ rather than secondary generalised, may have had primary generalised epilepsy. Treatment arms included 99 patients on 50 mg pregabalin three times daily (TDS) and 92 patients on 200 mg pregabalin TDS, while 97 patients were randomised to placebo. After a baseline assessment of eight weeks the trial was conducted over 12 weeks (including titration period of four and eight days). During the trial period patients were assessed weekly for the first two weeks and fortnightly thereafter. Median follow-up was 12 weeks (range one day to 12 weeks). Three time points were reported in the study, each at four-weekly intervals. For further details see Characteristics of included studies as well as text below.
Baulac et al (Baulac 2010) conducted a multicentre (97 sites in Europe, Canada and Australia) parallel trial on 434 patients. Randomised patients were between 16 and 82 years of age and had undergone an EEG within two years prior to randomisation. Treatment arms were 150 mg to 300 mg pregabalin twice daily (BD) (n = 152), 150 mg to 300 mg lamotrigine BD (n = 141) and 141 patients were randomised to the placebo arm. Following a six-week baseline period, treatment was conducted over 12 weeks following a five-week titration phase (one week for pregabalin and five weeks for lamotrigine). The lamotrigine titration phase started four weeks prior to the pregabalin titration phase so all patients were started the treatment phase on the same dose. Patient review time points and follow-up were not reported.
Beydoun et al (Beydoun 2005) randomised three-hundred and thirteen patients aged 17 to 82 years from 43 US and Canadian centres. Parallel groups included 111 individuals on 200 mg pregabalin TDS, 104 on 300 mg pregabalin BD and 98 on placebo. After a baseline assessment of eight weeks the trial was conducted over 12 weeks (including a one-week titration period). Follow-up occurred on weeks 2, 4, 8 and 12. Median follow-up was 12 weeks (range not reported). During the trial an interim analysis was carried out on 129 patients. This led to alteration of the statistical analysis.
Elger et al (Elger 2005) reported a multicentre (53 sites in Canada and Europe) parallel trial of 341 patients aged 17 to 78 years. Treatment arms were 150 to 600 mg pregabalin (n = 131) titrated to clinical response and adverse effects in 150 mg daily increments and fixed-dose pregabalin of 300 mg DB (n = 137). A total of 73 people received placebo. Patients were randomised to one of the three treatments using a 1:2:2 ratio. The trial ran over 12 weeks and followed a six-week baseline period. Patients were reviewed at 2, 4, 8 and 12 weeks into the study. Median follow-up was 12 weeks and over 58% of patients completed the study in each arm. Range of follow-up was not reported.
French et al (French 2003) published a multicentre (76 sites in US and Canada) parallel trial including 455 patients. Randomised patients were between 12 and 70 years of age, but not all had EEG and imaging data. Those with absence seizures and Lennox-Gastaut syndrome were excluded; however, the inclusion of some patients with primary generalised epilepsy could not be ruled out. Participants were randomised into one of five treatment arms; 50 mg/day (n = 88), 150 mg/day (n = 88), 300 mg/day (n = 90) and 600 mg/day (n = 89) pregabalin in a DB regimen, and 100 patients were in the placebo group. Baseline assessment occurred over eight weeks and trial duration was 12 weeks with no titration period. Follow-up occurred on weeks 2, 4, 8 and 12. Median follow-up was 12 weeks (range one day to 12 weeks). Around 83% of patients completed the study.
Lee et al (Lee 2009) conducted a multicentre (9 sites in Korea) parallel trial consisting of two treatment arms. A total of 178 patients, aged 18 years and above, were randomised to either 75 mg to 300 mg pregabalin BD (n = 119) or placebo (n = 59) using a 2:1 ratio. Following a six-week baseline period, treatment was conducted over 12 weeks with a one-week taper period at the end. Patients were assessed at weeks 2, 4, 6, 8 and 12 with a follow-up visit at week 13. Eighty-eight per cent of patients randomised completed the study.
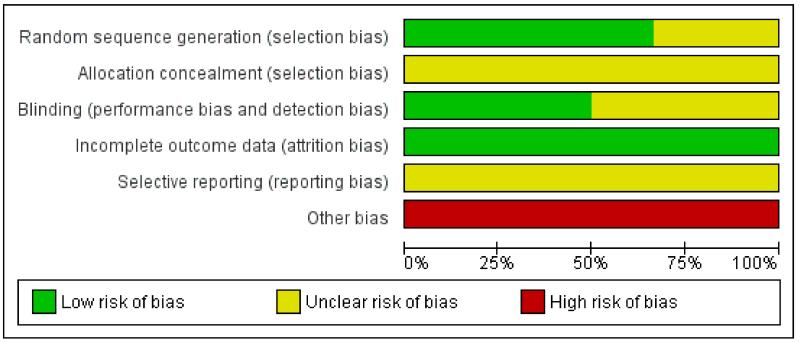
Risk of bias in included studies
All six included studies were subject to an assessment of bias on six domains. See ‘Risk of bias’ tables for each study for further details.
Randomisation: four of the six studies used an adequate method of allocation concealment by using computer-generated identification numbers and block sizes of five or six. Two of the studies did not provide details of method of randomisation (Baulac 2010; Lee 2009).
Concealment: methods employed to prevent foreknowledge of group assignment were not reported in any of the studies.
Blinding: all studies reported to be double blind and used identical tablets with identical packaging for all treatment groups. Further information regarding the blinding of key study personnel, participants and outcome assessors was not provided for all studies.
Missing data: all studies reported study attrition rates and all studies used an intention-to-treat analysis on randomised patients who took at least one dose of medication and using the last-observation-carried-forwards approach. That is, for participants failing to complete follow-up, seizure-frequency data were extrapolated from the last patient observation point for the whole treatment period, while for participants with no seizure data during the treatment period, baseline data were extrapolated. In the trial reports, a total of four patients were excluded from analyses, but these patients have been included in the denominator as non-responders for calculations in this review.
Outcome reporting: most of the included studies distinguished between the primary and secondary outcome variables. All studies reported their primary/secondary outcomes within the articles; however, no trial protocols were available for examination in order to compare reported outcomes.
Other bias: in two of the studies, patients with primary generalised epilepsy may have been included in the trials possibly leading to bias within the results (Arroyo 2004; French 2003). All studies were sponsored by the same pharmaceutical company indicating the possibility of reporting bias.
See Figure 1 for ‘Risk of bias’ graph.
Figure 1.
Effects of interventions
Intention-to-treat analysis
As mentioned above, the six trials included a total of four patients who were randomised (one to placebo and three to treatment) but not included in the analysis, as neither took first dose of trial medication. In our primary intention-to-treat analysis, these participants are included in the denominator and assumed to be non-responders.
Pregabalin versus placebo control
Primary outcome measure: 50% seizure reduction
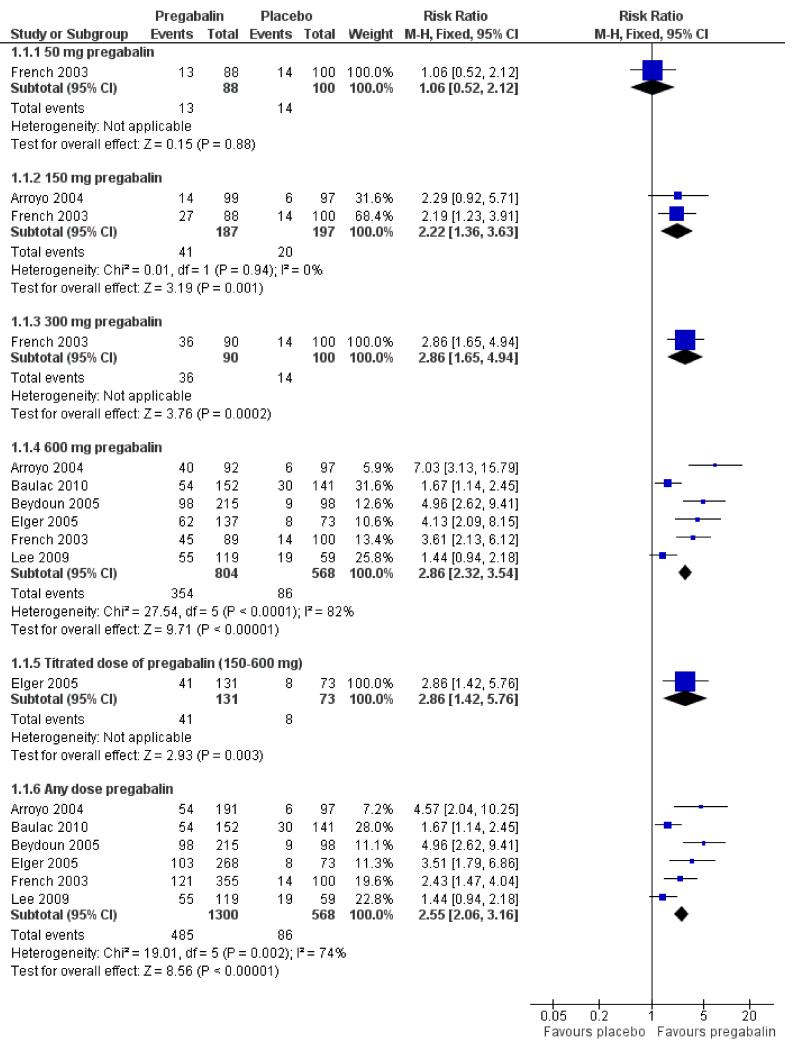
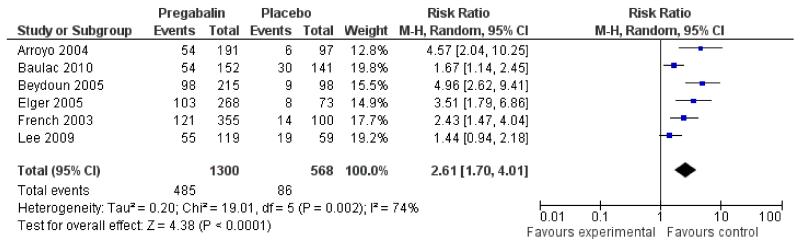
An intention-to-treat analysis pooling across doses (50 mg to 600 mg/day) (Analysis 1.1) shows evidence of heterogeneity (I2 74%). A sensitivity analysis showed two studies contributing largely to the heterogeneity (Baulac 2010; Lee 2009) therefore a random effects model was employed for this outcome. See Discussion for further details. Those participants allocated pregabalin were significantly more likely to achieve a 50% or greater reduction in seizure frequency (RR 2.61; 95% CI 1.70 to 4.01) (Figure 2; Figure 3). Subgroup analyses assessing the effect of individual doses show no significant effect for 50 mg pregabalin (RR 1.06; 95% CI 0.52 to 2.12). Higher doses of pregabalin were significantly associated with 50% or greater reduction in seizure frequency (150 mg: RR 2.22; 95% CI 1.36 to 3.63; 300 mg: RR 2.86; 95% CI 1.65 to 4.94; 600 mg: RR 2.86; 95% CI 2.32 to 3.54; titrated 150 to 600mg: RR 2.86; 95% CI 1.42 to 5.76) (Analysis 1.1; Analysis 1.2; Summary of findings for the main comparison).
Figure 2. Forest plot of comparison: 1 Pregabalin versus placebo: 50% seizure reduction - ITT, outcome: 1.1 50% responders, ITT.
Figure 3. Forest plot of comparison: 1 Pregabalin versus placebo: 50% seizure reduction - ITT, outcome: 1.2 50% responders (random effects).
A best-case analysis (all drop-outs assumed to be responders to treatment) pooling across doses (50 mg to 600 mg/day) showed participants allocated to pregabalin were significantly more likely to achieve a 50% or greater reduction in seizure frequency (RR 3.58; 95% CI 2.38 to 5.39) (Analysis 1.3; Analysis 1.4). Subgroup analyses assessing the effect of individual doses show significant effects for all pregabalin doses (50 mg: RR 1.87; 95% CI 1.03 to 3.40; 150 mg: RR 2.61; 95% CI 1.63 to 4.19; 300 mg: RR 4.37; 95% CI 2.61 to 7.29; 600 mg: RR 4.32; 95% CI 2.60 to 7.17; titrated 105 to 600mg: RR 5.02; 95% CI 2.56 to 9.82).
A worst-case analysis (all drop-outs assumed to be responders to control) pooling across doses (50 mg to 600 mg/day) showed no significant differences between pregabalin and placebo (RR 1.40; 95% CI 0.65 to 3.00) (Analysis 1.5). Subgroup analyses by dose showed one dose (50 mg) to be significantly associated with seizure reduction in the placebo group compared to pregabalin (RR 0.55; 95% CI 0.30 to 0.99). There were no significant differences between group for all other doses (150 mg: RR 1.32; 95% CI 0.88 to 1.96; 300 mg: RR 1.48; 95% CI 0.98 to 2.23; 600 mg: RR 1.64; 95% CI 0.78 to 3.44; titrated 150 to 600mg: RR 0.35; 95% CI 0.27 to 0.46).
Dose regression analysis for 50% response
We fitted a generalised linear mixed model (logit link) to estimate the effect of dose (details in Methods). This method estimates an OR as opposed to an RR. Dose was standardised by its standard deviation (277 mg). The odds of response (50% reduction in seizure frequency) more than doubled (OR 2.12; 95% CI 1.76 to 2.54 with estimated between-study standard deviation of 0.49 (standard error (SE) 0.22)) for each 279 mg increase in dose of pregabalin. This translates into an estimated doubling of odds of response with an increase in dose from 300 mg to 600 mg.
Secondary outcome measures
Seizure freedom
Three trials (Arroyo 2004; Beydoun 2005; French 2003) gave data for the proportion of patients free of seizures for the last eight weeks of the treatment phase, while a fourth trial (Elger 2005) gave the number free of seizures for the last 12 weeks. Baulac 2010 provided percentages of patients free of seizures over the 17-week study period and Lee 2009 gave proportions over the 12-week double-blind period and any 28-day period. An analysis pooling across doses (50 mg/day to 600 mg/day) showed no evidence of heterogeneity (I2 0%). Pregabalin was significantly associated with freedom from seizures (RR 2.59; 95% CI 1.05 to 6.36; Analysis 2.1).
Treatment withdrawal for any reason
An analysis pooling across doses (50 mg/day to 600 mg/day) showed no evidence of heterogeneity (I2 0%). Participants allocated pregabalin were significantly more likely to have withdrawn from treatment (RR 1.39; 95% CI 1.13 to 1.72; Analysis 3.1). Subgroup analyses assessing the individual doses showed no significant effect for 50 mg/day pregabalin (RR 0.87; 95% CI 0.40 to 1.89), 150 mg/day pregabalin (RR 0.72; 95% CI 0.41 to 1.28), 300 mg/day daily dose (RR 1.62; 95% CI 0.85 to 3.10) and 150 to 600 mg titrated dose of pregabalin (RR 1.02; 95% CI 0.61 to 1.71). The only dose associated with significant withdrawal for all reasons was 600 mg/day (RR 1.65; 95% CI 1.33 to 2.04) (Analysis 3.1).
Treatment withdrawal due to adverse effects
An analysis pooling across doses (50 mg/day to 600 mg/day) showed no evidence of heterogeneity (I2 0%). Participants allocated pregabalin were significantly more likely to withdraw from treatment for adverse effects (RR 2.69; 95% CI 1.88 to 3.86; Analysis 4.1). Subgroup analyses assessing treatment withdrawal with differing doses of pregabalin showed a higher withdrawal rate with higher doses (50 mg/day: RR 1.36; 95% CI 0.43 to 4.31; 150 mg/day: RR 1.02; 95% CI 0.45 to 2.32; 300 mg/day: RR 2.89; 95% CI 1.07 to 7.78; 600 mg/day: RR 3.39; 95% CI 2.36 to 4.87; Analysis 4.1). In Elger 2005, one treatment group had the dose titrated up to 150/day to 600 mg/day according to clinical response. The RR for withdrawal for this group was 1.78 (95% CI 0.68 to 4.67; Analysis 4.1).
Adverse events
In addition to the five pre-specified adverse effects, weight gain and headache were among the most common adverse effects reported. Analyses pooling across doses (50 mg/day to 600 mg/day) indicated that ataxia (RR 3.90; 99% CI 2.05 to 7.42; Analysis 5.1), dizziness (RR 3.06; 99% CI 2.16 to 4.34; Analysis 5.3), somnolence (RR 2.08; 99% CI 1.45 to 2.99; Analysis 5.5) and weight gain (RR 4.92; 99% CI 2.41 to 10.03; Analysis 5.6) were significantly associated with pregabalin, while fatigue (RR 1.28; 99% CI 0.88 to 1.86; Analysis 5.2), headache (RR 0.75; 99% CI 0.52 to 1.08; Analysis 5.7) and nausea (RR 1.14; 99% CI 0.51 to 2.53; Analysis 5.4) were not.
Pregabalin versus lamotrigine
Only one included study (Baulac 2010) compared pregabalin with an active control drug (lamotrigine), therefore the pooling of data could not be undertaken for this comparison.
Primary outcome measure: 50% seizure reduction
Within this study, participants allocated to pregabalin were significantly more likely to achieve a 50% or greater reduction in seizure frequency (RR 1.47; 95% CI 1.03 to 2.12) than those allocated lamotrigine (Analysis 6.1). A best-case analysis (all drop-outs assumed to be responders to treatment) revealed a significant increase in seizure reduction in favour of the pregabalin group (RR 2.73; 95% CI 1.99 to 3.74). A worst-case analysis (all drop-outs assumed to be responders to control) revealed a significant increase in seizure reduction in favour of the control group (RR 0.68; 95% CI 0.52 to 0.88) (Analysis 6.6).
Secondary outcome measures
Seizure freedom
No significant differences were found between pregabalin and lamotrigine for seizure frequency (RR 1.39; 95% CI 0.40 to 4.83; Analysis 6.2).
Treatment withdrawal for any reason
No significant differences were found between pregabalin and lamotrigine for withdrawal from the study due to any reason (RR 1.07; 95% CI 0.75 to 1.52; Analysis 6.3).
Treatment withdrawal due to adverse effects
No significant differences were found between pregabalin and lamotrigine for withdrawal from the study due to adverse events (RR 0.96; 95% CI 0.57 to 1.60; Analysis 6.4).
Adverse events
Within this study, patients allocated to pregabalin were significantly more likely to experience dizziness (RR 2.94; 99% CI 1.32 to 6.52) than patients allocated lamotrigine (Analysis 6.5). No other differences were found for the remaining adverse events between pregabalin and lamotrigine (ataxia: RR 1.72; 99% CI 0.54 to 5.55; fatigue: RR 1.72; 99% CI 0.77 to 3.83; somnolence: RR 1.99; 99% CI 0.91 to 4.33; weight gain: RR 4.33; 99% CI 0.86 to 21.68; headache: RR 0.52; 99% CI 0.26 to 1.05; (Analysis 6.5)).
DISCUSSION
This systematic review is one in a series of reviews analysing effects of new AEDs on treatment-resistant localisation-related epilepsy. We identified six industry-sponsored (Pfizer Ltd.) randomised placebo-controlled parallel trials. All studies were of good methodological quality and most were randomised using suitable sequence generation methods; all studies did not report methods of concealing allocation; all were reported to be double blinded and had few patients lost to follow-up. Summary trial data were taken from the relevant publications and individual patient data were not obtained. The approach to analysis for all of the included trials used the last-observation-carried-forwards approach. For participants failing to complete follow-up, seizure frequency data were extrapolated to the whole treatment period, while for participants with no seizure data during the treatment period, baseline data were extrapolated. While this approach may help minimise bias due to losses to follow-up (and is preferred by drug regulatory authorities), its use must be taken into consideration when interpreting the results of this systematic review.
The included studies tested doses ranging from 50 mg/day to 600 mg/day. The results showed that pregabalin, when used as add-on treatment, can reduce seizure frequency in individuals with treatment-resistant localisation-related epilepsy. In the analysis, when all doses of pregabalin were pooled, the RR for a 50% or greater reduction in seizure frequency was 2.61 (95% CI 1.70 to 4.01), thus demonstrating that out of 100 people with refractory epilepsy 40 are likely to have their seizures reduced when taking pregabalin compared to 15 out of 100 people not taking pregabalin. The large heterogeneity statistic associated with this result led to a sensitivity analysis that revealed two studies to be contributing largely. There were three main reasons for this: one study Lee 2009 was conducted in Korea and used a non-Western population sample and two studies, Baulac 2010 and Lee 2009, were conducted at later times and therefore treatment regimens may have been different from earlier therapies. Both of these trials had the 150 mg/day to 300 mg/dayTD and 75 mg/day to 300 mg/day TD treatment arms, therefore heterogeneity could also be due to the range of doses administered within the studies. Heterogeneity between studies was not found for any of the other outcomes.
A generalised linear mixed-model analysis showed evidence of a dose effect, suggesting that using pregabalin as an add-on treatment at 600 mg dose doubles the odds of reducing seizures compared to 300 mg. Participants taking pregabalin were also found to be more likely to be free of seizures than controls.
Participants were more likely to withdraw for any reason or because of adverse effects from pregabalin than from placebo. In both cases, treatment withdrawal was more likely on higher doses. Although the assessment of adverse events showed that ataxia, dizziness, somnolence and weight gain were significantly associated with pregabalin, these adverse events were not documented using a checklist or other systematic approach, but required physician reporting, so event rates may have been underestimated.
The one study (Baulac 2010) that examined the effectiveness of pregabalin in comparison to another add-on drug (lamotrigine) showed that pregabalin was more effective in reducing seizure frequency than lamotrigine. Participants taking pregabalin were no more likely to be free of seizures than participants taking lamotrigine. Participants taking pregabalin were not more likely to withdraw from the study than participants taking lamotrigine. This was found for both any reason for withdrawal and due to adverse events. Only dizziness was found to be significantly more prevalent when taking pregabalin.
In summary, pregabalin (150 mg/day to 600 mg/day) as an add-on drug can effectively reduce seizures by 50% or more in people with partial epilepsy. However, the addition of pregabalin doubled seizure freedomhad four significant adverse effects, ataxia, dizziness, somnolence and weight gain.
AUTHORS’ CONCLUSIONS
Implications for practice
In the short term (12 weeks) 150 mg/day to 600 mg/day of pregabalin given in a three times regimen can significantly reduce seizure frequency and significantly increase seizure freedom rates in people with treatment-resistant partial epilepsy. Adverse events significantly associated with pregabalin were ataxia, dizziness, somnolence and weight gain. There are currently no data regarding cost-effectiveness, longer-term effectiveness of pregabalin versus placebo and, more importantly, versus other adjunctive treatments.
Implications for research
To improve clinical decisions, further clinical trials are required in adults or children, or both with drug-refractory partial epilepsy. These trials should:
compare efficacy and tolerability of pregabalin with other adjunctive treatments;
be of long-term duration (at least 12 months);
assess seizure freedom rates, quality of life and health economic outcomes;
establish cost-effectiveness and compare it with that of other AEDs.
Further data are also necessary regarding pregnancy outcomes, which will require the recruitment of women taking pregabalin to ongoing pregnancy registries.
PLAIN LANGUAGE SUMMARY.
Pregabalin add-on for drug-resistant partial epilepsy
Use of pregabalin in combination with other antiepileptic drugs can reduce the frequency of seizures, but has some adverse effects.
Approximately one in 400 people have epileptic seizures that continue despite antiepileptic drug treatment. This review summarises data from six trials that included a total of 2009 participants. In addition to their usual antiepileptic drugs, participants were randomised to take pregabalin (an antiepileptic drug) or control drug. Results showed that patients taking pregabalin were two to three times more likely to reduce their seizure frequency by more than 50% than those taking placebo and two to three times more likely to increase seizure freedom over a 12-week interval. Pregabalin was shown to be effective across a range of doses (150 mg to 600 mg) with increasing effectiveness at higher doses. Side effects associated with pregabalin included co-ordination problems, dizziness, sleepiness, and weight gain. There are no data regarding the longer-term effectiveness of pregabalin; this should be investigated in future studies.
ACKNOWLEDGEMENTS
We acknowledge Dora Lozsadi for contributions made in the original review.
Appendix 1. CENTRAL search strategy
#1 (pregabalin or Lyrica)
#2 MeSH descriptor Epilepsy explode all trees
#3 MeSH descriptor Seizures explode all trees
#4 epilep* or seizure* or convulsion*
#5 (#2 OR #3 OR #4)
#6 (#1 AND #5)
Appendix 2. MEDLINE search strategy
The most recent version of this review used the following search strategy, which is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011).
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp animals/not humans.sh.
8 not 9
exp Epilepsy/
exp Seizures/
(epilep$ or seizure$ or convuls$).tw.
11 or 12 or 13
(pregabalin or lyrica).tw.
10 and 14 and 15
Earlier versions of this review employed the following search strategy. It was based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0, updated March 2011) (Higgins 2011).
randomized controlled trial.pt.
controlled clinical trial.pt.
exp Randomized Controlled Trials/
exp Random Allocation/
exp Double-Blind Method/
exp Single-Blind Method/
1 or 2 or 3 or 4 or 5 or 6
(animals not humans).sh.
7 not 8
clinical trial.pt.
Clinical Trial/
(clin$ adj trial$).ab,ti.
((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ab,ti.
exp PLACEBOS/
placebo$.ab,ti.
random$.ab,ti.
exp Research Design/
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
(animals not humans).sh.
18 not 19
9 or 20
epilep$.tw.
exp EPILEPSY/
seizure$.tw.
exp SEIZURES/
convulsion$.tw.
22 or 23 or 24 or 25 or 26
Lyrica.tw.
pregabalin.tw.
28 or 29
21 and 27 and 30
CHARACTERISTICS OF STUDIES
Characteristics of included studies
[ordered by study ID]
| Methods | Randomised double-blind placebo (PCB) controlled parallel multicentre (45 in Europe, Australia and South Africa) trial. 3 treatment arms: 1 PCB, 2 PGB. Patients randomised in blocks of 6, each allocated unique ID number. All patients received 2 capsules TDS but 2 capsule sizes were used (no further information available). Duration of baseline period: 8 weeks. 12-week treatment period included 4- to 8-day titration period | |
| Participants | Adults aged 17 to 73 years (mean 37 years), 50.5% male, all with treatment-resistant partial epilepsy. Patients were on 1 to 4 baseline AEDs. 344 patients screened, 288 patients randomised: 97 patients to PCB (mean baseline 28-day seizure frequency: 23. 5), 99 patients to 50 mg/day PGB TDS (mean baseline 28-day seizure frequency: 26.2) and 92 patients to 200 mg/day PGB TDS (mean baseline 28-day seizure frequency: 19. 3) | |
| Interventions | Group 1: PCB Group 2: PGB 50 mg TDS (4-day titration phase) Group 3: PGB 200 mg TDS (8-day titration phase) |
|
| Outcomes | Primary outcome: reduction in seizure frequency compared to baseline (response ratio) Secondary outcomes: responder rate, seizure freedom, change in seizure frequency, ad verse events |
|
| Notes | Study used capsules of 2 sizes, containing 25 mg PGB or PCB (size 1# = small capsules) ; and 100 mg PGB or PCB (size 4# = large capsules). It is stated that patients received 2 capsules TDS. One patient excluded from ITT in PCB arm, as failed to take study drugs | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random computer-generated code used stratified by centre using block size of 6 |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medication presented in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in methods section of paper were reported in the results; however, there was no protocol available to check a priori outcomes |
| Other bias | High risk | As an EEG was not required to confirm the above, some of the 18 patients included who were stated to have 'generalised seizures' rather than secondary generalised, may have had primary generalised epilepsy Sponsored by the same pharmaceutical company (Pfizer Ltd.) as with all the other included trials |
| Methods | Randomised double-blind PCB and active drug controlled parallel multicentre (97 in Europe, Canada and Australia) trial. 3 treatment arms: 1 PCB, 1 PGB and 1 LTG. Patients randomised to 1 of 3 treatment arms (no further information available). Duration of baseline period: 6 weeks. 12-week treatment period with 5-week titration phase before treatment (1 week of titration for PGB and 5 weeks of titration for LTG) | |
| Participants | Adults aged 16 to 82 years (mean 39.4 years), 48.5% male, all with treatment-resistant partial epilepsy confirmed by history and recent EEG. Patients were on 1 to 3 baseline AEDs. 546 patients screened, 434 patients randomised: 141 patients to PCB (mean baseline 28-day seizure frequency: 16.38), 152 patients to 150 mg to 300 mg PGB BD (mean baseline 28-day seizure frequency: 21.32), and 141 patients to 150 mg to 300 mg LTG BD (mean baseline 28-day seizure frequency: 21.80) | |
| Interventions | Group 1: PCB Group 2: PGB 150 mg to 300 mg BD (1-week titration phase) Group 3: LTG 150 mg to 300 mg BD (5-week titration phase) |
|
| Outcomes | Primary outcome: change in seizure frequency compared to baseline (response ratio) Secondary outcomes: responder rate, seizure freedom, adverse events |
|
| Notes | One patient randomised to the PCB group failed to take > 1 dose of medication, and therefore was excluded from ITT analysis. No information provided on methods of randomisation, concealment or blinding | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details of concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Same number of capsules administered per study day per group. No further details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT analysis employed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in methods section of paper were reported in the results; however, there was no protocol available to check a priori outcomes |
| Other bias | High risk | Sponsored by same pharmaceutical company (Pfizer Ltd.) as with all the other included trials |
| Methods | Randomised double-blind PCB controlled parallel multicentre (43 in USA and Canada) trial. 3 treatment arms: 1 PCB, 2 PGB. Patients randomised in blocks of 6, each allocated unique ID number. All patients received TDS regimen of blinded capsules (no further information available). Duration of baseline period: 8 weeks. 1-week titration period and 11-week treatment period | |
| Participants | Adults aged 17 to 82 years (mean 39.1 years), 50.2% male, all with treatment-resistant partial epilepsy confirmed by history and recent EEG. Patients were on 1 to 4 baseline AEDs. 378 patients screened, 313 patients randomised: 98 patients to PCB (mean baseline 28-day seizure frequency: 25.1), 104 patients to 300 mg PGB BD (mean baseline 28-day seizure frequency: 21.5), and 111 patients to 200 mg PGB TDS (mean baseline 28-day seizure frequency: 21.3) | |
| Interventions | Group 1: PCB Group 2: 300 mg PGB BD (1-week titration phase) Group 3: 200 mg PGB TDS (1-week titration phase) |
|
| Outcomes | Primary outcome: reduction in seizure frequency compared to baseline (response ratio) Secondary outcomes: responder rate, median percentage change in seizure frequency |
|
| Notes | One patient randomised to the 300 mg BD group failed to take tablets, and therefore was excluded from ITT analysis. Blinding was broken with 1 patient in the PCB arm when she became pregnant | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised in blocks of 6 and al located unique ID number |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients received identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT analysis employed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in methods section of paper were reported in the results; however, there was no protocol available to check a priori outcomes |
| Other bias | High risk | Sponsored by same pharmaceutical company (Pfizer Ltd.) as with all the other included trials |
| Methods | Randomised double-blind PCB controlled parallel multicentre (53 in Europe and Canada) trial. 3 treatment arms: 1 PCB, 2 PGB. Patients randomised in blocks of 5, each allocated unique ID number. All regimens mimicked control group using identical capsules (no further information available). Duration of baseline period: 6 weeks. 12-week treatment period | |
| Participants | Adults aged 18 to 78 years (mean 40.5 years), 49.9% male, all with treatment-resistant partial epilepsy confirmed by personal and family history as well as recent EEG. Patients were on 1 to 5 baseline AEDs. 400 patients screened, 341 patients randomised: 73 patients to PCB (median baseline 28-day seizure frequency: 8.7), 137 patients to 300 mg PGB BD fixed (median baseline 28-day seizure frequency: 10), and 131 patients to PGB flexible dosing (median baseline 28-day seizure frequency: 9.33) | |
| Interventions | Group 1: PCB Group 2: 300 mg PGB DB fixed dose Group 3: 75 mg to 300 mg PGB BD flexible titration at physician’s discretion |
|
| Outcomes | Primary outcome: reduction in seizure frequency compared to baseline (response ratio) Secondary outcomes: responder rate, median percentage change in seizure frequency and reduction of GTCS in those completing the study, adverse events |
|
| Notes | In PGB titration and PCB groups, patients were included with seizure frequency ofover 120 a day. Documenting seizures at this frequency is difficult and may be unreliable. Medium length of follow-up not reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised using a 1:2:2 ratio and block sizes of 5 |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study medication presented in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT analysis employed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in methods section of paper were reported in the results; however, there was no protocol available to check a priori outcomes |
| Other bias | High risk | Sponsored by same pharmaceutical company as with all the other included trials |
| Methods | Randomised double-blind PCB-controlled parallel multicentre (71 in the USA and 5 in Canada) trial. 5 treatment arms: 1 PCB, 4 PGB. Patients randomised in blocks of 5, each allocated unique ID number. Capsule sizes varied (no further information available). Duration of baseline period: 8 weeks. There was no titration; 12-week treatment period | |
| Participants | Patients 12 years and above (range 12 to 75 years, mean 38.4 years), 48.1% male, all with treatment-resistant partial epilepsy. Patients were on 1 to 4 baseline AEDs. 586 patients screened, 455 patients randomised: 100 patients to PCB (mean baseline seizure frequency: 22.3); 88 patients to 50 mg PGB (mean baseline seizure frequency: 27.4); 88 patients to 150 mg PGB (mean baseline 28-day seizure frequency: 23.1); 90 patients to 300 mg PGB (mean baseline 28-day seizure frequency: 19.1) and 89 patients to 600 mg PGB (mean baseline 28-day seizure frequency: 18.6) | |
| Interventions | Group 1: PCB Group 2: 50 mg PGB daily Group 3: 150 mg PGB Group 4: 300 mg PGB Group 5: 600 mg PGB daily |
|
| Outcomes | Primary outcome: reduction in seizure frequency compared to baseline (response ratio) Secondary outcomes: responder rate, pairwise comparisons with PCB, adverse events |
|
| Notes | Blinding broken for interim analysis (data obtained were only known to committee who were not involved in further running of study) and for 1 patients who developed visual field defect. 2 patients were excluded from ITT analysis (1 withdrew consent, 1 had AEDs changed during baseline period). Seizure frequency and responder rate were calculated from data collected from seizure diaries and mean calculated over a 4-week period | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer-generated randomised schedule using block sizes of 5 |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study medication presented in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT analysis employed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in methods section of paper were reported in the results; however, there was no protocol available to check a priori outcomes |
| Other bias | High risk | Possibility of the inclusion of patients with primary generalised epilepsy Sponsored by same pharmaceutical company as with all the other included trials |
| Methods | Randomised double-blind PCB-controlled parallel multicentre (9 in Korea) trial. 2 treatment arms: 1 PCB, 1 PGB. Patients randomised to 1 of 2 treatment arms (no further information available). Duration of baseline period: 6 weeks. 12-week treatment period (no further details provided) | |
| Participants | Patients 18 years and above (mean 34.2 years), 48.3% male, all with treatment-resistant partial epilepsy. Patients were on 1 to 3 baseline AEDs. 209 patients screened, 178 patients randomised: 59 patients to PCB (mean baseline 28-day seizure frequency: 13. 2) and 119 patients to 150 mg to 600 mg PGB (mean baseline 28-day seizure frequency 13.2) | |
| Interventions | Group 1: PCB Group 2: 150 mg/day to 600 mg/day |
|
| Outcomes | Primary outcome: change in seizure frequency (response ratio) Secondary outcomes: responder rate, seizure freedom, anxiety/depression, sleep, quality of life, adverse events |
|
| Notes | All randomised patients included in ITT analysis. No information provided on methods of randomisation, concealment or blinding | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients randomised using 2:1 ratio. No further information given |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT analysis employed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in methods section of paper were reported in the results; however, there was no protocol available to check a priori outcomes |
| Other bias | High risk | Sponsored by same pharmaceutical company (Pfizer Ltd.) as with all the other included trials |
AED: antiepileptic drug; BD: twice daily; EEG: electroencephalogram; GTCS: generalised tonic-clonic seizures; ITT: intention-to- treat analysis; LEV: levetiracetam; LTG: lamotrigine; PCB: placebo; PGB: pregabalin; TDS: three times a day
Characteristics of studies awaiting assessment
[ordered by study ID]
| Methods | Randomised observational controlled study. 4 treatment arms: 1 LEV fast rate, 2 LEV slow rate, 3 PGB fast rate, 4 PGB slow rate dosage |
| Participants | 128 patients with refractory partial epilepsy (32 in each treatment arm) |
| Interventions | Group 1: starting dose of 1000 mg BD LEV fast rate with weekly increments of 500 mg Group 2: starting dose of 500 mg BD LEV slow rate with weekly increments of 250 mg Group 3: starting dose of 300 mg BD PGB fast rate with weekly increments of 150 mg Group 4: starting dose of 150 mg BD PGB slow rate with weekly increments of 75 mg |
| Outcomes | Rate of withdrawals and continuation to maximum dose Incidence of adverse events |
| Notes | Study reported in abstract form only. Further details of study are unavailable |
| Methods | Randomised cross-over trial consisting of 2 treatment arms: 1 PGB, 2 LEV. Patients randomised to groups using 1:1 ratio. Study was open-label. Long-term study duration of minimum 6 months |
| Participants | 28 adults aged 19 to 62 years, 54% male. Patients currently taking different AED without maintaining good seizure control, stabilised to therapeutic association of valproate and lamotrigine |
| Interventions | Group 1: starting dose of 150 mg to target dose of 600 mg PGB Group 2: starting dose of 1000 mg to target dose of 3000 mg LEV |
| Outcomes | Seizure freedom, seizure reduction, withdrawals, adverse events |
| Notes | Study reported in abstract only. Further details of study are unavailable |
LEV: levetiracetam
DATA AND ANALYSES
Comparison 1. Pregabalin versus placebo: 50% seizure reduction - ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% responders, ITT | 6 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.52, 2.12] |
| 1.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 2.22 [1.36, 3.63] |
| 1.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 95% CI) | 2.86 [1.65, 4.94] |
| 1.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 95% CI) | 2.86 [2.32, 3.54] |
| 1.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 95% CI) | 2.86 [1.42, 5.76] |
| 1.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 95% CI) | 2.55 [2.06, 3.16] |
| 2 50% responders (random effects) | 6 | 1868 | Risk Ratio (M-H, Random, 95% CI) | 2.61 [1.70, 4.01] |
| 3 50% responders, Best Case Analysis | 6 | 4206 | Risk Ratio (M-H, Fixed, 95% CI) | 3.72 [3.27, 4.23] |
| 3.1 50mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 95% CI) | 1.87 [1.03, 3.40] |
| 3.2 150mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 2.61 [1.63, 4.19] |
| 3.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 95% CI) | 4.37 [2.61, 7.29] |
| 3.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 95% CI) | 4.09 [3.34, 5.01] |
| 3.5 Titrated dose of pregabalin (150-600mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 95% CI) | 5.02 [2.56, 9.82] |
| 3.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 95% CI) | 3.60 [2.93, 4.43] |
| 4 50% responders, Best Case Analysis Random Effects | 6 | 3240 | Risk Ratio (M-H, Random, 95% CI) | 1.50 [0.92, 2.45] |
| 4.1 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Random, 95% CI) | 1.64 [0.78, 3.44] |
| 4.2 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Random, 95% CI) | 1.40 [0.65, 3.00] |
| 5 50% responders, Worst Case Analysis | 6 | 4206 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.96, 1.14] |
| 5.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 95% CI) | 0.55 [0.30, 0.99] |
| 5.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 1.32 [0.88, 1.96] |
| 5.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [0.98, 2.23] |
| 5.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 95% CI) | 1.24 [1.08, 1.43] |
| 5.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.27, 0.46] |
| 5.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.94, 1.23] |
Comparison 2. Pregabalin versus placebo: seizure freedom - ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure freedom post titration | 6 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 95% CI) | 3.40 [0.14, 82.52] |
| 1.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.01, 7.92] |
| 1.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 600 mg pregabalin | 6 | 1880 | Risk Ratio (M-H, Fixed, 95% CI) | 2.45 [1.02, 5.91] |
| 1.5 Titrated dose of pregabalin (150-600mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 95% CI) | 5.05 [0.28, 92.42] |
| 1.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 95% CI) | 2.59 [1.05, 6.36] |
Comparison 3. Pregabalin versus placebo: treatment withdrawal (any reason) - ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment withdrawal any reason | 6 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.40, 1.89] |
| 1.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.41, 1.28] |
| 1.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 95% CI) | 1.62 [0.85, 3.10] |
| 1.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 95% CI) | 1.65 [1.33, 2.04] |
| 1.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.61, 1.71] |
| 1.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 95% CI) | 1.39 [1.13, 1.72] |
Comparison 4. Pregabalin versus placebo: treatment withdrawal (adverse events) - ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment withdrawal for adverse events | 6 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [0.43, 4.31] |
| 1.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.45, 2.32] |
| 1.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 95% CI) | 2.89 [1.07, 7.78] |
| 1.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 95% CI) | 3.39 [2.36, 4.87] |
| 1.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 95% CI) | 1.78 [0.68, 4.67] |
| 1.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 95% CI) | 2.69 [1.88, 3.86] |
Comparison 5. Pregabalin versus placebo: adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ataxia | 6 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 1.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 99% CI) | 1.14 [0.14, 9.00] |
| 1.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 99% CI) | 1.98 [0.56, 7.01] |
| 1.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 99% CI) | 3.33 [0.62, 17.81] |
| 1.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 99% CI) | 4.93 [2.56, 9.48] |
| 1.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 2.23 [0.44, 11.26] |
| 1.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 99% CI) | 3.90 [2.05, 7.42] |
| 2 Fatigue | 6 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 2.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 99% CI) | 0.71 [0.17, 2.94] |
| 2.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 99% CI) | 1.09 [0.50, 2.39] |
| 2.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 99% CI) | 1.53 [0.49, 4.76] |
| 2.4 600 mg pregabalin | 6 | 1503 | Risk Ratio (M-H, Fixed, 99% CI) | 1.32 [0.89, 1.95] |
| 2.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 1.23 [0.49, 3.04] |
| 2.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 99% CI) | 1.28 [0.88, 1.86] |
| 3 Dizziness | 6 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 3.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 99% CI) | 1.01 [0.31, 3.33] |
| 3.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 99% CI) | 2.04 [0.99, 4.22] |
| 3.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 99% CI) | 3.46 [1.39, 8.62] |
| 3.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 99% CI) | 3.57 [2.50, 5.09] |
| 3.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 2.97 [1.01, 8.77] |
| 3.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 99% CI) | 3.06 [2.16, 4.34] |
| 4 Nausea | 3 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 4.1 150 mg pregabalin | 1 | 196 | Risk Ratio (M-H, Fixed, 99% CI) | 1.31 [0.34, 5.00] |
| 4.2 600 mg pregabalin | 3 | 712 | Risk Ratio (M-H, Fixed, 99% CI) | 1.18 [0.51, 2.75] |
| 4.3 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 1.11 [0.12, 10.05] |
| 4.4 Any dose pregabalin | 3 | 942 | Risk Ratio (M-H, Fixed, 99% CI) | 1.14 [0.51, 2.53] |
| 5 Somnolence | 6 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 5.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 99% CI) | 0.93 [0.31, 2.78] |
| 5.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 99% CI) | 1.26 [0.58, 2.74] |
| 5.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 99% CI) | 1.62 [0.63, 4.12] |
| 5.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 99% CI) | 2.42 [1.68, 3.50] |
| 5.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 2.32 [0.77, 7.04] |
| 5.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 99% CI) | 2.08 [1.45, 2.99] |
| 6 Weight gain | 6 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 6.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 99% CI) | 3.40 [0.05, 224.69] |
| 6.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 99% CI) | 3.85 [0.64, 23.35] |
| 6.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 99% CI) | 14.43 [0.34, 620.87] |
| 6.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 99% CI) | 5.61 [2.74, 11.47] |
| 6.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 2.79 [0.84, 9.29] |
| 6.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 99% CI) | 4.92 [2.41, 10.03] |
| 7 Headache | 6 | Risk Ratio (M-H, Fixed, 99% CI) | Subtotals only | |
| 7.1 50 mg pregabalin | 1 | 188 | Risk Ratio (M-H, Fixed, 99% CI) | 0.52 [0.16, 1.77] |
| 7.2 150 mg pregabalin | 2 | 384 | Risk Ratio (M-H, Fixed, 99% CI) | 0.53 [0.24, 1.17] |
| 7.3 300 mg pregabalin | 1 | 190 | Risk Ratio (M-H, Fixed, 99% CI) | 0.43 [0.12, 1.57] |
| 7.4 600 mg pregabalin | 6 | 1372 | Risk Ratio (M-H, Fixed, 99% CI) | 0.77 [0.51, 1.15] |
| 7.5 Titrated dose of pregabalin (150-600 mg) | 1 | 204 | Risk Ratio (M-H, Fixed, 99% CI) | 1.25 [0.45, 3.50] |
| 7.6 Any dose pregabalin | 6 | 1868 | Risk Ratio (M-H, Fixed, 99% CI) | 0.75 [0.52, 1.08] |
Comparison 6. Pregabalin versus Lamotrigine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% responders, ITT | 1 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 1.47 [1.03, 2.12] |
| 2 Seizure freedom | 1 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 1.39 [0.40, 4.83] |
| 3 Treatment withdrawal (any reason) | 1 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 1.07 [0.75, 1.52] |
| 4 Treatment withdrawal (adverse events) | 1 | 304 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.57, 1.60] |
| 5 Adverse events | 1 | 1758 | Risk Ratio (M-H, Fixed, 99% CI) | 1.57 [1.12, 2.20] |
| 5.1 Ataxia | 1 | 293 | Risk Ratio (M-H, Fixed, 99% CI) | 1.72 [0.54, 5.55] |
| 5.2 Fatigue | 1 | 293 | Risk Ratio (M-H, Fixed, 99% CI) | 1.72 [0.77, 3.83] |
| 5.3 Dizziness | 1 | 293 | Risk Ratio (M-H, Fixed, 99% CI) | 2.94 [1.32, 6.52] |
| 5.4 Somnolence | 1 | 293 | Risk Ratio (M-H, Fixed, 99% CI) | 1.99 [0.91,4.33] |
| 5.5 Weight gain | 1 | 293 | Risk Ratio (M-H, Fixed, 99% CI) | 4.33 [0.86, 21.68] |
| 5.6 Headache | 1 | 293 | Risk Ratio (M-H, Fixed, 99% CI) | 0.52 [0.26, 1.05] |
| 6 50% responders, Best and Worst Case Analysis | 1 | 586 | Risk Ratio (M-H, Fixed, 95% CI) | 1.32 [1.09, 1.60] |
| 6.1 Best Case | 1 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 2.73 [1.99, 3.74] |
| 6.2 Worst Case | 1 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.52, 0.88] |
WHAT’S NEW
Last assessed as up-to-date: 12 June 2012.
| Date | Event | Description |
|---|---|---|
| 12 June 2012 | New search has been performed | Two new studies were included in this update of the original review |
HISTORY
Protocol first published: Issue 1, 2006
Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 7 August 2009 | Amended | Copyedits made at editorial base. |
| 16 September 2008 | Amended | Converted to new review format. |
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
Pregabalin versus placebo: 50% seizure reduction - ITT for drug-resistant partial epilepsy.
| Patient or population: patients with drug-resistant partial epilepsy | ||||||
|---|---|---|---|---|---|---|
| Settings: | ||||||
| Intervention: Pregabalin versus placebo: 50% seizure reduction - ITT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect
(95% CI) |
No of Participants
(studies) |
Quality of the evidence
(GRADE) |
Comments | |
|
|
||||||
| Assumed risk | Corresponding risk | |||||
|
|
||||||
| Control | Pre-gabalin versus placebo: 50% seizure reduction - ITT | |||||
|
| ||||||
| 50% responders, ITT - any dose pregabalin | 15 per 100 |
40 per 100
(26 to 61) |
RR 2.61
(1.7 to 4.01) |
1868 (6 studies) |
⊕⊕⊕○ moderate 1,1 |
|
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval;
RR: Risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
High heterogeneity statistic.
All studies sponsored by the same pharmaceutical company all reporting significant findings.
Footnotes
DECLARATIONS OF INTEREST
AM has been sponsored to attend a conference and has had research funding from Pfizer Ltd. Also a consortium of phramaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to University of Liverpool.
JP has no conflict of interest.
KH has acted as expert witness as a statistician in a number of legal cases including anti-epileptic drug cases.
SOURCES OF SUPPORT
- No sources of support supplied
- National Institute for Health Research, UK, Not specified.
This review presents independent research commissioned by the National Institute for Health Research (NIHR). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
Protocol for last up-to-date review was amended for this review to include interventions comparing pregabalin to other active AEDs.
The method of analysis for examining dose-regression has been changed for this review due to advances in techniques for analysis binary data. A generalised linear mixed model using the software package STATA SE version 11 was employed as opposed to the generalised linear model using Splus.
References to studies included in this review
- Arroyo S, Anhut H, Kugler AR, Lee CM, Knapp LE, Garofalo EA, et al. Pregabalin add-on treatment: a randomized, double-blind, placebo-controlled, dose-response study in adults with partial seizures. Epilepsia. 2004;45(1):20–7. doi: 10.1111/j.0013-9580.2004.31203.x. CN-00453016. [ published and unpublished data ] [DOI] [PubMed] [Google Scholar]
- Baulac M, Leon T, O’Brien T, Whalen E, Barrett J. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partialonset seizures. Epilepsy Research. 2010;91(1):10–9. doi: 10.1016/j.eplepsyres.2010.05.008. CN-00769723. [ published data only ] [DOI] [PubMed] [Google Scholar]
- Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA. Pregabalin 1008-009 Study Group. Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology. 2005;64(3):475–80. doi: 10.1212/01.WNL.0000150932.48688.BE. CN-00508438. [ *published and unpublished data ] [DOI] [PubMed] [Google Scholar]
- Elger CE, Brodie MJ, Anhut H, Lee CM, Barrett JA. Pregabalin add-on treatment in patients with partial seizures: a novel evaluation of flexible-dose and fixed-dose treatment in a double-blind, placebo-controlled study. Epilepsia. 2005;46(12):1926–36. doi: 10.1111/j.1528-1167.2005.00341.x. CN-00532656. [ published and unpublished data ] [DOI] [PubMed] [Google Scholar]
- French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology. 2003;60(10):1631–7. doi: 10.1212/01.wnl.0000068024.20285.65. CN-00431856. [ published and unpublished data ] [DOI] [PubMed] [Google Scholar]
- Lee BI, Yi S, Hong SB, Kim MK, Lee SA, Lee SK, et al. Pregabalin add-on therapy using a flexible, optimized dose schedule in refractory partial epilepsies: A double-blind, randomized, placebo-controlled, multicenter trial. Epilepsia. 2009;50(3):464–74. doi: 10.1111/j.1528-1167.2008.01954.x. CN-00688320. [ published data only ] [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study
References to studies awaiting assessment
- Russi A, Tarancon T. Comparative dosage adjustment of levetiracetam and pregabalin as adjunctive therapy for refractory partial epilepsy with polytherapy. A prospective observational randomized study. Epilepsia. 2006;47(Suppl 4):172. [ published data only ] [Google Scholar]
- Tata M, Guizzaro A, Daniele O, Natale E. Open label, randomised, cross-over trial in epilepsy patients with cryptogenic focal, refractory epilepsy, comparing levetiracetam and pregabalin as add-on therapy. Epilepsia. 2007;484(Suppl 7):69–70. [ published data only ] [Google Scholar]
Additional references
- Brodie MJ, Wilson EA, Wesche DL, Alvey CW, Randinitis EJ, Posvar EL, et al. Pregabalin drug interaction studies: lack of effect on the pharmacokinetics of carbamazepine, phenytoin, lamotrigine, and valproate in patients with partial epilepsy. Epilepsia. 2005;46:1407–13. doi: 10.1111/j.1528-1167.2005.19204.x. [DOI] [PubMed] [Google Scholar]
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346(8968):140–4. doi: 10.1016/s0140-6736(95)91208-8. [DOI] [PubMed] [Google Scholar]
- Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, et al. Inhibition of neuronal (Ca2+) influx by gabapentin and pregabalin in human neocortex. Neuropharmacology. 2002;42(2):229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences. Demos Publications; New York: 1990. [Google Scholar]
- The Cochrane Collaboration . In: Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011] Higgins JPT, Green S, editors. John Wiley & Sons, Ltd; Chichester, UK: 2011. [Google Scholar]
- Commission on Antiepileptic Drugs of the International League Against Epilepsy Guidelines for clinical evaluation of antiepileptic drugs. Epilepsia. 1989;30(4):400–8. [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J, The Cochrane Collaboration . Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org [Google Scholar]
- Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta-analysis of clinical trials with binary outcomes. Statistics in Medicine. 2000;19:3417–32. doi: 10.1002/1097-0258(20001230)19:24<3417::aid-sim614>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Villeneuve N. Quality-of-life scales for patients with drug-resistant partial epilepsy. Reviews of Neurology (Paris) 2004;160(Spec No.1):5S376–93. [PubMed] [Google Scholar]
References to other published versions of this review
- Lozsadi D, Hemming K, Marson AG. Pregabalin add-on for drug-resistant partial epilepsy. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD005612.pub2. DOI: 10.1002/14651858.CD005612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]